Effect of Hippophae rhamnoides L. Leaves Treatment on the Antioxidant Capacity, Total Phenol Content and Sensory Profile of Moschofilero Wines Vinified with and without Added Sulphites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Collection and Preparation of Plant Material
2.3. Vinification of Wine Samples
2.4. H. rhamnoides Leaves Treatment
2.5. Main Oenological Attributes
2.6. Sensory Evaluation
2.7. DPPH Assay
2.8. Ferric Reducing Antioxidant Power (FRAP) Assay
2.9. Determination of Total Phenol Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Main Oenological Parameters
3.2. Antioxidant Capacity
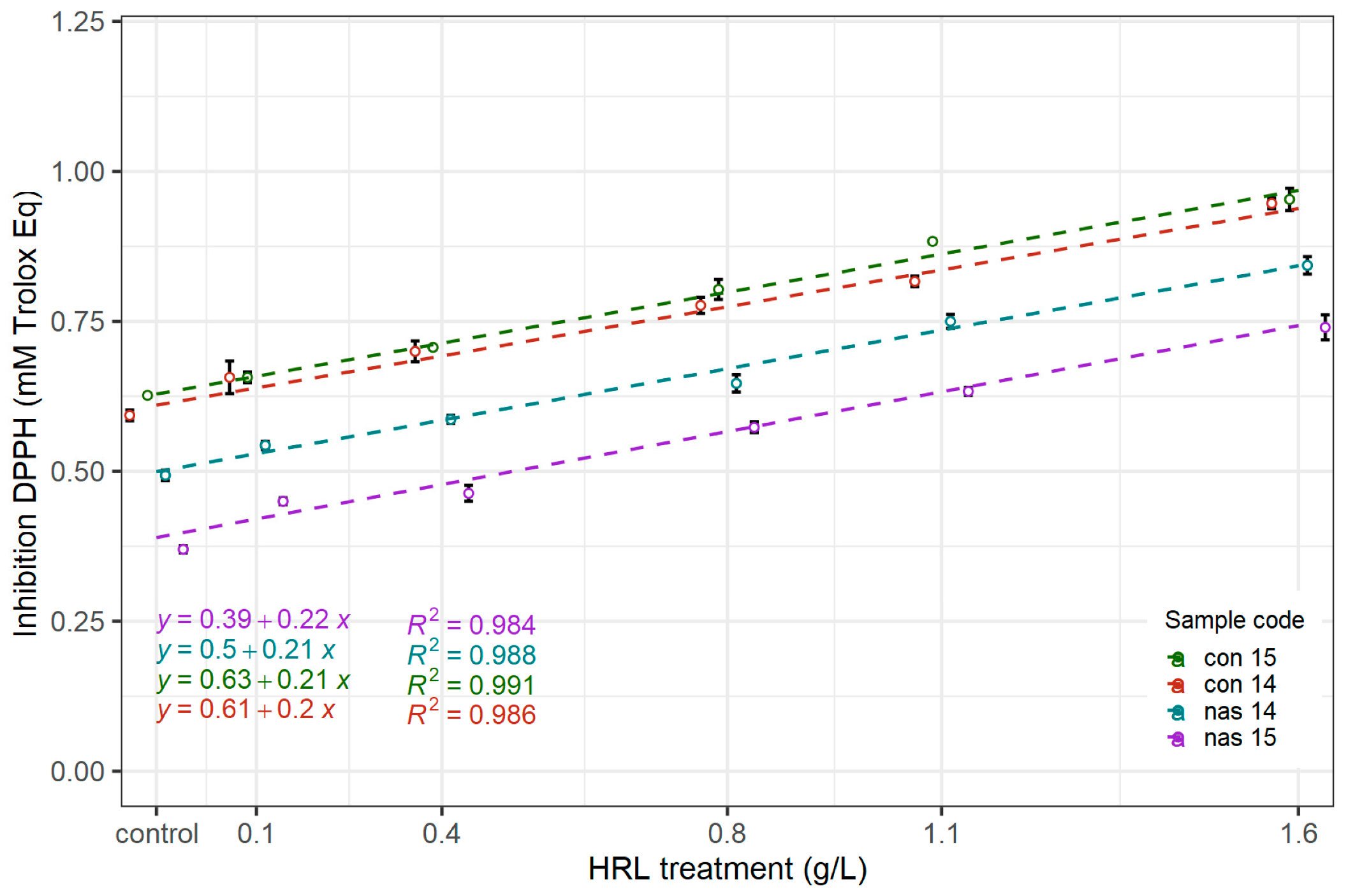
3.2.1. Radical Scavenging Activity as Determined by the Inhibition of the DPPH Radical Assay
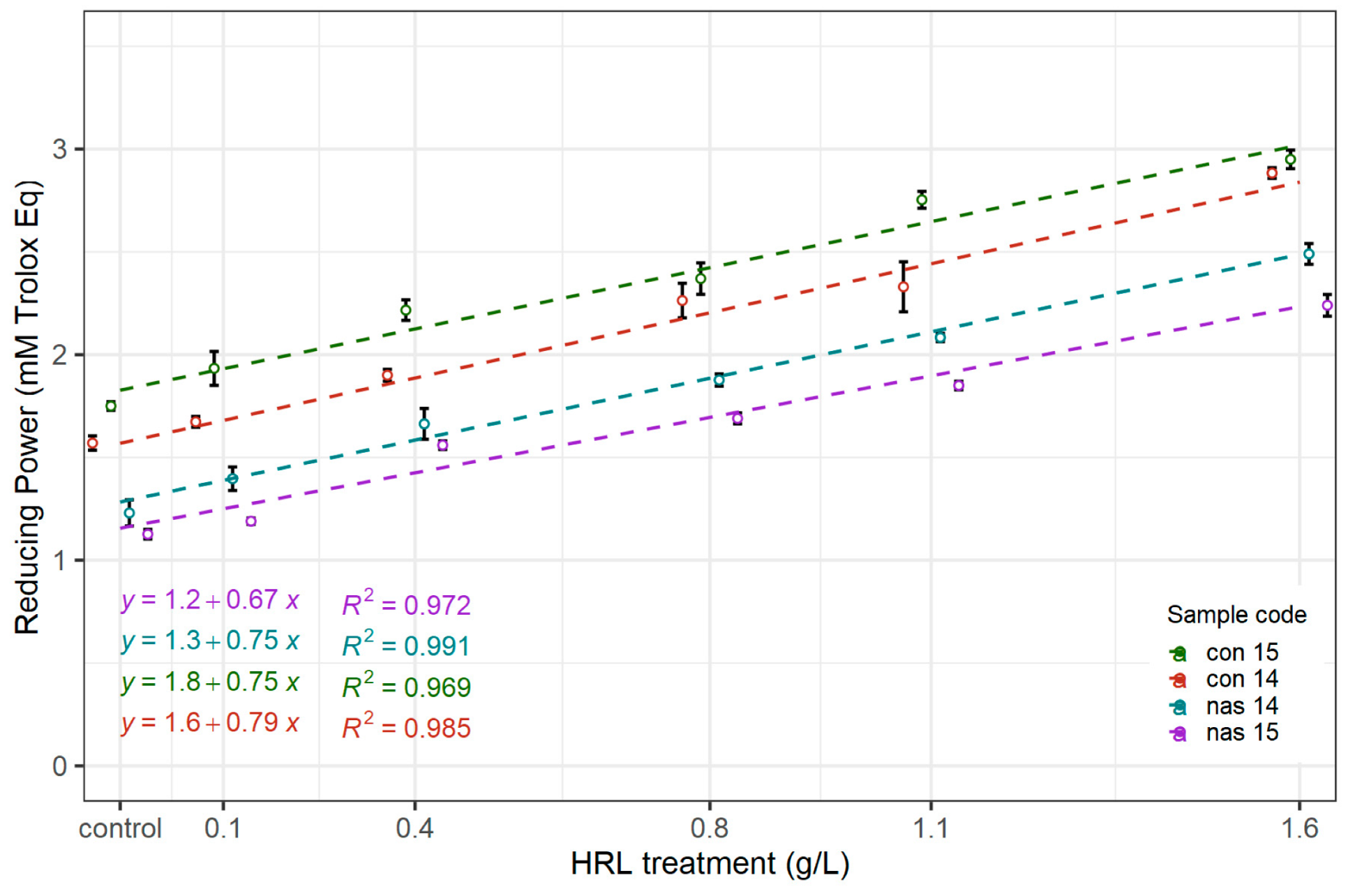
3.2.2. Reducing Capacity as Determined by the FRAP Assay
3.3. Total Phenol Content
3.4. Correlation Analysis
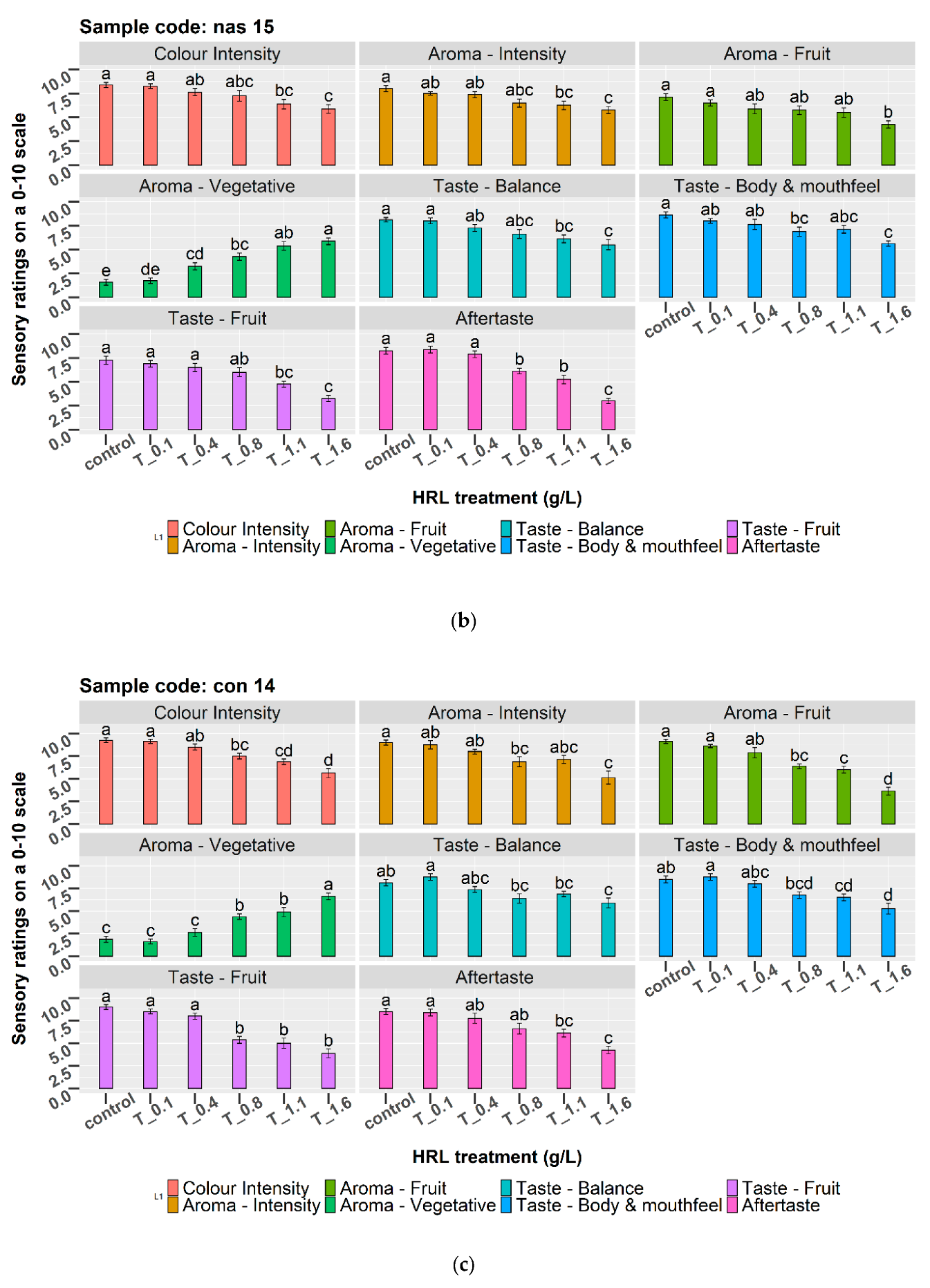
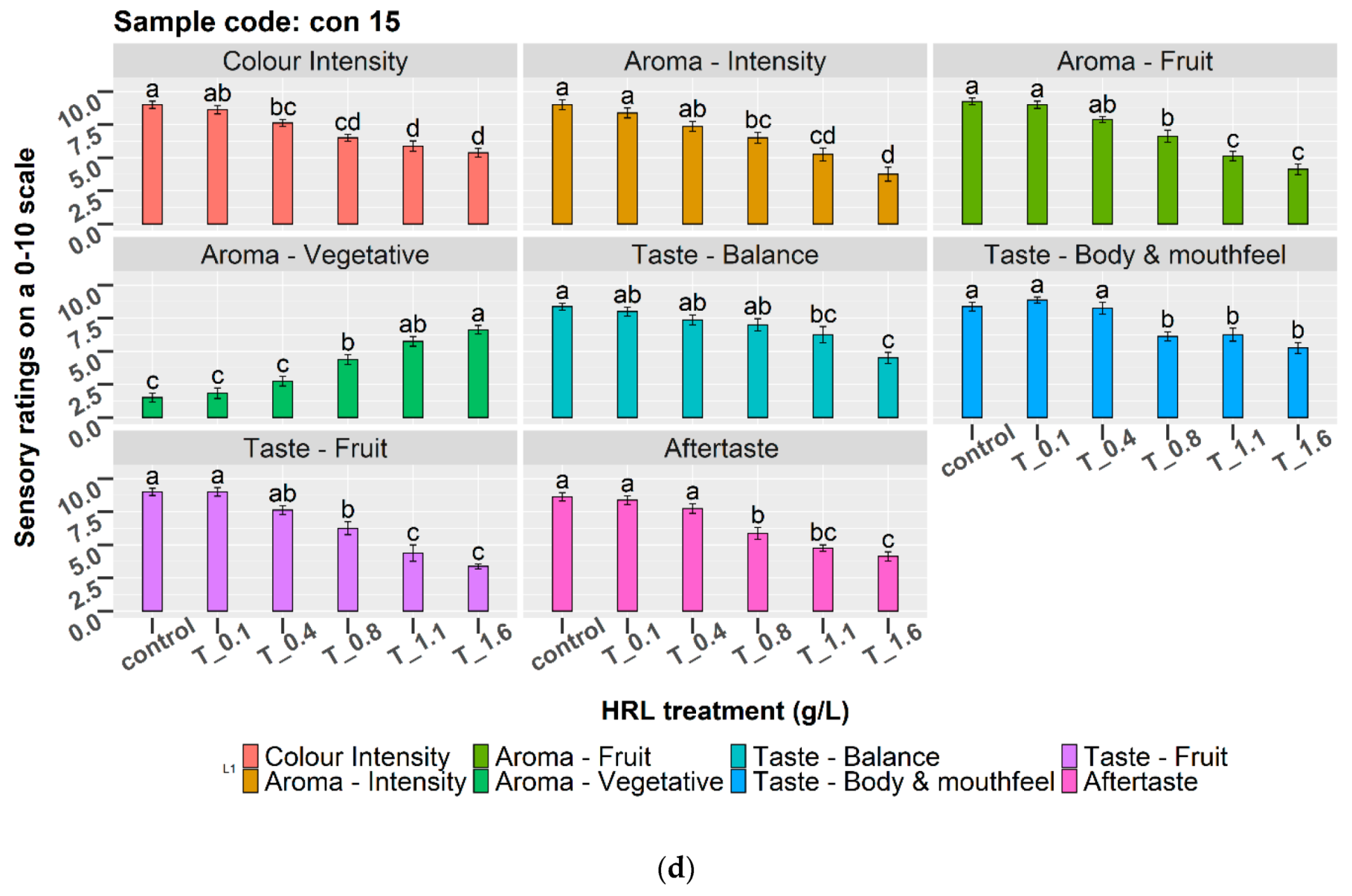
3.5. Sensory Evaluation
3.6. Possible Use in Winemaking
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H.; Košmerl, T.; Poklar Ulrih, N.; Cigić, B. Contribution of SO2 to antioxidant potential of white wine. Food Chem. 2015, 174, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, C.D.; Piva, A.; Martuscelli, M.; Mastrocola, D.; Sacchetti, G. Effect of sulfites on the in vitro antioxidant activity of wines. Ital. J. Food Sci. 2015, 27, 505–512. [Google Scholar]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Folin-Ciocalteu, FRAP, and DPPH• assays for measuring polyphenol concentration in white wine. Am. J. Enol. Vitic. 