Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains
Abstract
Featured Application
Abstract
1. Introduction
2. Material and Methods
2.1. Barley Grains
2.2. Mycotoxin Standards
2.3. Sample Preparation
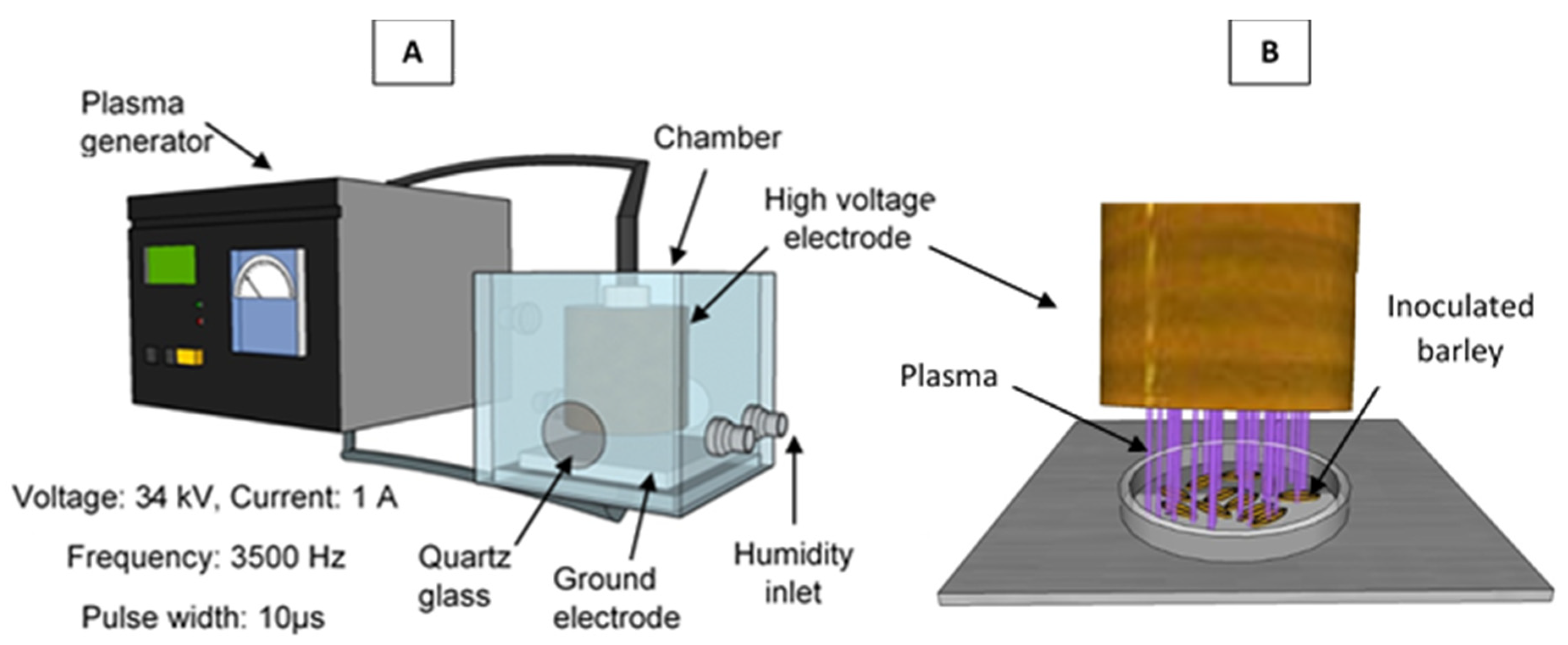
2.4. ACP Treatment
2.5. Extraction and Quantification of DON
2.6. Cold Plasma Diagnostics
2.7. Measurements of Ozone, Nitrous Gas, and Hydrogen Peroxide Concentration
2.8. Effect of Moisture Content of Barley Grains and Environmental RH
2.9. Post-Treatment Storage of Barley Grains on DON Degradation by ACP
2.10. Effect of Steeping of Barley Grains on DON Degradation by ACP
2.11. Quality Parameters of Barley Grains after ACP
