1. Introduction
Dental erosion is defined as a chemical (acid-related) loss of dental hard tissue with a process independent from bacteria [
1,
2]. Concerning the origin of the erosion-causing acid, a distinction between exogenous and endogenous erosion could be made [
3]: extrinsic factors include the dietary regimen and the use of some medications, while intrinsic factors are mostly correlated to diseases such as gastroesophageal reflux (GERD), often affecting diabetic and obese individuals, bulimia nervosa (BN) and alcoholism [
2]. The initial stage of dental erosion, that is a reversible process, is characterized by the softening of the enamel due to acidic demineralization of hydroxyapatite (HA) [
4]. For this reason, the partially demineralized HA crystals may re-establish the original conformation if exposed to a favorable oral environment, at this stage [
2]. The intrinsic remineralization due to the chemical composition of saliva is well documented, in fact at physiological pH, saliva is supersaturated with phosphoprotein-stabilized Ca
2+ and PO
43− ions, which are bioavailable for hard tissue development and maintenance, establishing an homeostatic equilibrium. However, salivary remineralization is a slow process, affecting only the surface of mild eroded tooth and improving neither the aesthetics nor the structural properties, in case of subsurface lesions [
5]. Indeed, in case of patients affected by chronic GERD and BN, the self salivary remineralization process results inadequate: as the acidic impact persists, additional dissolution of the enamel matrix occurs, resulting in the layer-by-layer inorganic material loss, decrease of the enamel thickness and, occasionally, in dentin exposure [
4]. This chronic situation, often leading to a painful experience for patients, requires professional dental assistance and, at the same time, the use of specific remineralizing and wear-protecting agents [
6]. Fluorinated toothpastes are the most commonly used remineralizing agents that convert hydroxyapatite (HA) into the stronger fluoro-hydroxyapatite, which is more resistant to the acid wear [
7].
Despite the use of remineralizing agents able to limit the inconveniences of drastic dental erosion, it is considered necessary the development of new combined strategies with the aim to avert hard tissue loss. To exert an antierosive effect, conventional fluorides (such as sodium fluoride (NaF)) appear to require an intensive and unrealistic fluoridation regimen [
4]. On the other hand, the increase in prevalence of dental erosion among population, despite the use of fluoride-containing toothpastes, points out the need for more effective compounds [
5]. Stannous fluoride (SnF
2), titanium tetrafluoride, amine fluoride, HA nanoparticles, SnF
2 with amorphous calcium phosphate, zinc-carbonate-HA nanoparticles, potassium nitrate, chitosan, and various proteins, are only some examples of the developed components of toothpastes which efficacy in preventing enamel and dentin erosion have been investigated in the last years [
8,
9,
10,
11]. Under these considerations, the combination between the remineralizing capacity and the promotion of surface resistance become an advisable goal for contemporary commercial products, towards an efficient treatment of patients afflicted by chronic dental erosions.
At the state of the art, several studies showed the performances of different suitable agents with anti-erosive properties or remineralizing activity [
12], but to the authors’ knowledge, there is a lack of information regarding the combined use of these products. The present study aims to investigate the remineralization capability and the tooth-surface protecting attitude achievable combining two commonly used home-devices in case of teeth characterized by severe enamel erosion conditions. The toothpaste Elmex Erosion Protection (Elm-EP, Colgate-Palmolive Co., New York, USA), based on the ChitoActive complex (Chitosan/AmF/SnCl
2) and the GC Tooth Mousse (GC-TM, GC Co., Tokyo, Japan) containing milk-derived casein-phospholipid and amorphous calcium phosphate (CPP-ACP), were specifically chosen because of their differences both in composition and mode of action: Elm-EP is a toothpaste based on chitosan (0.5%) and enriched with amine fluoride, sodium fluoride (1400 ppm total F
−) and SnCl
2 (3500 ppm) [
11]. Biopolymer chitosan, derived from chemical deacetylation of chitin, has been largely studied for use in preventive dentistry. Its anti-inflammatory, antimicrobial and biofilm inhibition properties have been confirmed as its ability to adhere to hydroxyapatite and to prevent dental wear [
13,
14,
15]. Nevertheless, chitosan does not contribute to the remineralization of existing dental lesions [
16]. On the other hand, chitosan microparticles act as carriers for the delivery of F
− in the oral cavity [
17]. Fluoride ions, derived from NaF and AmF, displace hydroxyapatite hydroxide groups forming fluoro-hydroxyapatite playing a double role in the surface-protection process: first, fluoride acts as a catalyst, assisting in the remineralization of enamel with phosphate ions dissolved in saliva; secondly the decreased solubility of fluoro-hydroxyapatite crystals ensures a superior resistance to acid erosion [
2]. Finally, the Sn
2+ ions interacting with hydroxyapatite may lead to the precipitation of a protective layer of insoluble mixed tin-salts which inhibits mineral loss protecting the tooth surface [
18].