2015, 66, 463–471. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology; John and Wiley and Sons: Hoboken, NJ, USA, 2006; Volume 2. [Google Scholar]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Marcon, Â.R.; Carnieli, G.J.; Vanderlinde, R. Effect of glutathione addition in sparkling wine. Food Chem. 2014, 159, 391–398. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Smith, M.E.; Smith, P.A.; Wilkes, E.N. Formation of hydrogen sulfide in wine: Interactions between copper and sulfur dioxide. Molecules 2016, 21, 1214. [Google Scholar] [CrossRef]
- Chagas, R.; Ferreira, L.M.; Laia, C.A.T.; Monteiro, S.; Ferreira, R.B. The challenging SO2-mediated chemical build-up of protein aggregates in wines. Food Chem. 2016, 192, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Lustrato, G.; Alfano, G.; Belli, C.; Grazia, L.; Iorizzo, M.; Ranalli, G. Scaling-up in industrial winemaking using low electric current as an alternative to sulfur dioxide addition. J. Appl. Microbiol. 2006, 101, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Sonni, F.; Bastante, M.J.C.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: Influence on volatile composition of white wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD-MS/MS characterization of phenolic compounds in white wine stored without added sulfite. Food Res. Int. 2014, 66, 207–215. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) So exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Ma, X.; Moilanen, J.; Laaksonen, O.; Yang, W.; Tenhu, E.; Yang, B. Phenolic compounds and antioxidant activities of tea-type infusions processed from sea buckthorn (Hippophaë rhamnoides) leaves. Food Chem. 2019, 272, 1–11. [Google Scholar] [CrossRef]