2.12. Germination of Barley Grains after ACP
2.13. Statistical Analysis
3. Results and Discussion
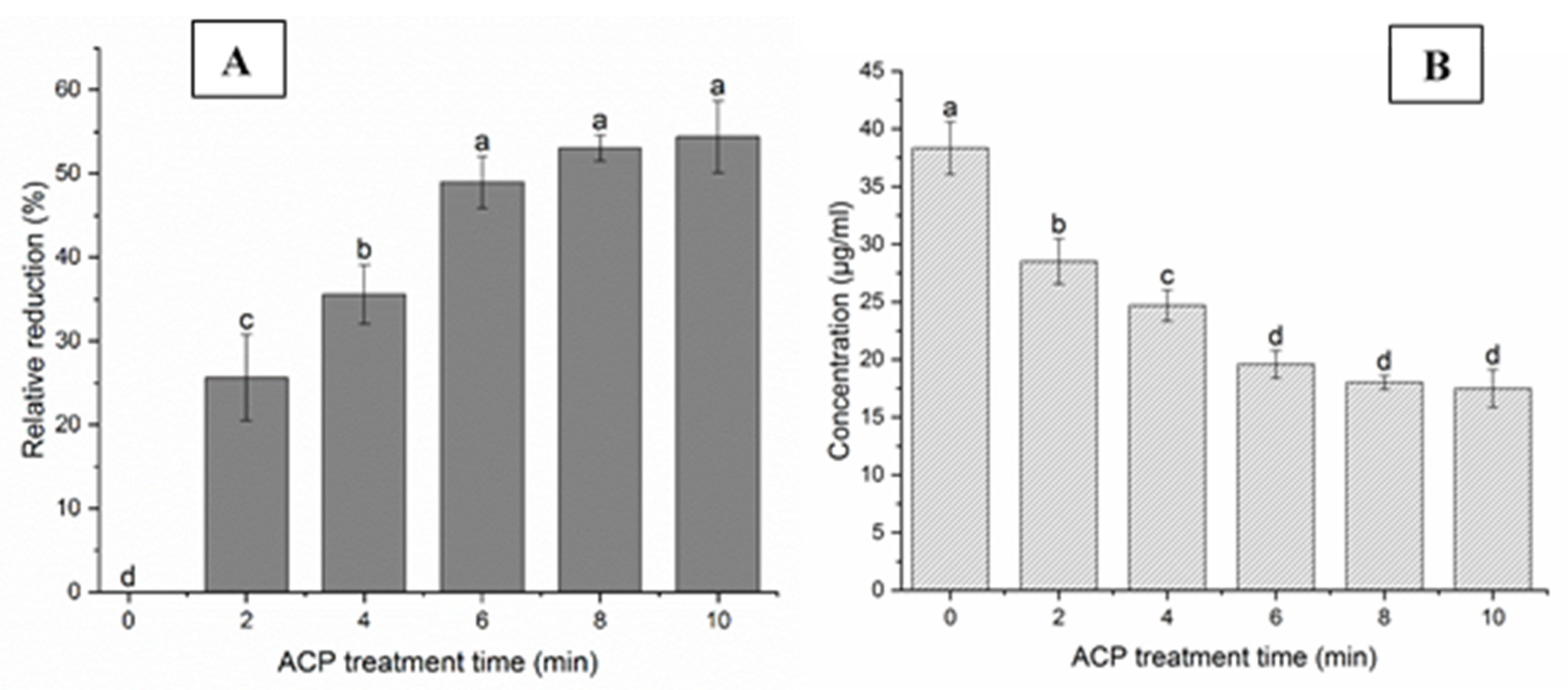
3.1. Effect of ACP Treatment on Deoxynivalenol Degradation on Barley Grains
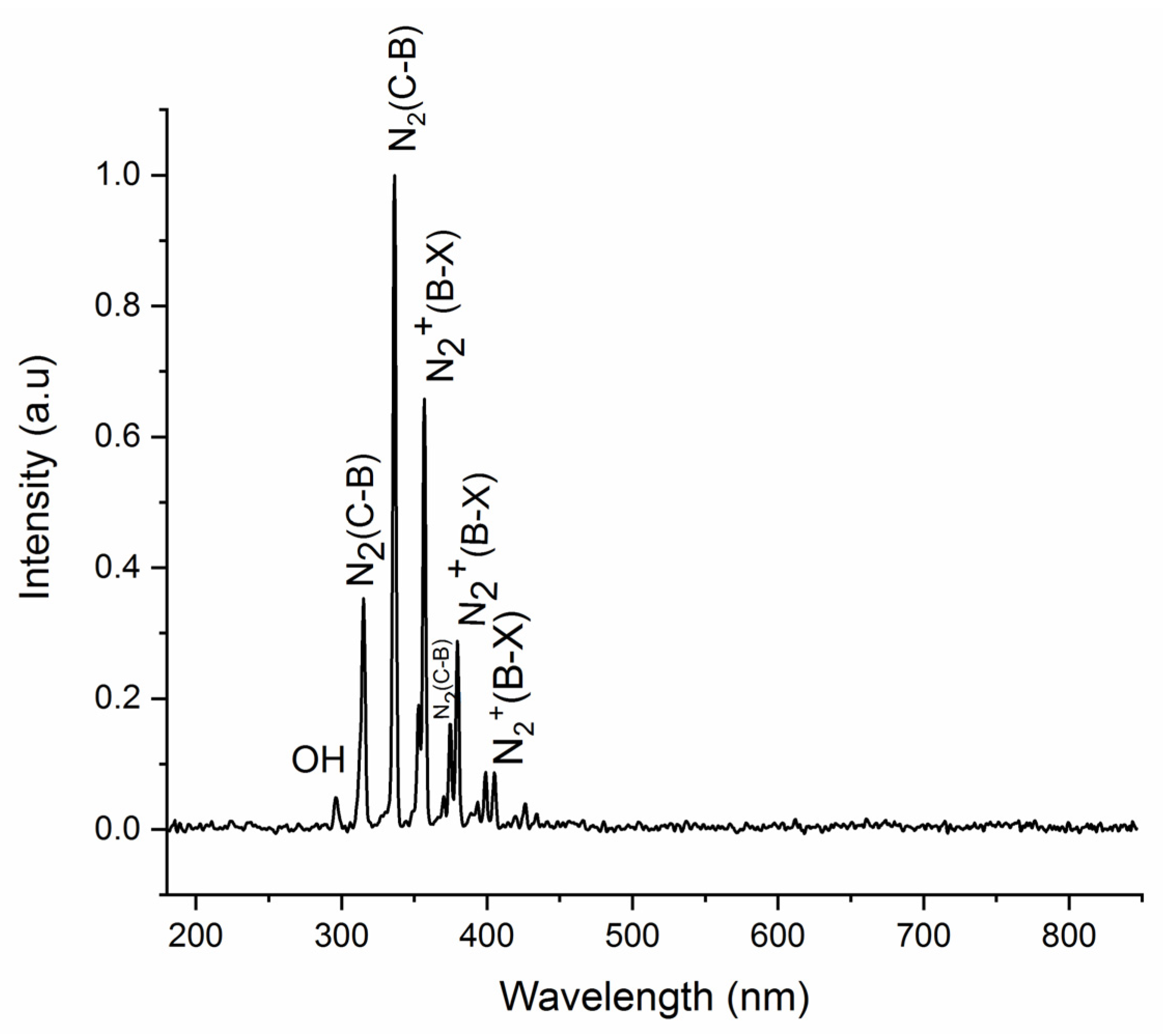
3.2. Cold Plasma Diagnostics
3.3. Ozone, Nitrous Gas, and Hydrogen Peroxide Concentration in DBD ACP
3.4. Effect of MC of Barley Grains and Environmental RH on DON Degradation by ACP
3.5. Effect of Post-Treatment Storage of Barley Grains on DON Degradation by ACP
3.6. Effect of Steeping the Barley Grains on DON Degradation by ACP
3.7. Effect of ACP Treatment on Quality Parameters of Barley Grains
