Bioactive Glasses and Glass/Polymer Composites for Neuroregeneration: Should We Be Hopeful?
Abstract
:1. Introduction
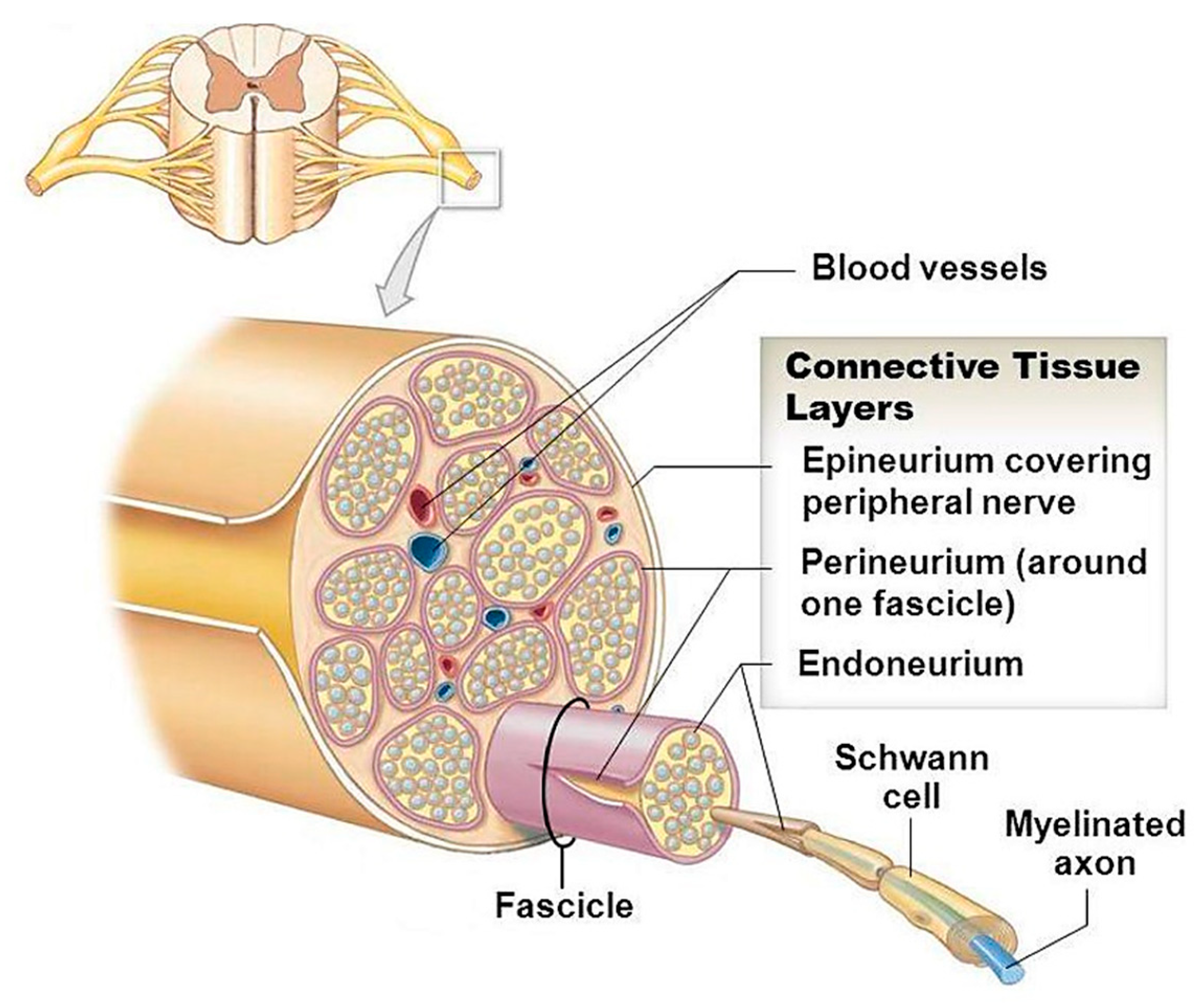
2. The Structure of Peripheral Nerves—Looking at What We Want to Regenerate
3. Post-Injury Scenario—A Short Overview
4. Silicate-Based BGs in Peripheral Nerve Regeneration
5. Phosphate-Based BGs in Peripheral Nerve Regeneration
6. Borate-Based BGs in Peripheral Nerve Regeneration
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mietto, B.S.; Mostacada, K.; Martinez, A.M.B. Neurotrauma and inflammation: CNS and PNS responses. Mediat. Inflamm. 2015, 2015, 251204. [Google Scholar] [CrossRef]
- El Seblani, N.; Welleford, A.; Quintero, J.E.; van Horne, C.; Gerhardt, G.A. Invited Review: Utilizing Peripheral Nerve Regenerative Elements to Repair Damage in the CNS. J. Neurosci. Methods 2020, 335, 108623. [Google Scholar] [CrossRef] [PubMed]
- Naghavi Alhosseini, S.; Moztarzadeh, F.; Kargozar, S.; Dodel, M.; Tahriri, M. Development of polyvinyl alcohol fibrous biodegradable scaffolds for nerve tissue engineering applications: In vitro study. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 474–480. [Google Scholar] [CrossRef]
- Novajra, G.; Baino, F.; Raimondo, S.; Lousteau, J.; Milanese, D.; Vitale-Brovarone, C. Bioactive glasses for nerve regeneration. In Bioactive Glasses; RSC Publishing: London, UK, 2016; pp. 420–441. [Google Scholar]
- Dodla, M.C.; Alvarado-Velez, M.; Mukhatyar, V.J.; Bellamkonda, R.V. Peripheral nerve regeneration. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1223–1236. [Google Scholar]
- Matejčík, V. Peripheral nerve reconstruction by autograft. Injury 2002, 33, 627–631. [Google Scholar] [CrossRef]
- Cerqueira, S.R.; Lee, Y.-S.; Cornelison, R.C.; Mertz, M.W.; Wachs, R.A.; Schmidt, C.E.; Bunge, M.B. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials 2018, 177, 176–185. [Google Scholar] [CrossRef]
- O’Rourke, C.; Day, A.; Murray-Dunning, C.; Thanabalasundaram, L.; Cowan, J.; Stevanato, L.; Grace, N.; Cameron, G.; Drake, R.; Sinden, J. An allogeneic ‘off the shelf’therapeutic strategy for peripheral nerve tissue engineering using clinical grade human neural stem cells. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nishiura, Y.; Brandt, J.; Nilsson, A.; Kanje, M.; Dahlin, L. Addition of cultured Schwann cells to tendon autografts and freeze–thawed muscle grafts improves peripheral nerve regeneration. Tissue Eng. 2004, 10, 157–164. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Zhang, L.; Yu, D. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biom. Sci. Polym. Ed. 2020, 31, 519–548. [Google Scholar] [CrossRef]
- Amani, H.; Kazerooni, H.; Hassanpoor, H.; Akbarzadeh, A.; Pazoki-Toroudi, H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3524–3539. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hsu, S.-H. Biomaterials and neural regeneration. Neural Regen. Res. 2020, 15, 1243. [Google Scholar]
- Hench, L.L. Bioactive Ceramics. Ann. N. Y. Acad. Sci. 1988, 523, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Baino, F.; Verné, E. Glass-based coatings on biomedical implants: A state-of-the-art review. Biomed. Glasses 2017, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Baino, F. Using Bioactive Glasses for the Management of Burns: Hope or Hype? Front. Bioeng. Biotechnol. 