- Yogendra Kumar, M.S.; Tirpude, R.J.; Maheshwari, D.T.; Bansal, A.; Misra, K. Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves in vitro. Food Chem. 2013, 141, 3443–3450. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Wani, T.A.; Wani, S.M.; Ahmad, M.; Ahmad, M.; Gani, A.; Masoodi, F.A. Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): A review. Cogent Food Agric. 2016, 2, 1128519. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Yogendra Kumar, M.S.; Gupta, A. Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2010, 48, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Ahmad, F.; Gore, D.D.; Tikoo, K.; Bansal, A.; Jachak, S.M.; Jena, G. Therapeutic potential of seabuckthorn: A patent review (2000–2018). Expert Opin. Ther. Pat. 2019, 29, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Ekholm, A.; Scheewe, P.; Rumpunen, K. Changes in content of major Phenolic compounds during leaf development of sea buckthorn (Hippophaë rhamnoides L.). Agric. Food Sci. 2014, 23, 207–219. [Google Scholar] [CrossRef]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J. UHPLC/PDA–ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaë rhamnoides L. varieties. Phytochem. Anal. 2013, 24, 484–492. [Google Scholar] [CrossRef]
- Pop, R.M.; Buzoianu, A.D.; Rati, I.V.; Socaciu, C. Untargeted metabolomics for sea buckthorn (Hippophae Rhamnoides ssp. carpatica) berries and leaves: Fourier transform infrared spectroscopy as a rapid approach for evaluation and discrimination. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 545–550. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical composition and biological activity of berries and leaves from four romanian sea buckthorn (Hippophae Rhamnoides L.) varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef]
- Saggu, S.; Divekar, H.M.; Gupta, V.; Sawhney, R.C.; Banerjee, P.K.; Kumar, R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: A dose dependent study. Food Chem. Toxicol. 2007, 45, 609–617. [Google Scholar] [CrossRef]
- Tulsawani, R. Ninety day repeated gavage administration of Hipphophae rhamnoides extract in rats. Food Chem. Toxicol. 2010, 48, 2483–2489. [Google Scholar] [CrossRef]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Shatunova, S.A.; Petrov, A.S. Production and analysis of non-traditional beer supplemented with sea buckthorn. Agron. Res. 2017, 15, 1831–1845. [Google Scholar]
- Guan, T.T.Y.; Cenkowski, S.; Hydamaka, A. Effect of Drying on the Nutraceutical Quality of Sea Buckthorn (Hippophae rhamnoides L. ssp. sinensis) Leaves. J. Food Sci. 2006, 70, E514–E518. [Google Scholar] [CrossRef]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bittová, M.; Krejzová, E.; Roblová, V.; Kubán, P.; Kubáň, V. Monitoring of HPLC profiles of selected polyphenolic compounds in sea buckthorn (Hippophaë rhamnoides L.) plant parts during annual growth cycle and estimation of their antioxidant potential. Cent. Eur. J. Chem. 2014, 12, 1152–1161. [Google Scholar] [CrossRef]
