Potential of Chrozophora tinctoria Seed Oil as a Biodiesel Resource
Abstract
:Featured Application
Abstract
1. Introduction
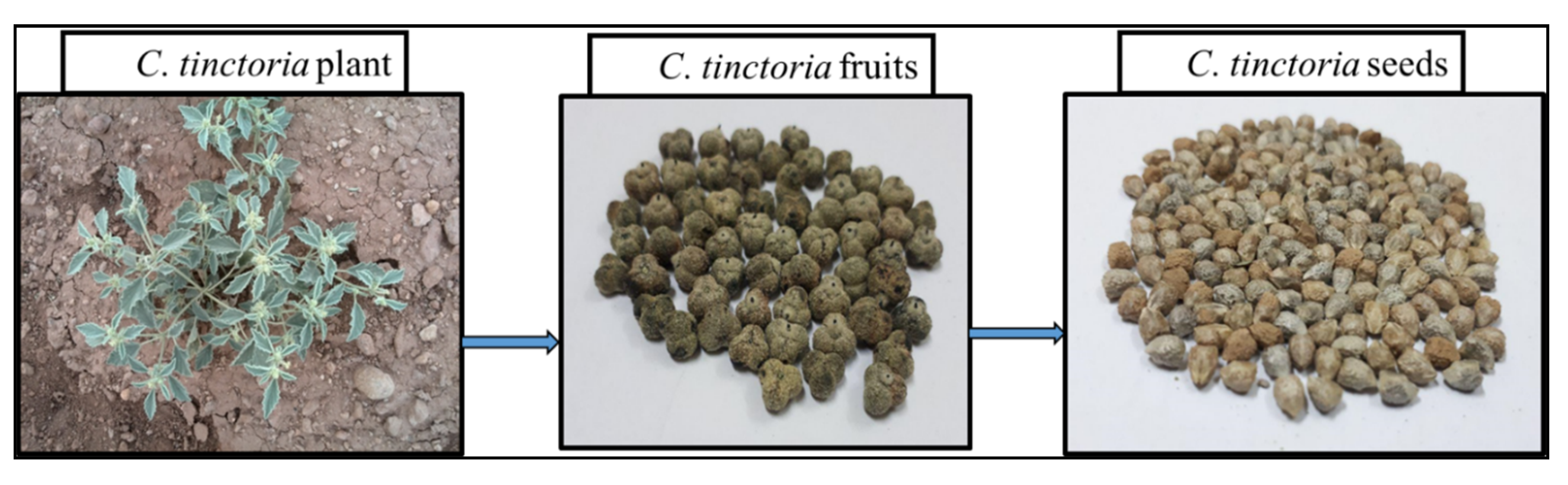
1.1. Why Chrozophora tinctoria?
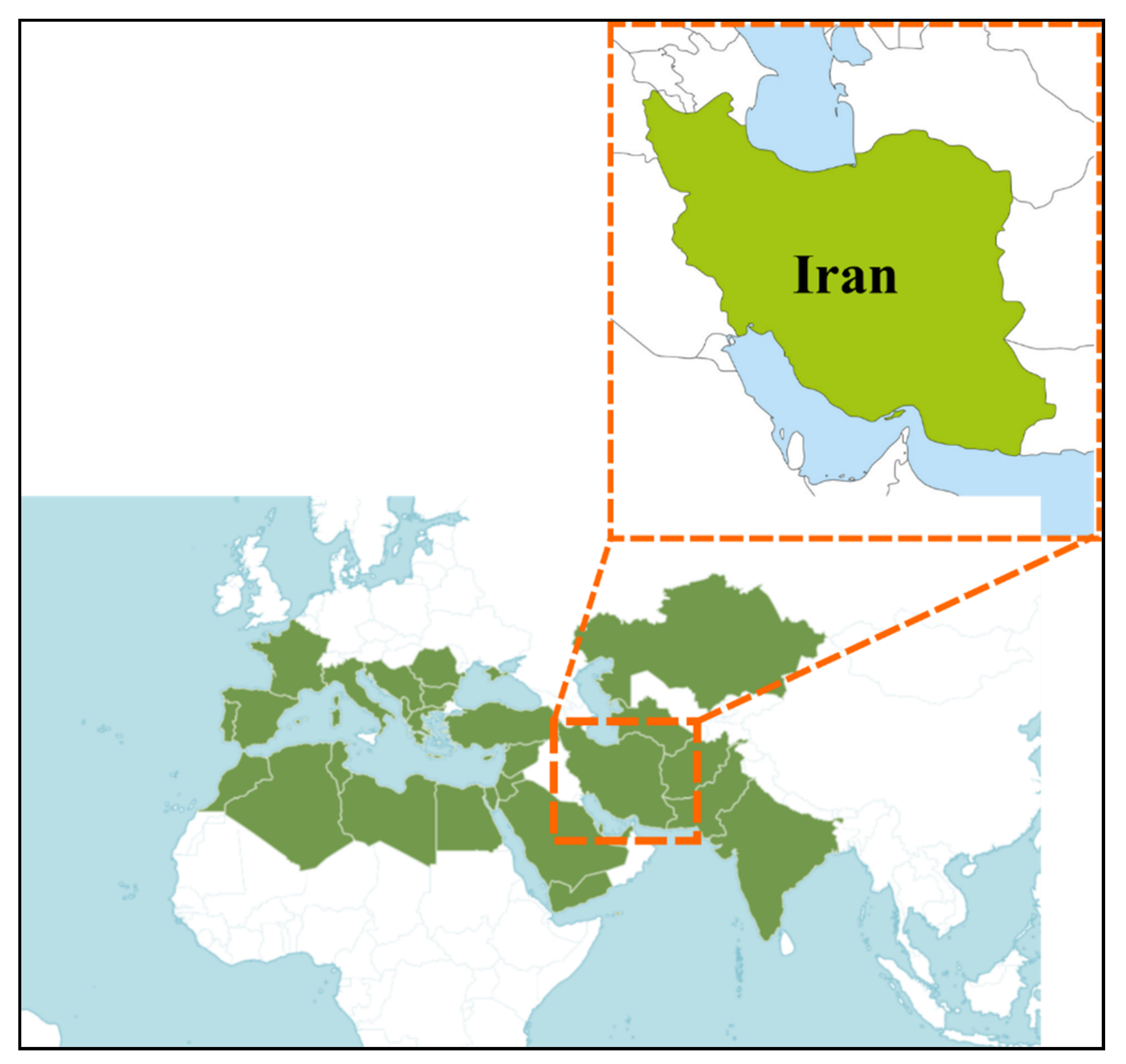
1.2. C. tinctoria in the World and Iran
2. Materials and Methods
2.1. Raw Materials
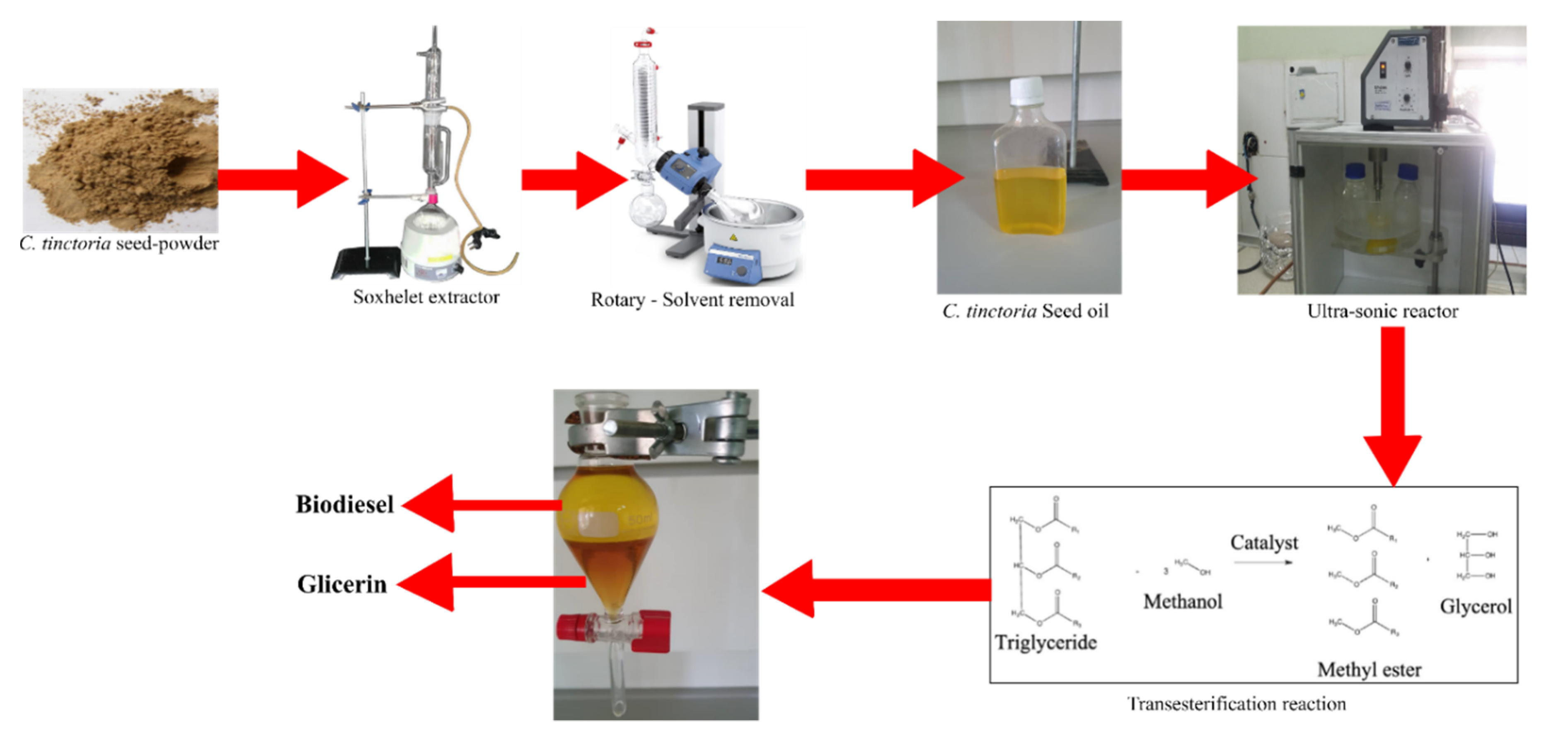
2.2. Methodology
3. Results and Discussion
3.1. Seed Oil Properties
3.2. Fatty Acid Profile
3.3. C. tinctoria Biodiesel Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Sidik, N.A.C.; Azmi, W.H. The effect of combustion management on diesel engine emissions fueled with biodiesel-diesel blends. Renew. Sustain. Energy Rev. 2017, 73, 307–331. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Xue, J. Combustion characteristics, engine performances and emissions of waste edible oil biodiesel in diesel engine. Renew. Sustain. Energy Rev. 2013, 23, 350–365. [Google Scholar] [CrossRef]
- Shahid, E.M.; Jamal, Y. Production of biodiesel: A technical review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Makareviciene, V.; Skorupskaite, V.; Levisauskas, D.; Andruleviciute, V.; Kazancev, K. The optimization of biodiesel fuel production from microalgae oil using response surface methodology. Int. J. Green Energy 2014, 11, 527–541. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Demirbas, A.; Karslioglu, S. Biodiesel production facilities from vegetable oils and animal fats. Energy Sources Part A 2007, 29, 133–141. [Google Scholar] [CrossRef]
- Yusuf, N.; Kamarudin, S.K.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Canakci, M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef]
