Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine
Abstract
1. Introduction
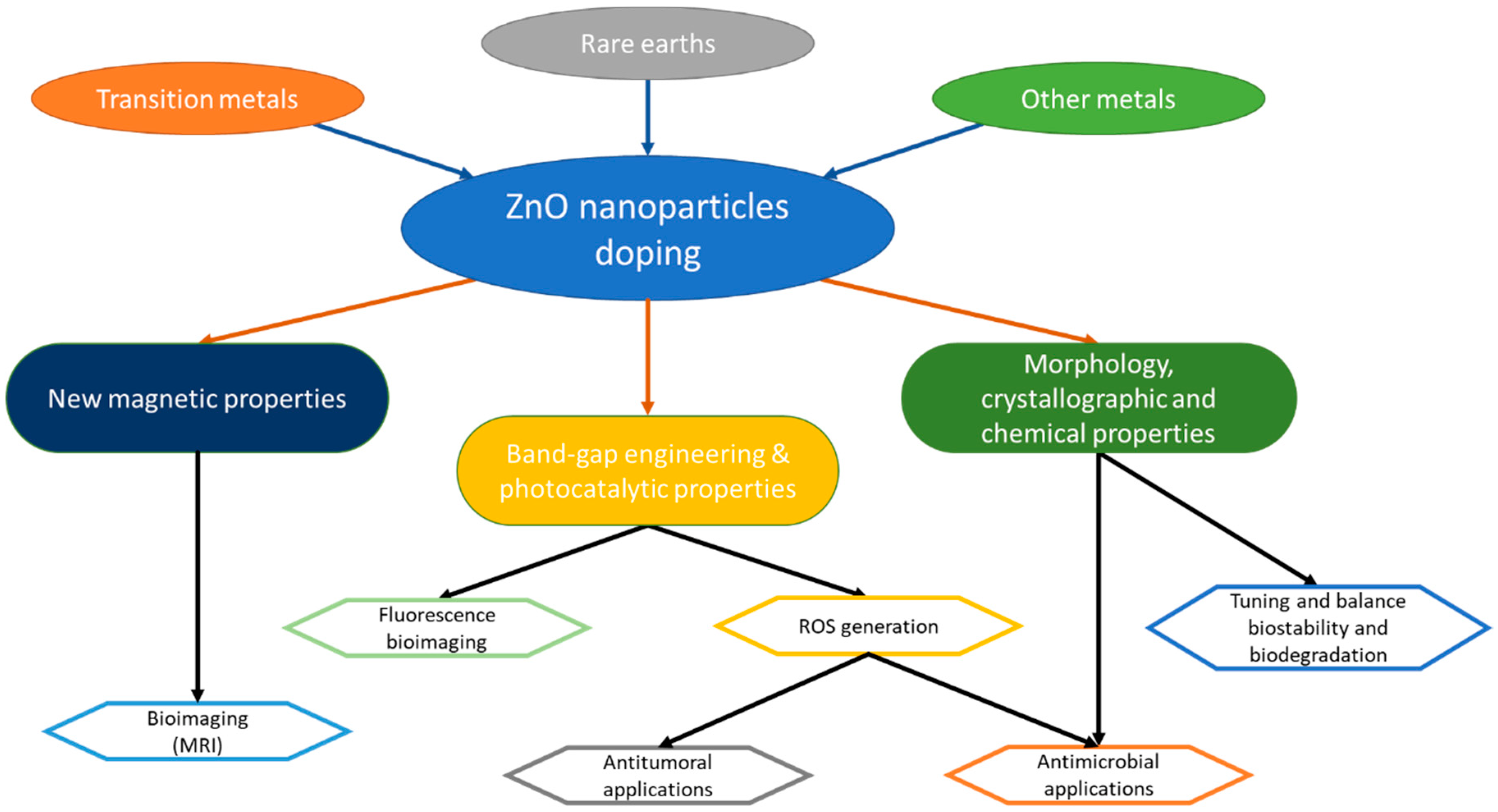
2. Materials for Doping ZnO Nanoparticles (NPs)
2.1. Rare Earth (RE) Elements
2.2. Transition Metals (TM) Elements
2.3. Other Elements
3. Synthesis Methods and Characterization
3.1. Wet Chemical Methods
3.2. Combustion Methods
3.3. Other Techniques
4. Use of Doped ZnO NPs in the Biomedical Field
4.1. Biological Behavior
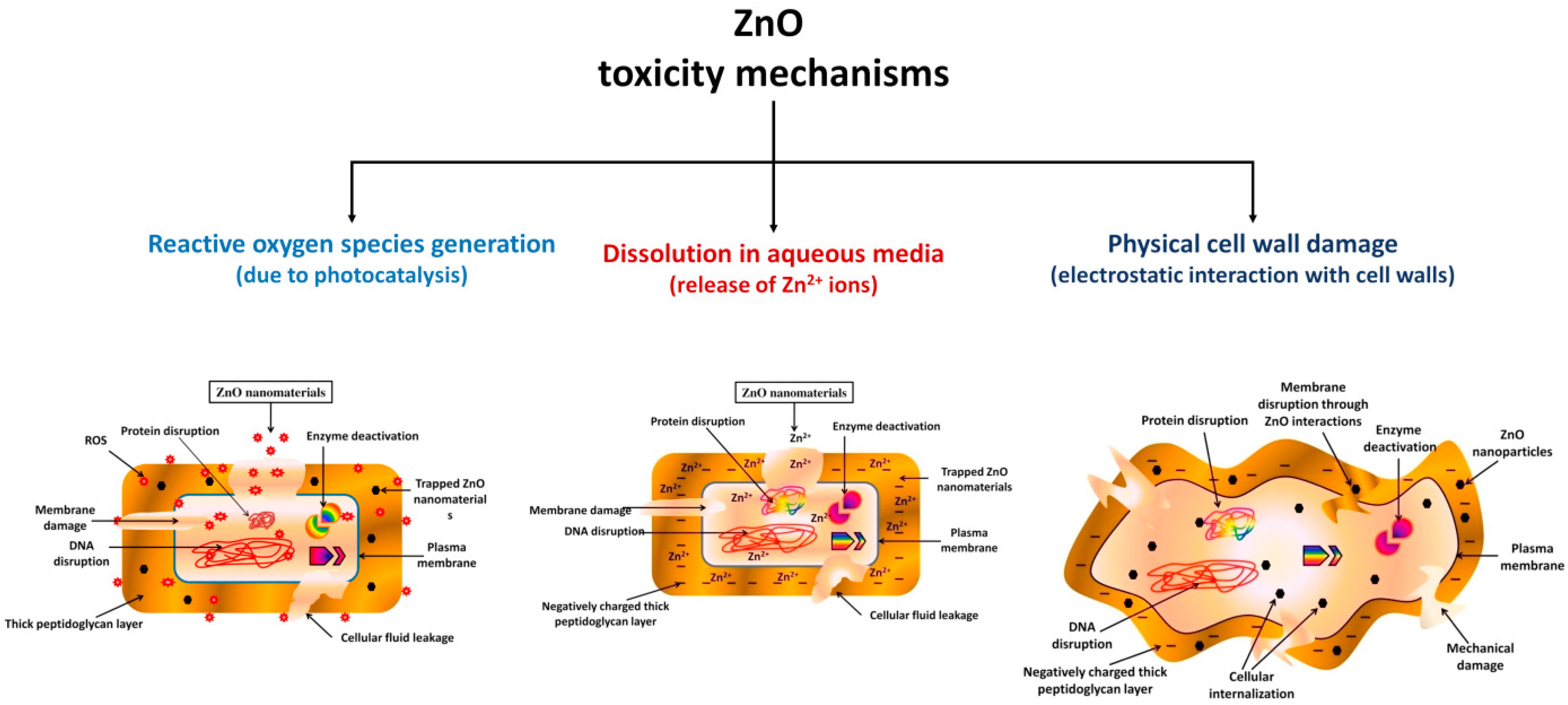
4.2. Antimicrobial Agents
4.3. Nanotools for Photobiomaging
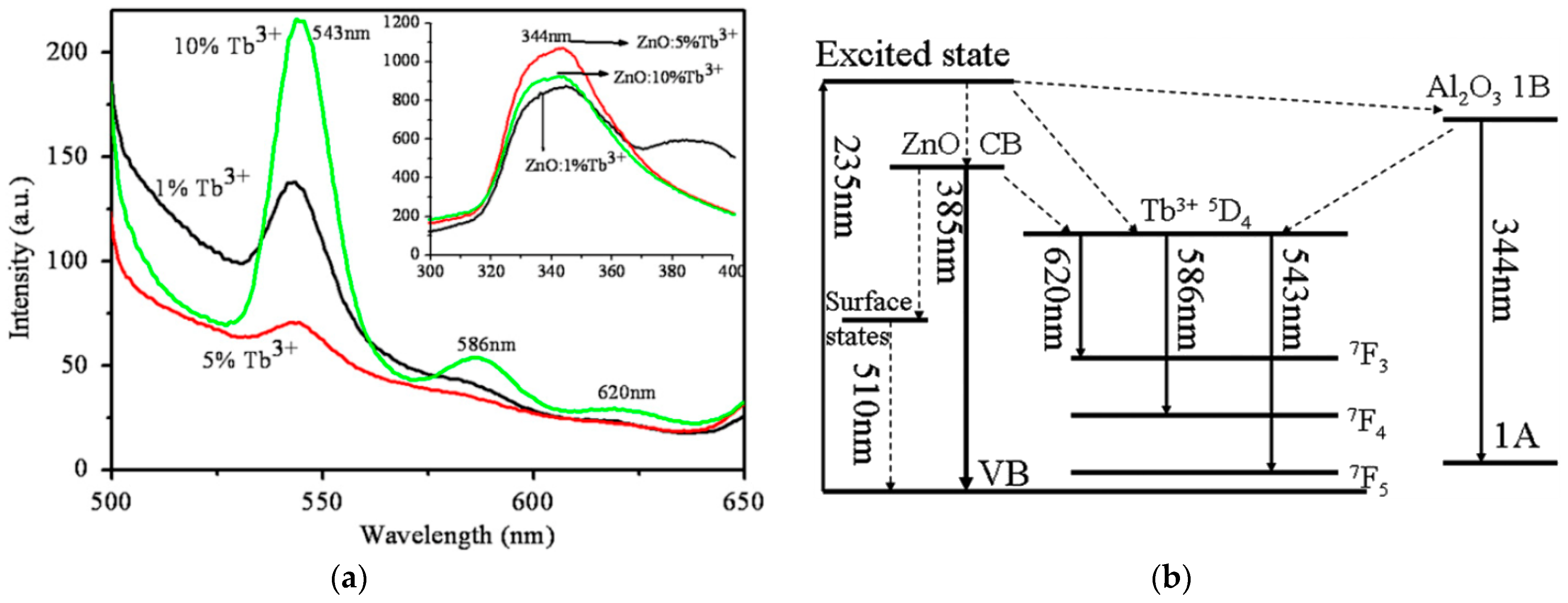
4.3.1. Photoluminescence Properties
4.3.2. Exploiting Room Temperature Ferromagnetism for Imaging
| Ref. | Dopant | Dopant Concentration | Bandgap (eV) | Dopant Concentration | Saturation Magnetization (emu/g) |
|---|---|---|---|---|---|
| [259,260,261] | Cu | 0, 10% | 3.35, 3.30, respectively | 0.05–0.20 mol.% | 0.011–0.063 |
| [180,262] | Fe | Zn1−xFexO (x = 0, 0.01, 0.04, 0.05, 0.06) | 3.243, 3.236, 3.216, 3.197, 3.195, respectively | x = 0.20 | 1.74 |
| [263] | Cr | 0.00 to 4.63 at.% | from 3.26 to 3.15 | 2.49 at.% | 4.86 |
| [175] | Co | 0, 5, 10 at.% | 3.10, 3.17, 3.24, respectively | 5 at.%, 10 at.% | 1.42, 1.75 |
| [264,265,266,267] | Mn | 0, 3, 5, 10, 15 mol.% | 3.31, 3.35, 3.38, 3.40, 3.42, respectively | 3.3 mol.%, 4.2 mol.% | 0.00123, 0.015 |
| [268] | Ni | Zn1−xNixO (x = 0, 0.05) | 3.28, 3.32, respectively | x = 0.05 | 2.9–2.8 |
| [269,270] | Al | 0, 2 at.% | 3.07, 3.12, respectively | 0.03 at.% | 0.012 |
| [138,271] | Mg | 0, 2.5%, 5%, 7.5% | 3.36, 3.27, 3.13, 3.04, respectively | 3% | 1.05 × 10−3 |
| [272] | Nd | ZnO, Zn0.97Nd0.03O | 3.34, 3.12, respectively | x = 0.03 | 0.67 |
| [273,274] | Sm | 0, 1, 3, 5 mol.% | 3.27, 3.25, 3.10, 3.05, respectively | 0%–8% | 0.45, 0.363, 1.694, 3.613 and 2.197 emu/cm3 |
| [275,276] | Eu | 0, 1, 3, 5 mol.% | 3.18, 3.05, 3.00, 2.94, respectively | 10% | 0.040 |
| [277] | Tb | Zn1−xTbxO (x = 0, 0.02, 0.05, 0.1) | 3.35, 3.31, 3.30, 3.28, respectively | x = 0, 0.02, 0.05, 0.1 | 0.0042, 0.0276, 0.0359, 0.0519 |
| [117,278] | Gd | 0, 3, 6% | 2.71, 2.74, 2.98, respectively | 1.1%, 3.5%, and 5.1% | 0.0001, 0.05, 0.0032 |
| [279,280] | La | 1, 5 wt.% | 3.12, 3.18, respectively | 0, 1 mol.% | 0.102, 0.232 |
| [281,282] | Ce | 0, 1, 3 and 5 at.% | 3.21, 3.10, 3.08, 2.96, respectively | 0, 0.96, 1.96, 2.52 and 3.12 at.% | 1.895 × 10−3, 31.612 × 10−3, 26.818 × 10−3, 26.136 × 10−3, 23.608 × 10−3 |
4.4. Doped ZnO as Therapeutics against Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Genchi, G.G.; Marino, A.; Tapeinos, C.; Ciofani, G. Smart Materials Meet Multifunctional Biomedical Devices: Current and Prospective Implications for Nanomedicine. Front. Bioeng. Biotechnol. 2017, 5, 80. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, J.; An, S.; Jiang, C. pH-sensitive drug-delivery systems for tumor targeting. Ther. Deliv. 2013, 4, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Boissenot, T.; Bordat, A.; Fattal, E.; Tsapis, N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J. Control. Release 2016, 241, 144–163. [Google Scholar] [CrossRef]
- Huh, A.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release Off. J. Control. Release Soc. 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Liu, L.; Xue, X.; Liang, X.-J. Nanoparticle-based drug delivery systems: What can they really do in vivo? F1000Research 2017, 6, 681. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Canavese, G.; Cauda, V. Surface Engineering of Nanostructured ZnO Surfaces. Adv. Mater. Interfaces 2017, 4, 1600758. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Hu, S.-H. Functional Nanoparticles for Tumor Penetration of Therapeutics. Pharmaceutics 2018, 10, 193. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Bugno, J.; Lantvit, D.; Burdette, J.E.; Hong, S. Prolonged blood circulation and enhanced tumor accumulation of folate-targeted dendrimer-polymer hybrid nanoparticles. J. Control. Release 2014, 191, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Birkedal, H.; Vorup-Jensen, T.; Jakobsen, M.H.; Østergaard, L.; Horsman, M.R.; Besenbacher, F.; Howard, K.A.; et al. Size-Dependent Accumulation of PEGylated Silane-Coated Magnetic Iron Oxide Nanoparticles in Murine Tumors. ACS Nano 2009, 3, 1947–1951. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Shargh, V.H.; Hondermarck, H.; Liang, M. Antibody-targeted biodegradable nanoparticles for cancer therapy. Nanomedicine 2015, 11, 63–79. [Google Scholar] [CrossRef]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.-Y.; Laird Forrest, M.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Boschi, F.; De Sanctis, F. Overview of the optical properties of fluorescent nanoparticles for optical imaging. Eur. J. Histochem. EJH 2017, 61, 2830. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Varna, M.; Xuan, H.V.; Fort, E. Gold nanoparticles in cardiovascular imaging. Wires Nanomed. Nanobiotechnol. 2018, 10, e1470. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Ahmad, M.Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Gold nanoparticles in theranostic oncology: Current state-of-the-art. Expert Opin. Drug Deliv. 2012, 9, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Gharatape, A.; Salehi, R. Recent progress in theranostic applications of hybrid gold nanoparticles. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef]
- Ren, X.; Chen, H.; Yang, V.; Sun, D. Iron oxide nanoparticle-based theranostics for cancer imaging and therapy. Front. Chem. Sci. Eng. 2014, 8, 253–264. [Google Scholar] [CrossRef]
- Martín-Saavedra, F.M.; Ruíz-Hernández, E.; Boré, A.; Arcos, D.; Vallet-Regí, M.; Vilaboa, N. Magnetic mesoporous silica spheres for hyperthermia therapy. Acta Biomater. 2010, 6, 4522–4531. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene Quantum Dots-Capped Magnetic Mesoporous Silica Nanoparticles as a Multifunctional Platform for Controlled Drug Delivery, Magnetic Hyperthermia, and Photothermal Therapy. Small 2017, 13, 1602225. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo- and magnetic hyperthermia therapy. Microporous Mesoporous Mater. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Fathy, M.M.; Al-Wafi, R.; Darwesh, R.; Abdel-dayem, U.A.; Aldhahri, M.; Noorwali, A.; Al-ghamdi, A.A. Multifunctional magnetic-gold nanoparticles for efficient combined targeted drug delivery and interstitial photothermal therapy. Int. J. Pharm. 2019, 554, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Xu, J.; Dong, S.; He, F.; Zhong, C.; Yang, G.; Bi, H.; Xu, M.; Hu, Y.; Yang, D.; et al. Mesoporous cerium oxide-coated upconversion nanoparticles for tumor-responsive chemo-photodynamic therapy and bioimaging. Chem. Sci. 2019, 10, 8618–8633. [Google Scholar] [CrossRef]
- Amirshaghaghi, A.; Yan, L.; Miller, J.; Daniel, Y.; Stein, J.M.; Busch, T.M.; Cheng, Z.; Tsourkas, A. Chlorin e6-Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Nanoclusters as a Theranostic Agent for Dual-Mode Imaging and Photodynamic Therapy. Sci. Rep. 2019, 9, 2613. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Zhang, J.; Linden, M.; Sahlgren, C. Mesoporous silica nanoparticles in tissue engineering—A perspective. Nanomedicine 2016, 11, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Anthony, S.P. Chapter 9—Antimicrobial studies of metal and metal oxide nanoparticles. In Surface Chemistry of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, UK, 2016; pp. 265–300. [Google Scholar]