- European Commission Comission delegated Regulation (EU). 2019/934 of 12 March 2019 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards wine-growing areas where the alcoholic strength may be increased, authorised oenological prac. Off. J. Eur. Union L. 2019, 149, 1–52. [Google Scholar]
- Scrimgeour, N.; Nordestgaard, S.; Lloyd, N.D.R.; Wilkes, E.N. Exploring the effect of elevated storage temperature on wine composition. Aust. J. Grape Wine Res. 2015, 21, 713–722. [Google Scholar] [CrossRef]
- OIV. OIV (Organisation International de la Vigne et du Vin) Sulfur Dioxide, (Iodometry), Methode OIV-MA-AS323-04B. Compend. Int. Methods Anal. Wines Musts 2009, 2, 291–294. [Google Scholar]
- Castillo-Sánchez, J.J.; Mejuto, J.C.; Garrido, J.; García-Falcón, S. Influence of wine-making protocol and fining agents on the evolution of the anthocyanin content, colour and general organoleptic quality of Vinhão wines. Food Chem. 2006, 97, 130–136. [Google Scholar] [CrossRef]
- Porgali, E.; Büyüktuncel, E. Determination of phenolic composition and antioxidant capacity of native red wines by high performance liquid chromatography and spectrophotometric methods. Food Res. Int. 2012, 45, 145–154. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl-hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Reactions involving iron in mediating catechol oxidation in model wine. Am. J. Enol. Vitic. 2013, 64, 316–324. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lee, E.J.; Nomura, N.; Patil, B.S.; Yoo, K.S. Measurement of total phenolic content in wine using an automatic Folin-Ciocalteu assay method. Int. J. Food Sci. Technol. 2014, 49, 2364–2372. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 2 May 2020).