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Can bioactive glasses be useful to accelerate the healing of epithelial tissues? Mater. Sci. Eng. C 2019, 97, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; O’shea, H.; Kehoe, S.; Boyd, D. Time-dependent evaluation of mechanical properties and in vitro cytocompatibility of experimental composite-based nerve guidance conduits. J. Mech. Behav. Biomed. Mater. 2011, 4, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Koudehi, M.F.; Fooladi, A.A.I.; Mansoori, K.; Jamalpoor, Z.; Amiri, A.; Nourani, M.R. Preparation and evaluation of novel nano-bioglass/gelatin conduit for peripheral nerve regeneration. J. Mater. Sci. 2014, 25, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, V.L.; Nigmatullin, R.; Ladino, B.; Taylor, C.; Boccaccini, A.; Knowles, J.; Claeyssens, F.; Haycock, J.; Roy, I. Modulation of neuronal cell affinity of composites scaffolds based on polyhydroxyalkanoates and bioactive glasses. Biom. Mater. 2020. [Google Scholar] [CrossRef]
- Shan, D.; Ma, C.; Yang, J. Enabling biodegradable functional biomaterials for the management of neurological disorders. Adv. Drug Deliv. Rev. 2019, 148, 219–238. [Google Scholar] [CrossRef]
- Marquardt, L.M.; Day, D.; Sakiyama-Elbert, S.E.; Harkins, A.B. Effects of borate-based bioactive glass on neuron viability and neurite extension. J. Biomed. Mater. Res. Part A 2014, 102, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.; Lee, G.S.; Kim, J.W.; Kim, M.S.; Ahn, H.S.; Lim, J.Y.; Kim, H.W.; Son, Y.J.; Knowles, J.C.; Hyun, J.K. Phosphate glass fibres promote neurite outgrowth and early regeneration in a peripheral nerve injury model. J. Tissue Eng. Regen. Med. 2015, 9, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhah, A.; Marquardt, L.M.; Sakiyama-Elbert, S.E.; Day, D.E.; Harkins, A.B. Fabrication and characterization of poly-(ε)-caprolactone and bioactive glass composites for tissue engineering applications. Mater. Sci. Eng. C 2015, 49, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Senger, J.-L.; Chan, K.M.; Macandili, H.; Chan, A.W.; Verge, V.M.; Jones, K.E.; Webber, C.A. Conditioning electrical stimulation promotes functional nerve regeneration. Exp. Neurol. 2019, 315, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Senger, J.-L.B.; Chan, K.M.; Webber, C.A. Conditioning electrical stimulation is superior to postoperative electrical stimulation, resulting in enhanced nerve regeneration and functional recovery. Exp. Neurol. 2020, 325, 113147. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Ubeda, A.; Sartori, M.; Azorin, J.M.; Felici, F.; Farina, D. Central nervous system modulates the neuromechanical delay in a broad range for the control of muscle force. J. Appl. Physiol. 2018, 125, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Noback, C.R.; Ruggiero, D.A.; Demarest, R.J.; Strominger, N.L. The Human Nervous System: Structure and Function; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Dalton, P.D.; Harvey, A.R.; Oudega, M.; Plant, G.W. Tissue engineering of the nervous system. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 583–625. [Google Scholar]
- Nicholls, K.; Furness, N.D. Peripheral nerve compression syndromes of the upper limb. Surgery 2019, 37, 288–293. [Google Scholar] [CrossRef]
- Johnson, E.O.; Zoubos, A.B.; Soucacos, P.N. Regeneration and repair of peripheral nerves. Injury 2005, 36, S24–S29. [Google Scholar] [CrossRef]
- Geuna, S.; Raimondo, S.; Ronchi, G.; Di Scipio, F.; Tos, P.; Czaja, K.; Fornaro, M. Histology of the peripheral nerve and changes occurring during nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 27–46. [Google Scholar]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef]
