Bioremediation Methods for the Recovery of Lead-Contaminated Soils: A Review
Abstract
:1. Introduction
2. Lead Toxic Effects
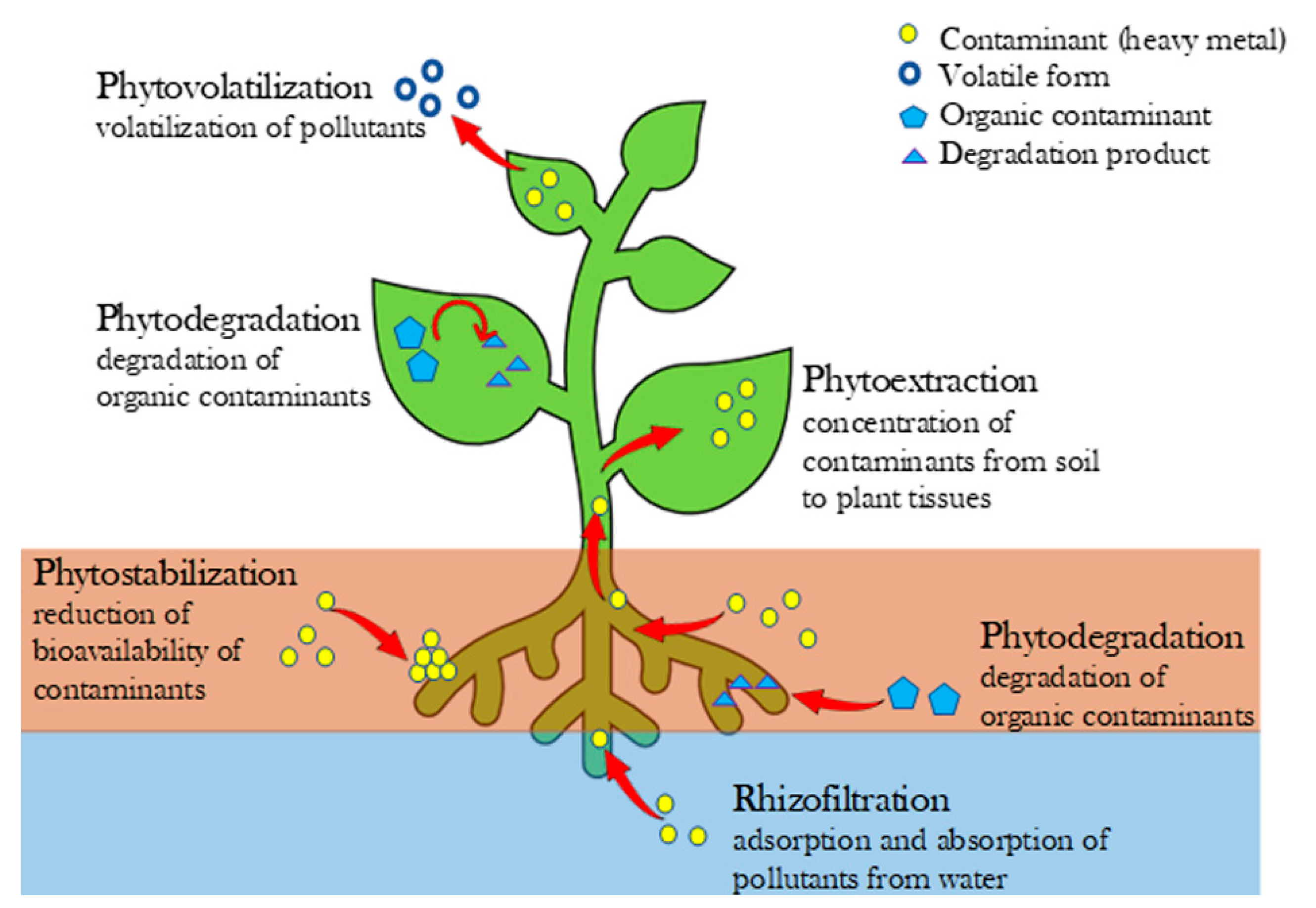
3. Phytoremediation
- (1)
- phytoextraction, when contaminants are concentrated from soil to plant tissues;
- (2)
- phytodegradation, if plants are able to degrade organic contaminants;
- (3)
- rhizofiltration, when exploiting the capability of plant’s roots to remove pollutants from contaminated water, adsorbing them into the extracellular negatively-charged residues present on the outer coatings of the roots and absorbing them through membrane proteins that act as carrier molecules [35];
- (4)
- phytostabilization, when plant’s roots are able to reduce the bioavailability of contaminants in the soil;
- (5)
- phytovolatilization by obtaining the pollutants’ volatilization.