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Proestos, C.; Sflomos, K.; Zoumpoulakis, P.; Tatarides, P.; Sinanoglou, V.J. Botanical Extracts Used as Wine Preservatives. Int. J. Agric. Sci. Food Technol. 2015, 007–011. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Psarra, E.; Makris, D.P.; Kallithraka, S.; Kefalas, P. Evaluation of the antiradical and reducing properties of selected Greek white wines: Correlation with polyphenolic composition. J. Sci. Food Agric. 2002, 82, 1014–1020. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Pappa, A.; Krokida, M.; Detsi, A.; Kefalas, P. Effects of Drying and Extraction Methods on the Quality and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides) Berries and Leaves. Dry. Technol. 2013, 31, 1063–1076. [Google Scholar] [CrossRef]
- Górnaś, P.; Šne, E.; Siger, A.; Segliņa, D. Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: The effect of harvest time, sex, drying and extraction methods. Ind. Crops Prod. 2014, 60, 1–7. [Google Scholar] [CrossRef]
- Li, T.S.C.; Beveridge, T.H.J. Sea Buckthorn (Hippophae rhamnoides L.): Production and Utilization; NRC Research Press: Ottawa, SO, USA, 2003. [Google Scholar]
- Morgenstern, A.; Ekholm, A.; Scheewe, P.; Rumpunen, K. Major phenolic compounds in processed sea buckthorn leaves. In Producing Sea Buckthorn of High Quality, Proceedings of the 3rd European Workshop on Sea Buckthorn EuroWorkS2014, Naantali, Finland, October 14–16, 2014; Kauppinen, S., Petruneva, E., Eds.; Natural Resources Institute Finland (Luke): Helsinki, Finland, 2015; Volume 31, pp. 74–77. Available online: http://urn.fi/URN:ISBN:978-952-326-035-1 (accessed on 2 May 2020).
- Roidaki, A.; Zoumpoulakis, P.G.; Proestos, C. Comparison of extraction methods for the determination of antioxidant activity in extracts of Hippophae rhamnoides L. and Lippia citriodora. The effect of. Austin J Nutr. Food Sci. 2015, 3, 1057–1064. [Google Scholar]
- Stratil, P.; Kubáň, V.; Fojtová, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2008, 26, 242–253. [Google Scholar] [CrossRef]
- Staško, A.; Brezová, V.; Mazúr, M.; Čertík, M.; Kaliňák, M.; Gescheidt, G. A comparative study on the antioxidant properties of Slovakian and Austrian wines. LWT Food Sci. Technol. 2008, 41, 2126–2135. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P.; Gkoulioti, A.; Marinos, V.; Tsoupeis, D.; Boutaris, I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Compos. Anal. 2008, 21, 614–621. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- González-Rompinelli, E.M.; Rodríguez-Bencomo, J.J.; García-Ruiz, A.; Sánchez-Patán, F.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. A winery-scale trial of the use of antimicrobial plant phenolic extracts as preservatives during wine ageing in barrels. Food Control 2013, 33, 440–447. [Google Scholar] [CrossRef]
- Singh, A.K.; Attrey, D.P.; Deep, P.; Dubey, S.; Naved, T.; Roy, B. Acute & sub-acute toxicity studies of pharmacologically active seabuckthorn leaf extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 415–419. [Google Scholar]


