Efficient Molecular Aggregation of Rhodamine 6G and Pseudoisocyanine by Light-Induced Force
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Sample Preparation
2.3. Aggregation Phenomenon
3. Results and Discussion
3.1. Rhodamine 6G
3.2. Pseudoisocyanine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J -aggregate formation of a water-soluble porphyrin in acidic aqueous media. J. Chem. Phys. 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Aggarwal, L.P.F.; Borissevitch, I.E. On the dynamics of the TPPS4 aggregation in aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Saito, K.; Serata, T.; Aida, H.; Uesu, Y. Morphology and thermochromic phase transition of merocyanine J -aggregate monolayers at the air–water and solid–water interfaces. J. Chem. Phys. 2001, 115, 1473–1484. [Google Scholar] [CrossRef]
- Bielejewski, M.; Łapiński, A.; Luboradzki, R.; Tritt-Goc, J. Solvent Effect on 1,2- O-(1-Ethylpropylidene)-α-d-glucofuranose Organogel Properties. Langmuir 2009, 25, 8274–8279. [Google Scholar] [CrossRef]
- Tian, Y.; Stepanenko, V.; Kaiser, T.E.; Würthner, F.; Scheblykin, I.G. Reorganization of perylene bisimide J-aggregates: From delocalized collective to localized individual excitations. Nanoscale 2012, 4, 218–223. [Google Scholar] [CrossRef]
- Villari, V.; Mineo, P.; Scamporrino, E.; Micali, N. Role of the hydrogen-bond in porphyrin J-aggregates. RSC Adv. 2012, 2, 12989. [Google Scholar] [CrossRef]
- Kobayashi, T. J-Aggregates; World Scientific: Singapore, 1996; ISBN 978-981-02-2737-1. [Google Scholar]
- Kobayashi, T. J-Aggregates: Volume 2; World Scientific: Singapore, 2012; ISBN 978-981-4365-74-1. [Google Scholar]
- Jelley, E.E. Spectral Absorption and Fluorescence of Dyes in the Molecular State. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Blau, W.J.; Fleming, A.J. Designer Nanotubes by Molecular Self-Assembly. Science 2004, 304, 1457. [Google Scholar] [CrossRef]
- Campione, M.; Capitani, G.C.; Raimondo, L.; Sassella, A. Porphyrin Nanowires with Epitaxially Locked Uniaxial Orientation. J. Phys. Chem. C 2015, 119, 18210–18215. [Google Scholar] [CrossRef]
- Morales-Curiel, L.F.; de León-Montiel, R.J. Photochemical dynamics under incoherent illumination: Light harvesting in self-assembled molecular J-aggregates. J. Chem. Phys. 2020, 152, 074304. [Google Scholar] [CrossRef] [Green Version]
