Bone Substitutes Scaffold in Human Bone: Comparative Evaluation by 3D Micro-CT Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomaterials
- Inorganic bovine bone (Bio-Oss®, Geistlich, Wolhusen, Switzerland);
- Dehydrated and deantigenated equine bone (Bio-Gen®, Biotek Srl, Milano, Italy);
- Reabsorbable nano-hydroxyapatite (HA; Apagen Resorb 400, Gruppo Stomygen Srl, Roma, Italy);
- Porous hydroxyapatite based (ENGIpore®, Finceramica, Faenza, Italia);
- Tricalcium phosphate (TCP; Bioset, Tiradix Srl, Vimercate, Italy);
- PLA/PGA copolymer-based bone filling material (SINTbone, Ghimas Spa, Bologna, Italia).
2.2. Micro-Computed Tomography Analysis
2.3. Preclinical Phase
2.4. Clinical Phase
2.5. Hystology
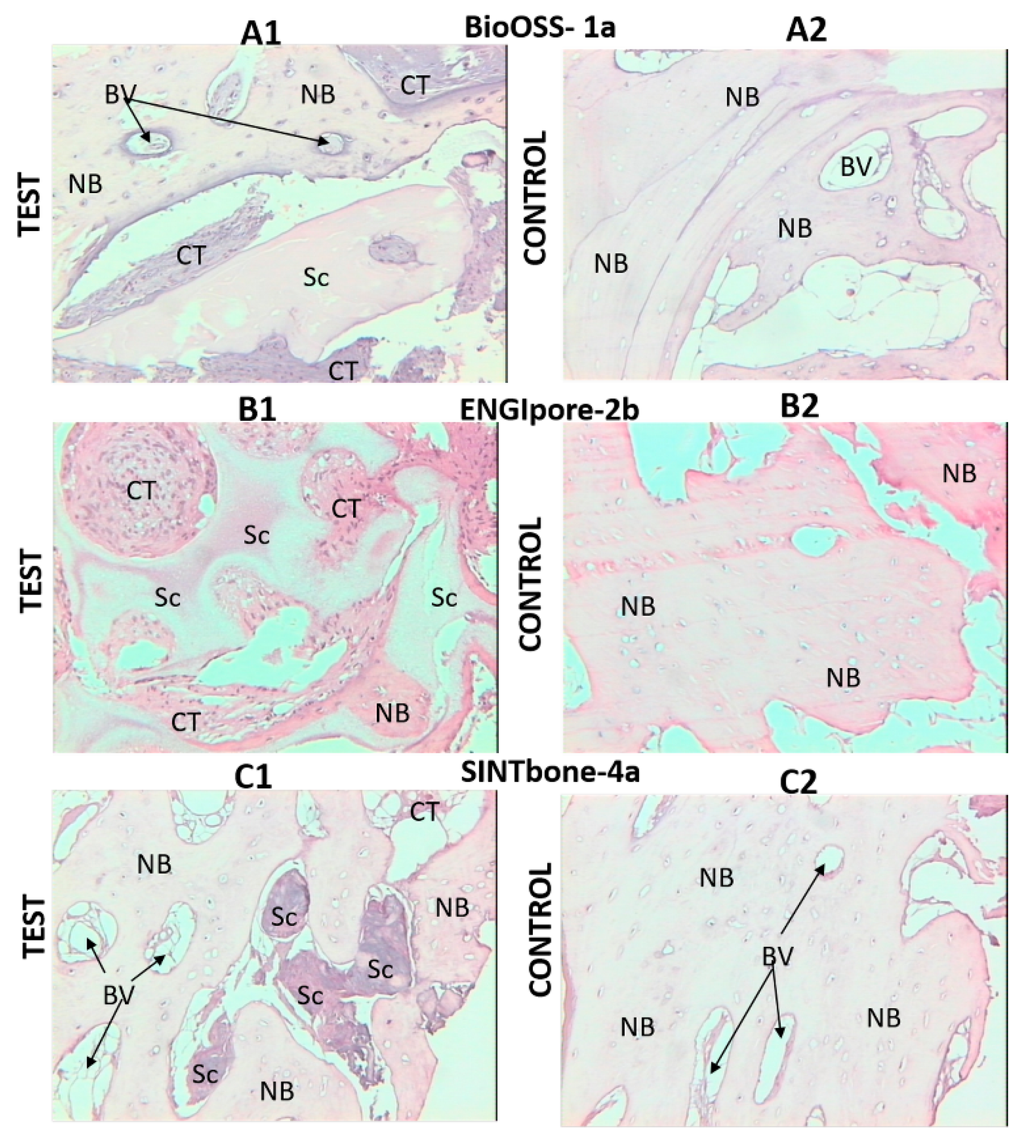
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- van Lenthe, G.H.; Hagenmüller, H.; Bohner, M.; Hollister, S.J.; Meinel, L.; Müller, R. Nondestructive micro-computed tomography for biological imaging and quantification of scaffold-bone interaction in vivo. Biomaterials 2007, 28, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Poologasundarampillai, G.; Atwood, R.C.; Bernard, D.; Lee, P.D. Non-destructive quantitative 3D analysis for the optimisation of tissue scaffolds. Biomaterials 2007, 28, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Muraglia, A.; Komlev, V.; Peyrin, F.; Rustichelli, F.; Crovace, A.; Cancedda, R. Tissue engineering of bone: Search for a better scaffold. Orthod. Craniofacial Res. 2005, 8, 277–284. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Xu, C.; Su, P.; Chen, X.; Meng, Y.; Yu, W.; Xiang, A.P.; Wang, Y. Biocompatibility and osteogenesis of biomimetic Bioglass-Collagen-Phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials 2011, 32, 1051–1058. [Google Scholar] [CrossRef]