| Sample Code | Variety | Region | Vintage Year | Vinification |
|---|---|---|---|---|
| nas 14 | Moschofilero | Arcadia | 2014 | No Sulphites added. 6 months maturation in stainless steel tanks |
| nas 15 | Moschofilero | Arcadia | 2015 | No Sulphites added. 6 months maturation in stainless steel tanks |
| con 14 | Moschofilero | Arcadia | 2014 | Conventional. 6 months maturation in stainless steel tanks |
| con15 | Moschofilero | Arcadia | 2015 | Conventional. 6 months maturation in stainless steel tanks |
| Compounds/Class | Range (mg/Kg DW) | References |
|---|---|---|
| Carotenoids | ||
| Zeaxanthin | 5–6 | [32] |
| Violaxanthin | 5–6 | [32] |
| Lutein | 8–11 | [32] |
| Zeaxanthin | ND–6 | [32] |
| β-Carotene | 7–9 | [32] |
| Flavonol Glucosides | ||
| Q-3-O-rutinoside/Rutin | 471–1310 | [24] |
| Q-3-O-galactoside | 205–458 | [24] |
| Q-3-O-sorphroside-7-O-rhamnoside | 301–359 | [25] |
| I-3-O-rutinoside | 107–496 | [25] |
| I-3-O-rutinoside-7-O-glucoside | 405–1146 | [25] |
| I-3-O-sophroside-7-O-rhamnoside | 250–446 | [25] |
| I-3-O-glucoside | 254–339 | [25] |
| I-3-O-glucoside-7-O-rhamnoside | 691–1110 | [25] |
| I-3-O-neohesperidoside | 585–1639 | [25] |
| K-3-O-glucoside | 56–101 | [24] |
| K-3-O-rutinoside | 291–894 | [25] |
| Quercetin | 332–1381 | [24] |
| Kaempferol | 2.8–4.1 | [24] |
| Gallic acid | 19.1–79.1 | [33] |
| Caffeic acid | 5.9–9.8 | [33] |
| p-coumaric acid | 8.4–13.4 | [33] |
| Ferulic acid | 7.2–15.1 | [33] |
| Ellagitanins | ||
| Pedunculagin | 220–510 | [18] |
| Stachyurin | 2400–4330 | [18] |
| Hippophaenin C | 1250–3360 | [18] |
| Casuarinin | 2820–4630 | [18] |
| Hippophaenin B | 930–1000 | [18] |
| Casuarictin | 3170–4700 | [18] |
| Code | HRL a | Free SO2 b | Total SO2 b | TA c | VA d | pH | D | %Vol | Extract e | Sugar b | A420 f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nas 14 | Ctrl | 0 | 9 | 4.9 | 0.65 | 3.40 | 0.991 | 11.6 | 19.36 | 1.9 | 0.075 |
| 1.6 | 0 | 10 | 5.0 | 0.72 | 3.41 | 0.991 | 11.6 | 19.92 | 2.2 | 0.145 | |
| nas 15 | Ctrl | 0 | 10 | 5.2 | 0.57 | 3.38 | 0.991 | 11.3 | 20.35 | 1.2 | 0.126 |
| 1.6 | 0 | 10 | 5.1 | 0.55 | 3.39 | 0.991 | 11.3 | 20.6 | 1.1 | 0.207 | |
| con 14 | Ctrl | 32 | 94 | 5.5 | 0.28 | 3.31 | 0.991 | 11.6 | 19.48 | 1.8 | 0.064 |
| 1.6 | 29 | 92 | 5.6 | 0.31 | 3.25 | 0.992 | 11.6 | 19.85 | 1.8 | 0.112 | |
| con 15 | Ctrl | 29 | 115 | 5.5 | 0.31 | 3.27 | 0.991 | 11.3 | 22.91 | 2.7 | 0.048 |
| 1.6 | 26 | 113 | 5.8 | 0.28 | 3.27 | 0.991 | 11.3 | 22.87 | 2.6 | 0.092 |
| Sample Code | HRL Dosage | AAR | FRAP | TPC | A420 |
|---|---|---|---|---|---|
| nas 14 | 0.00 | 0.49 a ± 0.01 | 1.23 a ± 0.09 | 233 a ± 7 | 0.075 a ± 0.004 |
| 0.13 | 0.55 ab ± 0.01 | 1.40 a ± 0.08 | 250 ab ± 7 | 0.082 a ± 0.003 | |
| 0.40 | 0.59 b ± 0.01 | 1.66 b ± 0.10 | 269 bc ± 7 | 0.092 b ± 0.003 | |
| 0.80 | 0.65 c ± 0.02 | 1.99 bc ± 0.04 | 280 cd ± 11 | 0.109 c ± 0.003 | |
| 1.07 | 0.75 d ± 0.02 | 2.09 c ± 0.03 | 301 d ± 8 | 0.115 c ± 0.003 | |