- Marino, A.; Genchi, G.G.; Mattoli, V.; Ciofani, G. Piezoelectric nanotransducers: The future of neural stimulation. Nano Today 2017, 14, 9–12. [Google Scholar] [CrossRef]
- Murillo, G.; Blanquer, A.; Vargas-Estevez, C.; Barrios, L.; Ibáñez, E.; Nogués, C.; Esteve, J. Electromechanical Nanogenerator–Cell Interaction Modulates Cell Activity. Adv. Mater. 2017, 29, 1605048. [Google Scholar] [CrossRef] [PubMed]
- Zwi-Dantsis, L.; Wang, B.; Marijon, C.; Zonetti, S.; Ferrini, A.; Massi, L.; Stuckey, D.J.; Terracciano, C.M.; Stevens, M.M. Remote Magnetic Nanoparticle Manipulation Enables the Dynamic Patterning of Cardiac Tissues. Adv. Mater. 2020, 32, 1904598. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ordered Mesoporous Materials in the Context of Drug Delivery Systems and Bone Tissue Engineering. Chem. A Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory Properties of Cerium Oxide Nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of Cerium oxide nanoparticles—A Review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef]
- Rosen, J.E.; Chan, L.; Shieh, D.-B.; Gu, F.X. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 275–290. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Hilger, I.; Kaiser, W.A. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine 2012, 7, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Racca, L.; Canta, M.; Dumontel, B.; Ancona, A.; Limongi, T.; Garino, N.; Laurenti, M.; Canavese, G.; Cauda, V. 12—Zinc Oxide Nanostructures in Biomedicine. In Smart Nanoparticles for Biomedicine; Ciofani, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 171–187. [Google Scholar]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Chapter 34—Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 425–431. [Google Scholar]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [PubMed]
- Laurenti, M.; Garino, N.; Canavese, G.; Hernandéz, S.; Cauda, V. Piezo- and Photocatalytic Activity of Ferroelectric ZnO:Sb Thin Films for the Efficient Degradation of Rhodamine-β dye Pollutant. ACS Appl. Mater. Interfaces 2020, 12, 25798–25808. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Lorenzoni, M.; Fontana, M.; Canavese, G.; Cauda, V.; Pirri, C.F. Evaluation of the piezoelectric properties and voltage generation of flexible zinc oxide thin films. Nanotechnology 2015, 26, 215704. [Google Scholar] [CrossRef]
- Laurenti, M.; Canavese, G.; Stassi, S.; Fontana, M.; Castellino, M.; Pirri, C.F.; Cauda, V. A porous nanobranched structure: An effective way to improve piezoelectricity in sputtered ZnO thin films. RSC Adv. 2016, 6, 76996–77004. [Google Scholar] [CrossRef]
- Liu, J.; Fernández-Serra, M.V.; Allen, P.B. First-principles study of pyroelectricity in GaN and ZnO. Phys. Rev. B 2016, 93, 081205. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Bagga, S.; Akhtar, J.; Mishra, S. Synthesis and applications of ZnO nanowire: A review. AIP Conf. Proc. 2018, 1989, 020004. [Google Scholar]
- Yi, G.-C.; Wang, C.; Park, W.I. ZnO nanorods: Synthesis, characterization and applications. Semicond. Sci. Technol. 2005, 20, S22–S34. [Google Scholar] [CrossRef]
- Li, Y.B.; Bando, Y.; Sato, T.; Kurashima, K. ZnO nanobelts grown on Si substrate. Appl. Phys. Lett. 2002, 81, 144–146. [Google Scholar] [CrossRef]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Pugliese, D.; Garino, N.; Sacco, A.; Bianco, S.; Bella, F.; Lamberti, A.; Gerbaldi, C. Multi-functional energy conversion and storage electrodes using flower-like Zinc oxide nanostructures. Energy 2014, 65, 639–646. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Mattoli, V. ZnO nanowire arrays as substrates for cell proliferation and differentiation. Mater. Sci. Eng. C 2012, 32, 341–347. [Google Scholar] [CrossRef]
- Lee, J.; Kang, B.S.; Hicks, B.; Chancellor, T.F.; Chu, B.H.; Wang, H.-T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, Y.-J.; Yeom, J.; Jeon, J.H.; Yi, G.-C.; Je, J.H.; Hahn, S.K. The Topographic Effect of Zinc Oxide Nanoflowers on Osteoblast Growth and Osseointegration. Adv. Mater. 2010, 22, 4857–4861. [Google Scholar] [CrossRef]
- Dumontel, B.; Canta, M.; Engelke, H.; Chiodoni, A.; Racca, L.; Ancona, A.; Limongi, T.; Canavese, G.; Cauda, V. Enhanced biostability and cellular uptake of zinc oxide nanocrystals shielded with a phospholipid bilayer. J. Mater. Chem. B 2017, 5, 8799–8813. [Google Scholar] [CrossRef] [PubMed]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; Rehman, I.u. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Garino, N.; Sanvitale, P.; Dumontel, B.; Laurenti, M.; Colilla, M.; Izquierdo-Barba, I.; Cauda, V.; Vallet-Regì, M. Zinc oxide nanocrystals as a nanoantibiotic and osteoinductive agent. RSC Adv. 2019, 9, 11312–11321. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Cauda, V. Gentamicin-Releasing Mesoporous ZnO Structures. Materials 2018, 11, 314. [Google Scholar] [CrossRef]
- Laurenti, M.; Lamberti, A.; Genchi, G.G.; Roppolo, I.; Canavese, G.; Vitale-Brovarone, C.; Ciofani, G.; Cauda, V. Graphene Oxide Finely Tunes the Bioactivity and Drug Delivery of Mesoporous ZnO Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 449–456. [Google Scholar] [CrossRef]
- Xiong, H.-M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered Controlled Drug Release from Mesoporous Silica Nanoparticles via Intracelluar Dissolution of ZnO Nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef]
- Bakrudeen, H.B.; Tsibouklis, J.; Reddy, B.S.R. Facile fabrication of mesoporous ZnO nanospheres for the controlled delivery of captopril. J. Nanoparticle Res. 2013, 15, 1505. [Google Scholar] [CrossRef]
- Kumar, V.B.; Kumar, K.; Gedanken, A.; Paik, P. Facile synthesis of self-assembled spherical and mesoporous dandelion capsules of ZnO: Efficient carrier for DNA and anti-cancer drugs. J. Mater. Chem. B 2014, 2, 3956–3964. [Google Scholar] [CrossRef]
- Patra, P.; Mitra, S.; Das Gupta, A.; Pradhan, S.; Bhattacharya, S.; Ahir, M.; Mukherjee, S.; Sarkar, S.; Roy, S.; Chattopadhyay, S.; et al. Simple synthesis of biocompatible biotinylated porous hexagonal ZnO nanodisc for targeted doxorubicin delivery against breast cancer cell: In vitro and in vivo cytotoxic potential. Colloids Surf. B Biointerfaces 2015, 133, 88–98. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Lin, Y.Y.; Huang, S.; Lem, K.W.; Nguyen, D.H.; Lee, D.S. Synthesis of water-dispersible zinc oxide quantum dots with antibacterial activity and low cytotoxicity for cell labeling. Nanotechnology 2013, 24, 475102. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Ding, H.; Xiong, H.-M. Folic acid functionalized ZnO quantum dots for targeted cancer cell imaging. Nanotechnology 2015, 26, 305702. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Li, B.; Chen, D.; Dong, X.; Wang, Y.; Gu, Y. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials 2015, 53, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lee, J.S.; Kim, D.; Zhu, L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef] [PubMed]
- Vighetto, V.; Ancona, A.; Racca, L.; Limongi, T.; Troia, A.; Canavese, G.; Cauda, V. The Synergistic Effect of Nanocrystals Combined With Ultrasound in the Generation of Reactive Oxygen Species for Biomedical Applications. Front. Bioeng. Biotechnol. 2019, 7, 374. [Google Scholar] [CrossRef]
- Dumontel, B.; Susa, F.; Limongi, T.; Canta, M.; Racca, L.; Chiodoni, A.; Garino, N.; Chiabotto, G.; Centomo, M.L.; Pignochino, Y.; et al. ZnO nanocrystals shuttled by extracellular vesicles as effective Trojan nano-horses against cancer cells. Nanomedicine 2019, 14, 2815–2833. [Google Scholar] [CrossRef]