- Zhang, P.-X.; Han, N.; Kou, Y.-H.; Zhu, Q.-T.; Liu, X.-L.; Quan, D.-P.; Chen, J.-G.; Jiang, B.-G. Tissue engineering for the repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 51. [Google Scholar] [PubMed]
- Ciaramitaro, P.; Mondelli, M.; Logullo, F.; Grimaldi, S.; Battiston, B.; Sard, A.; Scarinzi, C.; Migliaretti, G.; Faccani, G.; Cocito, D. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010, 15, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wiener Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and cellular mechanisms of axonal regeneration after spinal cord injury. Mol. Cell. Proteom. 2016, 15, 394–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-G.; Ma, J.-T.; Liu, H.-W.; Hu, M.; Huang, H.-T. ERK/MAPK and PI3K/AKT signal channels simultaneously activated in nerve cell and axon after facial nerve injury. Saudi J. Biol. Sci. 2017, 24, 1853–1858. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Guo, Q.; Qian, T.; Zhang, P.; Li, S.; Xue, C.; Gu, X. Overlapping mechanisms of peripheral nerve regeneration and angiogenesis following sciatic nerve transection. Front. Cell. Neurosci. 2017, 11, 323. [Google Scholar] [CrossRef]
- Gutmann, E.; Guttmann, L.; Medawar, P.; Young, J. The rate of regeneration of nerve. J. Exp. Biol. 1942, 19, 14–44. [Google Scholar] [CrossRef] [Green Version]
- Sunderland, S. Rate of regeneration in human peripheral nerves: Analysis of the interval between injury and onset of recovery. Arch. Neurol. Psychiatry 1947, 58, 251–295. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J. Neurosci. 1995, 15, 3876–3885. [Google Scholar] [CrossRef] [Green Version]
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Whiteman, D.R.; Voigt, C.; Miller, M.C.; Kim, H. No Difference in Outcomes Detected Between Decellular Nerve Allograft and Cable Autograft in Rat Sciatic Nerve Defects. JBJS 2019, 101, e42. [Google Scholar] [CrossRef] [PubMed]
- Roballo, K.C.S.; Bushman, J. Evaluation of the host immune response and functional recovery in peripheral nerve autografts and allografts. Transpl. Immunol. 2019, 53, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Boriani, F.; Savarino, L.; Fazio, N.; Pedrini, F.A.; Fini, M.; Aldini, N.N.; Martini, L.; Zini, N.; Bernardini, M.; Bolognesi, F. Auto-Allo Graft Parallel Juxtaposition for Improved Neuroregeneration in Peripheral Nerve Reconstruction Based on Acellular Nerve Allografts. Ann. Plast. Surg. 2019, 83, 318–325. [Google Scholar] [CrossRef]
- Liu, M.; Huang, C.; Zhao, Z.; Wang, A.; Li, P.; Fan, Y.; Zhou, G. Nano-hydroxyapatite (n-HA) Involved in The Regeneration of Rat Nerve Injury Triggered by Overloading Stretch. Med. Novel Technol. Devices 2019, 4, 100022. [Google Scholar] [CrossRef]
- Itoh, S.; Yamaguchi, I.; Suzuki, M.; Ichinose, S.; Takakuda, K.; Kobayashi, H.; Shinomiya, K.; Tanaka, J. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003, 993, 111–123. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, G.; Hou, Y.; Kuang, G.; Jia, Z.; Li, P.; Fan, Y. Effect of nano-hydroxyapatite-coated magnetic nanoparticles on axonal guidance growth of rat dorsal root ganglion neurons. J. Biom. Mater. Res. Part A 2015, 103, 3066–3071. [Google Scholar] [CrossRef]
- Zhong, D.; Cao, Y.; Li, C.-J.; Li, M.; Rong, Z.-J.; Jiang, L.; Guo, Z.; Lu, H.-B.; Hu, J.-Z. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 2020, 1535370219895491. [Google Scholar] [CrossRef]