- Balat, M. Potential alternatives to edible oils for biodiesel production—A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Atabani, A.; Badruddin, I.A.; Badarudin, A.; Khayoon, M.; Triwahyono, S. Recent scenario and technologies to utilize non-edible oils for biodiesel production. Renew. Sustain. Energy Rev. 2014, 37, 840–851. [Google Scholar]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Ayoob, A.K.; Fadhil, A.B. Valorization of waste tires in the synthesis of an effective carbon based catalyst for biodiesel production from a mixture of non-edible oils. Fuel 2020, 264, 116754. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.; Lim, S.; Lee, H. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Malani, R.S.; Shinde, V.; Ayachit, S.; Goyal, A.; Moholkar, V.S. Ultrasound–assisted biodiesel production using heterogeneous base catalyst and mixed non–edible oils. Ultrason. Sonochem. 2019, 52, 232–243. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A. Optimization of biodiesel production from mixture of edible and nonedible vegetable oils. Biocatal. Agric. Biotechnol. 2016, 8, 112–120. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Dubey, A.M.; Pal, A. Performance and emission characteristics of a transportation diesel engine operated with non-edible vegetable oils biodiesel. Case Stud. Therm. Eng. 2016, 8, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Devi, A.; Das, V.K.; Deka, D. Ginger extract as a nature based robust additive and its influence on the oxidation stability of biodiesel synthesized from non-edible oil. Fuel 2017, 187, 306–314. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Ong, H.C.; Mofijur, M.; Silitonga, A.; Gumilang, D.; Kusumo, F.; Mahlia, T. Physicochemical Properties of Biodiesel Synthesised from Grape Seed, Philippine Tung, Kesambi, and Palm Oils. Energies 2020, 13, 1319. [Google Scholar] [CrossRef] [Green Version]
- Corral Bobadilla, M.; Fernández Martínez, R.; Lostado Lorza, R.; Somovilla Gómez, F.; Vergara González, E.P. Optimizing Biodiesel Production from Waste Cooking Oil Using Genetic Algorithm-Based Support Vector Machines. Energies 2018, 11, 2995. [Google Scholar] [CrossRef] [Green Version]