Differently, CG-TM has been indicated as fluoride-free remineralizing agent, containing milk-derived multiphosphorylated casein phosphopeptides (CPP) able to form stable complexes with calcium and phosphate ions [
12]. CPP is a biomimetic saliva with a significantly greater calcium-stabilizing capacity than salivary proteins, due to higher content of its phosphoseryl residues. CPP-amorphous calcium phosphate (CPP-ACP) nanocomplexes are readily soluble in saliva, acting as Ca
2+ and PO
43− reservoir, creating a diffusion gradient and a supersaturated level of ions in the oral cavity. Low pH conditions facilitate the release of Ca
2+ and PO
43− ions, inhibiting demineralization and contributing positively to the remineralization by precipitation of the released ions [
5]. Published literature and systematic reviews on the CPP-ACP performances reached sometimes conflicting conclusions: some reviews suggest that CPP-ACP had significant remineralizing and caries preventive effects; conversely, other systematic reviews conclude that the evidence to support its long-term remineralizing action or synergistic effect with fluoride is limited [
19,
20]. However, it has been suggested that the reason for the variability of the results obtained by using CPP-ACP, could be correlated to its remineralization mechanism, that involves an enhanced remineralization of enamel sub-surface lesions compared to predominantly surface-only remineralization produced by fluoride containing agents [
21].
Given the different specific action of the two compounds and the absence of studies regarding their combined use, the present protocol for the evaluation of their potential synergic activity was designed. The treatment efficacy, in terms of remineralization potential and surface protection, was evaluated by means of profilometric and SEM analysis in an ex vivo model. Differences in surface resistance to acid attacks, before and after the treatment, were assessed by quantifying the release of Ca2+ using complexometric titration. The results obtained with the combined use of Elm-EP (Elm-EP, Colgate-Palmolive Co., New York, USA) and CG-TM (GC-TM, GC Co., Tokyo, Japan) were compared with those obtained using the sole Elm-EP or only artificial remineralizing saliva.
2. Materials and Methods
2.1. Generality
Table 1 shows the composition of the two considered remineralizing agents, Elm-EP and GC-TM, together with their suggested use as indicated by the manufacturer.
During the experimental procedures, the wear condition of the specimen surfaces was evaluated quantitatively, by the profilometric analysis of the surface roughness (Sa, µm), and qualitatively, by SEM analysis, at four different stages namely:
T0 = baseline point/preparation of the specimens;
T1 = first erosive test;
T2 = 15 days of remineralization protocol;
T3 = second erosive test.
In order to preserve the original specimens during the experimental protocol, for each considered stage, epoxy resin clones (EpoThin Epoxy Resin+Hardener, Buehler, Lake Bluff, Illinois, USA) were prepared by using silicone molds (Type 3 impression material, AquasilUltra XLV; light-bodied consistency; Dentsply, York, Pennsylvania, USA) and used in the quantitative and qualitative analysis.