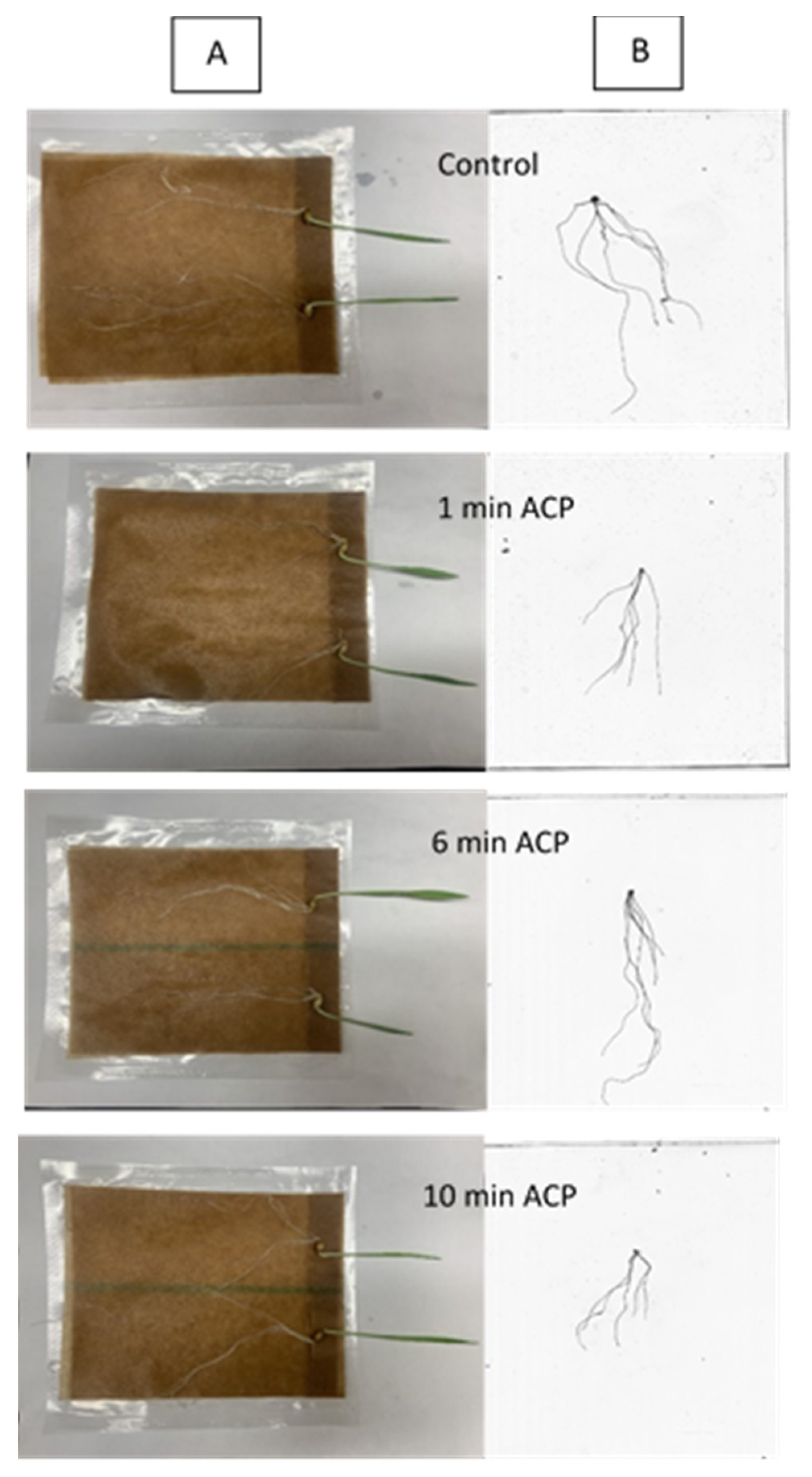
3.8. Effect of ACP Treatment on Germination of Barley Grains
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misra, N.; Yadav, B.; Roopesh, M.; Jo, C. Cold Plasma for Effective Fungal and Mycotoxin Control in Foods: Mechanisms, Inactivation Effects, and Applications. Compr. Rev. Food Sci. Food Saf. 2018, 18, 106–120. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Alizadeh, A.; Braber, S.; Akbari, P.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol and Its Modified Forms: Are There Major Differences? Toxins 2016, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, A.; Horsley, R.; Jack, M.M.; Kobielush, B.; Ryu, D.; Tittlemier, S.; Wilson, W.W.; Abbas, H.; Abel, S.; Harrison, G.; et al. DON Occurrence in Grains: A North American Perspective. Cereal Foods World 2015, 60, 32–56. [Google Scholar] [CrossRef]
- Bretz, M.; Beyer, M.; Cramer, B.; Knecht, A.; Humpf, H.-U. Thermal Degradation of theFusariumMycotoxin Deoxynivalenol. J. Agric. Food Chem. 2006, 54, 6445–6451. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Schmale, D.G., III; Munkvold, G.P. Mycotoxins in Crops: A Threat to Human and Domestic Animal Health. Available online: https://www.apsnet.org/edcenter/disimpactmngmnt/topc/Mycotoxins/Pages/EconomicImpact.aspx (accessed on 31 March 2020).
