Biomechanical Study of Proximal Femur for Designing Stems for Total Hip Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Geometric Model
2.2. Finite Element Model
2.3. Boundary Conditions
2.4. Post-Processing
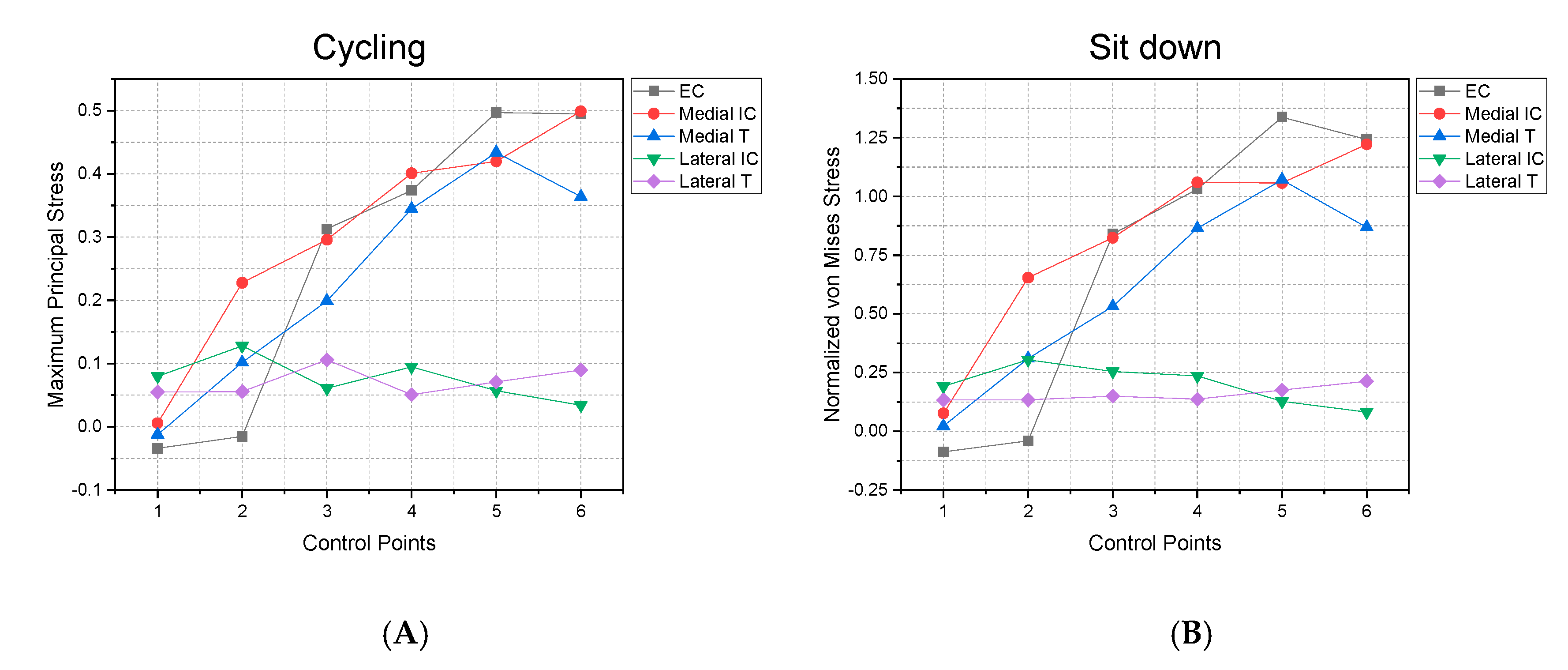
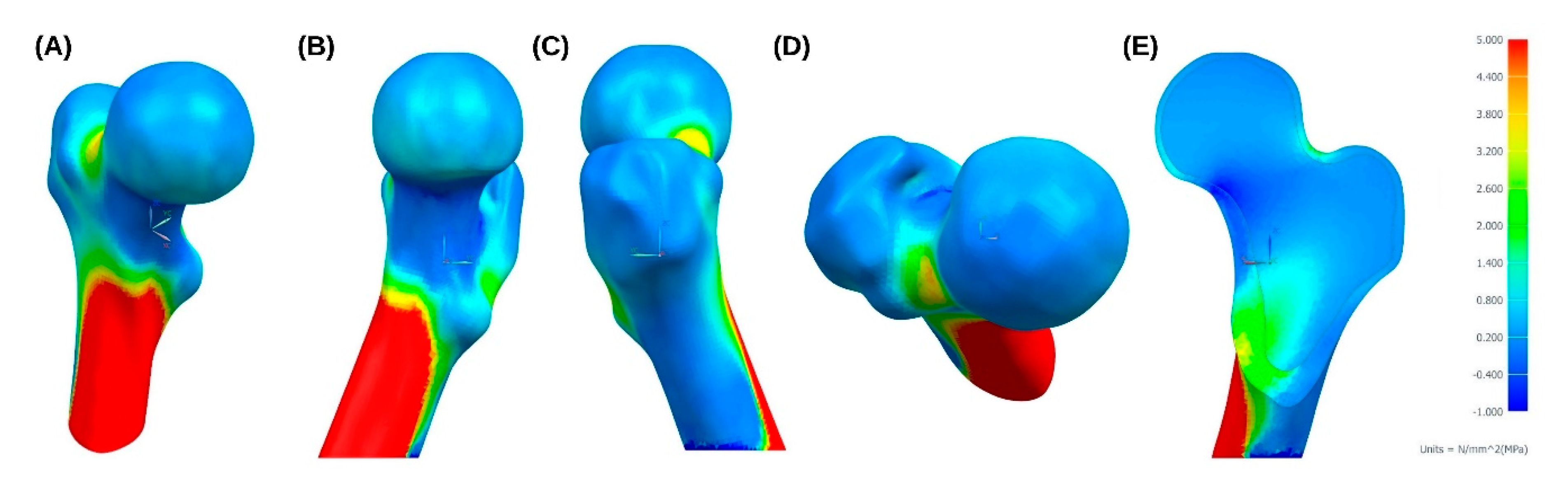
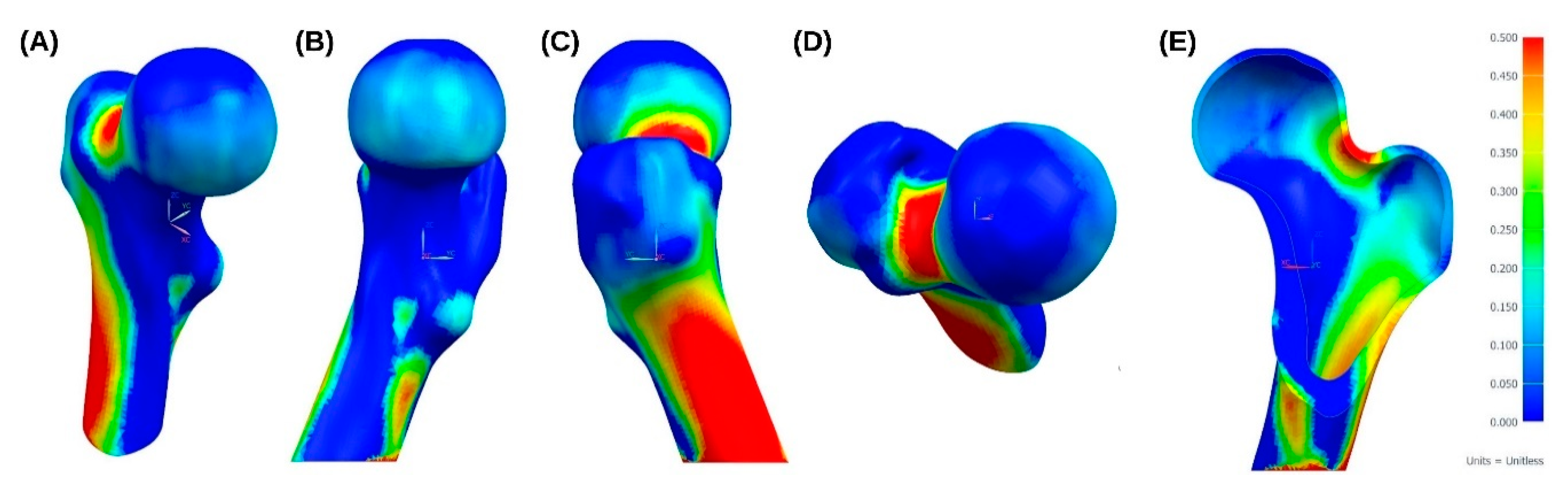
3. Results
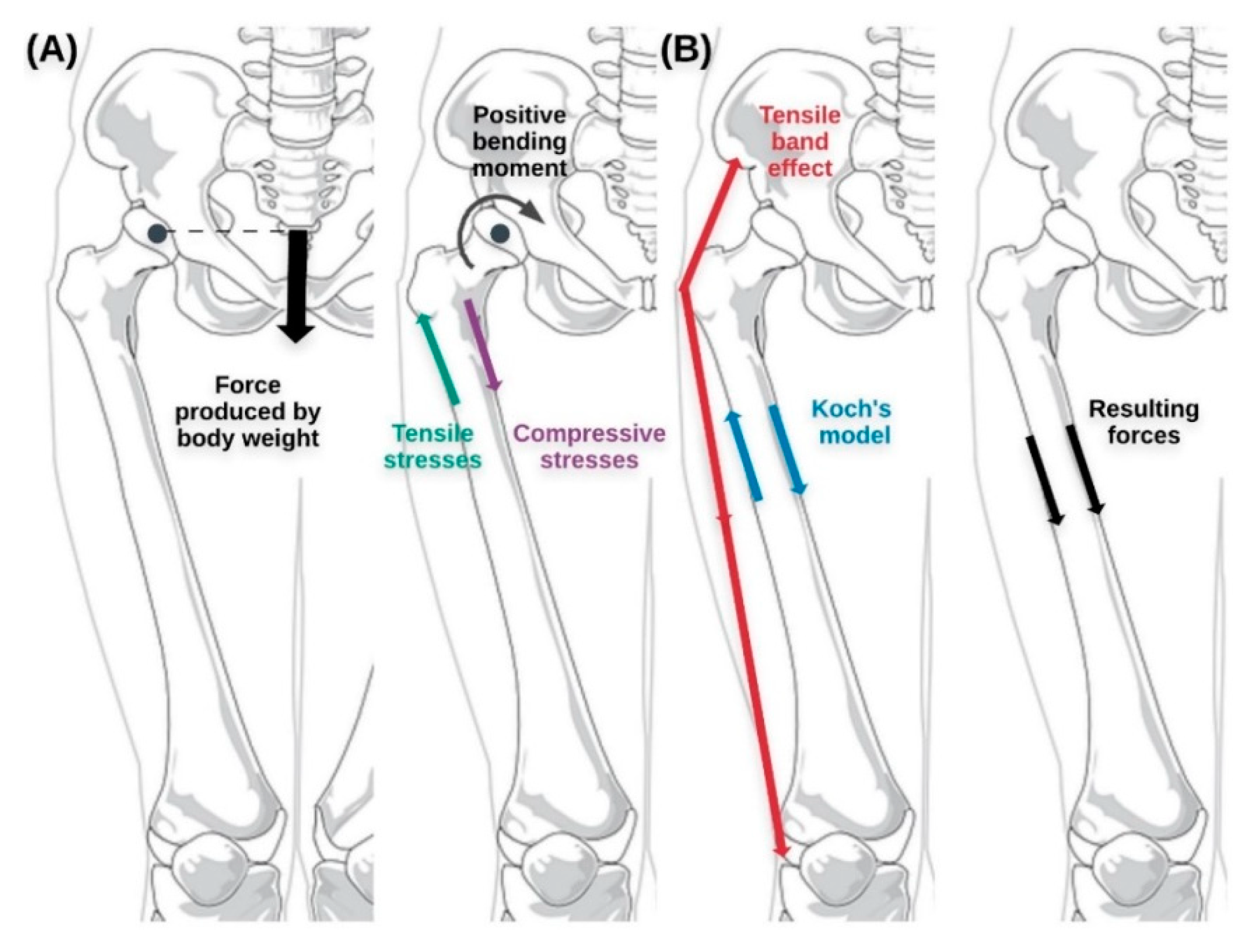
4. Discussion and Future Proposals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), 131–139. [Google Scholar] [CrossRef] [PubMed]
- Johannesdottir, F.; Bouxsein, M.L. Bone structure and biomechanics. In Encyclopedia of Endocrine Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–30. [Google Scholar]
- Taichman, R.S. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005, 105, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.K.; Thompson, W.R.; Warden, S.J. Bone biology. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–52. [Google Scholar]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ferrer Cañabate, J.; Tovar, I.; Martínez, P. Osteoprotegerina y Sistema RANKL/RANK: ¿el futuro fel metabolismo óseo? Anales de Medicina Interna 2002, 19, 5–8. [Google Scholar] [CrossRef][Green Version]
- Caetano-Lopes, J.; Canhão, H.; Fonseca, J.E. Osteoblasts and bone formation. Acta Reumatol. Port. 2007, 32, 103–110. [Google Scholar]
- Arboleya, L.; Castañeda, S. Osteoclastos: Mucho más que células remodeladoras del hueso. Rev. Osteoporos. Metab. Miner. 2014, 6, 109–121. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Raisz, L.G.; Martin, T.J. Principles of Bone Biology, 3rd ed.; Academic Press/Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Bozal, C.B. Osteocytes as mechanosensors in bone. Actual. Osteol. 2013, 9, 176–193. [Google Scholar]
