Biodegradation of Total Petroleum Hydrocarbons in Soil: Isolation and Characterization of Bacterial Strains from Oil Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Bacterial Isolation and Incubation
2.2. Optimization of Bacterial Growth Conditions
2.3. Biodegradation of TPH in the Soil
2.4. Identification of the Isolated Strains
3. Results and Discussion
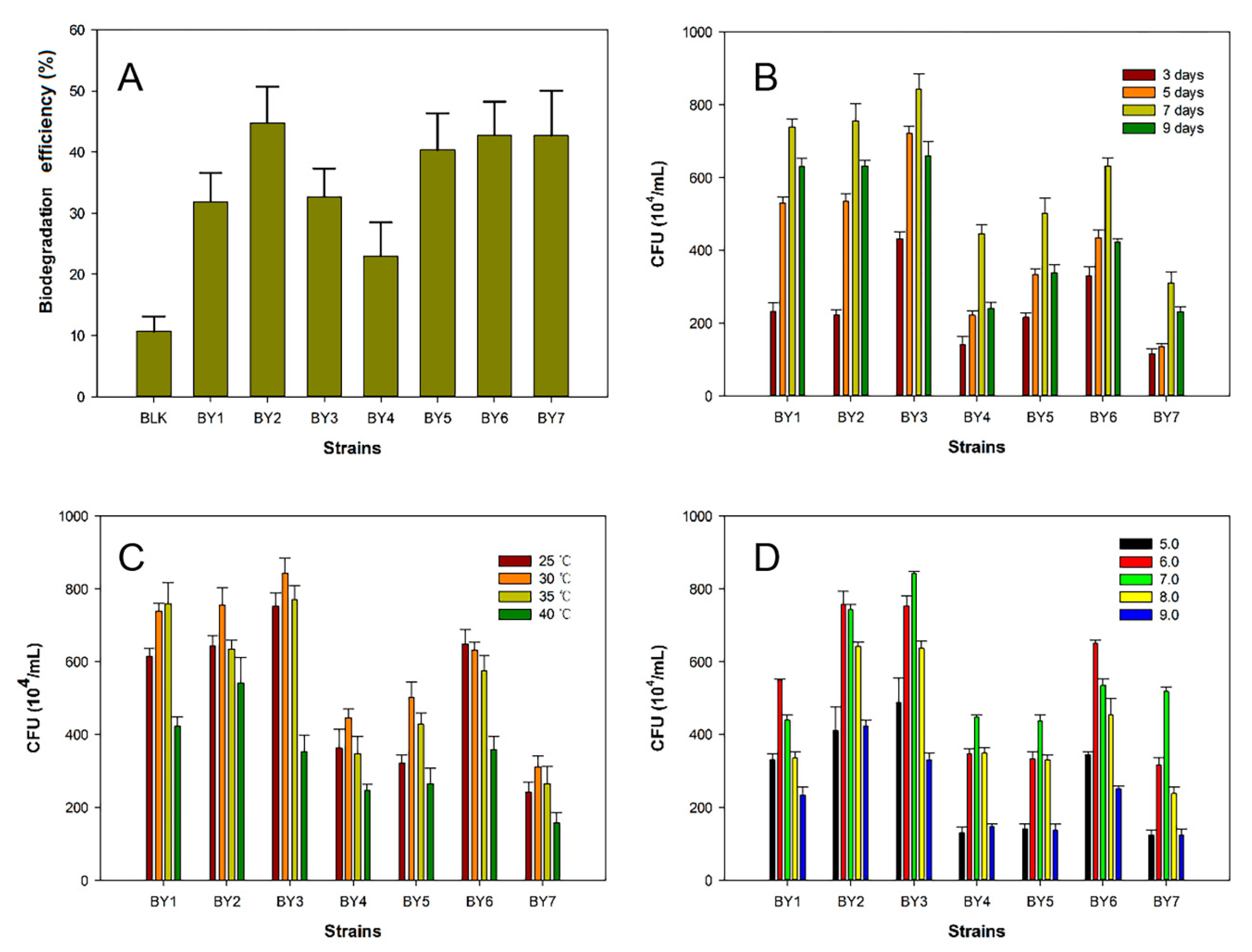
3.1. Biodegradation Efficiency of the Isolated Strains in BP Medium
3.2. Optimization of Growth Conditions of the Isolated Strains in BP Medium
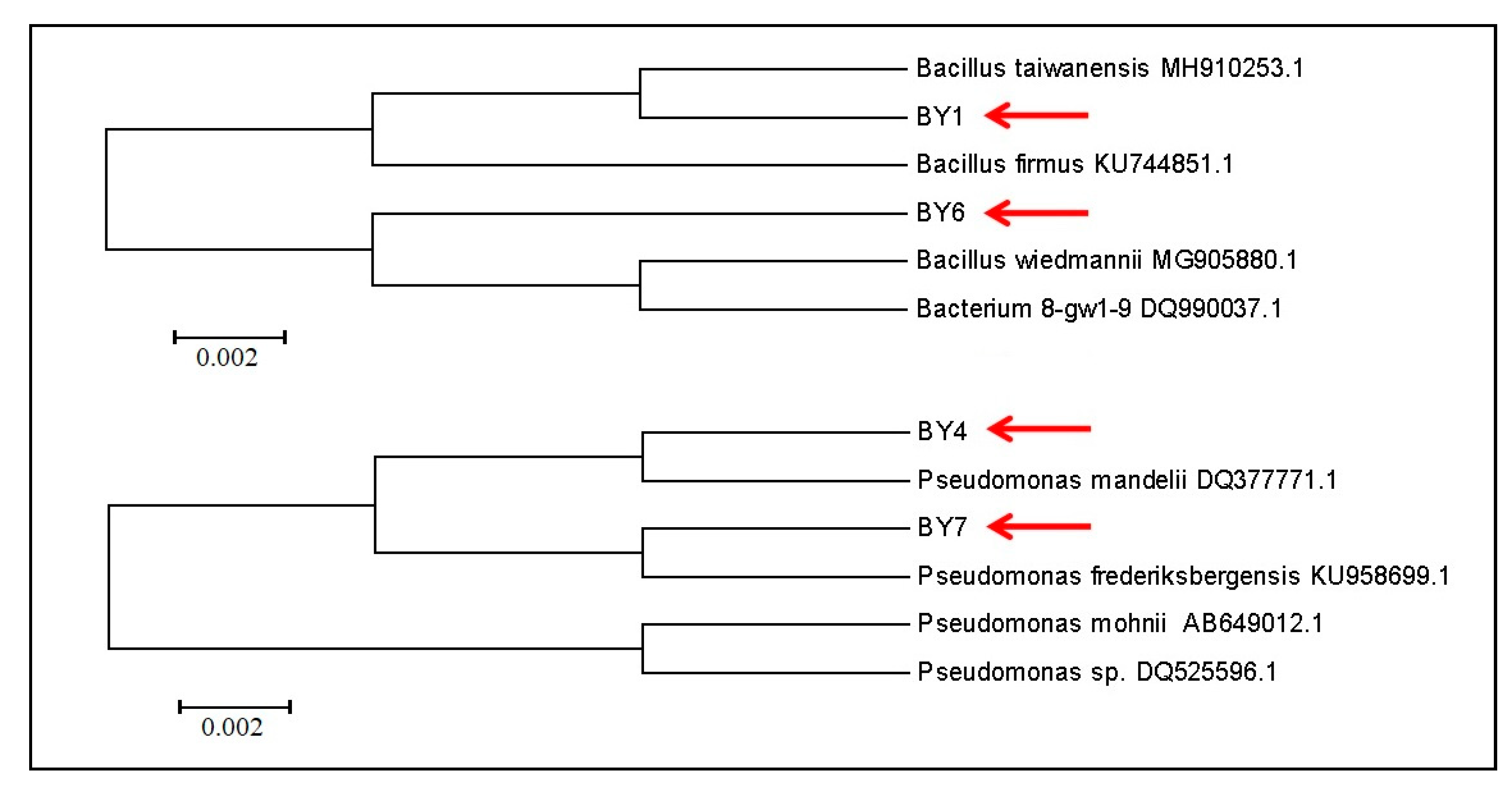
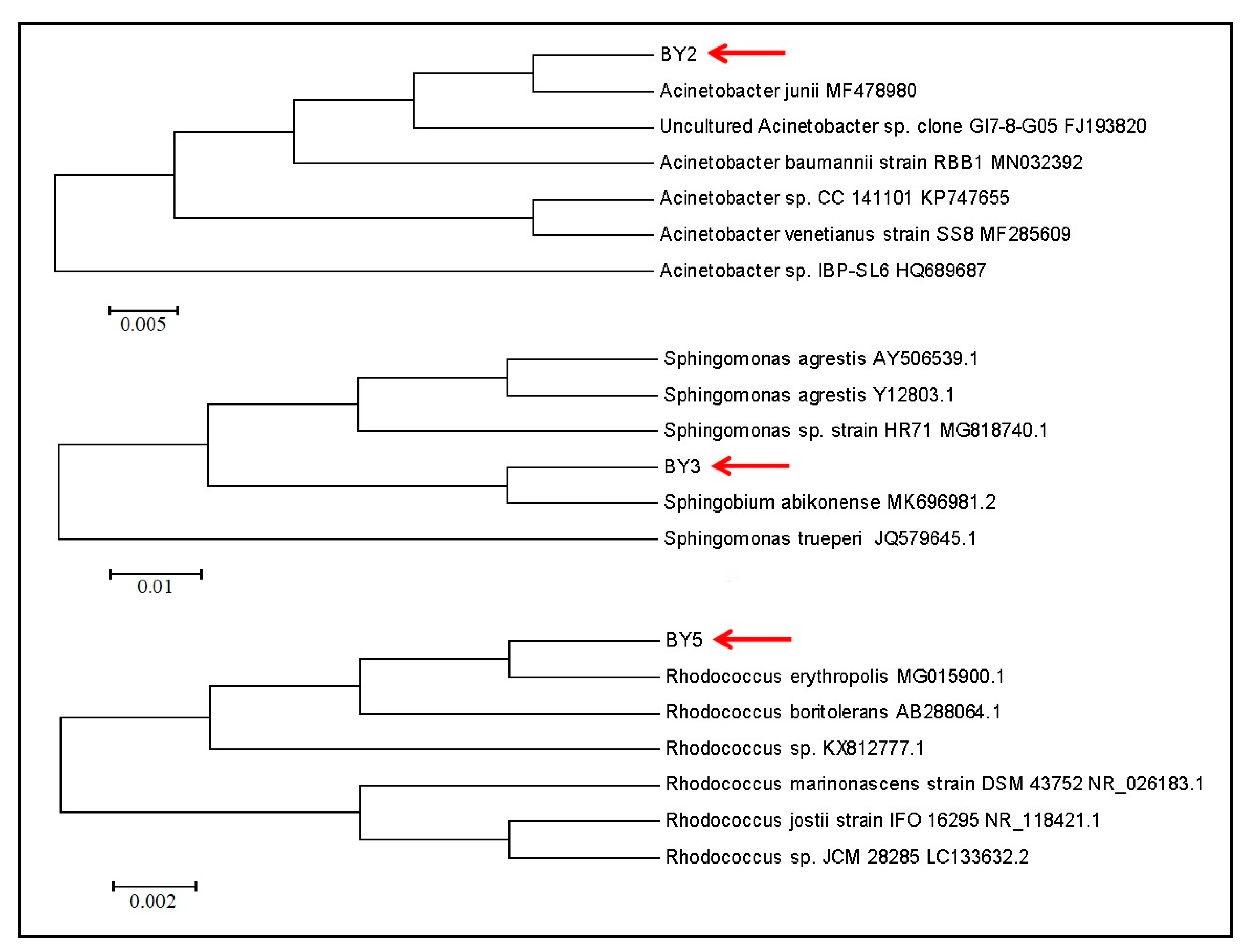
3.3. Isolated Strains Identification
3.4. Determination of the Biodegradation Efficiency of the Isolated Strains Exposed to Native Soils
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akopyan, A.V.; Fedorov, R.A.; Anisimov, A.V.; Eseva, E.A.; Karakhanov, E.A. Peroxide Oxidative Desulfurization of Crude Petroleum. Pet. Chem. 2018, 57, 1132–1136. [Google Scholar] [CrossRef]
- Corma, A.; Corresa, E.; Mathieu, Y.; Sauvanaud, L.; Al-Bogami, S.; Al-Ghrami, M.S.; Bourane, A. Crude oil to chemicals: Light olefins from crude oil. Catal. Sci. Technol. 2017, 7, 12–46. [Google Scholar] [CrossRef]
- Zeneli, A.; Kastanaki, E.; Simantiraki, F.; Gidarakos, E. Monitoring the biodegradation of TPH and PAHs in refinery solid waste by biostimulation and bioaugmentation. J. Environ. Chem. Eng. 2019, 7, 103054. [Google Scholar] [CrossRef]
- McIntosh, P.; Schulthess, C.P.; Kuzovkina, Y.A.; Guillard, K. Bioremediation and phytoremediation of total petroleum hydrocarbons (TPH) under various conditions. Int. J. Phytoremediation 2017, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Q.; Chen, S.; Yu, W.; Zhao, C.; Chen, H. Optimization for microbial degradation of petroleum hydrocarbon (TPH) by Enterobacter sp. S-1 using response surface methodolog. Pet. Sci. Technol. 2019, 37, 821–828. [Google Scholar] [CrossRef]