- Feizollahi, E.; Misra, N.; Roopesh, M. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit. Rev. Food Sci. Nutr. 2020, 1–24. [Google Scholar] [CrossRef]
- Misra, N.; Schlüter, O.; Cullen, P.J. Cold Plasma in Food and Agriculture: Fundamentals and Applications; Academic Press: Amsterdam, The Netherland, 2016; pp. 223–254. [Google Scholar]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Rosa, M.D.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.-J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process. Polym. 2016, 15, 1600056. [Google Scholar] [CrossRef]
- Kříž, P.; Petr, B.; Zbynek, H.; Jaromir, K.; Pavel, O.; Petr, S.; Miroslav, D.; Bartos, P.; Havelka, Z.; Kadlec, J.; et al. Influence of Plasma Treatment in Open Air on Mycotoxin Content and Grain Nutriments. Plasma Med. 2015, 5, 145–158. [Google Scholar] [CrossRef]
- Bosch, L.T.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Chigbo, E.O.J. Selected Physical Properties of Soybean In Relation To Storage Design. Int. J. Eng. Res. Appl. 2016, 6, 71–75. [Google Scholar]
- Lim, C.W.; Tai, S.H.; Lee, L.M.; Chan, S.H. Analytical method for the accurate determination of tricothecenes in grains using LC-MS/MS: A comparison between MRM transition and MS3 quantitation. Anal. Bioanal. Chem. 2011, 403, 2801–2806. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.; Von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Van Gils, K.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Butscher, D.; Van Loon, H.; Waskow, A.; Von Rohr, P.R.; Schuppler, M. Plasma inactivation of microorganisms on sprout seeds in a dielectric barrier discharge. Int. J. Food Microbiol. 2016, 238, 222–232. [Google Scholar] [CrossRef]
- Technical, A. Crude Protein--Combustion Method. AACC Int. Approv. Methods 2009, 3. [Google Scholar] [CrossRef]
- Technical, A. beta-Glucan Content of Barley and Oats--Rapid Enzymatic Procedure. AACC Int. Approv. Methods 2009, 3. [Google Scholar] [CrossRef]
- Technical, A. Moisture--Air-Oven Method, Drying at 135 degrees. AACC Int. Approv. Methods 2009, 3. [Google Scholar] [CrossRef]
- Wang, L.; Shao, H.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Luo, Y.; Chen, Z. Effect of Ozone Treatment on Deoxynivalenol and Wheat Quality. PLoS ONE 2016, 11, e0147613. [Google Scholar] [CrossRef]
- Alexandre, A.P.S.; Paredes, R.S.V.; Santos, A.S.; Costa, N.S.; Canniatti-Brazaca, S.G.; Calori-Domingues, M.A.; Augusto, P. Ozone treatment to reduce deoxynivalenol (DON) and zearalenone (ZEN) contamination in wheat bran and its impact on nutritional quality. Food Addit. Contam. Part A 2018, 35, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Schoenbach, K.; Kogelschatz, U.; Vidmar, R.; Kuo, S.; Schmidt, M.; Behnke, J.; Yukimura, K.; Stoffels, E. Current applications of atmospheric pressure air plasmas. In Non-Equilibrium Air Plasmas at Atmospheric Pressure, Series in Plasma Physics; Institute of Physics Publishing: Bristol, UK; Philadelphia, PA, USA, 2005; pp. 537–672. [Google Scholar]