- Moon, S.-H.; Choi, W.J.; Choi, S.-W.; Kim, E.H.; Kim, J.; Lee, J.-O.; Kim, S.H. Anti-cancer activity of ZnO chips by sustained zinc ion release. Toxicol. Rep. 2016, 3, 430–438. [Google Scholar] [CrossRef]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-Coated Zinc Oxide Nanoparticles as Innovative ROS-Generators for Photodynamic Therapy in Cancer Cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef]
- Racca, L.; Limongi, T.; Vighetto, V.; Dumontel, B.; Ancona, A.; Canta, M.; Canavese, G.; Garino, N.; Cauda, V. Zinc Oxide Nanocrystals and High-Energy Shock Waves: A New Synergy for the Treatment of Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 577. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, M.; Kim, D.H. In vitro toxicity of zinc oxide nanoparticles: A review. J. Nanoparticle Res. 2015, 17, 158. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Bashir, H.; Riaz, S.; Naseem, S. Optical properties and antibacterial activity of V doped ZnO used in solar cells and biomedical applications. Mater. Res. Bull. 2019, 115, 121–129. [Google Scholar] [CrossRef]
- Han, C.; Duan, L.; Zhao, X.; Hu, Z.; Niu, Y.; Geng, W. Effect of Fe doping on structural and optical properties of ZnO films and nanorods. J. Alloy. Compd. 2019, 770, 854–863. [Google Scholar] [CrossRef]
- Laurenti, M.; Castellino, M.; Perrone, D.; Asvarov, A.; Canavese, G.; Chiolerio, A. Lead-free piezoelectrics: V3+ to V5+ ion conversion promoting the performances of V-doped Zinc Oxide. Sci. Rep. 2017, 7, 41957. [Google Scholar] [CrossRef]
- Laurenti, M.; Canavese, G.; Sacco, A.; Fontana, M.; Bejtka, K.; Castellino, M.; Pirri, C.F.; Cauda, V. Nanobranched ZnO Structure: P-Type Doping Induces Piezoelectric Voltage Generation and Ferroelectric–Photovoltaic Effect. Adv. Mater. 2015, 27, 4218–4223. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Rivera, V.F.; Auras, F.; Motto, P.; Stassi, S.; Canavese, G.; Celasco, E.; Bein, T.; Onida, B.; Cauda, V. Length-Dependent Charge Generation from Vertical Arrays of High-Aspect-Ratio ZnO Nanowires. Chem. A Eur. J. 2013, 19, 14665–14674. [Google Scholar] [CrossRef]
- Cauda, V.; Stassi, S.; Lamberti, A.; Morello, M.; Fabrizio Pirri, C.; Canavese, G. Leveraging ZnO morphologies in piezoelectric composites for mechanical energy harvesting. Nano Energy 2015, 18, 212–221. [Google Scholar] [CrossRef]
- Stassi, S.; Cauda, V.; Ottone, C.; Chiodoni, A.; Pirri, C.F.; Canavese, G. Flexible piezoelectric energy nanogenerator based on ZnO nanotubes hosted in a polycarbonate membrane. Nano Energy 2015, 13, 474–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jeong, S.-Y.; Cho, C.R.; Park, C.H. Study of diluted magnetic semiconductor: Co-doped ZnO. Appl. Phys. Lett. 2002, 81, 4020–4022. [Google Scholar] [CrossRef]
- Fukumura, T.; Jin, Z.; Ohtomo, A.; Koinuma, H.; Kawasaki, M. An oxide-diluted magnetic semiconductor: Mn-doped ZnO. Appl. Phys. Lett. 1999, 75, 3366–3368. [Google Scholar] [CrossRef]
- Sato, K.; Katayama-Yoshida, H. First principles materials design for semiconductor spintronics. Semicond. Sci. Technol. 2002, 17, 367–376. [Google Scholar] [CrossRef]
- Xiao, J.; Kuc, A.; Pokhrel, S.; Schowalter, M.; Parlapalli, S.; Rosenauer, A.; Frauenheim, T.; Mädler, L.; Pettersson, L.; Heine, T. Evidence for Fe2+ in Wurtzite Coordination: Iron Doping Stabilizes ZnO Nanoparticles. Small 2011, 7, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Luo, J.; Yang, Y.; Wang, X.; Zeng, F. Giant piezoresponse and promising application of environmental friendly small-ion-doped ZnO. Sci. China Technol. Sci. 2012, 55, 421–436. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Berruti, I.; Sordello, F.; Calza, P.; Paganini, M.C. Rare earth ions doped ZnO: Synthesis, characterization and preliminary photoactivity assessment. J. Solid State Chem. 2018, 264, 42–47. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Ibrahim, A.A.; Bouzid, H.; Al-Sayari, S.A. Highly efficient photocatalyst based on Ce doped ZnO nanorods: Controllable synthesis and enhanced photocatalytic activity. Chem. Eng. J. 2013, 229, 225–233. [Google Scholar] [CrossRef]
- Kumar, V.; Ntwaeaborwa, O.M.; Soga, T.; Dutta, V.; Swart, H.C. Rare Earth Doped Zinc Oxide Nanophosphor Powder: A Future Material for Solid State Lighting and Solar Cells. ACS Photonics 2017, 4, 2613–2637. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Yang, L.; Wang, Z. Synthesis and luminescence properties of ZnO:Tb3+ nanotube arrays via electrodeposited method. Phys. B Condens. Matter 2010, 405, 3200–3204. [Google Scholar] [CrossRef]
- Christman, J.A.; Woolcott, R.R.; Kingon, A.I.; Nemanich, R.J. Piezoelectric measurements with atomic force microscopy. Appl. Phys. Lett. 1998, 73, 3851–3853. [Google Scholar] [CrossRef]
- Goel, S.; Sinha, N.; Yadav, H.; Joseph, A.J.; Kumar, B. Experimental investigation on the structural, dielectric, ferroelectric and piezoelectric properties of La doped ZnO nanoparticles and their application in dye-sensitized solar cells. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 91, 72–81. [Google Scholar] [CrossRef]
- Dakhel, A.A.; El-Hilo, M. Ferromagnetic nanocrystalline Gd-doped ZnO powder synthesized by coprecipitation. J. Appl. Phys. 2010, 107, 123905. [Google Scholar] [CrossRef]
- Barui, S.; Gerbaldo, R.; Garino, N.; Brescia, R.; Laviano, F.; Cauda, V. Facile Chemical Synthesis of Doped ZnO Nanocrystals Exploiting Oleic Acid. Nanomaterials 2020, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Murmu, P.P.; Kennedy, J.; Ruck, B.J.; Markwitz, A.; Williams, G.V.M.; Rubanov, S. Structural and magnetic properties of low-energy Gd implanted ZnO single crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2012, 272, 100–103. [Google Scholar] [CrossRef]
- Djerdj, I.; Jagličić, Z.; Arčon, D.; Niederberger, M. Co-Doped ZnO nanoparticles: Minireview. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef]
- Yang, Y.C.; Song, C.; Wang, X.H.; Zeng, F.; Pan, F. Cr-substitution-induced ferroelectric and improved piezoelectric properties of Zn1−xCrxO films. J. Appl. Phys. 2008, 103, 074107. [Google Scholar] [CrossRef]
- Li, M.; Pokhrel, S.; Jin, X.; Mädler, L.; Damoiseaux, R.; Hoek, E.M.V. Stability, Bioavailability, and Bacterial Toxicity of ZnO and Iron-Doped ZnO Nanoparticles in Aquatic Media. Environ. Sci. Technol. 2011, 45, 755–761. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y. Structural, optical and magnetic properties of Mn-doped ZnO thin films prepared by sol–gel method. J. Magn. Magn. Mater. 2013, 334, 52–58. [Google Scholar] [CrossRef]
- Hassan, I.A.; Sathasivam, S.; Nair, S.P.; Carmalt, C.J. Antimicrobial Properties of Copper-Doped ZnO Coatings under Darkness and White Light Illumination. ACS Omega 2017, 2, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Dietl, T. A ten-year perspective on dilute magnetic semiconductors and oxides. Nat. Mater. 2010, 9, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Omri, K.; El Ghoul, J.; Lemine, O.M.; Bououdina, M.; Zhang, B.; El Mir, L. Magnetic and optical properties of manganese doped ZnO nanoparticles synthesized by sol–gel technique. Superlattices Microstruct. 2013, 60, 139–147. [Google Scholar] [CrossRef]
- Dabir, F.; Esfahani, H.; Bakhtiargonbadi, F.; Khodadadi, Z. Study on microstructural and electro-optical properties of sol–gel derived pure and Al/Cu-doped ZnO thin films. J. Sol-Gel Sci. Technol. 2020. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Mädler, L.; et al. Use of a Rapid Cytotoxicity Screening Approach To Engineer a Safer Zinc Oxide Nanoparticle through Iron Doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef]