- Czarny, J.; Staninska-Pięta, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolniewicz, A.; Marecik, R.; Ławniczak, Ł.; Chrzanowski, Ł. Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard. Mater. 2020, 383, 121168. [Google Scholar] [CrossRef]
- Iwabuchi, N.; Sunairi, M.; Anzai, H.; Nakajima, M.; Harayama, S. Relationships between Colony Morphotypes and Oil Tolerance in Rhodococcus rhodochrous. Appl. Environ. Microbiol. 2000, 66, 5073–5077. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Alrumman, S.A.; Al-Amari, J.A. 16S rDNA Phylogenetic and RAPD–PCR Analyses of Petroleum Polycyclic Aromatic Hydrocarbons-Degrading Bacteria Enriched from Oil-Polluted Soils. Arabian J. Sci. Eng. 2015, 41, 2095–2106. [Google Scholar] [CrossRef]
- Luong, T.M.; Ponamoreva, O.N.; Nechaeva, I.A.; Petrikov, K.V.; Delegan, Y.A.; Surin, A.K.; Linklater, D.; Filonov, A.E. Characterization of biosurfactants produced by the oil-degrading bacterium Rhodococcus erythropolis S67 at low temperature. World J. Microbiol. Biotechnol. 2018, 34, 20. [Google Scholar] [CrossRef]
- Ohadi, M.; Dehghannoudeh, G.; Shakibaie, M.; Banat, I.M.; Pournamdari, M.; Forootanfar, H. Isolation, characterization, and optimization of biosurfactant production by an oil-degrading Acinetobacter junii B6 isolated from an Iranian oil excavation site. Biocatal. Agric. Biotechnol. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V. Bioremediation of polycyclic aromatic hydrocarbon (PAH) compounds: (acenaphthene and fluorene) in water using indigenous bacterial species isolated from the Diep and Plankenburg rivers, Western Cape, South Africa. Braz. J. Microbiol. 2017, 48, 314–325. [Google Scholar] [CrossRef]
- Owabor, C.N.; Agarry, S.E.; Azeez, T.O. Development of a transport model for the microbial degradation of polycyclic aromatic hydrocarbons in a saturated porous medium. J. Niger. Assoc. Math. Phys. 2010, 11, 317–324. [Google Scholar]