- (a)
- accumulators that concentrate metals in above-ground tissues;
- (b)
- indicators that regulate uptake and transport of metals so that the internal concentration reflects the external concentration;
- (c)
- excluders for which an accumulation of heavy metals occurs in the roots, but the entry and transport in the aerial parts is limited [32].
3.1. Phytoremediation Capability of Spontaneously Grown Plants
- (a)
- diluted solution of strong acids (HCl and HNO3), acetic acid, and acidified CaCl2;
- (b)
- chelating agent solutions (EDTA and DTPA), mix of low molecular weight organic acids, (LMWOAs), and neutral salt solution (MgCl2);
- (c)
- neutral salt solutions and bidistilled water (BDW).
3.2. Phytoremediation Capability of Plants in Spiked Soils
3.3. In Field Phytoremediation
3.4. Limitation and Disadvantages of Phytoremediation
- soil preparation time, period of planting, and need for irrigation depend on the frequency and intensity of rainfall as well as snowmelt;
- fluctuations in air temperature that influence plants growth performance and are affected in turn by sunlight;
- natural (vegetation) or artificial (neighboring buildings) shading that can modify plants developing capability;
- the growing season length must be taken into account in the forecast of necessary times for recovery since phytoremediation processes are more likely to be active during this period;
- wind affects evaporation and can damage plants with dispersion of volatiles substances and debris;
- all the factors described above are influenced by regional and local weather patterns.
3.5. Phytoremediation By-Products
4. Bioremediation via Fungi and Bacteria
- (a)
- the concentration and bioavailability of the contaminants;
- (b)
- the characteristics of the treated site (e.g., pH, the redox potential, and the oxygen content);
- (c)
- availability of nutrients;
- (d)
- humidity;
- (e)
- temperature (it influences the metabolism of microorganisms) [62].
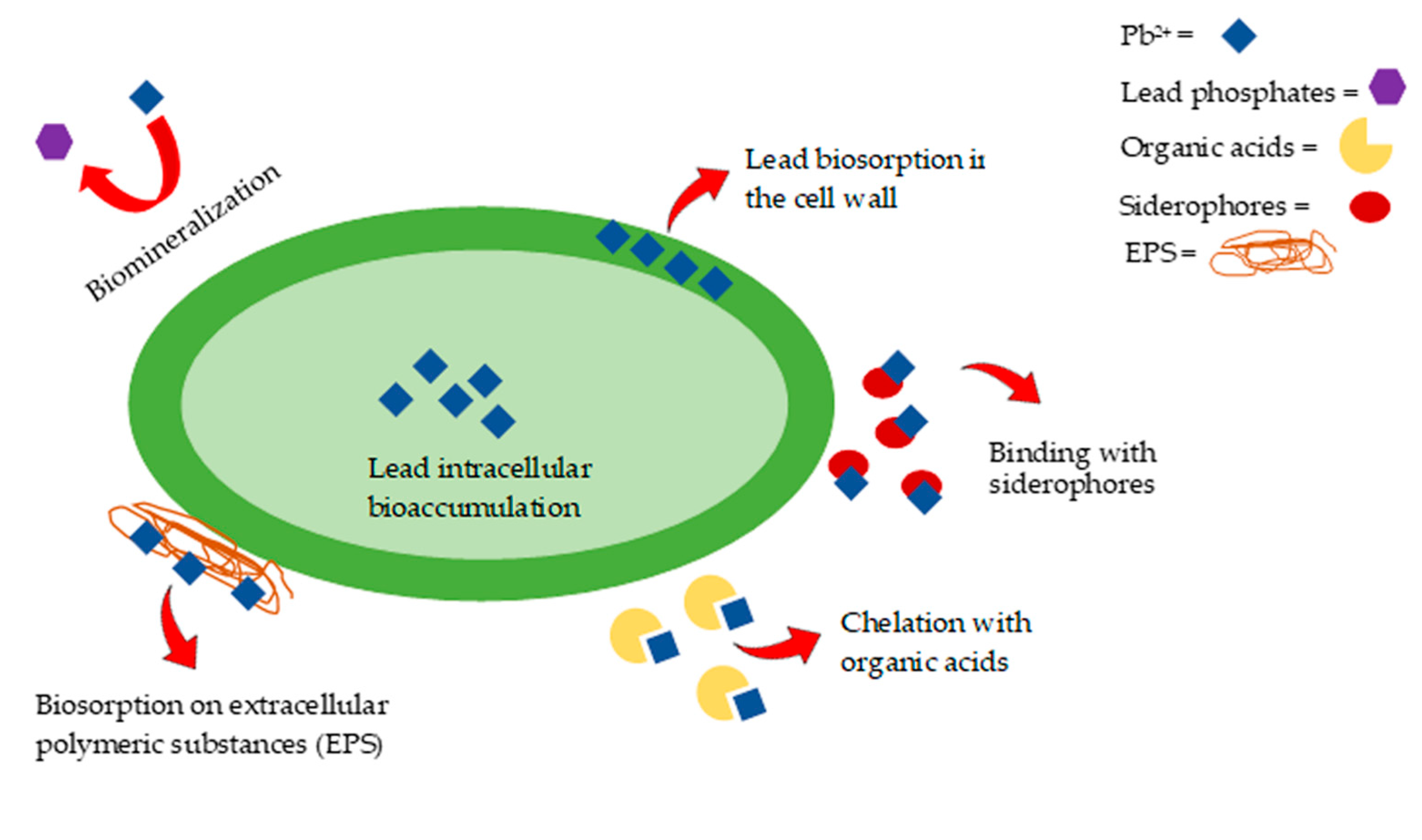
4.1. Bioremediation by Fungi
- (1)