- Luo, J.T.; Yang, Y.C.; Zhu, X.Y.; Chen, G.; Zeng, F.; Pan, F. Enhanced electromechanical response of Fe-doped ZnO films by modulating the chemical state and ionic size of the Fe dopant. Phys. Rev. B 2010, 82, 014116. [Google Scholar] [CrossRef]
- Srinivasulu, T.; Saritha, K.; Reddy, K.T.R. Synthesis and characterization of Fe-doped ZnO thin films deposited by chemical spray pyrolysis. Mod. Electron. Mater. 2017, 3, 76–85. [Google Scholar] [CrossRef]
- Young Ahn, G.; Park, S.-I.; Sung Kim, C. Enhanced ferromagnetic properties of diluted Fe doped ZnO with hydrogen treatment. J. Magn. Magn. Mater. 2006, 303, e329–e331. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Shah, I.; Riaz, S.; Naseem, S. Effect of Co doping on the physical properties of Co-doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 5953–5961. [Google Scholar] [CrossRef]
- Ouarez, L.; Chelouche, A.; Touam, T.; Mahiou, R.; Djouadi, D.; Potdevin, A. Au-doped ZnO sol-gel thin films: An experimental investigation on physical and photoluminescence properties. J. Lumin. 2018, 203, 222–229. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kroon, R.E.; Swart, H.C. Photocatalytic and biological applications of Ag and Au doped ZnO nanomaterial synthesized by combustion. Vacuum 2018, 157, 508–513. [Google Scholar] [CrossRef]
- Verma, R.; Chauhan, A.; Shandilya, M.; Li, X.; Kumar, R.; Kulshrestha, S. Antimicrobial potential of ag-doped ZnO nanostructure synthesized by the green method using moringa oleifera extract. J. Environ. Chem. Eng. 2020, 103730. [Google Scholar] [CrossRef]
- Namgung, G.; Ta, Q.T.H.; Yang, W.; Noh, J.-S. Diffusion-Driven Al-Doping of ZnO Nanorods and Stretchable Gas Sensors Made of Doped ZnO Nanorods/Ag Nanowires Bilayers. ACS Appl. Mater. Interfaces 2019, 11, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-T.; Chang, S.-J.; Ji, L.-W.; Hsiao, Y.-J.; Tang, I.T. Fast Detection and Flexible Microfluidic pH Sensors Based on Al-Doped ZnO Nanosheets with a Novel Morphology. ACS Omega 2019, 4, 19847–19855. [Google Scholar] [CrossRef] [PubMed]
- Pradeev raj, K.; Sadaiyandi, K.; Kennedy, A.; Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.B.; Aziz, F.A.; Rafique, R.F.; Thamiz Selvi, R.; Rathina bala, R. Influence of Mg Doping on ZnO Nanoparticles for Enhanced Photocatalytic Evaluation and Antibacterial Analysis. Nanoscale Res. Lett. 2018, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Brahma, S.; Liu, C.P.; Huang, J.-L. Enhancement of the piezoelectric coefficient in hexagonal MgxZn1-xO films at lower Mg compositions. J. Alloy. Compd. 2017, 728, 1248–1253. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.M.; Müller, R.H. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Liu, C.; Yu, A.; Peng, M.; Song, M.; Liu, W.; Zhang, Y.; Zhai, J. Improvement in the Piezoelectric Performance of a ZnO Nanogenerator by a Combination of Chemical Doping and Interfacial Modification. J. Phys. Chem. C 2016, 120, 6971–6977. [Google Scholar] [CrossRef]
- Yang, Y.; Pradel, K.C.; Jing, Q.; Wu, J.M.; Zhang, F.; Zhou, Y.; Zhang, Y.; Wang, Z.L. Thermoelectric Nanogenerators Based on Single Sb-Doped ZnO Micro/Nanobelts. ACS Nano 2012, 6, 6984–6989. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Myles, A.; Quilty, B.; McCormack, D.E.; Fagan, R.; Hinder, S.J.; Dionysiou, D.D.; Pillai, S.C. Antibacterial properties of F-doped ZnO visible light photocatalyst. J. Hazard. Mater. 2017, 324, 39–47. [Google Scholar] [CrossRef]
- Martínez Julca, M.A.; Rivera, I.; Perales-Pérez, O.; Bailón, S.; Pérez, M. Li-Doped ZnO Nanoparticles as Novel Direct Generator of Singlet Oxygen for Potential Photodynamic Therapy Applications. MRS Proc. 2015, 1784, mrss15-2136565. [Google Scholar] [CrossRef]
- Shi, R.; Yang, P.; Wang, J.; Zhang, A.; Zhu, Y.; Cao, Y.; Ma, Q. Growth of flower-like ZnO via surfactant-free hydrothermal synthesis on ITO substrate at low temperature. CrystEngComm 2012, 14, 5996–6003. [Google Scholar] [CrossRef]
- Gao, T.; Wang, T.H. Synthesis and properties of multipod-shaped ZnO nanorods for gas-sensor applications. Appl. Phys. A 2005, 80, 1451–1454. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Choy, W.C.H.; Cheah, K.W.; Chan, W.K. Visible photoluminescence in ZnO tetrapod and multipod structures. Appl. Phys. Lett. 2004, 84, 2635–2637. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of Arrayed Nanorods and Nanowires of ZnO from Aqueous Solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Sun, X.W.; Lo, G.Q.; Kwong, D.L.; Wang, J.X. Improved dye-sensitized solar cells with a ZnO-nanoflower photoanode. Appl. Phys. Lett. 2007, 90, 263501. [Google Scholar] [CrossRef]
- Yadav, H.; Sinha, N.; Goel, S.; Kumar, B. Eu-doped ZnO nanoparticles for dielectric, ferroelectric and piezoelectric applications. J. Alloy. Compd. 2016, 689, 333–341. [Google Scholar] [CrossRef]
- Xiong, Y.; Yi, W.Y.; Yang, G.W.; Yang, Y.H. Elongated hexagonal ZnO micro-fence optical resonator. Curr. Appl. Phys. 2019, 19, 984–986. [Google Scholar] [CrossRef]
- Abinaya, C.; Marikkannan, M.; Manikandan, M.; Mayandi, J.; Suresh, P.; Shanmugaiah, V.; Ekstrum, C.; Pearce, J.M. Structural and optical characterization and efficacy of hydrothermal synthesized Cu and Ag doped zinc oxide nanoplate bactericides. Mater. Chem. Phys. 2016, 184, 172–182. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Opel, M.; Nielsen, K.W.; Bauer, S.; Goennenwein, S.T.B.; Cezar, J.C.; Schmeisser, D.; Simon, J.; Mader, W.; Gross, R. Nanosized superparamagnetic precipitates in cobalt-doped ZnO. Eur. Phys. J. B 2008, 63, 437. [Google Scholar] [CrossRef]
- Samanta, A.; Goswami, M.N.; Mahapatra, P.K. Fe-doped ZnO nanoparticles as novel photonic and multiferroic semiconductor. Mater. Chem. Phys. 2020, 240, 122180. [Google Scholar] [CrossRef]
- Pascariu, P.; Tudose, I.V.; Suchea, M.; Koudoumas, E.; Fifere, N.; Airinei, A. Preparation and characterization of Ni, Co doped ZnO nanoparticles for photocatalytic applications. Appl. Surf. Sci. 2018, 448, 481–488. [Google Scholar] [CrossRef]
- Isik, M.; Gasanly, N.M. Gd-doped ZnO nanoparticles: Synthesis, structural and thermoluminescence properties. J. Lumin. 2019, 207, 220–225. [Google Scholar] [CrossRef]
- Panwar, A.; Yadav, K.L. A novel one-pot synthesis of hierarchical europium doped ZnO nanoflowers. Mater. Lett. 2015, 142, 30–34. [Google Scholar] [CrossRef]
- Iqbal, J.; Wang, B.; Liu, X.; Yu, D.; He, B.; Yu, R. Oxygen-vacancy-induced green emission and room-temperature ferromagnetism in Ni-doped ZnO nanorods. New J. Phys. 2009, 11, 063009. [Google Scholar] [CrossRef]
- Sinha, N.; Goel, S.; Joseph, A.J.; Yadav, H.; Batra, K.; Gupta, M.K.; Kumar, B. Y-doped ZnO nanosheets: Gigantic piezoelectric response for an ultra-sensitive flexible piezoelectric nanogenerator. Ceram. Int. 2018, 44, 8582–8590. [Google Scholar] [CrossRef]
- Goel, S.; Sinha, N.; Yadav, H.; Godara, S.; Joseph, A.J.; Kumar, B. Ferroelectric Gd-doped ZnO nanostructures: Enhanced dielectric, ferroelectric and piezoelectric properties. Mater. Chem. Phys. 2017, 202, 56–64. [Google Scholar] [CrossRef]
- Sharma, N.; Jandaik, S.; Kumar, S.; Chitkara, M.; Sandhu, I.S. Synthesis, characterisation and antimicrobial activity of manganese- and iron-doped zinc oxide nanoparticles. J. Exp. Nanosci. 2016, 11, 54–71. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Yuan, Q.; Lu, L. Fluorescence-enhanced gadolinium-doped zinc oxide quantum dots for magnetic resonance and fluorescence imaging. Biomaterials 2011, 32, 1185–1192. [Google Scholar] [CrossRef]