- Bello-Akinosho, M.; Makofane, R.; Adeleke, R.; Thantsha, M.; Pillay, M.; Chirima, G.J. Potential of Polycyclic Aromatic Hydrocarbon-Degrading Bacterial Isolates to Contribute to Soil Fertility. Biomed. Res. Int. 2016, 2016, 5798593. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.M.; Vidigal, P.M.P.; Pylro, V.S.; Morais, D.K.; Leite, L.R.; Roesch, L.F.W.; Tótola, M.R. Draft genome of Nocardia farcinica TRH1, a linear and polycyclic aromatic hydrocarbon-degrading bacterium isolated from the coast of Trindade Island, Brazil. Braz. J. Microbiol. 2017, 48, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, G.; Liao, X.; Yan, X. Interactions between Pteris vittata L. genotypes and a polycyclic aromatic hydrocarbon (PAH)-degrading bacterium (Alcaligenes sp.) in arsenic uptake and PAH-dissipation. Environ. Pollut. 2017, 230, 862–870. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, Q.; Wu, Y.; Lin, X. Oxidation of polycyclic aromatic hydrocarbons using Bacillus subtilis CotA with high laccase activity and copper independence. Chemosphere 2016, 148, 1–7. [Google Scholar] [CrossRef]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotox Environ. Saf. 2020, 194, 18. [Google Scholar] [CrossRef]
- Ako, S.E.; Akum, E.A.; Nkenfou, C.N.; Pokam, T.B.; Assob, J.C.N. Fecal Gram stain morphotype and their distribution patterns in a Cameroonian cohort with and without HIV infection. Sci. Afr. 2020, 8, e00376. [Google Scholar] [CrossRef]
- Lane, D. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; John Wiley & Sons: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Jesitha, K.; Nimisha, K.M.; Manjusha, C.M.; Harikumar, P.S. Biodegradation of Endosulfan by Pseudomonas fluorescens. Environ. Process. 2015, 2, 225–240. [Google Scholar] [CrossRef]