- the chelation between the fungus and lead by means of active functional groups, such as carboxylic or hydroxyl groups present on the surface of the fungus cell walls,
- (2)
- an enhanced decomposition of organic matter with the consequent augmentation of the humic substances content and capability to immobilize Pb,
- (3)
- an increase in pH with the resulting reduced solubility of the metal [74].
4.2. Bioremediation by Bacteria
4.3. Bioremediation of Organic Lead
5. Bioaugmentation-Assisted Phytoremediation
6. Conclusions
Funding
Conflicts of Interest
References
- Mahaffey, K.R. Introduction: Advances in Lead Research: Implications for Environmental Health. Environ. Health Perspect. 1990, 89, 3. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Directive 2002/95/EC; European Commission: Brussels, Belgium, 2002; pp. 19–23. [Google Scholar]
- Ross, S.M. (Ed.) Toxic Metals in Soil-Plant Systems; Wiley: Chichester, UK, 1994. [Google Scholar]
- Tong, S.; von Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem of global dimensions. Bull. World Health Organ. 2000, 78, 1068–1077. [Google Scholar]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, E.G.; Pacyna, J.M.; Fudala, J.; Strzelecka-Jastrzab, E.; Hlawiczka, S.; Panasiuk, D.; Nitter, S.; Pregger, T.; Pfeiffer, H.; Friedrich, R. Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmos. Environ. 2007, 41, 8557–8566. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M. Soil lead bioavailability and in situ remediation of lead-contaminated soils: A review. Environ. Prog. 2004, 23, 78–93. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley & Sons: Chichester, UK, 1979. [Google Scholar]
- Nriagu, J.O. Lead orthophosphates—II. Stability of cholopyromophite at 25 °C. Geochim. Cosmochim. Acta 1973, 37, 367–377. [Google Scholar] [CrossRef]
- Johnson, F.M. The genetic effects of environmental lead. Mutat. Res./Rev. Mutat. Res. 1998, 410, 123–140. [Google Scholar] [CrossRef]
- Casas, J.S.; Sordo, J. Lead: Chemistry, Analytical Aspects, Environmental Impact and Health Effects; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Available online: http://www.atsdr.cdc.gov (accessed on 25 March 2020).