- Fang, Z.; Ge, X.; Chen, X.; Xu, Y.; Yuan, W.-E.; Ouyang, Y. Enhancement of sciatic nerve regeneration with dual delivery of vascular endothelial growth factor and nerve growth factor genes. J. Nanobiotechnology 2020, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.; Banijamali, S.; Baino, F.; Kargozar, S.; Hill, R.G. Calcium carbonate: Adored and ignored in bioactivity assessment. Acta Biomater. 2019, 91, 35–47. [Google Scholar] [CrossRef]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biom. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Zhao, X.; Li, H.; Yuan, W.-E.; Fan, C. Asymmetrical 3D nanoceria channel for severe neurological defect regeneration. iScience 2019, 12, 216–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.-W.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Papke, J.B.; Mohammadkhah, A.; Day, D.E.; Harkins, A.B. Effects of chemically doped bioactive borate glass on neuron regrowth and regeneration. Ann. Biomed. Eng. 2016, 44, 3468–3477. [Google Scholar] [CrossRef]
- Zhang, X.; Kehoe, S.; Adhi, S.; Ajithkumar, T.; Moane, S.; O’Shea, H.; Boyd, D. Composition–structure–property (Zn2+ and Ca2+ ion release) evaluation of Si–Na–Ca–Zn–Ce glasses: Potential components for nerve guidance conduits. Mater. Sci. Eng. C 2011, 31, 669–676. [Google Scholar] [CrossRef]
- Bunting, S.; Di Silvio, L.; Deb, S.; Hall, S. Bioresorbable Glass Fibres Facilitate Peripheral Nerve Regeneration. J. Hand Surg. 2005, 30, 242–247. [Google Scholar] [CrossRef]
- Parthasarathy, K.S.; Cheng, Y.-C.N.; McAllister II, J.P.; Shen, Y.; Li, J.; Deren, K.; Haacke, E.M.; Auner, G.W. Biocompatibilities of sapphire and borosilicate glass as cortical neuroprostheses. Magn. Reson. Imaging 2007, 25, 1333–1340. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Samadikuchaksaraie, A.; Hill, R.G.; Shah, P.A.; Milan, P.B.; Mozafari, M.; Fathi, M.; Joghataei, M.T. Synthesis, physico-chemical and biological characterization of strontium and cobalt substituted bioactive glasses for bone tissue engineering. J. Non-Cryst. Solids 2016, 449, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Meyers, J.L. Bioactive Glass Treatment of Inflammation in Skin Conditions. Google Patents US6423343B1, 23 July 2002. [Google Scholar]
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peştean, C.; Nagy, A.; Tăbăran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biom. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Boccafoschi, F.; Bosetti, M.; Cannas, M. Adhesion and differentiation of neuronal cells on Zn-doped bioactive glasses. J. Biomater. Appl. 2014, 28, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Dun, G.; Jin, D.; Du, Y. Development of polypyrrole/collagen/nano-strontium substituted bioactive glass composite for boost sciatic nerve rejuvenation in vivo. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3423–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehnavi, N.; Parivar, K.; Goodarzi, V.; Salimi, A.; Nourani, M.R. Systematically engineered electrospun conduit based on PGA/collagen/bioglass nanocomposites: The evaluation of morphological, mechanical, and bio-properties. Polym. Adv. Technol. 2019, 30, 2192–2206. [Google Scholar] [CrossRef]
- Souza, M.T.; Peitl, O.; Zanotto, E.D.; Boccaccini, A.R. Novel Double-Layered Conduit Containing Highly Bioactive Glass Fibers for Potential Nerve Guide Application. Int. J. Appl. Glass Sci. 2016, 7, 183–194. [Google Scholar] [CrossRef]
- Navarro, M.; del Valle, S.; Martínez, S.; Zeppetelli, S.; Ambrosio, L.; Planell, J.A.; Ginebra, M.P. New macroporous calcium phosphate glass ceramic for guided bone regeneration. Biomaterials 2004, 25, 4233–4241. [Google Scholar] [CrossRef]