- Kim, T.R.; Kim, M.S.; Goh, T.S.; Lee, J.S.; Kim, Y.H.; Yoon, S.Y.; Lee, C.S. Evaluation of structural and mechanical properties of porous artificial bone scaffolds fabricated via advanced TBA-based freeze-gel casting technique. Appl. Sci. 2019, 9, 1965. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Z.; Li, Y.; Jin, L.Y.; Quinn, J.M.W.; Komesaroff, P.A. A new sol-gel process for producing Na2O-containing bioactive glass ceramics. Acta Biomater. 2010, 6, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamjid, E.; Bohlouli, M.; Mohammadi, S.; Alipour, H.; Nikkhah, M. Sustainable drug release from highly porous and architecturally engineered composite scaffolds prepared by 3D printing. J. Biomed. Mater. Res. Part A 2020, 108, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choi, B.; Wu, B.; Lee, M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 045003. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Jiang, J.; Lv, F.; Xia, X.; Ma, X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J. Nanobiotechnology 2020, 18, 39. [Google Scholar] [CrossRef] [Green Version]
- Pecci, R.; Baiguera, S.; Ioppolo, P.; Bedini, R.; Del Gaudio, C. 3D printed scaffolds with random microarchitecture for bone tissue engineering applications: Manufacturing and characterization. J. Mech. Behav. Biomed. Mater. 2020, 103, 103583. [Google Scholar] [CrossRef]
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Traini, T.; Piattelli, A.; Caputi, S.; Degidi, M.; Mangano, C.; Scarano, A.; Perrotti, V.; Iezzi, G. Regeneration of human bone using different bone substitute biomaterials. Clin. Implant Dent. Relat. Res. 2015, 17, 150–162. [Google Scholar] [CrossRef]
- Bai, Y.; Sha, J.; Kanno, T.; Miyamoto, K.; Hideshima, K.; Matsuzaki, Y. Comparison of the Bone Regenerative Capacity of Three-Dimensional Uncalcined and Unsintered Hydroxyapatite/Poly-d/l-Lactide and Beta-Tricalcium Phosphate Used as Bone Graft Substitutes. J. Investig. Surg. 2019, 1–14. [Google Scholar] [CrossRef]
- Sha, J.; Kanno, T.; Miyamoto, K.; Bai, Y.; Hideshima, K.; Matsuzaki, Y. Application of a Bioactive/Bioresorbable Three-Dimensional Porous Uncalcined and Unsintered Hydroxyapatite/Poly-D/L-lactide Composite with Human Mesenchymal Stem Cells for Bone Regeneration in Maxillofacial Surgery: A Pilot Animal Study. Materials 2019, 12, 705. [Google Scholar] [CrossRef] [Green Version]