- Ghaemi, B.; Mashinchian, O.; Mousavi, T.; Karimi, R.; Kharrazi, S.; Amani, A. Harnessing the Cancer Radiation Therapy by Lanthanide-Doped Zinc Oxide Based Theranostic Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, J.; Thakur, S.; Sharma, S.; Shrivastava, V. Antibacterial study of silver doped zinc oxide nanoparticles against Staphylococcus aureus and Bacillus subtilis. Drug Invent. Today 2013, 5, 50–54. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Varaprasad, K.; Venugopal, S.K.; Arun, L.; Hameed, A.S.H. Synergistic Antibacterial Effect of the Magnesium-Doped ZnO Nanoparticles with Chloramphenicol. BioNanoScience 2020, 10, 106–111. [Google Scholar] [CrossRef]
- Sun, G.; Cao, M.; Wang, Y.; Hu, C.; Liu, Y.; Ren, L.; Pu, Z. Anionic surfactant-assisted hydrothermal synthesis of high-aspect-ratio ZnO nanowires and their photoluminescence property. Mater. Lett. 2006, 60, 2777–2782. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, D.; Zhang, K.; Lu, A.; Wang, L.; Peng, D.-L. Au–ZnO hybrid nanoflowers, nanomultipods and nanopyramids: One-pot reaction synthesis and photocatalytic properties. Nanoscale 2014, 6, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Sinha, N.; Ray, G.; Bhandari, S.; Godara, S.; Kumar, B. Synthesis and enhanced properties of cerium doped ZnO nanorods. Ceram. Int. 2014, 40, 12337–12342. [Google Scholar] [CrossRef]
- Batra, K.; Sinha, N.; Goel, S.; Yadav, H.; Joseph, A.J.; Kumar, B. Enhanced dielectric, ferroelectric and piezoelectric performance of Nd-ZnO nanorods and their application in flexible piezoelectric nanogenerator. J. Alloy. Compd. 2018, 767, 1003–1011. [Google Scholar] [CrossRef]
- Gazzali, P.M.M.; Rajan, S.; Chandrasekaran, G. Low-Temperature Magnetic Properties of Vanadium-Doped ZnO Nanoparticles. J. Supercond. Nov. Magn. 2018, 31, 2817–2828. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, D.; Lin, F.; Shi, W.; Ma, X. Fe-doped ZnO magnetic semiconductor by mechanical alloying. J. Alloy. Compd. 2007, 436, 30–33. [Google Scholar] [CrossRef]
- Chanda, A.; Gupta, S.; Vasundhara, M.; Joshi, S.R.; Mutta, G.R.; Singh, J. Study of structural, optical and magnetic properties of cobalt doped ZnO nanorods. RSC Adv. 2017, 7, 50527–50536. [Google Scholar] [CrossRef]
- Singhal, S.; Kaur, J.; Namgyal, T.; Sharma, R. Cu-doped ZnO nanoparticles: Synthesis, structural and electrical properties. Phys. B Condens. Matter 2012, 407, 1223–1226. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, M.K.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and photocatalytic activity of Gd doped ZnO nanoparticles for enhanced degradation of methylene blue under visible light. Mater. Sci. Semicond. Process. 2019, 103, 104622. [Google Scholar] [CrossRef]
- Beltrán, J.J.; Barrero, C.A.; Punnoose, A. Understanding the role of iron in the magnetism of Fe doped ZnO nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 15284–15296. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Riaz, S.; Kayani, Z.N.; Naseem, S. Structural and Magnetic Properties of Iron Doped ZnO Nanoparticles. Mater. Today Proc. 2015, 2, 5384–5389. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Z.; Zhang, L.; Wang, X.; Yang, H.; Jiang, J. Optical and Magnetic Properties of Fe Doped ZnO Nanoparticles Obtained by Hydrothermal Synthesis. J. Nanomater. 2014, 2014, 792102. [Google Scholar] [CrossRef]
- Gilbert, B.; Fakra, S.C.; Xia, T.; Pokhrel, S.; Mädler, L.; Nel, A.E. The Fate of ZnO Nanoparticles Administered to Human Bronchial Epithelial Cells. ACS Nano 2012, 6, 4921–4930. [Google Scholar] [CrossRef]
- Mahmoudi Khatir, N.; Abdul-Malek, Z.; Zak, A.K.; Akbari, A.; Sabbagh, F. Sol–gel grown Fe-doped ZnO nanoparticles: Antibacterial and structural behaviors. J. Sol-Gel Sci. Technol. 2016, 78, 91–98. [Google Scholar] [CrossRef]
- Moontragoon, P.; Pinitsoontorn, S.; Thongbai, P. Mn-doped ZnO nanoparticles: Preparation, characterization, and calculation of electronic and magnetic properties. Microelectron. Eng. 2013, 108, 158–162. [Google Scholar] [CrossRef]
- Lima, M.K.; Fernandes, D.M.; Silva, M.F.; Baesso, M.L.; Neto, A.M.; de Morais, G.R.; Nakamura, C.V.; de Oliveira Caleare, A.; Hechenleitner, A.A.W.; Pineda, E.A.G. Co-doped ZnO nanoparticles synthesized by an adapted sol–gel method: Effects on the structural, optical, photocatalytic and antibacterial properties. J. Sol-Gel Sci. Technol. 2014, 72, 301–309. [Google Scholar] [CrossRef]
- Nair, M.G.; Nirmala, M.; Rekha, K.; Anukaliani, A. Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. Mater. Lett. 2011, 65, 1797–1800. [Google Scholar] [CrossRef]
- Pal, S.; Yu Kyung, T.; Song, J. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Pandey, L.M. Synthesis, characterization and antibacterial activity of aluminum doped zinc oxide. Mater. Today Proc. 2019, 18, 1388–1400. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Xiao, G.; Liu, H.; Wang, M. Synthesis and morphology control of ZnO nanostructures in microemulsions. J. Colloid Interface Sci. 2009, 333, 465–473. [Google Scholar] [CrossRef]
- Wang, J.; Shi, N.; Qi, Y.; Liu, M. Reverse micelles template assisted fabrication of ZnO hollow nanospheres and hexagonal microtubes by a novel fast microemulsion-based hydrothermal method. J. Sol-Gel Sci. Technol. 2010, 53, 101–106. [Google Scholar] [CrossRef]
- Ishizumi, A.; Takahashi, Y.; Yamamoto, A.; Kanemitsu, Y. Fabrication and optical properties of Eu3+-doped ZnO nanospheres and nanorods. Mater. Sci. Eng. B 2008, 146, 212–215. [Google Scholar] [CrossRef]
- Ishizumi, A.; Fujita, S.; Yanagi, H. Influence of atmosphere on photoluminescence properties of Eu-doped ZnO nanocrystals. Opt. Mater. 2011, 33, 1116–1119. [Google Scholar] [CrossRef]
- Jayakumar, O.; Gopalakrishnan, I.K.; Kadam, R.M.; Vinu, A.; Asthana, A.; Tyagi, A.K. Magnetization and structural studies of Mn doped ZnO nanoparticles: Prepared by reverse micelle method. J. Cryst. Growth 2007, 300, 358–363. [Google Scholar] [CrossRef]
- Saif, M.; Hafez, H.; Nabeel, A.I. Photo-induced self-cleaning and sterilizing activity of Sm3+ doped ZnO nanomaterials. Chemosphere 2012, 90, 840–847. [Google Scholar] [CrossRef]
- Singh, N.; Madhav, H.; Yadav, S.; Jaiswar, G. Impact of vanadium-, sulfur-, and dysprosium-doped zinc oxide nanoparticles on various properties of PVDF/functionalized-PMMA blend nanocomposites: Structural, optical, and morphological studies. J. Appl. Polym. Sci. 2019, 136, 47116. [Google Scholar] [CrossRef]
- Mishra, A.K.; Das, D. Investigation on Fe-doped ZnO nanostructures prepared by a chemical route. Mater. Sci. Eng. B 2010, 171, 5–10. [Google Scholar] [CrossRef]
- Pandiyarajan, T.; Udayabhaskar, R.; Karthikeyan, B. Role of Fe doping on structural and vibrational properties of ZnO nanostructures. Appl. Phys. A 2012, 107, 411–419. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Vahdat Vasei, H.; Masoudpanah, S.M.; Habibollahzadeh, M. Different morphologies of ZnO via solution combustion synthesis: The role of fuel. Mater. Res. Bull. 2020, 125, 110784. [Google Scholar] [CrossRef]
- Sonali, S.; Jena, I.; Rout, S.K. A comparative study of synthesis of ZnO nano particles: Hydrothermal and modified combustion routes. Mater. Today Proc. 2020, 2, 826, in press. [Google Scholar] [CrossRef]
- Silambarasan, M.; Saravanan, S.; Ohtani, N.; Soga, T. Structural and optical studies of pure and Ni-doped ZnO nanoparticles synthesized by simple solution combustion method. Jpn. J. Appl. Phys. 2014, 53, 05FB16. [Google Scholar] [CrossRef]
- Jongprateep, O.; Deedit, P.; Puranasamriddhi, R.; Meesombad, K. Synthesis of nanoparticulate Ti-doped ZnO by solution combustion technique. J. Met. Mater. Miner. 2018, 28, 104–108. [Google Scholar]