- Neel, E.A.; Knowles, J. Physical and biocompatibility studies of novel titanium dioxide doped phosphate-based glasses for bone tissue engineering applications. J. Mater. Sci. 2008, 19, 377–386. [Google Scholar]
- Vitale-Brovarone, C.; Ciapetti, G.; Leonardi, E.; Baldini, N.; Bretcanu, O.; Verné, E.; Baino, F. Resorbable glass–ceramic phosphate-based scaffolds for bone tissue engineering: Synthesis, properties, and in vitro effects on human marrow stromal cells. J. Biomater. Appl. 2011, 26, 465–489. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Mattu, C.; Baino, F.; Vitale-Brovarone, C.; Ciardelli, G. Bioresorbable glass effect on the physico-chemical properties of bilayered scaffolds for osteochondral regeneration. Mater. Lett. 2012, 89, 74–76. [Google Scholar] [CrossRef]
- Bretcanu, O.; Baino, F.; Verné, E.; Vitale-Brovarone, C. Novel resorbable glass-ceramic scaffolds for hard tissue engineering: From the parent phosphate glass to its bone-like macroporous derivatives. J. Biomater. Appl. 2014, 28, 1287–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, N.-Y.; Knowles, J.C.; Lee, G.-S.; Kim, J.-W.; Kim, H.-W.; Son, Y.-J.; Hyun, J.K. Effects of phosphate glass fiber–collagen scaffolds on functional recovery of completely transected rat spinal cords. Acta Biomater. 2012, 8, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Novajra, G.; Lousteau, J.; Milanese, D.; Raimondo, S.; Fornaro, M. Phosphate glass fibres and their role in neuronal polarization and axonal growth direction. Acta Biomater. 2012, 8, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Kermani, F.; Mollazadeh Beidokhti, S.; Hamzehlou, S.; Verné, E.; Ferraris, S.; Baino, F. Functionalization and Surface Modifications of Bioactive Glasses (BGs): Tailoring of the Biological Response Working on the Outermost Surface Layer. Materials 2019, 12, 3696. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.-S.; Hwang, J.-Y.; Kim, M.S.; Lee, J.-Y.; Kim, J.-W.; Kim, H.-S.; Shin, U.S.; Knowles, J.C.; Kim, H.-W.; Hyun, J.K. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015, 13, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Dzondo-Gadet, M.; Mayap-Nzietchueng, R.; Hess, K.; Nabet, P.; Belleville, F.; Dousset, B. Action of boron at the molecular level. Biol. Trace Elem. Res. 2002, 85, 23–33. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Hupa, L.; Jokic, B.; Detsch, R.; Grünewald, A.; Boccaccini, A.R. Angiogenic potential of boron-containing bioactive glasses: In vitro study. J. Mater. Sci. 2017, 52, 8785–8792. [Google Scholar] [CrossRef]
- Acaroz, U.; Ince, S.; Arslan-Acaroz, D.; Gurler, Z.; Kucukkurt, I.; Demirel, H.H.; Arslan, H.O.; Varol, N.; Zhu, K. The ameliorative effects of boron against acrylamide-induced oxidative stress, inflammatory response, and metabolic changes in rats. Food Chem. Toxicol. 2018, 118, 745–752. [Google Scholar] [CrossRef]
- Kızılay, Z.; Erken, H.A.; Çetin, N.K.; Aktaş, S.; Abas, B.İ.; Yılmaz, A. Boric acid reduces axonal and myelin damage in experimental sciatic nerve injury. Neural Regen. Res. 2016, 11, 1660–1665. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Ricotti, L.; Moscato, S.; Bertoni, G.; Falqui, A.; Berrettini, S.; Petrini, M.; Mattoli, V.; et al. Enhancement of Neurite Outgrowth in Neuronal-Like Cells following Boron Nitride Nanotube-Mediated Stimulation. ACS Nano 2010, 4, 6267–6277. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Sher Waris, T.; Shahzadi, L.; Anwar Chaudhry, A.; Ur Rehman, I.; Yar, M. Boron for tissue regeneration-it’s loading into chitosan/collagen hydrogels and testing on chorioallantoic membrane to study the effect on angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 525–534. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Buettner, T.; Pacheco, V.M.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Thyparambil, N.J.; Gutgesell, L.C.; Bromet, B.A.; Flowers, L.E.; Greaney, S.; Day, D.E.; Semon, J.A. Bioactive borate glass triggers phenotypic changes in adipose stem cells. J. Mater. Sci. Mater. Med. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Schuhladen, K.; Wang, X.; Hupa, L.; Boccaccini, A.R. Dissolution of borate and borosilicate bioactive glasses and the influence of ion (Zn, Cu) doping in different solutions. J. Non-Cryst. Solids 2018, 502, 22–34. [Google Scholar] [CrossRef]
- Shafaghi, R.; Rodriguez, O.; Phull, S.; Schemitsch, E.H.; Zalzal, P.; Waldman, S.D.; Papini, M.; Towler, M.R. Effect of TiO2 doping on degradation rate, microstructure and strength of borate bioactive glass scaffolds. Mater. Sci. Eng. C 2020, 107, 110351. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Li, J.; Roberts, S.; Semon, J.A.; Park, J.; Day, D.E.; Leu, M.C. Near-field electrospinning of a polymer/bioactive glass composite to fabricate 3D biomimetic structures. Int. J. Bioprinting 2019, 5. [Google Scholar] [CrossRef]
- Tang, Y.; Pang, L.; Wang, D. Preparation and characterization of borate bioactive glass cross-linked PVA hydrogel. J. Non-Cryst. Solids 2017, 476, 25–29. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Semon, J.A.; Bindbeutel, A.T.; Day, D.E.; Leu, M.C. Bioprinting with bioactive glass loaded polylactic acid composite and human adipose stem cells. Bioprinting 2020, 18, e00075. [Google Scholar] [CrossRef]
- Kolan, K.; Liu, Y.; Baldridge, J.; Murphy, C.; Semon, J.; Day, D.; Leu, M. Solvent Based 3D Printing of Biopolymer/Bioactive Glass Composite and Hydrogel for Tissue Engineering Applications. Procedia CIRP 2017, 65, 38–43. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. Int. J. Appl. Ceramic Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 022001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.; Garino, J.; Flaitz, C.; Ducheyne, P. Excretion of resorption products from bioactive glass implanted in rabbit muscle. J. Biomed. Mater. Res. Part A 2005, 75, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Garino, J.; Ducheyne, P. Silicon excretion from bioactive glass implanted in rabbit bone. Biomaterials 2002, 23, 213–217. [Google Scholar] [CrossRef]
- Modglin, V.C.; Brown, R.F.; Jung, S.B.; Day, D.E. Cytotoxicity assessment of modified bioactive glasses with MLO-A5 osteogenic cells in vitro. J. Mater. Sci. 2013, 24, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, M.; Chen, S.; Sun, B.; Guo, Y.; He, C.; Mo, X.; Zhu, B.; You, Z. Molecularly engineered metal-based bioactive soft materials–neuroactive magnesium ion/polymer hybrids. Acta Biomater. 2019, 85, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, G.; Luk, K.D.; Cheung, K.M.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cell. Phys. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, A.; Yao, P.; Sun, X.; Chen, C.; Mo, C.; Shi, L.; Chen, Y.; Liu, Q. Gadolinium promoted proliferation in mouse embryo fibroblast NIH3T3 cells through Rac and PI3K/Akt signaling pathways. Biometals 2014, 27, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rouabhia, M.; Wang, Z.; Roberge, C.; Shi, G.; Roche, P.; Li, J.; Dao, L.H. Electrically conductive biodegradable polymer composite for nerve regeneration: Electricity-stimulated neurite outgrowth and axon regeneration. Artif. Organs 2007, 31, 13–22. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.; Kim, H.W.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. Biofactors 2019, 45, 135–151. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Budel, S.; Baughman, K.; Gould, G.; Song, K.-H.; Strittmatter, S.M. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J. Neurotrauma 2009, 26, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Madura, T.; Tomita, K.; Terenghi, G. Ibuprofen improves functional outcome after axotomy and immediate repair in the peripheral nervous system. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1641–1646. [Google Scholar] [CrossRef] [PubMed]






| BG Composition/Synthesis Route | Applied Construct | Remarks | Ref (s) |
|---|---|---|---|
| 64 SiO2, 26 CaO, 5 MgO and 5 P2O5 (mol%), sol–gel method | Silicate glass nanoparticles/gelatin nanocomposites | - The composites showed no significant cytotoxicity - The composite showed great potential in the regeneration of myelinated axons in damaged sciatic nerves | [20] |
| 45S5 BG: 45.0 SiO2, 24.5 Na2O, 24.5 CaO, and 6.0 P2O5 (wt%) Borate glass: 53 SiO2, 20 CaO, 6 Na2O, 4 P2O5, 12 K2O, 5% MgO (wt%), melt-derived method | Silicate glass/polyhydroxyalkanoate (PHA) composites | - The composites exhibited good biocompatibility - The constructs improved the mechanical properties needed for the regeneration of peripheral nerves - The constructs supported growth and neuronal differentiation | [21] |
| Bioglass 45S5 (45% SiO2, 24% Na2O, 24.5% CaO and 6% P2O5) (wt%), melt-derived method | Silicate glass (45S5) fibers | - The glass fibers provided a suitable substrate for the adhesion and growth of rat Schwann cells - Axonal regeneration occurred through a Silastic conduit filled with Bioglass fibers - Axonal regrowth was observed in the Bioglass-fiber-treated rats comparable to an autograft 4 weeks after implantation in a 0.5 cm interstump gap in the sciatic nerves | [60] |
| 45S5 BG: 45% SiO2, 24% Na2O, 24.5% CaO and 6% P2O5 (wt%) substituted with Zn (5, 10, and 20 wt%) | Silicate glass (45S5) doped with Zn | - Bioglasses doped with low concentration of Zn supported cell adhesion and proliferation of undifferentiated SK-N-BE human neuroblastoma cells - Bioglasses doped with high Zn concentration lightly induced adhesion and phenotype characterization of the cells | [67] |
| Commercial nano-sized 45S5 BG | Silicate glass nanoparticles/polyglycolic acid (PGA), collagen electrospun composites | - The glass-containing nanofibers showed good compatibility with rats’ mesenchymal stem cells (MSCs) - The composite constructs provided a better substrate for cell adhesion and proliferation as compared to glass-free matrixes | [69] |
| SiO2–Na2O–K2O–MgO–CaO–P2O5 | Silicate glass microfibers incorporated in nanofibrous PCL | - Significant improvements in the mechanical properties and wettability were observed in the case of glass/polymer composites than the polymer matrix alone - The composites showed increased bioactivity | [70] |
| 50 P2O5, 40 CaO, 5 Na2O, 5 Fe2O3 (mol%), melt-derived method | Phosphate glass/collagen scaffolds | - Stimulated neurite outgrowth along the fibers in vitro - Enhanced axons extending along the scaffold at 7 days post-surgery - Caused recovery of plantar muscle atrophy at 8 weeks post-implantation - No significant differences in the case of functional capacity between the experimental groups and controls (collagen scaffolds lacking BGs) 12 weeks after implantation | [24] |