- Doblaré, M.; García, J.M.; Gómez, M.J. Modelling bone tissue fracture and healing: A review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. Das Gesetz der Transformation der Knochen; Pro Business: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Skedros, J.G.; Brand, R.A. Biographical sketch: Georg hermann von meyer (1815–1892). Clin. Orthop. Relat. Res. 2011, 469, 3072–3076. [Google Scholar] [CrossRef]
- Hammer, A. The paradox of wolff’s theories. Ir. J. Med. Sci. 2015, 184, 13–22. [Google Scholar] [CrossRef]
- Koch, J.C. The laws of bone architecture. Am. J. Anat. 1917, 21, 177–298. [Google Scholar] [CrossRef]
- Rybicki, E.F.; Simonen, F.A.; Weis, E.B. On the mathematical analysis of stress in the human femur. J. Biomech. 1972, 5, 203–215. [Google Scholar] [CrossRef]
- Fetto, J.F.; Bettinger, P.; Austin, K. Reexamination of hip biomechanics during unilateral stance. Am. J. Orthop. 1995, 24, 605–612. [Google Scholar]
- Santori, N.; Lucidi, M.; Santori, F.S. Proximal load transfer with a stemless uncemented femoral implant. J. Orthopaed. Traumatol. 2006, 7, 154–160. [Google Scholar] [CrossRef]
- Rho, J.Y.; Hobatho, M.C.; Ashman, R.B. Relations of mechanical properties to density and CT numbers in human bone. Med. Eng. Phys. 1995, 17, 347–355. [Google Scholar] [CrossRef]
- Keyak, J.H.; Kaneko, T.S.; Tehranzadeh, J.; Skinner, H.B. Predicting proximal femoral strength using structural engineering models. Clin. Orthop. Relat. Res. 2005, 437, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Pithioux, M. Lois de Comportement et Modèles de Rupture des os Longs en Accidentologie. Ph.D. Thesis, Université de la Méditerranée, Marseille, France, 2000. [Google Scholar]