- Wanless, E.J.; Davey, T.W.; Ducker, W.A. Surface Aggregate Phase Transition. Langmuir 1997, 13, 4223–4228. [Google Scholar] [CrossRef]
- Pang, P.; Miao, X.; Ying, L.; Kong, G.; Che, C.; Deng, W. Halogen-Bond-Controlled Self-Assembly of Regioisomeric Phenanthridine Derivatives into Nanowires and Nanosheets. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Yeom, J.; Yeom, B.; Chan, H.; Smith, K.W.; Dominguez-Medina, S.; Bahng, J.H.; Zhao, G.; Chang, W.-S.; Chang, S.-J.; Chuvilin, A.; et al. Chiral templating of self-assembling nanostructures by circularly polarized light. Nat. Mater. 2015, 14, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garetz, B.A.; Aber, J.E.; Goddard, N.L.; Young, R.G.; Myerson, A.S. Nonphotochemical, Polarization-Dependent, Laser-Induced Nucleation in Supersaturated Aqueous Urea Solutions. Phys. Rev. Lett. 1996, 77, 3475–3476. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Yoshikawa, H.; Masuhara, H. Two-Photon Fluorescence Spectroscopy of Individually Trapped Pseudoisocyanine J-Aggregates in Aqueous Solution. J. Phys. Chem. B 2006, 110, 17906–17911. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshikawa, H.; Asahi, T.; Masuhara, H. Laser microfixation of highly ordered J aggregates on a glass substrate. Appl. Phys. Lett. 2007, 91, 041102. [Google Scholar] [CrossRef]
- Adachi, H.; Takano, K.; Hosokawa, Y.; Inoue, T.; Mori, Y.; Matsumura, H.; Yoshimura, M.; Tsunaka, Y.; Morikawa, M.; Kanaya, S.; et al. Laser Irradiated Growth of Protein Crystal. Jpn. J. Appl. Phys. 2003, 42, L798–L800. [Google Scholar] [CrossRef]
- Adachi, H.; Murakami, S.; Niino, A.; Matsumura, H.; Takano, K.; Inoue, T.; Mori, Y.; Yamaguchi, A.; Sasaki, T. Membrane Protein Crystallization Using Laser Irradiation. Jpn. J. Appl. Phys. 2004, 43, L1376–L1378. [Google Scholar] [CrossRef]
- Okutsu, T.; Furuta, K.; Terao, M.; Hiratsuka, H.; Yamano, A.; Ferté, N.; Veesler, S. Light-Induced Nucleation of Metastable Hen Egg-White Lysozyme Solutions. Cryst. Growth Des. 2005, 5, 1393–1398. [Google Scholar] [CrossRef]
- Veesler, S.; Furuta, K.; Horiuchi, H.; Hiratsuka, H.; Ferté, N.; Okutsu, T. Crystals from Light: Photochemically Induced Nucleation of Hen Egg-White Lysozyme. Cryst. Growth Des. 2006, 6, 1631–1635. [Google Scholar] [CrossRef]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Ashkin, A.; Dziedzic, J.M.; Smith, P.W. Continuous-wave self-focusing and self-trapping of light in artificial Kerr media. Opt. Lett. 1982, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, F.; Reihani, S.N.S. Optimized optical trapping of gold nanoparticles. Opt. Express 2010, 18, 551. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Gordon, R. Optical Trapping of 12 nm Dielectric Spheres Using Double-Nanoholes in a Gold Film. Nano Lett. 2011, 11, 3763–3767. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Ren, Y.; Shang, W.; Zhu, W.; Han, L.; Lu, H.; Mei, T.; Premaratne, M.; Zhao, J. Sub-10 nm particle trapping enabled by a plasmonic dark mode. Opt. Lett. 2018, 43, 3413. [Google Scholar] [CrossRef]
- Arita, Y.; Tkachenko, G.; McReynolds, N.; Marro, N.; Edwards, W.; Kay, E.R.; Dholakia, K. Invited Article: Optical trapping of ultrasmooth gold nanoparticles in liquid and air. APL Photonics 2018, 3, 070801. [Google Scholar] [CrossRef]
- Niinomi, H.; Sugiyama, T.; Tagawa, M.; Murayama, K.; Harada, S.; Ujihara, T. Enantioselective amplification on circularly polarized laser-induced chiral nucleation from a NaClO3 solution containing Ag nanoparticles. CrystEngComm 2016, 18, 7441–7448. [Google Scholar] [CrossRef]
- Miyauchi, K.; Tawa, K.; Kudoh, S.N.; Taguchi, T.; Hosokawa, C. Surface plasmon-enhanced optical trapping of quantum-dot-conjugated surface molecules on neurons cultured on a plasmonic chip. Jpn. J. Appl. Phys. 2016, 55, 06GN04. [Google Scholar] [CrossRef]
- Iida, T.; Nishimura, Y.; Tamura, M.; Nishida, K.; Ito, S.; Tokonami, S. Submillimetre Network Formation by Light-induced Hybridization of Zeptomole-level DNA. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Niinomi, H.; Sugiyama, T.; Tagawa, M.; Maruyama, M.; Ujihara, T.; Omatsu, T.; Mori, Y. Plasmonic Heating-Assisted Laser-Induced Crystallization from a NaClO3 Unsaturated Mother Solution. Cryst. Growth Des. 2017, 17, 809–818. [Google Scholar] [CrossRef]
- Juan, M.L.; Righini, M.; Quidant, R. Plasmon nano-optical tweezers. Nat. Photonics 2011, 5, 349–356. [Google Scholar] [CrossRef]
- Gao, D.; Ding, W.; Nieto-Vesperinas, M.; Ding, X.; Rahman, M.; Zhang, T.; Lim, C.; Qiu, C.-W. Optical manipulation from the microscale to the nanoscale: FundamenTals, advances and prospects. Light Sci. Appl. 2017, 6, e17039. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Gurunatha, K.L.; Choi, H.-K.; Mohr, D.A.; Ertsgaard, C.T.; Gordon, R.; Oh, S.