2.2. Dental Specimens Preparation
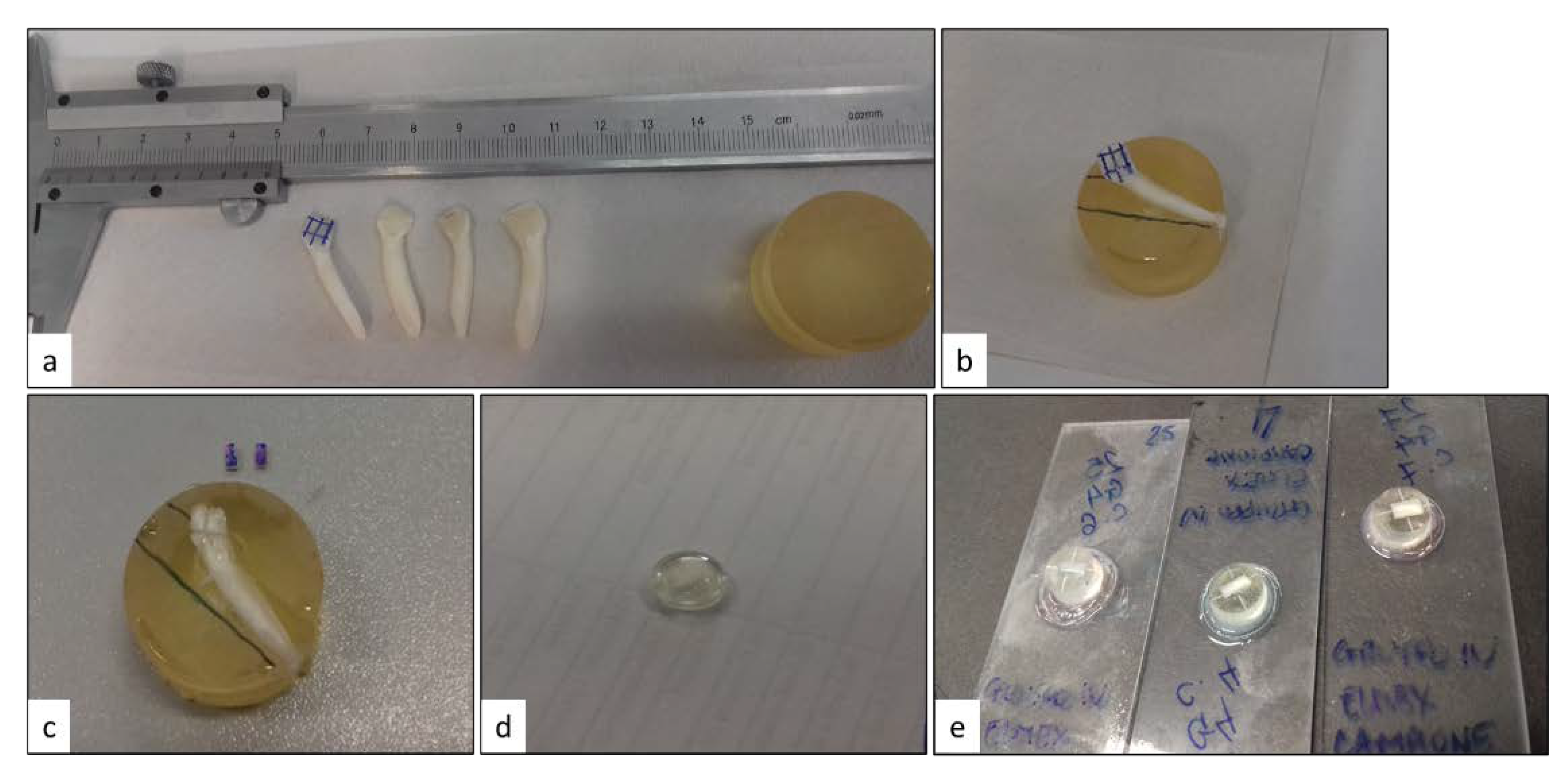
Twenty-four enamel specimens were obtained from 3-years-old cattle incisor teeth, by longitudinally sectioning the buccal-lingual fragments with a microtome (Micromet Remet, Bologna, Italy) equipped with a 0.5 mm thickness diamond blade. The buccal sections were discarded and from each lingual section, two parallelepipeds of an average size of 4 × 2 × 3 mm were obtained. The parallelepipeds were embedded in epoxy resin cylindrical supports, carefully leaving the dental surface exposed. The opposite surface of the resin support was fixed onto a glass slide (
Figure 1). The surface of each specimen was polished for one minute with wet sand-paper of decreasing abrasive grain (# 800, # 1200, # 2400). The surface conditions were verified using a 50x optical microscope (Leica MZ16, Leica Microsystems Srl, Milan, Italy), equipped with a digital camera (Leica DFC320, Leica Microsystems Srl, Milan, Italy), in order to eliminate specimens with defects and cracks. The obtained specimens were then randomly divided in three groups (N = 8), which underwent different treatments (see following section) after the first erosive challenge at stage T1.
2.3. Erosive Challenge and Ca2+ Titration
The specimens were subjected to the erosive challenge twice (T1 and T3) individually placing them into HCl aqueous solution (0.01 M, pH 2, 10 mL) for 60 min at 37 °C.