- Chang, M.B.; Wu, S.-J. Experimental Study on Ozone Synthesis via Dielectric Barrier Discharges. Ozone Sci. Eng. 1997, 19, 241–254. [Google Scholar] [CrossRef]
- Hong, Z.; Hiett, K.L.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C.; Keener, K.M. In-Package Air Cold Plasma Treatment of Chicken Breast Meat: Treatment Time Effect. J. Food Qual. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Patil, S.; Moiseev, T.; Misra, N.; Cullen, P.J.; Mosnier, J.-P.; Keener, K.; Bourke, P. Influence of high voltage atmospheric cold plasma process parameters and role of relative humidity on inactivation of Bacillus atrophaeus spores inside a sealed package. J. Hosp. Infect. 2014, 88, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, P.; Cheng, Y.; Peng, P.; Liu, J.; Ma, Y.; Liu, Y.; Ruan, R. Deoxynivalenol Decontamination in Raw and Germinating Barley Treated by Plasma-Activated Water and Intense Pulsed Light. Food Bioprocess Technol. 2018, 12, 246–254. [Google Scholar] [CrossRef]
- Feizollahi, E.; Arshad, M.; Yadav, B.; Ullah, A.; Roopesh, M.S. Degradation of deoxynivalenol by atmospheric-pressure cold plasma and hurdle treatments. Food Eng. Rev. under review.
- Abramson, D.; House, J.D.; Nyachoti, C.M. Reduction of Deoxynivalenol in Barley by Treatment with Aqueous Sodium Carbonate and Heat. Mycopathologia 2005, 160, 297–301. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Govindan, B.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M. Cold plasma treatment of ready-to-eat ham: Influence of process conditions and storage on inactivation of Listeria innocua. Food Res. Int. 2019, 123, 276–285. [Google Scholar] [CrossRef]
- Machala, Z.; Janda, M.; Hensel, K.; Jedlovský, I.; Leštinská, L.; Foltin, V.; Martisovits, V.; Morvová, M. Emission spectroscopy of atmospheric pressure plasmas for bio-medical and environmental applications. J. Mol. Spectrosc. 2007, 243, 194–201. [Google Scholar] [CrossRef]
- Walsh, J.L.; Liu, D.X.; Iza, F.; Rong, M.Z.; Kong, M.G. Contrasting characteristics of sub-microsecond pulsed atmospheric air and atmospheric pressure helium–oxygen glow discharges. J. Phys. D Appl. Phys. 2010, 43, 32001. [Google Scholar] [CrossRef]
- Morgan, N. Atmospheric pressure dielectric barrier discharge chemical and biological applications. Int. J. Phys. Sci. 2009, 4, 885–892. [Google Scholar]
- Lee, G.J.; Sim, G.B.; Choi, E.H.; Kwon, Y.-W.; Kim, J.Y.; Jang, S.; Kim, S.H. Optical and structural properties of plasma-treated Cordyceps bassiana spores as studied by circular dichroism, absorption, and fluorescence spectroscopy. J. Appl. Phys. 2015, 117, 023303. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2018, 52, 034002. [Google Scholar] [CrossRef]
- Locke, B.R.; Shih, K.-Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci. Technol. 2011, 20, 34006. [Google Scholar] [CrossRef]
- Shi, H.; Cooper, B.; Stroshine, R.L.; Ileleji, K.E.; Keener, K.M. Structures of Degradation Products and Degradation Pathways of Aflatoxin B1 by High-Voltage Atmospheric Cold Plasma (HVACP) Treatment. J. Agric. Food Chem. 2017, 65, 6222–6230. [Google Scholar] [CrossRef]