- Schreck, E.; Foucault, Y.; Sarret, G.; Sobanska, S.; Cecillon, L.; Castrec-Rouelle, M.; Uzu, G.; Dumat, C. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: Mechanisms involved for lead. Sci. Total Environ. 2012, 427–428, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.; Corzo, A.; Leigh, R.A.; Sanders, D. Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 1994, 5, 683–694. [Google Scholar] [CrossRef]
- Huang, J.W.; Grunes, D.L.; Kochian, L.V. Voltage-dependent Ca2+ influx into right-side-out plasma membrane vesicles isolated from wheat roots: Characterization of a putative Ca2+ channel. Proc. Natl. Acad. Sci. USA 1994, 91, 3473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Qados, A.M.S. Phytoremediation of Pb and Cd by native tree species grown in the Kingdom of Saudi Arabia. Agric. Biol. J. N. Am. 2015, 6, 8–21. [Google Scholar] [CrossRef]
- Ribeiro de Souza, S.C.; Adrian Lopez de Andrade, S.; Anjos de Souza, L.; Schiavinato, M.A. Lead tolerance and phytoremediation potential of Brazilian leguminous tree species at the seedling stage. J. Environ. Manag. 2012, 110, 299–307. [Google Scholar] [CrossRef]
- McBride, M.B. Arsenic and Lead Uptake by Vegetable Crops Grown on Historically Contaminated Orchard Soils. Appl. Environ. Soil Sci. 2013, 2013, 283472. [Google Scholar] [CrossRef] [Green Version]
- Saumel, I.; Kotsyuk, I.; Holscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Finster, M.E.; Gray, K.A.; Binns, H.J. Lead levels of edibles grown in contaminated residential soils: A field survey. Sci. Total Environ. 2004, 320, 245–257. [Google Scholar] [CrossRef]
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Weigert, P. Metal loads of food of vegetable origin including mushrooms. In Metals and Their Compounds in the Environment; Merian, E., Ed.; Wiley-VCH: Weinheim, Germany, 1991; pp. 449–468. [Google Scholar]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull. Environ. Contam. Toxicol. 2006, 77, 312–318. [Google Scholar] [CrossRef]
- Lacatusu, R.; Lacatusu, A.-R. Vegetable and fruits quality within heavy metals polluted areas in Romania. Carpathian J. Earth Environ. Sci. 2008, 3, 115–129. [Google Scholar]
- Farooq, M.; Anwar, F.; Rashid, U. Appraisal of heavy metal contents in different vegetables grown in the vicinity of an industrial area. Pak. J. Bot. 2008, 40, 2099–2106. [Google Scholar]
- Zhu, Y.G.; Chen, S.B.; Yang, J.C. Effects of soil amendments on lead uptake by two vegetable crops from a lead-contaminated soil from Anhui, China. Environ. Int. 2004, 30, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, V.; Swamy, A.V.V.S. Phytoremediation of Pb and Ni Contaminated Soils Using Catharanthus roseus (L.). Univers. J. Environ. Res. Technol. 2013, 3, 465–472. [Google Scholar]
- Lago-Vila, M.; Arenas-Lago, D.; Rodriguez-Seijo, A.; Andrade, M.L.; Vega, F.A. Ability of Cytisus scoparius for phytoremediation of soils from a Pb/Zn mine: Assessment of metal bioavailability and bioaccumulation. J. Environ. Manag. 2019, 235, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals--concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, S.; Kirchner, G. Environmental processes affecting plant root uptake of radioactive trace elements and variability of transfer factor data: A review. J. Environ. Radioact. 2002, 58, 97–112. [Google Scholar] [CrossRef]
- Liu, J.-G.; Qu, P.; Zhang, W.; Dong, Y.; Li, L.; Wang, M.-X. Variations among rice cultivars in subcellular distribution of Cd: The relationship between translocation and grain accumulation. Environ. Exp. Bot. 2014, 107, 25–31. [Google Scholar] [CrossRef]