- Annibali, S.; Bellavia, D.; Ottolenghi, L.; Cicconetti, A.; Cristalli, M.P.; Quaranta, R.; Pilloni, A. Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: Preliminary data. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Mangano, C.; Valbonetti, L.; Marruchella, G.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mauro, A.; Bedini, R.; Turriani, M.; et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS ONE 2013, 8, e63256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, A.; Cheok, C.; Teoh, S.H.; Zhang, Z.Y.; Buser, D.; Bosshardt, D.D. Lateral ridge augmentation using a PCL-TCP scaffold in a clinically relevant but challenging micropig model. Clin. Oral Implants Res. 2012, 23, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2019, 13, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Barbetta, A.; Bedini, R.; Pecci, R.; Dentini, M. Role of X-ray microtomography in tissue engineering. Ann. Ist. Super. Sanità 2012, 48, 10–18. [Google Scholar]
- Grande, N.M.; Plotino, G.; Gambarini, G.; Testarelli, L.; D’Ambrosio, F.; Pecci, R.; Bedini, R. Present and future in the use of micro-CT scanner 3D analysis for the study of dental and root canal morphology. Ann. Ist. Super. Sanita 2012, 48, 26–34. [Google Scholar]
- Campioni, I.; Cacciotti, I.; Gupta, N. Additive manufacturing of reconstruction devices for maxillofacial surgery: Design and accuracy assessment of a mandibular plate prototype. Ann. Ist. Super. Sanità 2020, 56, 10–18. [Google Scholar]
- Irie, M.S.; Rabelo, G.D.; Spin-Neto, R.; Dechichi, P.; Borges, J.S.; Soares, P.B.F. Use of Micro-Computed Tomography for Bone Evaluation in Dentistry. Braz. Dent. J. 2018, 29, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Bedini, R.; Pecci, R.; Marinozzi, F.; Bini, F.; Rizzo, G.; Campioni, I. Valutazione Morfometrica e Strutturale Della Architettura del Tessuto Osseo Trabecolare del Collo del Femore: Analisi Microtomografica; Istituto Superiore di Sanità: Roma, Italy, 2018; Volume 18/7, Rapporti ISTISAN; Available online: http://old.iss.it/binary/publ/cont/18_7_web.pdf (accessed on 15 April 2020).