- Pithioux, M.; Lasaygues, P.; Chabrand, P. An Alternative ultrasonic method for measuring the elastic properties of cortical bone. J. Biomech. 2002, 35, 961–968. [Google Scholar] [CrossRef]
- Peng, L.; Bai, J.; Zeng, X.; Zhou, Y. Comparison of isotropic and orthotropic material property assignments on femoral finite element models under two loading conditions. Med. Eng. Phys. 2006, 28, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tellache, M.; Pithioux, M.; Chabrand, P.; Hochard, C. Femoral neck fracture prediction by anisotropic yield criteria. Eur. J. Comput. Mech. 2009, 18, 33–41. [Google Scholar] [CrossRef][Green Version]
- Bergmann, G.; Bender, A.; Dymke, J.; Duda, G.; Damm, P. Standardized loads acting in hip implants. PLoS ONE 2016, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, I.; Chandraphak, S.; Mahakkanukrauh, P. Femoral neck-shaft angle in humans: Variation relating to climate, clothing, lifestyle, sex, age and side. J. Anat. 2013, 223, 133–151. [Google Scholar] [CrossRef]
- Mu Jung, J.; Sang Kim, C. Analysis of stress distribution around total hip stems custom-designed for the standardized Asian femur configuration. Biotechnol. Biotechnol. Equip. 2014, 28, 525–532. [Google Scholar] [CrossRef]
- Rabbani, M.; Saidpour, H. stress analysis of a total hip replacement subjected to realistic loading conditions. J. Robot. Mech. Eng. Res. 2015, 1, 18–23. [Google Scholar]
- Schileo, E.; Taddei, F.; Cristofolini, L.; Viceconti, M. Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J. Biomech. 2008, 41, 356–367. [Google Scholar] [CrossRef]
- Ojeda, C. Estudio de la Influencia de Estabilidad Primaria en el Diseño de Vástagos de Prótesis Femorales Personalizadas: Aplicación a Paciente Específico. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2009. [Google Scholar]