- Yadav, B.K.; Siebel, M.A.; van Bruggen, J.J.A. Rhizofiltration of a Heavy Metal (Lead) Containing Wastewater Using the Wetland Plant Carex pendula. Clean-Soil Air Water 2011, 39, 467–474. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders -strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Rotkittikhun, P.; Kruatrachue, M.; Chaiyarat, R.; Ngernsansaruay, C.; Pokethitiyook, P.; Paijitprapaporn, A.; Baker, A.J. Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ. Pollut. 2006, 144, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Islam, E.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q. Comparison of synthetic chelators and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J. Hazard. Mater. 2008, 153, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bassegio, C.; Campagnolo, M.A.; Schwantes, D.; Goncalves Junior, A.C.; Manfrin, J.; Schiller, A.D.P.; Bassegio, D. Growth and accumulation of Pb by roots and shoots of Brassica juncea L. Int. J. Phytoremediation 2020, 22, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Sugawara, K.; Huang, Y.; Chien, M.F.; Inoue, C. Arsenic, lead and cadmium removal potential of Pteris multifida from contaminated water and soil. Int. J. Phytoremediation 2018, 20, 1187–1193. [Google Scholar] [CrossRef]
- Huang, Y.; Xi, Y.; Gan, L.; Johnson, D.; Wu, Y.; Ren, D.; Liu, H. Effects of lead and cadmium on photosynthesis in Amaranthus spinosus and assessment of phytoremediation potential. Int. J. Phytoremediation 2019, 21, 1041–1049. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Gurajala, H.K.; Cao, X.; Tang, L.; Ramesh, T.M.; Lu, M.; Yang, X. Comparative assessment of Indian mustard (Brassica juncea L.) genotypes for phytoremediation of Cd and Pb contaminated soils. Environ. Pollut. 2019, 254, 113085. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Mwegoha, W.J.S. The use of phytoremediation technology for abatment soil and groundwater pollution in Tanzania: Opportunities and challenges. J. Sustain. Dev. Afr. 2008, 10, 140–156. [Google Scholar]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Ma, L.Q.; Fayiga, A.O.; Zillioux, E.J. Phytoremediation of arsenic-contaminated groundwater by the arsenic hyperaccumulating fern Pteris vittata L. Int. J. Phytoremediation 2004, 6, 35–47. [Google Scholar] [CrossRef]
- Erakhrumen, A.A.; Agbontalor, A. Phytoremediation:an environmentally sound technology for pollution prevention, control and remediation in developing countries. Educ. Res. Rev. 2007, 2, 151–156. [Google Scholar]
- Plume Focus Area. Summary Report of a Workshop on Phytoremediation Research Needs; Energy, U.D.O., Ed.; Plume Focus Area: Santa Rosa, CA, USA, 1994. [Google Scholar]
- Agency, U.S.E.P. (Ed.) EPA/600/R-99/107: Introduction to Phytoremediation; EPA: Washington, DC, USA, 2002.
- Ginneken, L.; Meers, E.; Guisson, R.; Ruttens, A.; Elst, K.; Tack, F.; Vangronsveld, J.; Diels, L.; Dejonghe, W. Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J. Environ. Eng. Landsc. Manag. 2007, 15, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Witters, N.; Mendelsohn, R.O.; Van Slycken, S.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenergy 2012, 39, 454–469. [Google Scholar] [CrossRef]
- RECONnet. Tecniche di Fitorimedio Nella Bonifica dei siti Contaminati; Consiglio Nazionale delle Ricerche: Rome, Italy, 2017. [Google Scholar]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 10, 1383–1398. [Google Scholar] [CrossRef] [Green Version]
- Willscher, S.; Mirgorodsky, D.; Jablonski, L.; Ollivier, D.; Merten, D.; Büchel, G.; Wittig, J.; Werner, P. Field scale phytoremediation experiments on a heavy metal and uranium contaminated site, and further utilization of the plant residues. Hydrometallurgy 2013, 131–132, 46–53. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Ivanov, K. Heavy Metal Accumulation and Distribution in Oil Crops. Commun. Soil Sci. Plant Anal. 2011, 35, 2551–2566. [Google Scholar] [CrossRef]