- Franco, A.; Alves, T.E.P. Room temperature ferromagnetism in combustion reaction prepared iron-doped zinc oxide nanoparticles. Mater. Sci. Semicond. Process. 2013, 16, 1804–1807. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Manshian, B.B.; Pokhrel, S.; Himmelreich, U.; Tämm, K.; Sikk, L.; Fernández, A.; Rallo, R.; Tamm, T.; Mädler, L.; Soenen, S.J. In Silico Design of Optimal Dissolution Kinetics of Fe-Doped ZnO Nanoparticles Results in Cancer-Specific Toxicity in a Preclinical Rodent Model. Adv. Healthc. Mater. 2017, 6, 1601379. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased Dissolution of ZnO by Iron Doping Yields Nanoparticles with Reduced Toxicity in the Rodent Lung and Zebrafish Embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Shuang, D.; Wang, J.B.; Zhong, X.L.; Yan, H.L. Raman scattering and cathodoluminescence properties of flower-like manganese doped ZnO nanorods. Mater. Sci. Semicond. Process. 2007, 10, 97–102. [Google Scholar] [CrossRef]
- Liu, L.Q.; Xiang, B.; Zhang, X.Z.; Zhang, Y.; Yu, D.P. Synthesis and room temperature ferromagnetism of FeCo-codoped ZnO nanowires. Appl. Phys. Lett. 2006, 88, 063104. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 2010, 123, 585–594. [Google Scholar] [CrossRef]
- Cobianu, C.; Dumbravescu, N.; Serban, B.-C.; Buiu, O.; Romanitan, C.; Comanescu, F.; Danila, M.; Marinescu, R.; Avramescu, V.; Ionescu, O. Sonochemically synthetized ZnO-Graphene nanohybrids and its characterization. Rev. Adv. Mater. Sci. 2020, 59, 176–187. [Google Scholar] [CrossRef]
- Khataee, A.; Darvishi Cheshmeh Soltani, R.; Hanifehpour, Y.; Safarpour, M.; Gholipour Ranjbar, H.; Joo, S.W. Synthesis and Characterization of Dysprosium-Doped ZnO Nanoparticles for Photocatalysis of a Textile Dye under Visible Light Irradiation. Ind. Eng. Chem. Res. 2014, 53, 1924–1932. [Google Scholar] [CrossRef]
- Xiong, H.M.; Shchukin, D.G.; Möhwald, H.; Xu, Y.; Xia, Y.Y. Sonochemical synthesis of highly luminescent zinc oxide nanoparticles doped with magnesium (II). Angew. Chem. 2009, 121, 2765–2769. [Google Scholar] [CrossRef]
- Khataee, A.; Karimi, A.; Arefi-Oskoui, S.; Soltani, R.D.C.; Hanifehpour, Y.; Soltani, B.; Joo, S.W. Sonochemical synthesis of Pr-doped ZnO nanoparticles for sonocatalytic degradation of Acid Red 17. Ultrason. Sonochem. 2015, 22, 371–381. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Dimitrievska, M.R.; Finčur, N.L.; Đačanin, L.R.; Gúth, I.O.; Abramović, B.F.; Lukić-Petrović, S.R. Effect of annealing temperature on structural and optical properties of Mg-doped ZnO nanoparticles and their photocatalytic efficiency in alprazolam degradation. Ceram. Int. 2014, 40, 1545–1552. [Google Scholar] [CrossRef]
- Ivill, M.; Pearton, S.J.; Rawal, S.; Leu, L.; Sadik, P.; Das, R.; Hebard, A.F.; Chisholm, M.; Budai, J.D.; Norton, D.P. Structure and magnetism of cobalt-doped ZnO thin films. New J. Phys. 2008, 10, 065002. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Ahamed, M.; Kumar, S.; Khan, M.; Ahmad, J.; Alrokayan, S. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845. [Google Scholar]
- Rasmussen, J.; Martinez, E.; Louka, P.; Wingett, D. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles to Escherichia coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger, M.-A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Wu, Y.; Tok, A.; Zeng, X.T.; Lim, c.s.; Kwek, L.; Boey, F. A dual-colored bio-marker made of doped ZnO nanocrystals. Nanotechnology 2008, 19, 345605. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press Ltd.: Cebelcor, Brussels, 1966. [Google Scholar]
- Feng, X.; Zhang, S.; Lou, X. Controlling silica coating thickness on TiO2 nanoparticles for effective photodynamic therapy. Colloids Surf. B Biointerfaces 2013, 107, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Choo, E.S.G.; Li, L.; Ding, J.; Xue, J. Synthesis of ZnO Nanoparticles with Tunable Emission Colors and Their Cell Labeling Applications. Chem. Mater. 2010, 22, 3383–3388. [Google Scholar] [CrossRef]
- Baber, O.; Jang, M.; Barber, D.; Powers, K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhal. Toxicol. 2011, 23, 532–543. [Google Scholar] [CrossRef]
- Bellido, E.; Hidalgo Crespo, T.; Lozano, M.; Guillevic, M.; Simón-Vázquez, R.; Santander-Ortega, M.; González-Fernández, A.; Serre, C.; Alonso, M.; Horcajada, P. Heparin-Engineered Mesoporous Iron Metal-Organic Framework Nanoparticles: Toward Stealth Drug Nanocarriers. Adv. Healthc. Mater. 2015, 4, 1246–1257. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of Active Oxygen Generated from Ceramic Powders Having Antibacterial Activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Demirel, R.; Suvacı, E.; Şahin, İ.; Dağ, S.; Kılıç, V. Antimicrobial activity of designed undoped and doped MicNo-ZnO particles. J. Drug Deliv. Sci. Technol. 2018, 47, 309–321. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Suwanboon, S.; Sangkanu, S.; Sukhoom, A.; Wudtipan, J.; Srijan, K.; Kaewtaro, S. Synthesis, photocatalytic and antibacterial activities of ZnO particles modified by diblock copolymer. Powder Technol. 2011, 212, 432–438. [Google Scholar] [CrossRef]
- Khan, S.A.; Noreen, F.; Kanwal, S.; Iqbal, A.; Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C 2018, 82, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Cele, Z.; Ndlandla, L.; Somboro, A.; Gyamfi, D.; Balogun, M. Synthesis, characterization and antimicrobial activities of quaternary chitosan-based materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 430, 012048. [Google Scholar] [CrossRef]
- Daksh, D.; Agrawal, Y. Comparative Study of Pure and Ni/Co, Gd3+, and Tb3+ Doped Zinc Oxide Nanoparticles for Photocatalytic Degradation and Antibacterial Activities. J. Adv. Microsc. Res. 2016, 11, 113–123. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.-H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B Environ. 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Röder, R.; Geburt, S.; Zapf, M.; Franke, D.; Lorke, M.; Frauenheim, T.; da Rosa, A.L.; Ronning, C. Transition Metal and Rare Earth Element Doped Zinc Oxide Nanowires for Optoelectronics. Physica Status Solidi 2019, 256, 1800604. [Google Scholar] [CrossRef]
- He, L.; Meng, J.; Feng, J.; Yao, F.; Zhang, L.; Zhang, Z.; Liu, X.; Zhang, H. Investigation of 4f-Related Electronic Transitions of Rare-Earth Doped ZnO Luminescent Materials: Insights from First-Principles Calculations. ChemPhysChem 2020, 21, 51–58. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Xiong, H.-M. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, P.; Zhang, L.; Liang, Z.; Zhao, H.; Wang, Z. Effects of oxygen partial pressure on the structural and optical properties of undoped and Cu-doped ZnO thin films prepared by magnetron co-sputtering. Mater. Lett. 2016, 164, 509–512. [Google Scholar] [CrossRef]
- Ning, G.-H.; Zhao, X.-P.; Li, J. Structure and optical properties of MgxZn1−xO nanoparticles prepared by sol–gel method. Opt. Mater. 2004, 27, 1–5. [Google Scholar] [CrossRef]
- Dutta, S.; Chattopadhyay, S.; Jana, D.; Banerjee, A.; Manik, S.; Pradhan, S.K.; Sutradhar, M.; Sarkar, A. Annealing effect on nano-ZnO powder studied from positron lifetime and optical absorption spectroscopy. J. Appl. Phys. 2006, 100, 114328. [Google Scholar] [CrossRef]
- Viswanatha, R.; Sapra, S.; Sen Gupta, S.; Satpati, B.; Satyam, P.V.; Dev, B.N.; Sarma, D.D. Synthesis and Characterization of Mn-Doped ZnO Nanocrystals. J. Phys. Chem. B 2004, 108, 6303–6310. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Singh, M.; Singh, R. ZnO Based Diluted Magnetic Semiconductors for Spintronic Device Applications: A Review. Int. J. Emerg. Res. Manag. Technol. 2015, 4, 16–20. [Google Scholar]