| 1.60 | 0.84 e ± 0.02 | 2.49 d ± 0.07 | 337 e ± 5 | 0.145 d ± 0.003 | |
| nas 15 | 0.00 | 0.37 a ± 0.01 | 1.13 a ± 0.03 | 220 a ± 4 | 0.126 a ± 0.007 |
| 0.13 | 0.45 b ± 0.01 | 1.19 a ± 0.02 | 227 a ± 10 | 0.127 a ± 0.003 | |
| 0.40 | 0.46 b ± 0.02 | 1.56 b ± 0.03 | 235 a ± 7 | 0.142 b ± 0.004 | |
| 0.80 | 0.57 c ± 0.01 | 1.69 b ± 0.04 | 272 b ± 6 | 0.173 c ± 0.005 | |
| 1.07 | 0.63 d ± 0.01 | 1.85 c ± 0.03 | 290 bc ± 8 | 0.181 c ± 0.002 | |
| 1.60 | 0.74 e ± 0.04 | 2.24 d ± 0.07 | 317 c ± 12 | 0.186 c ± 0.007 | |
| con 14 | 0.00 | 0.59 a ± 0.01 | 1.57 a ± 0.06 | 293 a ± 6 | 0.064 a ± 0.002 |
| 0.13 | 0.66 ab ± 0.04 | 1.68 ab ± 0.04 | 300 a ± 6 | 0.069 ab ± 0.004 | |
| 0.40 | 0.70 b ± 0.03 | 1.90 b ± 0.05 | 314 ab ± 6 | 0.076 b ± 0.002 | |
| 0.80 | 0.78 c ± 0.02 | 2.26 c ± 0.15 | 334 b ± 8 | 0.090 c ± 0.004 | |
| 1.07 | 0.81 c ± 0.01 | 2.33 c ± 0.21 | 340 b ± 20 | 0.099 d ± 0.004 | |
| 1.60 | 0.96d ± 0.02 | 2.88 d ± 0.05 | 383 c ± 14 | 0.110 e ± 0.002 | |
| con 15 | 0.00 | 0.62 a ± 0.01 | 1.75 a ± 0.04 | 305 a ± 8 | 0.048 a ± 0.001 |
| 0.13 | 0.65 ab ± 0.02 | 1.93 a ± 0.14 | 315 a ± 3 | 0.060 b ± 0.002 | |
| 0.40 | 0.71 b ± 0.01 | 2.22 b ± 0.09 | 317 a ± 9 | 0.066 c ± 0.001 | |
| 0.80 | 0.80 c ± 0.03 | 2.37 b ± 0.13 | 339 b ± 6 | 0.072 d ± 0.003 | |
| 1.07 | 0.88 d ± 0.00 | 2.75 c ± 0.07 | 367 c ± 6 | 0.079 e ± 0.003 | |
| 1.60 | 0.95 e ± 0.03 | 2.95 c ± 0.08 | 398 d ± 3 | 0.092 f ± 0.004 |
| DPPH | FRAP | TPC | |
|---|---|---|---|
| DPPH | 1 | ||
| FRAP | 0.96 | 1 | |
| TPC | 0.95 | 0.94 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzachristas, A.; Pasvanka, K.; Liouni, M.; Calokerinos, A.C.; Tataridis, P.; Proestos, C. Effect of Hippophae rhamnoides L. Leaves Treatment on the Antioxidant Capacity, Total Phenol Content and Sensory Profile of Moschofilero Wines Vinified with and without Added Sulphites. Appl. Sci. 2020, 10, 3444. https://doi.org/10.3390/app10103444
Tzachristas A, Pasvanka K, Liouni M, Calokerinos AC, Tataridis P, Proestos C. Effect of Hippophae rhamnoides L. Leaves Treatment on the Antioxidant Capacity, Total Phenol Content and Sensory Profile of Moschofilero Wines Vinified with and without Added Sulphites. Applied Sciences. 2020; 10(10):3444. https://doi.org/10.3390/app10103444
Chicago/Turabian StyleTzachristas, Alexandros, Konstantina Pasvanka, Maria Liouni, Antony C. Calokerinos, Panagiotis Tataridis, and Charalampos Proestos. 2020. "Effect of Hippophae rhamnoides L. Leaves Treatment on the Antioxidant Capacity, Total Phenol Content and Sensory Profile of Moschofilero Wines Vinified with and without Added Sulphites" Applied Sciences 10, no. 10: 3444. https://doi.org/10.3390/app10103444
APA StyleTzachristas, A., Pasvanka, K., Liouni, M., Calokerinos, A. C., Tataridis, P., & Proestos, C. (2020). Effect of Hippophae rhamnoides L. Leaves Treatment on the Antioxidant Capacity, Total Phenol Content and Sensory Profile of Moschofilero Wines Vinified with and without Added Sulphites. Applied Sciences, 10(10), 3444. https://doi.org/10.3390/app10103444