- Leung, M. Bioremediation:techniques for cleaning up a mess. J. Biotechnol. 2004, 2, 18–22. [Google Scholar]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugmention: A Review. Int. J. Environ. Bioremediation Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area. Appl. Environ. Microbiol. 2001, 67, 3127–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speight, J.G. Removal of Organic Compounds from the Environment. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; pp. 387–432. [Google Scholar] [CrossRef]
- Haleyur, N.; Shahsavari, E.; Jain, S.S.; Koshlaf, E.; Ravindran, V.B.; Morrison, P.D.; Osborn, A.M.; Ball, A.S. Influence of bioaugmentation and biostimulation on PAH degradation in aged contaminated soils: Response and dynamics of the bacterial community. J. Environ. Manag. 2019, 238, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Innemanová, P.; Filipová, A.; Michalíková, K.; Wimmerová, L.; Cajthaml, T. Bioaugmentation of PAH-contaminated soils: A novel procedure for introduction of bacterial degraders into contaminated soil. Ecol. Eng. 2018, 118, 93–96. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Zhang, X. Co-biodegradation of pyrene and other PAHs by the bacterium Acinetobacter johnsonii. Ecotoxicol. Environ. Saf. 2018, 163, 465–470. [Google Scholar] [CrossRef]
- Povedano-Priego, C.; Martín-Sánchez, I.; Jroundi, F.; Sánchez-Castro, I.; Merroun, M.L. Fungal biomineralization of lead phosphates on the surface of lead metal. Miner. Eng. 2017, 106, 46–54. [Google Scholar] [CrossRef]
- Rhee, Y.J.; Hillier, S.; Gadd, G.M. Lead transformation to pyromorphite by fungi. Curr. Biol. 2012, 22, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Skinner, H.C.W.; Jahren, A.H. Biomineralization. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 8, pp. 1–69. [Google Scholar]
- Ezzouhri, L.; Castro, E.; Moya, M.; Espinola, F.; Lairini, K. Heavy metal_tolerance of_filamentous fungi isolated from polluted sites in Tangier, Marocco. Afr. J. Microbiol. Res. 2009, 3, 35–48. [Google Scholar]
- Arwidsson, Z.; Johansson, E.; von Kronhelm, T.; Allard, B.; van Hees, P. Remediation of Metal Contaminated Soil by Organic Metabolites from Fungi I—Production of Organic Acids. Water Air Soil Pollut. 2009, 205, 215–226. [Google Scholar] [CrossRef]
- Iram, S.; Shabbir, R.; Zafar, H.; Javaid, M. Biosorption and Bioaccumulation of Copper and Lead by Heavy Metal-Resistant Fungal Isolates. Arab. J. Sci. Eng. 2015, 40, 1867–1873. [Google Scholar] [CrossRef]
- Huang, D.L.; Zeng, G.M.; Jiang, X.Y.; Feng, C.L.; Yu, H.Y.; Huang, G.H.; Liu, H.L. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. J. Hazard. Mater. 2006, 134, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.M.; Dubey, S.K. Lead resistant bacteria: Lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicol. Environ. Saf. 2013, 98, 1–7. [Google Scholar] [CrossRef]
- Tiquia-Arashiro, S.M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 2018, 102, 5437–5444. [Google Scholar] [CrossRef]
- Li, X.; Peng, W.; Jia, Y.; Lu, L.; Fan, W. Bioremediation of lead contaminated soil with Rhodobacter sphaeroides. Chemosphere 2016, 156, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Shao, W.; Zhang, K.; Huo, Y.; Zhu, J.; Li, M. Pb biosorption by Leclercia adecarboxylata: Protective and immobilized mechanisms of extracellular polymeric substances. Chem. Eng. J. 2019, 375. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D.; Fu, Q. Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. J. Microbiol. Biotechnol. 2012, 22, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.T.; Thomas, J.E.; Jing, T.W. Biological and chemical degradatiom of tetrarthyl lead in soil. Bull. Environ. Contam. Toxicol. 1994, 52. [Google Scholar] [CrossRef]
- Teeling, H.; Cypionka, H. Microbial degradation of tetraethyl lead in soil monitored by microcalorimetry. Appl. Microbiol. Biotechnol. 1997, 48, 275–279. [Google Scholar] [CrossRef]
- Blais, J.S.; Doige, C.A.; Marshall, W.D.; Knowles, R. Persistence and toxicity of alkyllead salts to anaerobic nitrogen transformations in soil. Arch. Environ. Contam. Toxicol. 1990, 19, 227–234. [Google Scholar] [CrossRef]