- Pearton, S.J.; Norton, D.P.; Ivill, M.P.; Hebard, A.F.; Zavada, J.M.; Chen, W.M.; Buyanova, I.A. Ferromagnetism in Transition-Metal Doped ZnO. J. Electron. Mater. 2007, 36, 462–471. [Google Scholar] [CrossRef]
- Ip, K.; Frazier, R.M.; Heo, Y.W.; Norton, D.P.; Abernathy, C.R.; Pearton, S.J.; Kelly, J.; Rairigh, R.; Hebard, A.F.; Zavada, J.M.; et al. Ferromagnetism in Mn- and Co-implanted ZnO nanorods. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2003, 21, 1476–1481. [Google Scholar] [CrossRef]
- Qi, J.; Yang, Y.; Zhang, L.; Chi, J.; Gao, D.; Xue, D. Room-temperature ferromagnetism in Er-doped ZnO thin films. Scr. Mater. 2009, 60, 289–292. [Google Scholar] [CrossRef]
- Tan, C.; Xu, D.; Zhang, K.; Tian, X.; Cai, W. Electronic and Magnetic Properties of Rare-Earth Metals Doped ZnO Monolayer. J. Nanomater. 2015, 2015, 329570. [Google Scholar] [CrossRef]
- Beltrán, J.J.; Osorio, J.A.; Barrero, C.A.; Hanna, C.B.; Punnoose, A. Magnetic properties of Fe doped, Co doped, and Fe+Co co-doped ZnO. J. Appl. Phys. 2013, 113, 17C308. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Liu, H.; Yang, J.; Liu, Y.; Liu, X.; Gao, M.; Wei, M.; Zhang, X.; Jiang, Y. Structural, optical and magnetic properties of Cu and V co-doped ZnO nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2013, 47, 1–5. [Google Scholar] [CrossRef]
- Xu, M.; Yuan, H.; You, B.; Zhou, P.F.; Dong, C.J.; Duan, M.Y. Structural, optical, and magnetic properties of (Co, Cu)-codoped ZnO films with different Co concentrations. J. Appl. Phys. 2014, 115, 093503. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Cong, M.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Nguyen, H.; Tinet, E.; Chauveau, T.; Geinguenaud, F.; Lalatonne, Y.; Michel, A.; Aid-Launais, R.; Journé, C.; Lefèbvre, C.; Simon-Yarza, T.; et al. Bimodal Fucoidan-Coated Zinc Oxide/Iron Oxide-Based Nanoparticles for the Imaging of Atherothrombosis. Molecules 2019, 24, 962. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Jin, X.; Yang, G.; Jiang, C.; Song, Z.; Sun, G. Biocompatible folate-modified Gd3+/Yb3+-doped ZnO nanoparticles for dualmodal MRI/CT imaging. RSC Adv. 2014, 4, 53561–53569. [Google Scholar] [CrossRef]
- Chiavacci, L.; da Silva, B.; Berbel Manaia, E.; Lepeltier, E.; Benoit, J.-P.; Lemaire, L. ZnO based Quantum Dots for Magnetic Resonance and Fluorescence Imaging. In Proceedings of the 4th World Congress on Recent Advances in Nanotechnology, Rome, Italy, 14–16 April 2019. [Google Scholar] [CrossRef]
- Bhuyan, T.; Khanuja, M.; Sharma, R.; Patel, S.; Reddy, M.R.; Anand, S.; Varma, A. A comparative study of pure and copper (Cu)-doped ZnO nanorods for antibacterial and photocatalytic applications with their mechanism of action. J. Nanoparticle Res. 2015, 17, 288. [Google Scholar] [CrossRef]
- Agarwal, D.C.; Singh, U.B.; Gupta, S.; Singhal, R.; Kulriya, P.K.; Singh, F.; Tripathi, A.; Singh, J.; Joshi, U.S.; Avasthi, D.K. Enhanced room temperature ferromagnetism and green photoluminescence in Cu doped ZnO thin film synthesised by neutral beam sputtering. Sci. Rep. 2019, 9, 6675. [Google Scholar] [CrossRef] [PubMed]
- Sahare, P.; Kumar, V. Optical and magnetic properties of Cu-doped ZnO nanoparticles. Int. J. Innov. Tech. Explor. Engin 2013, 3, 2278–3075. [Google Scholar]
- Felipe, S.; Tupan, L.; Valerio-Cuadros, I.M.; Oliveira, A.A.; Barco, R.; Ivashita, F.F.; Lopes, F.L.; Passamani, C.E.; Paesano, A. Spin-Glass Transitions in Zn1-xFexO Nanoparticles. Materials 2020, 13, 869. [Google Scholar] [CrossRef]
- Yılmaz, S.; Parlak, M.; Özcan, Ş.; Altunbaş, M.; McGlynn, E.; Bacaksız, E. Structural, optical and magnetic properties of Cr doped ZnO microrods prepared by spray pyrolysis method. Appl. Surf. Sci. 2011, 257, 9293–9298. [Google Scholar] [CrossRef]
- Tan, T.L.; Lai, C.W.; Abd Hamid, S.B. Tunable Band Gap Energy of Mn-Doped ZnO Nanoparticles Using the Coprecipitation Technique. J. Nanomater. 2014, 2014, 371720. [Google Scholar] [CrossRef]
- Dhanalakshmi, A.; Natarajan, B.; Ramadas, V.; Palanimurugan, A.; Thanikaikarasan, S. Structural, morphological, optical and antibacterial activity of rod-shaped zinc oxide and manganese-doped zinc oxide nanoparticles. Pramana 2016, 87, 57. [Google Scholar] [CrossRef]
- El-Hilo, M.; Dakhel, A.A. Structural and magnetic properties of Mn-doped ZnO powders. J. Magn. Magn. Mater. 2011, 323, 2202–2205. [Google Scholar] [CrossRef]
- Bououdina, M.; Omri, K.; El-Hilo, M.; El Amiri, A.; Lemine, O.M.; Alyamani, A.; Hlil, E.K.; Lassri, H.; El Mir, L. Structural and magnetic properties of Mn-doped ZnO nanocrystals. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 107–112. [Google Scholar] [CrossRef]
- Pal, B.; Sarkar, D.; Giri, P.K. Structural, optical, and magnetic properties of Ni doped ZnO nanoparticles: Correlation of magnetic moment with defect density. Appl. Surf. Sci. 2015, 356, 804–811. [Google Scholar] [CrossRef]
- Tyona, M.D.; Osuji, R.U.; Lokhande, C.D.; Ezema, F.I. Photovoltaic Properties of Aluminum Doped Zinc Oxide Electrodes Based on Variation of Aluminum Impurities in the Semiconductor. J. Mater. Phys. Chem. 2018, 6, 9–16. [Google Scholar]
- Gao, D.; Zhang, J.; Yang, G.; Zhang, J.; Shi, Z.; Qi, J.; Zhang, Z.; Xue, D. Ferromagnetism in ZnO Nanoparticles Induced by Doping of a Nonmagnetic Element: Al. J. Phys. Chem. C 2010, 114, 13477–13481. [Google Scholar] [CrossRef]
- Maru, A.; Kamble, H.; Kalarikkal, A.; Shah, R.; Bhanuse, P.; Pradhan, N. Mg doped ZnO Dilute Magnetic Oxides Prepared by Chemical Method. Int. J. Chem. Phys. Sci. 2020, 5, 44–49. [Google Scholar]
- Verma, K.C.; Goyal, N.; Kotnala, R.K. Lattice defect-formulated ferromagnetism and UV photo-response in pure and Nd, Sm substituted ZnO thin films. Phys. Chem. Chem. Phys. 2019, 21, 12540–12554. [Google Scholar] [CrossRef]
- Faraz, M.; Naqvi, F.K.; Shakir, M.; Khare, N. Synthesis of samarium-doped zinc oxide nanoparticles with improved photocatalytic performance and recyclability under visible light irradiation. New J. Chem. 2018, 42, 2295–2305. [Google Scholar] [CrossRef]
- Kumar, D.R.; Ranjith, K.S.; Nivedita, L.R.; Kumar, R.T.R. Effect of samarium doping on structural, optical and magnetic properties of vertically aligned ZnO nanorod arrays. J. Rare Earths 2017, 35, 1002–1007. [Google Scholar] [CrossRef]
- Vinoditha, U.; Sarojini, B.K.; Sandeep, K.M.; Narayana, B.; Maidur, S.R.; Patil, P.S.; Balakrishna, K.M. Defects-induced nonlinear saturable absorption mechanism in europium-doped ZnO nanoparticles synthesized by facile hydrothermal method. Appl. Phys. A 2019, 125, 436. [Google Scholar] [CrossRef]
- Yoon, H.; Hua Wu, J.; Hyun Min, J.; Sung Lee, J.; Ju, J.-S.; Keun Kim, Y. Magnetic and optical properties of monosized Eu-doped ZnO nanocrystals from nanoemulsion. J. Appl. Phys. 2012, 111, 07B523. [Google Scholar] [CrossRef]
- Aggarwal, N.; Vasishth, A.; Kaur, K.; Verma, N.K. Investigation of optical, electrical and magnetic properties of Tb-doped ZnO nanorods. J. Mater. Sci. Mater. Electron. 2019, 30, 4807–4812. [Google Scholar] [CrossRef]
- Obeid, M.M.; Jappor, H.R.; Al-Marzoki, K.; Al-Hydary, I.A.; Edrees, S.J.; Shukur, M.M. Unraveling the effect of Gd doping on the structural, optical, and magnetic properties of ZnO based diluted magnetic semiconductor nanorods. RSC Adv. 2019, 9, 33207–33221. [Google Scholar] [CrossRef]
- Shally, V. Room Temperature Ferromagnetism of La doped ZnO Nanorods.