- Berardinelli, A.; Vannini, L.; Ragni, L.; Guerzoni, M.E. Impact of Atmospheric Plasma Generated by a DBD Device on Quality-Related Attributes of “Abate Fetel” Pear Fruit. In Counteraction to Chemical and Biological Terrorism in East European Countries; Springer Science and Business Media LLC: Berlin, Germany, 2011; pp. 457–467. [Google Scholar]
- Falkenstein, Z.; Coogan, J.J. Microdischarge behaviour in the silent discharge of nitrogen—Oxygen and water—Air mixtures. J. Phys. D Appl. Phys. 1997, 30, 817–825. [Google Scholar] [CrossRef]
- Klockow, P.A.; Keener, K.M. Safety and quality assessment of packaged spinach treated with a novel ozone-generation system. LWT 2009, 42, 1047–1053. [Google Scholar] [CrossRef]
- Omurtag, G.Z.; Beyoglu, D. Occurrence of deoxynivalenol (vomitoxin) in beer in Turkey detected by HPLC. Food Control. 2007, 18, 163–166. [Google Scholar] [CrossRef]
- Alexandre, A.P.S.; Castanha, N.; Calori-Domingues, M.A.; Augusto, P. Ozonation of whole wheat flour and wet milling effluent: Degradation of deoxynivalenol (DON) and rheological properties. J. Environ. Sci. Health Part B 2017, 52, 516–524. [Google Scholar] [CrossRef]
- Butscher, D.; Zimmermann, D.; Schuppler, M.; Von Rohr, P.R. Plasma inactivation of bacterial endospores on wheat grains and polymeric model substrates in a dielectric barrier discharge. Food Control. 2016, 60, 636–645. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bajgai, T.R.; Raghavan, G.S.V.; Hashinaga, F.; Ngadi, M.O. Electrohydrodynamic Drying—A Concise Overview. Dry. Technol. 2006, 24, 905–910. [Google Scholar] [CrossRef]
- Wang, Y.; Thorup-Kristensen, K.; Jensen, L.S.; Magid, J. Vigorous Root Growth Is a Better Indicator of Early Nutrient Uptake than Root Hair Traits in Spring Wheat Grown under Low Fertility. Front. Plant Sci. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Sera, B.; Spatenka, P.; Sery, M.; Vrchotova, N.; Hruskova, I. Influence of Plasma Treatment on Wheat and Oat Germination and Early Growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Filatova, I.; Azharonok, V.; Lushkevich, V.; Zhukovsky, A.; Gadzhieva, G.; Spasic, K.; Zivkovic, S.; Puac, N.; Lazovic, S.; Malovic, G. Plasma seeds treatment as a promising technique for seed germination improvement. In Proceedings of the 31st International Conference on Phenomena in Ionized Gases, Granada, Spain, 14–19 July 2013; pp. 4–7. [Google Scholar]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of Atmospheric Pressure Air Plasma Pretreatment on the Seed Germination and Early Growth ofAndrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
| Crop Year | MC (g Water/100 g Sample) | aw | Grain Density (g/cm3) | Grain Dimensions | |
|---|---|---|---|---|---|
| Length (mm) | Width (mm) | ||||
| 2015 | 10.8 ± 1.7 | 0.450 ± 0.003 | 1.19 ± 0.09 | 9.32 ± 0.97 | 3.88 ± 0.02 |
| ACP Treatment Time(s) | O3 (ppm) | H2O2 (ppm) | NOx (ppm) |
|---|---|---|---|
| 60–80 | 600 | 100 | 400 |
| 360–380 | 675 | 150 | 470 |
| 600–620 | 675 | 200 | 480 |