For the titration of the loss Ca2+, 3 mL aliquot of the HCl solution used for the erosion of the specimens was placed into a vial and 2–3 drops of NH3/NH4Cl buffer solution were added to pH 10. Two drops of Eriochrome Black T was then added and immediately titrated against a standardized 0.001 M EDTA solution to the red-blue indicator end-point, using a glass graduated burette (50 mL, precision 0.1 ± 0.05 mL, Hirschmann, Baden-Württemberg, Germany). Titration was repeated three times for each specimen. The concentration of free calcium ions in solution was reported as mg/L.
2.4. Treatment of the Specimens
Specimen groups G0, G1 and G2 (N = 8) underwent the following treatments for 15 days after the first erosion:
G0 specimens were stored in remineralizing saliva prepared as previously reported [
22]. Briefly, 500 mL of artificial saliva contained: Na
2PO
4 0.170 g; sodium ascorbate 0.001 g; glucose 0.015 g, NaCl 0.290 g, CaCl
2 0.085 g, NH
4Cl 0.080 g, KCl 0.635 g, NaSCN 0.080 g, KH
2PO
4 0.165 g, urea 0.100 g and the final pH was adjusted to 7.
G1 specimens were brushed twice a day (at 9 a.m. and at 8 p.m.) with Elm-EP using a soft toothbrush (Elmex Sensitive, Colgate-Palmolive Co., New York, USA) and following an adequate modified Bass technique performing a “rolling” number (n = 5) for each specimen [
23,
24]. Before rinsing with water, Elm-EP was left for two minutes onto the specimens surface.
G2 specimens were first treated with Elm-EP as described before for G1, then a rice size quantity of GC-TM was applied to each surface and left for 5 min. The excess of paste was gently removed with a cotton swab.
G1 and G2 specimens were stored in artificial saliva when not retrieved for the treatments.
2.5. Profilometric Assessment
At each considered experimental stage (T0-T3) the resin clones were used for average surface roughness measurement (Sa, µm) by means of a profilometer (Talysurf CLI 1000; Taylor Hobson, AMETEK Inc, Berwyn, PA, USA) as previously described [
25,
26]. Approximatively in the middle area of each specimen a 0.5 × 0.5 mm surface was analyzed (along horizontal lines), with a 501 point/line resolution and 1 µm spacing; forward and return speeds were 200 µm/s and 500 µm/s respectively. The obtained data were processed with TalyMap Expert 4.1 profile software (Taylor Hobson Precision, Taylor Hobson, AMETEK Inc, Berwyn, PA, USA).
2.6. Scanning Electron Microscope Assessment
At any considered experimental stage (T0-T3), three resin clones per specimen group (G0-G2) were randomly chosen for surface qualitative evaluation by scanning electron microscopy. The clones were mounted on aluminum supports, coated with a carbon-conductive double-sided adhesive and then coated with a layer of gold with the metallizer (Sputter Coater K550X, Emitech, Quorum Technologies Ltd., Laughton, United Kingdom). The images were acquired with a scanning electron microscope (SEM, Quanta 250 SEM, FEI, Hillsboro, USA) operating in secondary electron acquisition mode. The working distance (WD) was modulated to obtain 5000× magnification; the acceleration voltage was set at 30 kV.
2.7. Statistical Analysis
Shapiro-Wilk test and Levene test were used for verifying the distribution normality and the variances homogeneity respectively. Statistically significant variations of the surface roughness parameter (Sa) were evaluated among different groups at the same experimental stage and among the same group at different stages, by means of parametric ANOVA analysis of variance. Multiple comparisons were determined by post-hoc Tukey test, with pre-set significance level p = 0.05. Statistically significant variations of the concentration of calcium (mg/L) in solution at T1 and T3 were evaluated by the non-parametric Kruskal-Wallis analysis. Pairwise comparison between dependent and independent groups of data was assessed by Wilcoxon and Mann-Whitney methods respectively, with pre-set significance level p = 0.05.
4. Discussion
In the present work, a possible advantage correlated to the synergic use of Elm-EP and GC-TM, two commonly used home-devices acting in different mode on eroded tooth surface, was investigated.