- Corral Bobadilla, M.; Lostado Lorza, R.; Escribano García, R.; Somovilla Gómez, F.; Vergara González, E.P. An improvement in biodiesel production from waste cooking oil by applying thought multi-response surface methodology using desirability functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, C.K.; Naik, C.V.; Herbinet, O.; Pitz, W.J.; Mehl, M.; Sarathy, S.M.; Curran, H.J. Detailed chemical kinetic reaction mechanisms for soy and rapeseed biodiesel fuels. Combust. Flame 2011, 158, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Kansedo, J.; Lee, K.T.; Bhatia, S. Cerbera odollam (sea mango) oil as a promising non-edible feedstock for biodiesel production. Fuel 2009, 88, 1148–1150. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R.N.M. Study of fuel properties of rubber seed oil based biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Aalam, C.S.; Saravanan, C. Effects of nano metal oxide blended Mahua biodiesel on CRDI diesel engine. Ain Shams Eng. J. 2017, 8, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Zhang, S. Production and characterization of biodiesel derived from Hodgsonia macrocarpa seed oil. Appl. Energy 2015, 146, 135–140. [Google Scholar] [CrossRef]
- Pradhan, R.; Naik, S.; Bhatnagar, N.; Swain, S. Moisture-dependent physical properties of Karanja (Pongamia pinnata) kernel. Ind. Crops Prod. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar]
- Benjumea, P.; Agudelo, J.; Agudelo, A. Basic properties of palm oil biodiesel–diesel blends. Fuel 2008, 87, 2069–2075. [Google Scholar] [CrossRef]
- Garnayak, D.; Pradhan, R.; Naik, S.; Bhatnagar, N. Moisture-dependent physical properties of jatropha seed (Jatropha curcas L.). Ind. Crops Prod. 2008, 27, 123–129. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.T.; Yusaf, T. Ailanthus altissima (tree of heaven) seed oil: Characterisation and optimisation of ultrasonication-assisted biodiesel production. Fuel 2018, 220, 621–630. [Google Scholar] [CrossRef]