- Li, L.; Shen, X.; Zhao, C.; Liu, Q.; Liu, X.; Wu, Y. Biodegradation of dibenzothiophene by efficient Pseudomonas sp. LKY-5 with the production of a biosurfactant. Ecotoxicol. Environ. Saf. 2019, 176, 50–57. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Zhu, Y.; Li, J.; Li, X. Biodegradation of seven phthalate esters by Bacillus mojavensis B1811. Int. Biodeterior. Biodegrad. 2018, 132, 200–207. [Google Scholar] [CrossRef]
- Mania, D.; Heylen, K.; van Spanning, R.J.; Frostegard, A. The nitrate-ammonifying and nosZ-carrying bacterium Bacillus vireti is a potent source and sink for nitric and nitrous oxide under high nitrate conditions. Environ. Microbiol. 2014, 16, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Baskar, S.; Baskar, R.; Paul, D.; Shouche, Y.S. Biomineralization Potential of Bacillus subtilis, Rummeliibacillus Stabekisii and Staphylococcus Epidermidis Strains In Vitro Isolated from Speleothems, Khasi Hill Caves, Meghalaya, India. Geomicrobiol. J. 2018, 35, 675–694. [Google Scholar] [CrossRef]
- Krikstaponis, A.; Meskys, R. Biodegradation of 7-Hydroxycoumarin in Pseudomonas mandelii 7HK4 via ipso-Hydroxylation of 3-(2,4-Dihydroxyphenyl)-propionic Acid. Molecules 2018, 23, 2613. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial Soil Bacterium Pseudomonas frederiksbergensis OS261 Augments Salt Tolerance and Promotes Red Pepper Plant Growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kushwaha, A.; Hans, N.; Gautam, A.; Rani, R. Evaluation of the cytotoxicity and interaction of lead with lead resistant bacterium Acinetobacter junii Pb1. Braz. J. Microbiol. 2019, 50, 223–230. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, J. Novosphingobium naphthae sp. nov., from oil-contaminated soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 3170–3176. [Google Scholar] [CrossRef]

| Composition | Content (g) | |
|---|---|---|
| LB medium | Tryptone | 10.0 |
| Yeast extract | 5.0 | |
| NaCl | 10.0 | |
| YM medium | Peptone | 5.0 |
| Malt extract | 3.0 | |
| Dextrose | 10.0 | |
| Yeast extract | 3.0 |
| Isolates | BY1 | BY2 | BY3 | BY4 | BY5 | BY6 | BY7 |
|---|---|---|---|---|---|---|---|