In order to mimic conditions of patients afflicted by diseases such as GERD and BN, the specimens were retrieved from frontal dental elements, as they represent the most affected elements by erosion. Furthermore, the acid experimental test was extended to 60 min to reach partially exposed dentine tubules, reproducing the situation of critically eroded tooth surfaces. The specimens were stored in artificial saliva both after preparation and during the entire treatment period reproducing the natural environment of the mouth. As negative reference group (G0), the specimens where stored in remineralizing saliva for 15 days, while as positive reference (G1) the specimens were treated with the sole toothpaste Elm-EP, which efficacy as anti-erosion agent has been largely assessed and reported in literature [
8,
9,
11]. The two products were applied on the specimen surfaces following the methodologies suggested by the manufacturer.
The results obtained in this study highlight that the combined use of Elm-EP and GC-TM for 15 days on critically eroded specimens, allow to greatly decrease the surface roughness. Even though, the Sa values of the specimens treated with Elm-EP or its combination with GC-TM were not statistically different, the calculated percentage of decrease in roughness at T2 compared to T1, resulted 42% and 50% for G1 and G2 respectively, confirming the promising action of the combined agents. Together with the morphologic analysis obtained by SEM evaluation, the profilometric analysis allows to speculate about the formation of a protective layer obtained by the Elm-EP active components in which some CPP-ACP clusters inserted, contributing to the increase of the surface uniformity.
Considering the severe erosion conditions applied to the specimens, not surprisingly, 15 days of treatment with the studied agents proved to be insufficient to restore the baseline Sa value, but as the action of both Elm-EP and GC-TM is time-dependent, it is possible to hypothesize a gradual decrease of the roughness by increasing the treatment period.
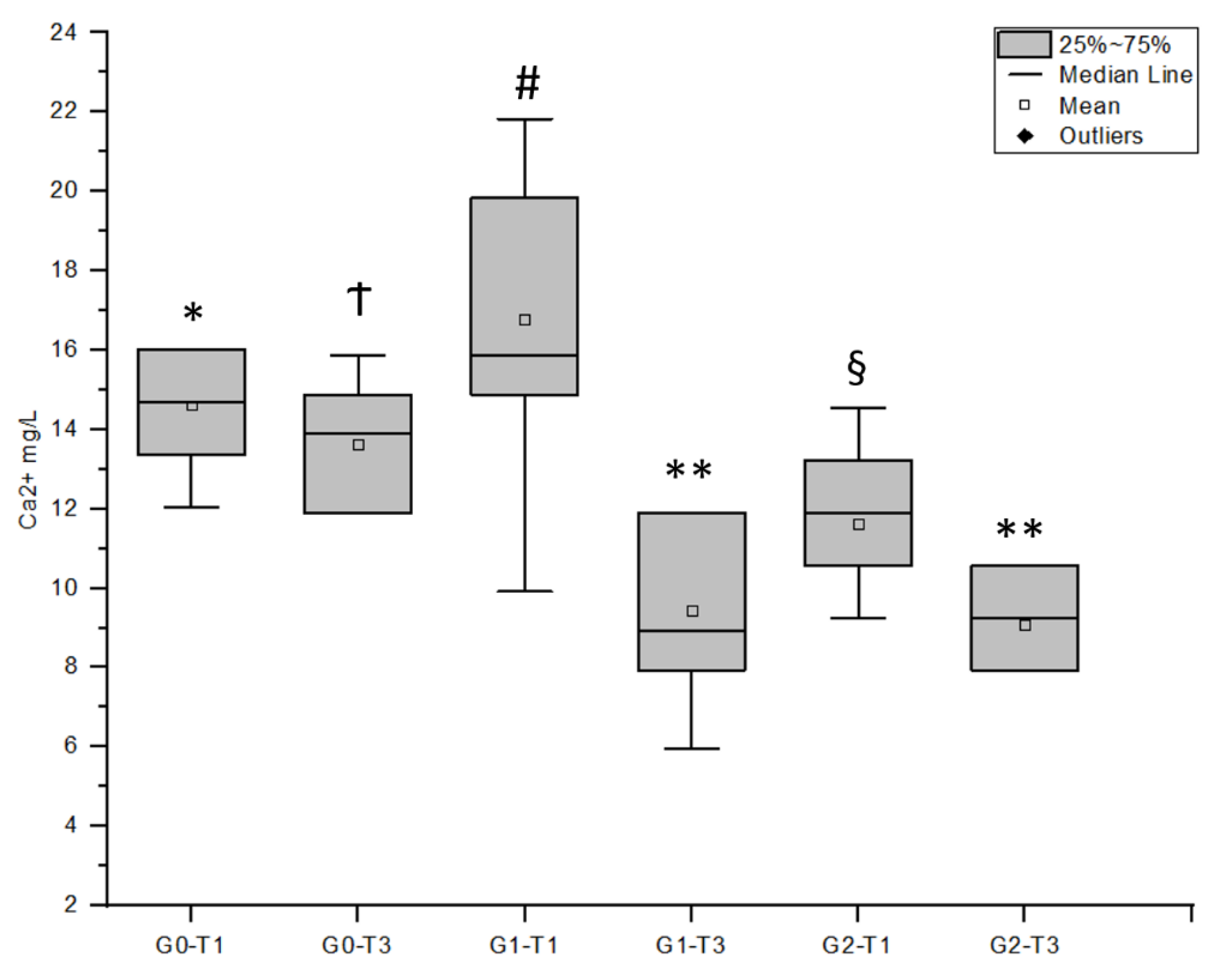
The evaluation and comparison of the released calcium concentration during the two acid attacks gave some information about the erosion-protecting attitude of the considered agents: the solubilized calcium titrated at T3, in case of the positive reference group G1, was greatly lower (44%) compared to that at T1, confirming the well-known ability of Elm-EP as anti-erosive system [
9]. However, G1 specimen surfaces appeared less homogeneous at T3 in comparison to the baseline and T2. Differently, in case of G2, the concentration of Ca
2+ lost during the second erosion was only 22%, lower than that titrated at T1. It is possible to speculate about this result comparing the SEM imagines regarding G2 at T2 and T3: after the second erosion, the most part of the CPP-ACP clusters, well visible at T2, disappeared from the specimen surfaces, suggesting their dissolution in the acid media. Bearing in mind that the CPP-ACP nanocomplexes are meaning to act as Ca