- Bedini, R.; Meleo, D.; Pecci, R. 3D microtomography characterization of dental implantology bone substitutes used in-vivo. Key Eng. Mater. 2013, 541, 97–113. [Google Scholar] [CrossRef]
- Meleo, D.; Bedini, R.; Pecci, R.; Mangione, F.; Pacifici, L. Microtomographic and morphometric characterization of a bioceramic bone substitute in dental implantology. Ann. Ist. Super. Sanita 2012, 48, 59–64. [Google Scholar] [PubMed]
- Bedini, R.; Meleo, D.; Pecci, R. Role of Benchtop Microtomographic Systems in Tissue Engineering. In Advanced High-Resolution Tomography in Regenerative Medicine; Giuliani, A., Cedola, A., Eds.; Fundamental Biomedical Technologies; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–50. ISBN 978-3-030-00367-8. [Google Scholar]
- Idrontino, G.; Valente, N.A. Intraoral and extraoral autologous bone block graft techniques: A review of the recent literature. Int. J. Contemp. Dent. Med. Rev. 2016, 2016. [Google Scholar]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef] [PubMed]

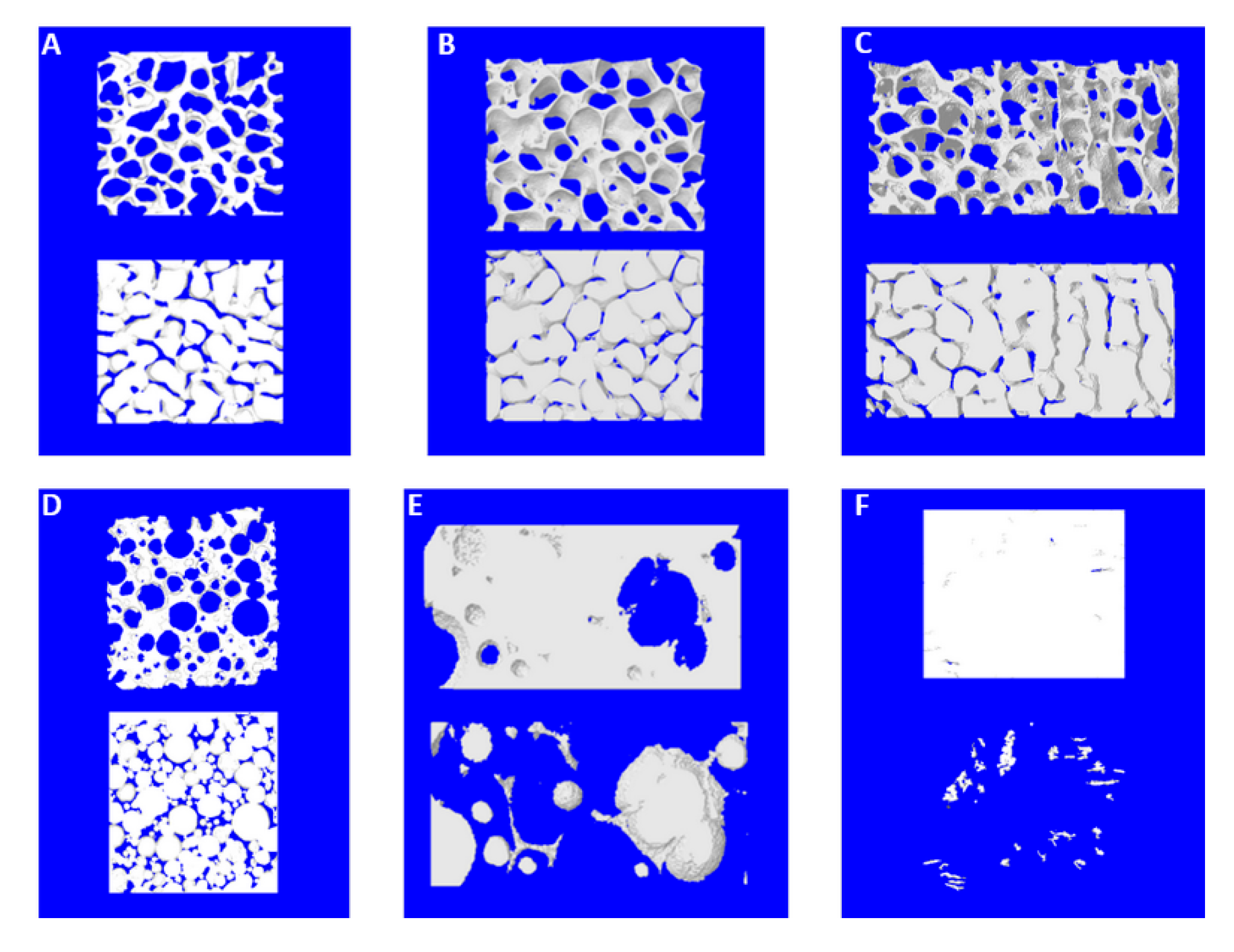
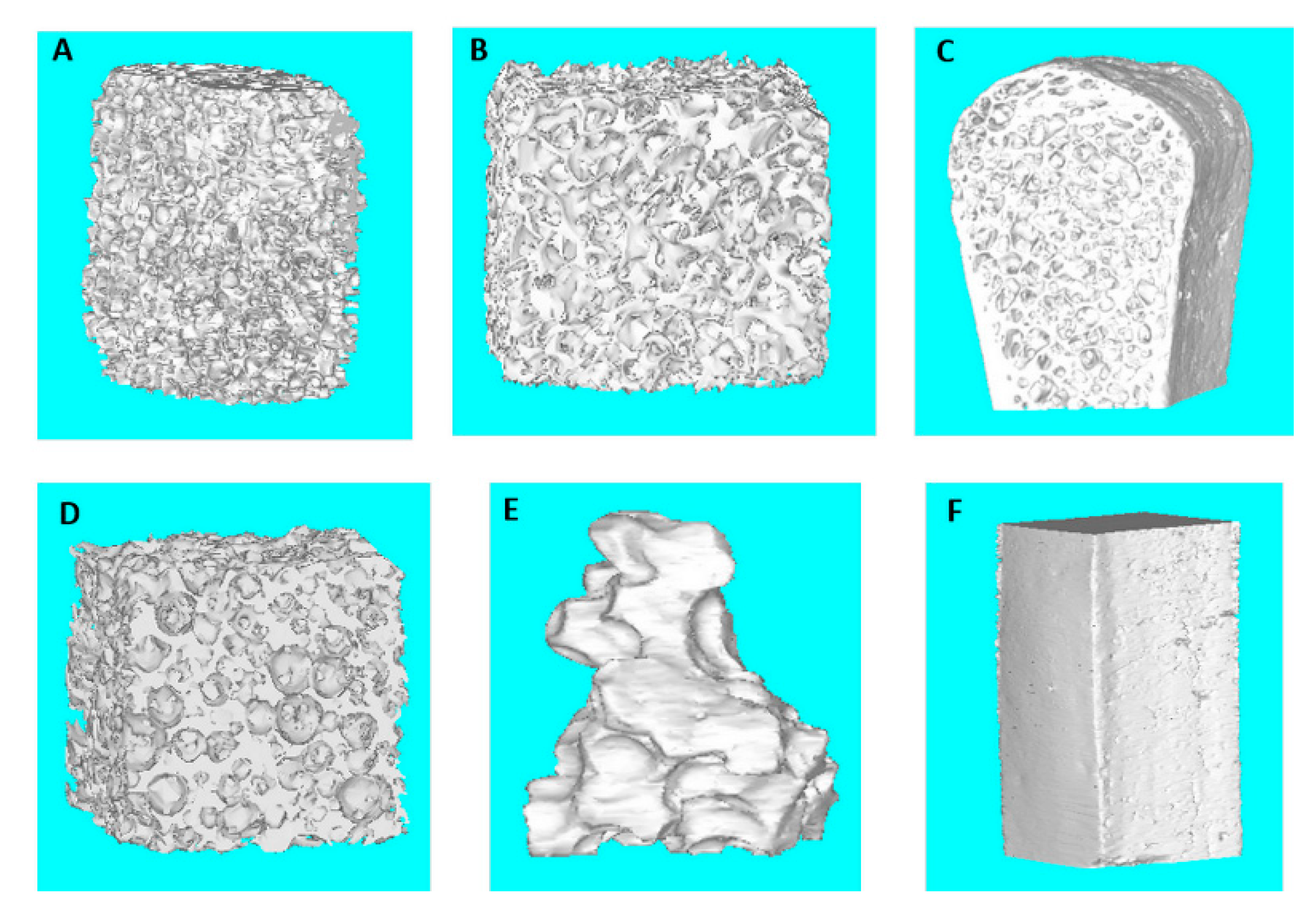
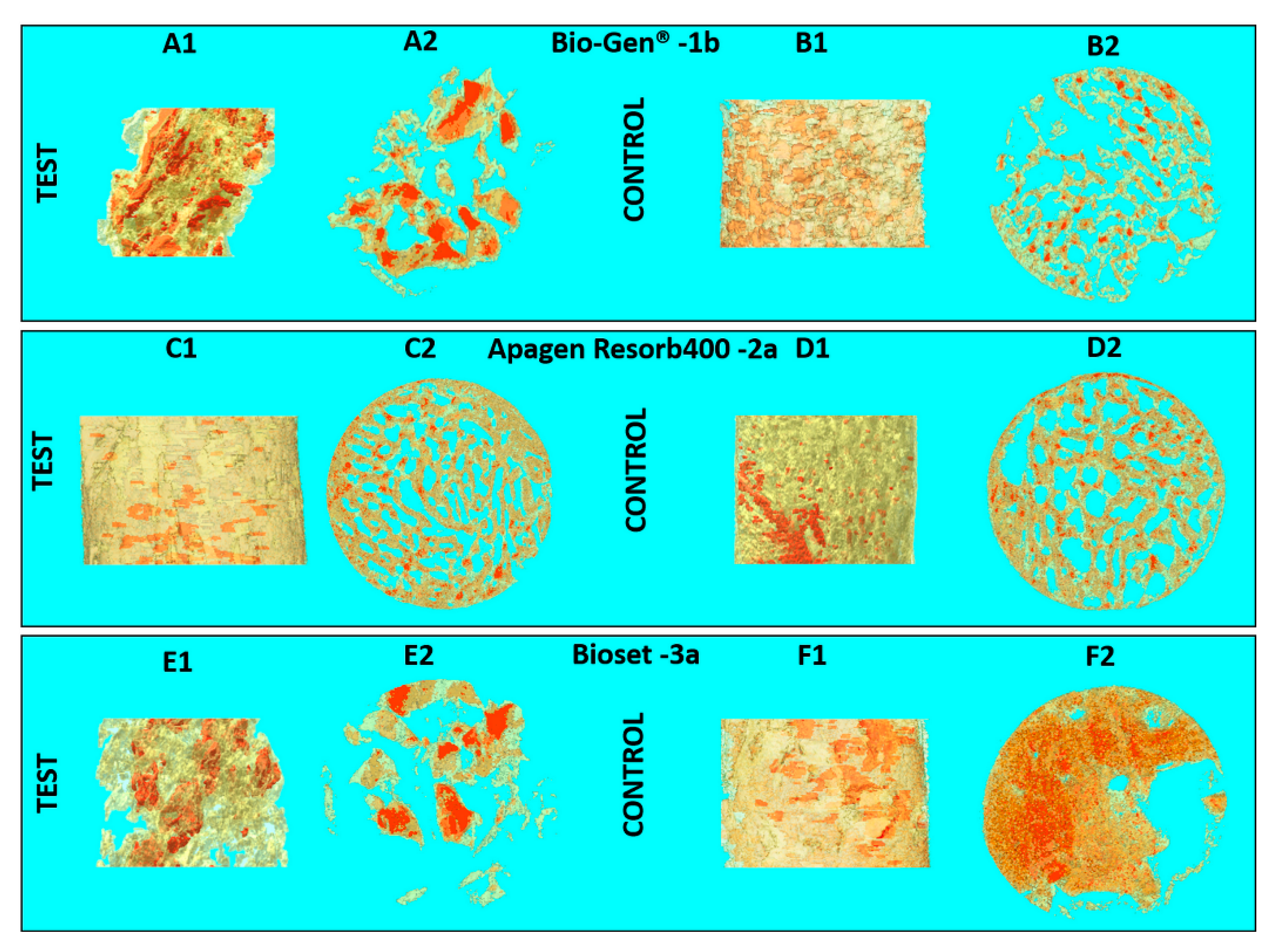
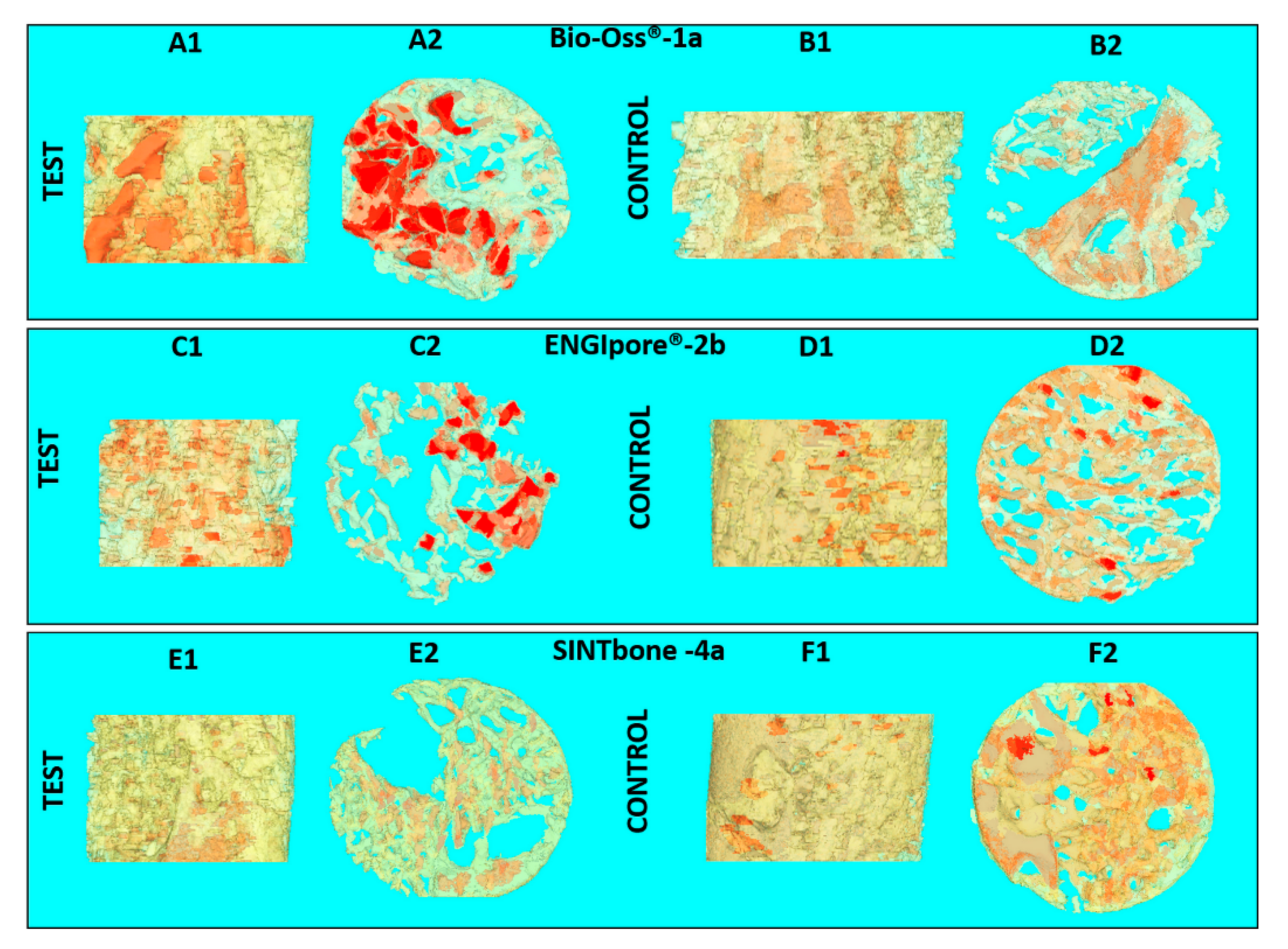
| Clinical Study | Groups | Label | Commercial Name | Origins and Description | Dimensions (mm) |
|---|---|---|---|---|---|
| Part 2 | 1-Bio-bone | 1a | Bio-Oss® | Animal Bovine bone, Block | 13.3 × 9 × 6.5 |
| Part 1 | 1b | Bio-Gen® | Animal Equine bone, Block | 10 × 10 × 10 | |
| Part 1 | 2-HA | 2a | Apagen 400 Resorb | Synthetic Nano-HA, Granular | Diameter 0.4 |