| 50 P2O5, 40 CaO, 5 Na2O, 5 Fe2O3 (mol%), melt-derived method | Phosphate glass fiber/collagen scaffolds | - The scaffolds induced axon growth from the proximal and distal stumps to the scaffold 12 weeks after implantation - The composites recovered locomotor and bladder functions at 8 weeks post-implantation - Endogenous BDNF levels were detected in the bladder at 12 weeks post-implantation | [76] |
| 50P2O5–30CaO–9Na2O–3SiO2–3MgO–(5– x)K2O–xTiO2 mol.% (x = 0, 2.5, 5) | Phosphate glass fibers with a diameter ranging between 25 and 82 µm | - The glass fibers provided a proper substrate for cell adhesion and proliferation - The aligned configuration of the fibers supported the growing axons of dorsal root ganglion (DRG) neurons along the fiber axis direction | [77] |
| 50P2O5–40CaO–5Na2O–5Fe2O3 (mol.%) | Phosphate glass microfibers–aminated carbon nanotubes (CNTs) incorporated into poly(L/D-lactic acid) (PLDLA) tubes | - Neurites of DRG outgrew along the aligned composite scaffolds - The constructs increased the number of regenerating axons and improved the electrophysiological functions | [79] |
| 13–93 B3 borate glass: 53B2O3, 20 CaO, 6Na2O, 12K2O, 5MgO, 4P2O (wt%) | Borate glass rods and microfiber/fibrin composites | - Borate glasses and the composite scaffolds improved neurite extension comparable to that of control fibrin scaffolds, clarifying the lack of significant effect of the glasses on neuronal health - Aligned glass scaffolds could guide neurite extension in an oriented manner | [23] |
| 13–93 B3 borate glass; 45S5 silicate glass; a blend of 13–93 B3 and 45S5 glasses | Borate and silicate glasses/PCL composites | - The composites containing 13–93 B3 borate glass exhibited a higher degradation rate than their counterparts containing only 45S5 silicate glass - None of the glasses caused adverse effects on neurite extension as compared to PCL alone - Neurite extension was increased in contact with PCL:45S5 PCL:13–93 B3 composites after 24 h of incubation | [25] |
| 13-93 B3 borate glass doped with Ag, Ce, Cu, Fe, Ga, iodine (I), Y, and Zn | Borate glass/PCL composite sheets | - Cu, Fe, Ga, Zn, and Sr-doped glasses promoted the survival and outgrowth of neurons as compared to undoped glasses - The Cu- and Ga-doped glasses showed the lowest average percent survival of support cells | [58] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargozar, S.; Mozafari, M.; Ghenaatgar-Kasbi, M.; Baino, F. Bioactive Glasses and Glass/Polymer Composites for Neuroregeneration: Should We Be Hopeful? Appl. Sci. 2020, 10, 3421. https://doi.org/10.3390/app10103421
Kargozar S, Mozafari M, Ghenaatgar-Kasbi M, Baino F. Bioactive Glasses and Glass/Polymer Composites for Neuroregeneration: Should We Be Hopeful? Applied Sciences. 2020; 10(10):3421. https://doi.org/10.3390/app10103421
Chicago/Turabian StyleKargozar, Saeid, Masoud Mozafari, Maryam Ghenaatgar-Kasbi, and Francesco Baino. 2020. "Bioactive Glasses and Glass/Polymer Composites for Neuroregeneration: Should We Be Hopeful?" Applied Sciences 10, no. 10: 3421. https://doi.org/10.3390/app10103421
APA StyleKargozar, S., Mozafari, M., Ghenaatgar-Kasbi, M., & Baino, F. (2020). Bioactive Glasses and Glass/Polymer Composites for Neuroregeneration: Should We Be Hopeful? Applied Sciences, 10(10), 3421. https://doi.org/10.3390/app10103421