-H. Low-Power Optical Trapping of Nanoparticles and Proteins with Resonant Coaxial Nanoaperture Using 10 nm Gap. Nano Lett. 2018, 18, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Ecarnot, A.; Magno, G.; Yam, V.; Dagens, B. Ultra-efficient nanoparticle trapping by integrated plasmonic dimers. Opt. Lett. 2018, 43, 455. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, M.; Peng, X.; Lissek, E.N.; Mao, Z.; Scarabelli, L.; Adkins, E.; Coskun, S.; Unalan, H.E.; Korgel, B.A.; et al. Opto-thermoelectric nanotweezers. Nat. Photonics 2018, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Nakata, K.; Suzuki, M.; Kobayashi, T.; Tokunaga, E. Nonlinear Absorption Spectroscopy of Porphyrin J-aggregates in Aqueous Solution: Evidence for Control of Degree of Association by Light-Induced Force. J. Phys. Soc. Jpn. 2017, 86, 044703. [Google Scholar] [CrossRef]
- Shirakawa, M.; Kobayashi, T.; Tokunaga, E. Solvent Effects in Highly Efficient Light-Induced Molecular Aggregation. Appl. Sci. 2019, 9, 5381. [Google Scholar] [CrossRef]
- Arden, J.; Deltau, G.; Huth, V.; Kringel, U.; Peros, D.; Drexhage, K.H. Fluorescence and lasing properties of rhodamine dyes. J. Lumin. 1991, 48–49, 352–358. [Google Scholar] [CrossRef]
- Arbeloa, F.L.; Gonzalez, I.L.; Ojeda, P.R.; Arbeloa, I.L. Aggregate formation of rhodamine 6G in aqueous solution. J. Chem. Soc. Faraday Trans. 2 1982, 78, 989. [Google Scholar] [CrossRef]
- Kamatani, H.; Kasai, H.; Okada, S.; Matsuda, H.; Oikawa, H.; Minami, N.; Kakuta, A.; Ono, K.; Mukoh, A.; Nakanishi, H. Preparation of J-Aggregated Microcrystals of Pseudoisocyanine. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1994, 252, 233–241. [Google Scholar] [CrossRef]
- Iwai, S.; Tanaka, M.; Mitsunaga, M.; Kobayashi, T.; Tokunaga, E. Excited-state absorption spectra for optically forbidden f-f transitions in an Eu3+:Y2SiO5 crystal and Eu3+ aqueous solution. J. Opt. Soc. Am. B 2008, 25, 1046. [Google Scholar] [CrossRef]
- Ishino, H.; Iwai, S.; Iwamoto, S.; Okumura, T.; Kobayashi, T.; Tokunaga, E. Nonlinear absorption microspectroscopy of single perylene nanocrystals with a multichannel double lock-in amplifier. Opt. Rev. 2010, 17, 337–340. [Google Scholar] [CrossRef]
- Chen, D.-M.; He, T.; Cong, D.-F.; Zhang, Y.-H.; Liu, F.-C. Resonance Raman Spectra and Excited-State Structure of Aggregated Tetrakis(4-sulfonatophenyl)porphyrin Diacid. J. Phys. Chem. A 2001, 105, 3981–3988. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakata, K.; Kuroda, R.; Kobayashi, T.; Tokunaga, E. Electrooptic Kerr effect of porphyrin H-aggregates in polymer films: Polymer specific spectral blue shift. Chem. Phys. 2016, 469–470, 88–96. [Google Scholar] [CrossRef]