2+ and PO
43− storage-release systems, it is plausible to consider that the most percentage of the calcium released and titrated at T3 originated from their dissolution instead of that of the tooth surface. Nevertheless, G2 specimens showed still occluded dentine tubule after the second drastic acid erosion, confirming, ones more, the remineralization process of the two combining agents.
The phenomenon of CPP-ACP dissolution, could also be correlated to the different percentage of roughness increase of G1 (26%) and G2 (76%) at T3: assuming that, thanks to the different remineralizing action of Elm-EP and GC-TM, a “mixed layer” nestling CPP-ACP clusters could form onto G2 specimen, in case of an acid attack, not only the CPP-ACP clusters on the surface would be removed, but also the ones included in the “mixed layer”, increasing the surface roughness.
Even though the tandem use of Elm-EP and GC-TM gave indeed promising results, the present study evidenced a poor stabilization of the CPP-ACP clusters onto the specimen surface in the considered conditions. To justify this trend, chemical and mechanical interaction between the active components of Elm-EP and GC-TM should be deeper studied. However, it is possible to speculate about some explanations: for instance, it has been previously reported that for obtaining optimal remineralization and protection results, CPP should bind to salivary pellicle and plaque bacteria [
27]. It is then possible, that the formation of a thin film of Elm-EP on the specimen surfaces would have limited the bonding process, decreasing the cluster stability to the acid attack. Moreover, the inclusion of the CPP-ACP nanocomplexes in the formation of the assumed “mixed layer” could be insufficient for their stability in comparison to the presence of the biologically complex matrix typical of an in vivo environment.
The complete dissolution of CPP-ACP nanocomplexes and the consequent limitation of the GC-TM protection efficiency, seem to be in disagreement with some results reported in literature, where the use of CPP-ACP-containing systems showed a clear effect against enamel demineralization in vitro [
28,
29]. However, it is important to keep in mind that data discrepancies among studies can be caused by the use of different experimental protocols, for instance, the use of bovine teeth model instead of human specimens. Even though bovine and human enamels possess many similarities, such as chemical compositions and physicochemical properties, the different arrangements of prisms and interprisms might result in some different outcomes [
30]. Moreover, bovine enamel is more porous than human enamel, probably because it consists of larger crystals [
31], and these morphological aspects may be correlated to the demineralization of bovine enamel which progresses about three times faster than that of human enamel. Therefore, the performance of the materials in human enamel may be different from the results of our study.