- Moosavi, S.A.; Aghaalikhani, M.; Ghobadian, B.; Fayyazi, E. Okra: A potential future bioenergy crop in Iran. Renew. Sustain. Energy Rev. 2018, 93, 517–524. [Google Scholar] [CrossRef]
- Kole, C.; Joshi, C.P.; Shonnard, D.R. Handbook of Bioenergy Crop Plants; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Golkar, P.; Taghizadeh, M.; Jalali, S.A.H. Determination of phenolic compounds, antioxidant and anticancer activity of Chrozophora tinctoria accessions collected from different regions of Iran. J. Food Biochem. 2019, 43, e13036. [Google Scholar] [CrossRef]
- Asghar Aliloo, A.; Mustafavi, S.-H.; Ezzati, F. Allelopathic potential of Chrozophora tinctoria on early growth of Barley and Wheat. Azarian J. Agric. 2015, 2, 12–18. [Google Scholar]
- Usman, H.; Musa, Y.; Ahmadu, A.; Tijjani, M. Phytochemical and antimicrobial effects of Chrozophora senegalensis. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Hazrati, S.; Nicola, S.; Khurizadeh, S.; Alirezalu, A.; Mohammadi, H. Physico-chemical properties and fatty acid composition of Chrozophora tinctoria seeds as a new oil source. Grasas y Aceites 2019, 70, 328. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. A review on lawsonia inermis: A potential medicinal plant. Int. J. Curr. Pharm. Res. 2019, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Van Welzen, P.C. Revision and phylogeny of subtribes Chrozophorinae and Doryxylinae (Euphorbiaceae) in Malesia and Thailand. Blumea-Biodivers. Evol. Biogeogr. Plants 1999, 44, 411–436. [Google Scholar]
- Delazar, A.; Talischi, B.; Nazemiyeh, H.; Rezazadeh, H.; Nahar, L.; Sarker, S. Chrozophorin: A new acylated flavone glucoside from Chrozophora tinctoria (Euphorbiaceae). Rev. Bras. Farmacogn. 2006, 16, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Chrozophora Tinctoria. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:340969-1#source-KBD (accessed on 17 April 2020).