- Toptygin, D.; Packard, B.Z.; Brand, L. Resolution of absorption spectra of rhodamine 6G aggregates in aqueous solution using the law of mass action. Chem. Phys. Lett. 1997, 277, 430–435. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Shapovalov, S.A.; Koval, V.L.; Shakhverdov, T.A.; Bochkaryov, Y.A. the surfactant-induced formation of J- and H-aggregates in aqueous pseudoisocyanine solutions. Dye. Pigment. 1992, 19, 33–40. [Google Scholar] [CrossRef]
- Brackmann, U. Lambdachrome Laser Dyes: Data Sheets, 3rd ed.; Lambda Physik GmbH: Göttingen, Germany, 2000. [Google Scholar]
- Rotomskis, R.; Augulis, R.; Snitka, V.; Valiokas, R.; Liedberg, B. Hierarchical Structure of TPPS4 J-Aggregates on Substrate Revealed by Atomic Force Microscopy. J. Phys. Chem. B 2004, 108, 2833–2838. [Google Scholar] [CrossRef]
- El-Hachemi, Z.; Escudero, C.; Acosta-Reyes, F.; Casas, M.T.; Altoe, V.; Aloni, S.; Oncins, G.; Sorrenti, A.; Crusats, J.; Campos, J.L.; et al. Structure vs. properties—Chirality, optics and shapes—In amphiphilic porphyrin J-aggregates. J. Mater. Chem. C 2013, 1, 3337. [Google Scholar] [CrossRef] [Green Version]
- Von Berlepsch, H.; Böttcher, C.; Dähne, L. Structure of J-Aggregates of Pseudoisocyanine Dye in Aqueous Solution. J. Phys. Chem. B 2000, 104, 8792–8799. [Google Scholar] [CrossRef]
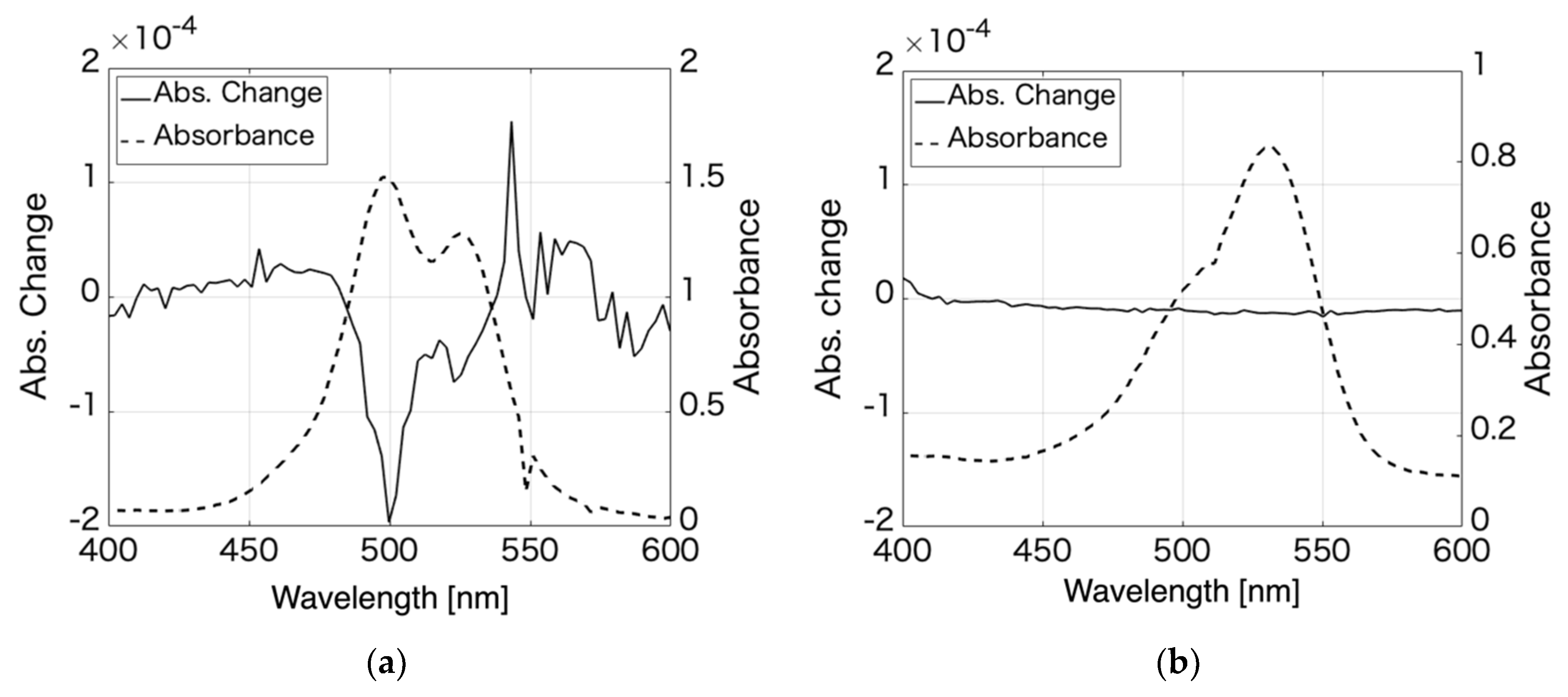
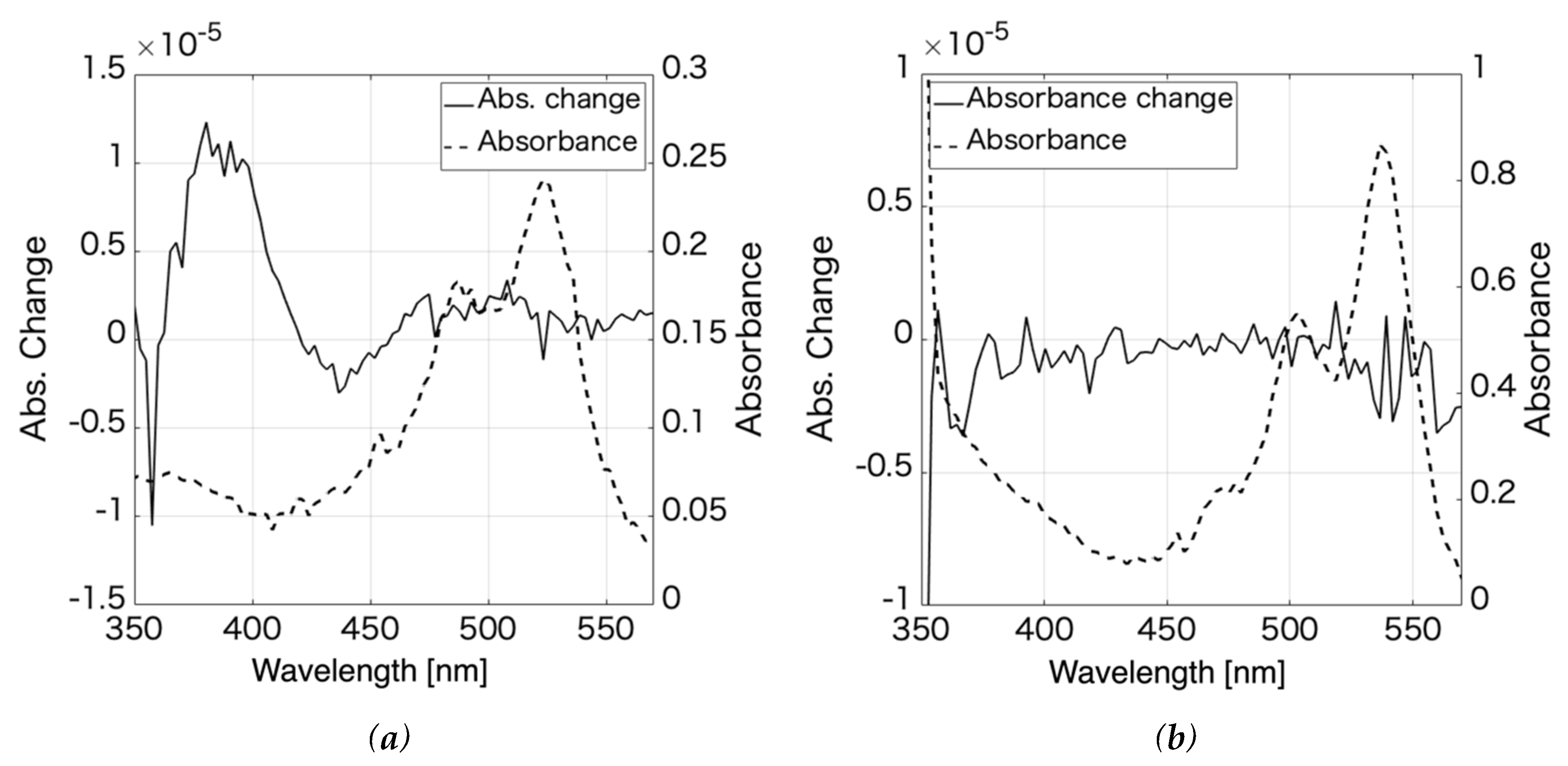
| Aggregation number | 1 | 2 | 3 | 4 |
| Absorption wavelength | 526 | 498 | 485 | 475 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirakawa, M.; Kobayashi, T.; Tokunaga, E. Efficient Molecular Aggregation of Rhodamine 6G and Pseudoisocyanine by Light-Induced Force. Appl. Sci. 2020, 10, 3563. https://doi.org/10.3390/app10103563
Shirakawa M, Kobayashi T, Tokunaga E. Efficient Molecular Aggregation of Rhodamine 6G and Pseudoisocyanine by Light-Induced Force. Applied Sciences. 2020; 10(10):3563. https://doi.org/10.3390/app10103563
Chicago/Turabian StyleShirakawa, Masayuki, Takayoshi Kobayashi, and Eiji Tokunaga. 2020. "Efficient Molecular Aggregation of Rhodamine 6G and Pseudoisocyanine by Light-Induced Force" Applied Sciences 10, no. 10: 3563. https://doi.org/10.3390/app10103563