| Biodegradation Efficiency (%) | 32.1 | 44.7 | 33.2 | 24.5 | 38.2 | 42.3 | 42.1 |
| Most Favored Temperature (°C) | 35 | 30 | 30 | 30 | 30 | 25 | 30 |
| Most Favored pH | 6.0 | 6.0 | 7.0 | 7.0 | 7.0 | 6.0 | 7.0 |
| Strains | Species | Description | Gram–Färbung Test (1) |
|---|---|---|---|
| BY1 | Bacillus sp. | 99.23% similarity to B. taiwanensis | + |
| BY2 | Acinetobactor sp. | 100.00% similarity to A. junii | + |
| BY3 | Sphingobium sp. | 100.00% similarity to S. abikonense | - |
| BY4 | Pseudomonas sp. | 99.86% similarity to P. mandelii | - |
| BY5 | Rhodococcus sp. | 100.00% similarity to R. erythropolis | - |
| BY6 | Bacillus sp. | 100.00% similarity to B. wiedmannii | - |
| BY7 | Pseudomonas sp. | 99.93% similarity to P. frederiksbergensis | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wu, B.; Zheng, J.; Chen, H.; Rao, P.; Yan, L.; Chai, F. Biodegradation of Total Petroleum Hydrocarbons in Soil: Isolation and Characterization of Bacterial Strains from Oil Contaminated Soil. Appl. Sci. 2020, 10, 4173. https://doi.org/10.3390/app10124173
Wang R, Wu B, Zheng J, Chen H, Rao P, Yan L, Chai F. Biodegradation of Total Petroleum Hydrocarbons in Soil: Isolation and Characterization of Bacterial Strains from Oil Contaminated Soil. Applied Sciences. 2020; 10(12):4173. https://doi.org/10.3390/app10124173
Chicago/Turabian StyleWang, Runkai, Baichun Wu, Jin Zheng, Hongkun Chen, Pinhua Rao, Lili Yan, and Fei Chai. 2020. "Biodegradation of Total Petroleum Hydrocarbons in Soil: Isolation and Characterization of Bacterial Strains from Oil Contaminated Soil" Applied Sciences 10, no. 12: 4173. https://doi.org/10.3390/app10124173
APA StyleWang, R., Wu, B., Zheng, J., Chen, H., Rao, P., Yan, L., & Chai, F. (2020). Biodegradation of Total Petroleum Hydrocarbons in Soil: Isolation and Characterization of Bacterial Strains from Oil Contaminated Soil. Applied Sciences, 10(12), 4173. https://doi.org/10.3390/app10124173