- Metcalfe, L.; Schmitz, A.; Pelka, J. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1966, 38, 514–515. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Biodiesel production from renewable feedstocks: Status and opportunities. Renew. Sustain. Energy Rev. 2012, 16, 4763–4784. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Hasni, K.; Ilham, Z.; Dharma, S.; Varman, M. Optimization of biodiesel production from Brucea javanica seeds oil as novel non-edible feedstock using response surface methodology. Energy Convers. Manag. 2017, 149, 392–400. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N. Production optimization and quality assessment of papaya (Carica papaya) biodiesel with response surface methodology. Energy Convers. Manag. 2018, 156, 103–112. [Google Scholar] [CrossRef]
| Physico-Chemical Parameters | Unit | Content |
|---|---|---|
| Oil content | % | 26 |
| Weight of 1000 seeds | g | 15 |
| Density | g/cm3 | 0.872 |
| Kinematic viscosity | mPa s | 4.32 |
| Acid value | Mg KOH/g oil | 2.5 |
| Iodine value | g I2/100 g oil | 170 |
| Water content | mg/g | 50 |
| Fatty Acid | Content (%) |
|---|---|
| Palmitic acid (C16:0) | 5.32 ± 0.047 |
| Palmitoleic acid (C16:1) | 0.11 ± 0.50 |
| Stearic acid (C18:0) | 3.15 ± 0.19 |
| Oleic acid (C18:1) | 13.99 ± 029 |
| Linoleic acid (C18:2) | 76.68 ± 0.16 |
| Linolenic acid (C18:3) | 0.67 ± 0.13 |
| ∑ UFA | 91.45 |
| ∑ SFA | 8.43 |
| ∑ MUFA | 14.10 |
| ∑ PUFA | 77.35 |
| ∑ UFA: ∑ SFA | 10.85 |
| ∑ MUFA: ∑ PUFA | 0.18 |
| Parameters | Method | Limitation | Units | Content |
|---|---|---|---|---|
| Density at 15 °C | ASTM D 4052 | 0.86–0.90 | g/cm3 | 0.862 |
| Kinematic Viscosity @ 40 °C | ASTMD445 | 1.9–6.0 | mm2/s | 3.74 |
| Flash Point, Closed Cup | D93 | >130 | °C | 169 |
| Cloud point | ASTM D6751 | −3 to 12 | °C | 4 |
| Pour point | ASTM D6751 | −15 to 10 | °C | −7 |
| Acid Number | ASTMD664 | 0.50 | mg | 0.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoseini, S.S.; Najafi, G.; Moazzez, A.F.; Hazrati, S.; Ebadi, M.T.; Yusaf, T. Potential of Chrozophora tinctoria Seed Oil as a Biodiesel Resource. Appl. Sci. 2020, 10, 3473. https://doi.org/10.3390/app10103473
Hoseini SS, Najafi G, Moazzez AF, Hazrati S, Ebadi MT, Yusaf T. Potential of Chrozophora tinctoria Seed Oil as a Biodiesel Resource. Applied Sciences. 2020; 10(10):3473. https://doi.org/10.3390/app10103473
Chicago/Turabian StyleHoseini, Seyed Salar, Gholamhassan Najafi, Armin Fattahpour Moazzez, Saeid Hazrati, Mohammad Taghi Ebadi, and Talal Yusaf. 2020. "Potential of Chrozophora tinctoria Seed Oil as a Biodiesel Resource" Applied Sciences 10, no. 10: 3473. https://doi.org/10.3390/app10103473