- Cheruvu, B.; Venkatarayappa, I.; Goswami, T. Stress shielding in cemented hip implants assessed from computed tomography. Biomed. J. Sci. Tech. Res. 2019, 18, 13637–13641. [Google Scholar]
- Gargiulo, P.; Gislason, M.K.; Edmunds, K.J.; Pitocchi, J.; Carraro, U.; Esposito, L.; Fraldi, M.; Bifulco, P.; Cesarelli, M.; Jónsson, H. CT-based bone and muscle assessment in normal and pathological conditions. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–134. [Google Scholar]
- Yao, Z.; Lin, T.-H.; Pajarinen, J.; Sato, T.; Goodman, S. Host response to orthopedic implants (metals and plastics). In Host Response to Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 315–373. [Google Scholar]
- Cooper, R.R. The Scientific Basis of Joint Replacement. JAMA 1977, 238, 2731. [Google Scholar] [CrossRef]
- Morales de Cano, J.; Vergara, P.; Valero, J.; Clos, R. Utilización de los vástagos metafisarios «Próxima» DePuy: Nuestra experiencia a más de cinco años. Acta Ortopédica Mexicana 2018, 32, 88–92. [Google Scholar] [PubMed]
- Shin, Y.-S.; Suh, D.-H.; Park, J.-H.; Kim, J.-L.; Han, S.-B. Comparison of specific femoral short stems and conventional-length stems in primary cementless total hip arthroplasty. Orthopedics 2016, 39, 311–317. [Google Scholar] [CrossRef] [PubMed]















| Properties | Cortical Bone | Trabecular Bone |
|---|---|---|
| (MPa) | 9753.3 | 3969.8 |
| (MPa) | 9753.3 | 3969.8 |
| (MPa) | 16255.6 | 6616.3 |
| (MPa) | 2835.7 | 2835.7 |
| (MPa) | 4063.9 | 4063.9 |
| (MPa) | 4063.9 | 4063.9 |
| 0.4 | 0.4 | |
| 0.25 | 0.25 | |
| 0.25 | 0.25 |
| Yield Limits (MPa) | Cortical Bone |
|---|---|
| 35.5 | |
| , | 21.3 |
| 71.1 | |
| , | 42.6 |
| 10.7 | |
| 17.8 |
| Tsai–Wu Coefficients | |
|---|---|
| (mm2/N) | 0.0141 |
| (mm2/N) | 0.0235 |
| (mm4/N2) | 3.96 × 10−4 |
| (mm4/N2) | 1.1 × 10−3 |