| Part 2 | 2b | ENGIpore® | Synthetic Porous HA, Block | 10 × 5 × 5 | |
| Part 1 | 3-TCP | 3a | Bioset TCP | Synthetic TCP, Granular | 2.8 × 3.3 × 3 |
| Part 2 | 4-polymer | 4a | SINTbone | Synthetic PLA/PGA, Block | 16 × 5.5 × 6 |
| Part 2 | Iliac crest bone | ICB | - | Human, Block | 9 × 15 × 10 |
| Group | Label | BV/TV (%) | BS/BV (mm−1) | Porosity (%) | Tb.Th (µm) | Tb.Sp (mm−1) |
|---|---|---|---|---|---|---|
| 1-Bio-bone | 1a | 28.73 | 18.30 | 71.27 | 0.22 | 0.51 |
| 1b | 26.59 | 14.05 | 73.41 | 0.27 | 0.68 | |
| 2-HA | 2b | 31.69 | 23.88 | 68.31 | 0.16 | 0.47 |
| 3-TCP | 3a | 24.13 | 11.09 | 75.87 | 0.28 | 0.52 |
| 4-polymer | 4a | 91.77 | 2.66 | 8.22 | 0.49 | 0.22 |
| Iliac crest bone | ICB | 39.43 | 11.69 | 60.57 | 0.31 | 0.61 |