- Jing, Y.D.; He, Z.L.; Yang, X.E. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ.-Sci. B 2007, 8, 192–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braud, A.; Jezequel, K.; Bazot, S.; Lebeau, T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 2009, 74, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Wang, X.; Yang, X.; Cui, Z. Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere 2019, 224, 716–725. [Google Scholar] [CrossRef]
| Category | Extractant | Conditions |
|---|---|---|
| Bidistilled water | BDW | - |
| Neutral salt solutions | Ca(NO3)2 | 0.1 M |
| MgCl2 | 1 M | |
| NH4NO3 | 1 M | |
| NaNO3 | 0.1 M | |
| Mg(NO3)2 | 0.5 M | |
| CaCl2 (no ac) * | 0.01 M | |
| CaCl2 (ac) ** | 0.01 M with HCl (0.1 M) | |
| Organic acids | LMWOA (low molecular weight organic acids) | acetic, lactic, citric, malic, and formic acids (ratio 4:2:1:1:1) solution with a total concentration of 10 mM |
| HOAc | 0.11 M | |
| Chelating agents | EDTA | Na2-EDTA 0.01 M + CH3COONH4 1 M |
| DTPA | 0.005 M DTPA + 0.1 M TEA + 0.01 M CaCl2 | |
| Diluted acids | HCl | 0.1 M |
| HNO3 | 0.5 M |
| Study | Plant Species | Results | |
|---|---|---|---|
| Phytoremediation capability of spontaneously grown plants | Lago-Vila et al. [31] | C. scoparius | Accumulated and concentrated Pb mainly in roots, so acting as a lead phytostabilizer (TF < 1, BF > 1) |
| Rotkittikhun et al. [37] | Herbs: S. arvensis Co. sumatrensis Cyperus sp. Shrubs: C. odoratum B. asiatica | Hyperaccumulators: accumulation in shoots 10–500 times more than usual plants (lead 5 mg/kg) shoot accumulation > 1000 mg/kg shoot/root quotient >1, Perennial shrubs are preferable | |
| Phytoremediation capability of plants in spiked soils | Alaboudi et al. [43] | H. annuus (sunflower) | BF < 1, so cannot be considered an accumulator but an excluder |
| Subhashini et al. [30] | C. roseus | Accumulator (BF > 1 and TF < 1 in roots) so it is useful for phytostabilization | |
| Abdul Qados [19] | Trees: C. erectus | Accumulated Pb mainly in shoots | |
| E. rostrata, A. saligna | Possible hyperaccumulators (high concentrations in the above-ground tissues, no reduction of biomass) | ||
| In-the-field phytoremediation | Gurajala et al. [44] | B. juncea L. (Indian mustard), 80 types | Genotypes IM-24 and IM-32 had TF > 1 and accumulated more Pb than other varieties |
| Study | Species | Mechanism | |
|---|---|---|---|
| Bioremediation by fungi | Rhee et al. [69] | P. javanicus M. anisopliae | Precipitation of lead as chloropyromorphite |
| Povedano-Priego et al. [68] | A. niger P. chrysogenum T. viride | Precipitation of lead phosphate and biosorption | |
| Arwidsson et al. [72] | A. niger P. bilaiae P. sp | Chelation with LMWOAs | |
| Iram et al. [73] | A. niger | Biosorption | |
| Huang et al. [74] | P. chrysosporium | Chelation with active functional groups Binding with humic substances, pH increase, and solubility reduction | |
| Bioremediation by bacteria | Li et al. [77] | R. sphaeroides | Immobilization as inert forms |
| Teng et al. [78] | L. adecarboxylata | Binding with EPS | |
| Achal et al. [79] | K. flava | Chelation by calcite |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoletto, M.; Calza, P.; Gaggero, E.; Malandrino, M.; Fabbri, D. Bioremediation Methods for the Recovery of Lead-Contaminated Soils: A Review. Appl. Sci. 2020, 10, 3528. https://doi.org/10.3390/app10103528
Rigoletto M, Calza P, Gaggero E, Malandrino M, Fabbri D. Bioremediation Methods for the Recovery of Lead-Contaminated Soils: A Review. Applied Sciences. 2020; 10(10):3528. https://doi.org/10.3390/app10103528
Chicago/Turabian StyleRigoletto, Monica, Paola Calza, Elisa Gaggero, Mery Malandrino, and Debora Fabbri. 2020. "Bioremediation Methods for the Recovery of Lead-Contaminated Soils: A Review" Applied Sciences 10, no. 10: 3528. https://doi.org/10.3390/app10103528