| Plasma Time (min) | MC (g Water/100 g Sample) | DON Reduction (%) |
|---|---|---|
| 0 | 9.5 ± 0.0 | 0 b |
| 6 | 9.5 ± 0.3 | 54.4 ± 2.2 a |
| 6 | 14.9 ± 0.3 | 49.8 ± 5.9 a |
| 6 | 15.7 ± 0.2 | 47.0 ± 5.9 a |
| RH (%) | ||
| 0 | 12 | 0 b |
| 6 | 12 | 62.9 ± 3.3 a |
| 6 | 60 | 65.7 ± 3.0 a |
| Treatment | DON Degradation Rate (%) |
|---|---|
| No treatment, no storage | 0 d |
| 0 min ACP, 24 h storage | 8.1 ± 2.7 c |
| 6 min ACP, no storage | 45.0 ± 0.9 b |
| 6 min ACP, 24 h storage | 53.6 ± 1.8 a |
| 6 min ACP, 10 min storage inside treatment chamber | 52.0 ± 1.6 a |
| Treatment | Solvent (ACN:Water) | Treatment Description | Reduction (%) |
|---|---|---|---|
| A | 100:0 | 10 min drying + 0 min ACP | 0 e |
| B | 100:0 | 10 min drying + 6 min ACP | 36.3 ± 5.4 c |
| C | 100:0 | 0 min drying + 6 min ACP | 66.3 ± 2.3 a |
| D | 20:80 | 10 min drying + 0 min ACP | 15.7 ± 4.6 d |
| E | 20:80 | 10 min drying +6 min ACP | 53.6 ± 1.0 b |
| F | 20:80 | 0 min drying + 6 min ACP | 65.6 ± 7.5 a |
| Treatment | N2 (%) | Protein (%) | Carbon (%) | β-Glucan (%) | MC (g Water/100 g Sample) |
|---|---|---|---|---|---|
| Control | 1.71 ± 0.02 a | 10.68 ± 0.15 a | 44.1 ± 0.2 a | 3.96 ± 0.14 a | 9.7 ± 0.1 a |
| 6 min ACP | 1.62 ± 0.05 a | 10.39 ± 0.33 a | 44.07 ± 0.21 a | 3.98 ± 0.08 a | 9.6 ± 0.0 a |
| 10 min ACP | 1.64 ± 0.05 a | 10.26 ± 0.29 a | 43.93 ± 0.55 a | 4.23 ± 0.25 a | 9.4 ± 0.2 a |
| Treatment | Average Root Length (cm) | Average Root Surface Area (cm2) | Average Root Diameter (mm) | Root Volume (cm3) | Shoot Length (cm) | Number of Roots | Germination Percentage (%) |
|---|---|---|---|---|---|---|---|
| Control | 44.2 ± 17.8 a | 6.37 ± 2.52 a | 0.462 ± 0.037 a | 0.073 ± 0.029 ab | 6.76 ± 1.72 a | 5.67 ± 0.64 a | 80 |
| 1 min ACP | 33.7 ± 19.4 ab | 4.88 ± 2.50 b | 0.495 ± 0.11 a | 0.058 ± 0.026 b | 6.40 ± 1.92 a | 5.16 ± 1.49 a | 83.3 |
| 6 min ACP | 42.2 ± 15.7 ab | 6.35 ± 2.23 a | 0.489 ± 0.060 a | 0.078 ± 0.029 a | 7.36 ± 1.62 a | 5.46 ± 0.84 a | 93.3 |
| 10 min ACP | 32.6 ± 20.0 b | 4.84 ± 2.50 b | 0.498 ± 0.066 a | 0.058 ± 0.027 b | 6.26 ± 2.07 a | 5.15 ± 1.26 a | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feizollahi, E.; Iqdiam, B.; Vasanthan, T.; Thilakarathna, M.S.; Roopesh, M.S. Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains. Appl. Sci. 2020, 10, 3530. https://doi.org/10.3390/app10103530
Feizollahi E, Iqdiam B, Vasanthan T, Thilakarathna MS, Roopesh MS. Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains. Applied Sciences. 2020; 10(10):3530. https://doi.org/10.3390/app10103530
Chicago/Turabian StyleFeizollahi, Ehsan, Basheer Iqdiam, Thava Vasanthan, Malinda S. Thilakarathna, and M. S. Roopesh. 2020. "Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains" Applied Sciences 10, no. 10: 3530. https://doi.org/10.3390/app10103530
APA StyleFeizollahi, E., Iqdiam, B., Vasanthan, T., Thilakarathna, M. S., & Roopesh, M. S. (2020). Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains. Applied Sciences, 10(10), 3530. https://doi.org/10.3390/app10103530