| Group | Label | Implant Site | BV/TV (%) | BS/BV (mm−1) | Porosity (%) | Tb.Th (µm) | Tb.Sp (mm−1) |
|---|---|---|---|---|---|---|---|
| 1-Bio-bone | 1a | Test | 19.80 | 95.59 | 80.20 | 0.08 | 0.37 |
| Control | 28.17 | 90.22 | 72.20 | 0.06 | 0.22 | ||
| 1b | Test | 10.93 | 39.35 | 89.06 | 0.12 | 0.70 | |
| Control | 23.15 | 61.21 | 76.85 | 0.08 | 0.28 | ||
| 2-HA | 2a | Test | 48.05 | 30.50 | 51.95 | 0.11 | 0.19 |
| Control | 52.21 | 29.20 | 47.79 | 0.16 | 0.13 | ||
| 2b | Test | 23.35 | 84.13 | 76.65 | 0.07 | 0.17 | |
| Control | 39.25 | 81.06 | 60.75 | 0.06 | 0.09 | ||
| 3-TCP | 3a | Test | 14.10 | 90.72 | 85.89 | 0.11 | 0.37 |
| Control | 44.84 | 45.46 | 51.46 | 0.08 | 0.15 | ||
| 4-polymer | 4a | Test | 30.99 | 44.15 | 69.01 | 0.13 | 0.27 |
| Control | 39.00 | 26.45 | 61.00 | 0.17 | 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedini, R.; Pecci, R.; Meleo, D.; Campioni, I. Bone Substitutes Scaffold in Human Bone: Comparative Evaluation by 3D Micro-CT Technique. Appl. Sci. 2020, 10, 3451. https://doi.org/10.3390/app10103451
Bedini R, Pecci R, Meleo D, Campioni I. Bone Substitutes Scaffold in Human Bone: Comparative Evaluation by 3D Micro-CT Technique. Applied Sciences. 2020; 10(10):3451. https://doi.org/10.3390/app10103451
Chicago/Turabian StyleBedini, Rossella, Raffaella Pecci, Deborah Meleo, and Ilaria Campioni. 2020. "Bone Substitutes Scaffold in Human Bone: Comparative Evaluation by 3D Micro-CT Technique" Applied Sciences 10, no. 10: 3451. https://doi.org/10.3390/app10103451