| (mm4/N2) | 3.168 × 10−3 |
| (mm4/N2) | 8.8 × 10−3 |
| Cycling | Sitting Down | Standing Up | Walking | Staying | Stairs Up | Knee Bending | Stairs Down | Jogging | ISO * Force | |
|---|---|---|---|---|---|---|---|---|---|---|
| −FX (N) | 299.5 | 714.4 | 1125.1 | 596.8 | 681.6 | 829.9 | 857 | 773.6 | 884.8 | - |
| FY (N) | −41.4 | −62.7 | 49.9 | 17.1 | −35.7 | −48.5 | −37.1 | −55.6 | −15 | - |
| −FZ (N) | 805.7 | 1931.5 | 2481.2 | 1931.5 | 2280.2 | 2763.6 | 2054.9 | 2611 | 3222 | 2300 |
| Cycling | Sitting Down | Standing Up | Walking | Staying | Stairs Up | Knee Bending | Stairs Down | Jogging | ISO Force | |
|---|---|---|---|---|---|---|---|---|---|---|
| Max. MPS (MPa) | 6.09 | 14.51 | 27.67 | 13.96 | 14.29 | 17.26 | 20 | 15.77 | 20.25 | 42 |
| Min. MPS (MPa) | −11.37 | −27.82 | −46.81 | −25.57 | −27.87 | −33.78 | −34.18 | −31.27 | −37.31 | −23.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solórzano, W.; Ojeda, C.; Diaz Lantada, A. Biomechanical Study of Proximal Femur for Designing Stems for Total Hip Replacement. Appl. Sci. 2020, 10, 4208. https://doi.org/10.3390/app10124208
Solórzano W, Ojeda C, Diaz Lantada A. Biomechanical Study of Proximal Femur for Designing Stems for Total Hip Replacement. Applied Sciences. 2020; 10(12):4208. https://doi.org/10.3390/app10124208
Chicago/Turabian StyleSolórzano, William, Carlos Ojeda, and Andres Diaz Lantada. 2020. "Biomechanical Study of Proximal Femur for Designing Stems for Total Hip Replacement" Applied Sciences 10, no. 12: 4208. https://doi.org/10.3390/app10124208
APA StyleSolórzano, W., Ojeda, C., & Diaz Lantada, A. (2020). Biomechanical Study of Proximal Femur for Designing Stems for Total Hip Replacement. Applied Sciences, 10(12), 4208. https://doi.org/10.3390/app10124208