- Young, S.-L.; Chen, H.-Z.; Kao, M.-C.; Kung, C.-Y.; Lin, C.-C.; Lin, T.-T.; Horng, L.; Shih, Y.-T.; Ou, C.-J.; Lin, C.-H. Magnetic properties of La-doped and Cu-doped ZnO nanowires fabricated by hydrothermal method. Int. J. Mod. Phys. B 2013, 27, 1362006. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Timpu, D.; Rotaru, A.; Sacarescu, L.; Ursu, L. New insights into structural and magnetic properties of Ce doped ZnO nanoparticles. J. Alloy. Compd. 2018, 757, 60–69. [Google Scholar] [CrossRef]
- Jayachandraiah, C.; Krishnaiah, G. Influence of cerium dopant on magnetic and dielectric properties of ZnO nanoparticles. J. Mater. Sci. 2017, 52, 7058–7066. [Google Scholar] [CrossRef]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro. Int. J. Oncol. 2010, 37, 1583–1590. [Google Scholar]
- Zangeneh, M.; Nedaei, H.A.; Mozdarani, H.; Mahmoudzadeh, A.; Salimi, M. Enhanced cytotoxic and genotoxic effects of gadolinium-doped ZnO nanoparticles on irradiated lung cancer cells at megavoltage radiation energies. Mater. Sci. Eng. C 2019, 103, 109739. [Google Scholar] [CrossRef]
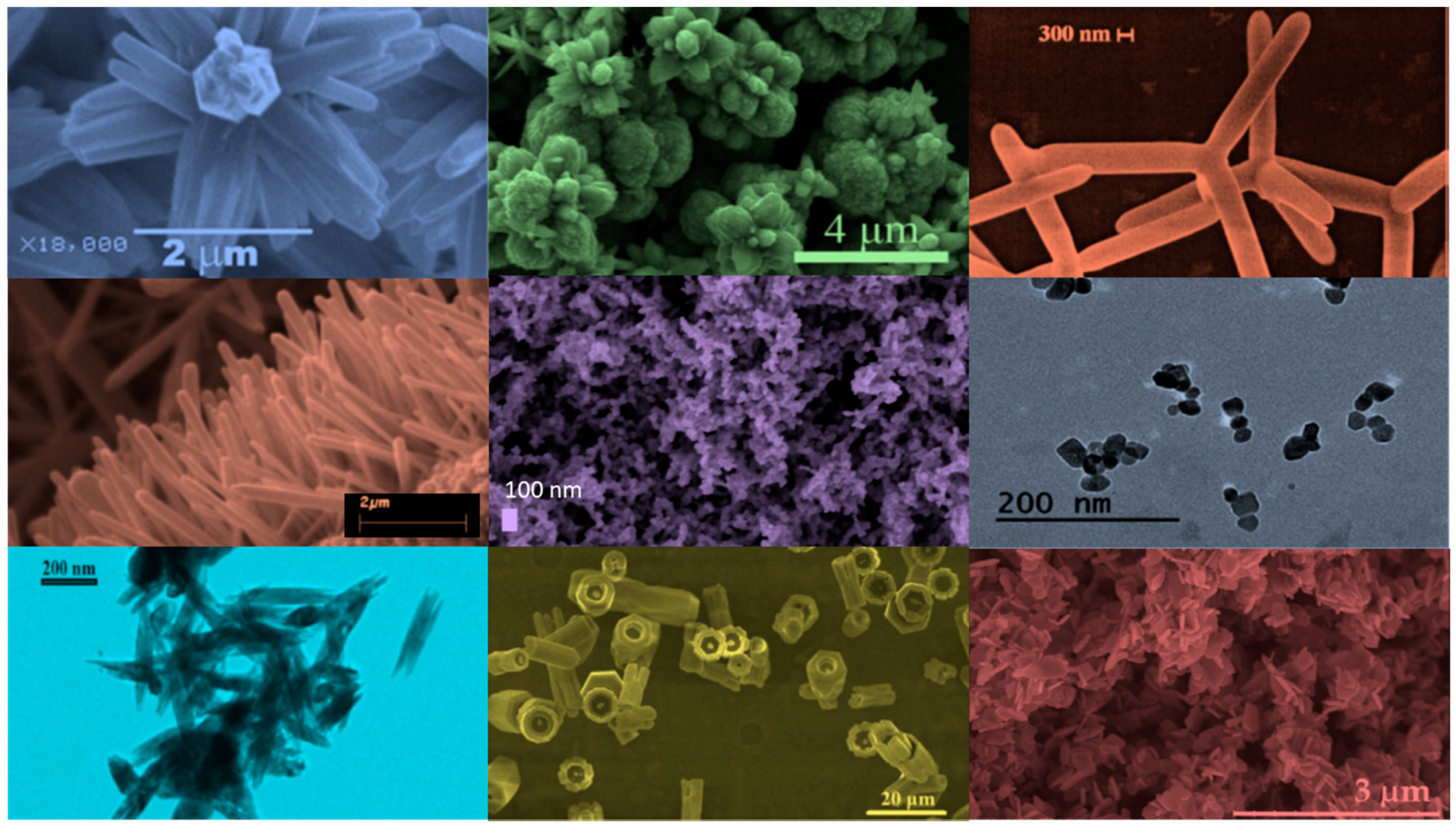
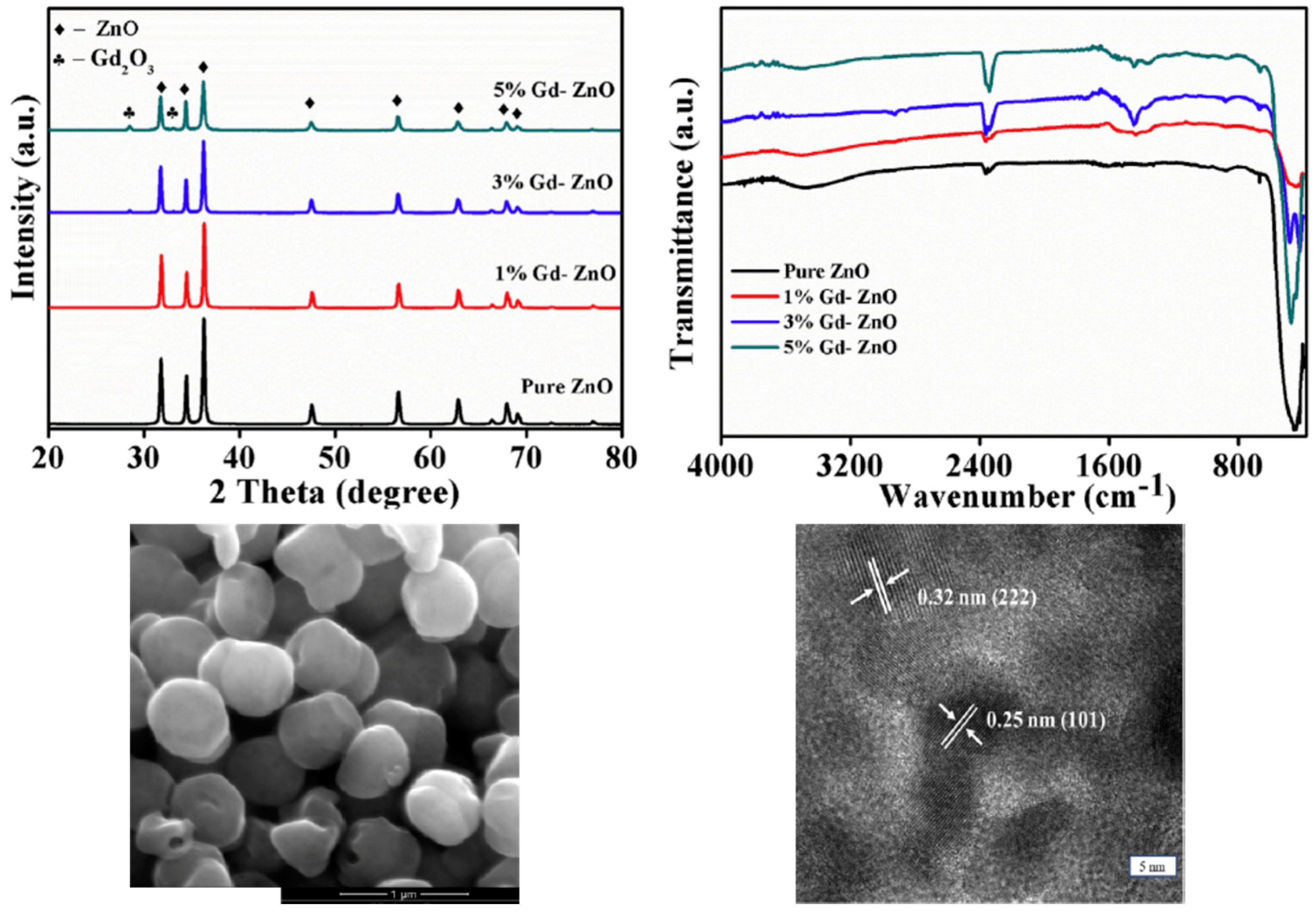
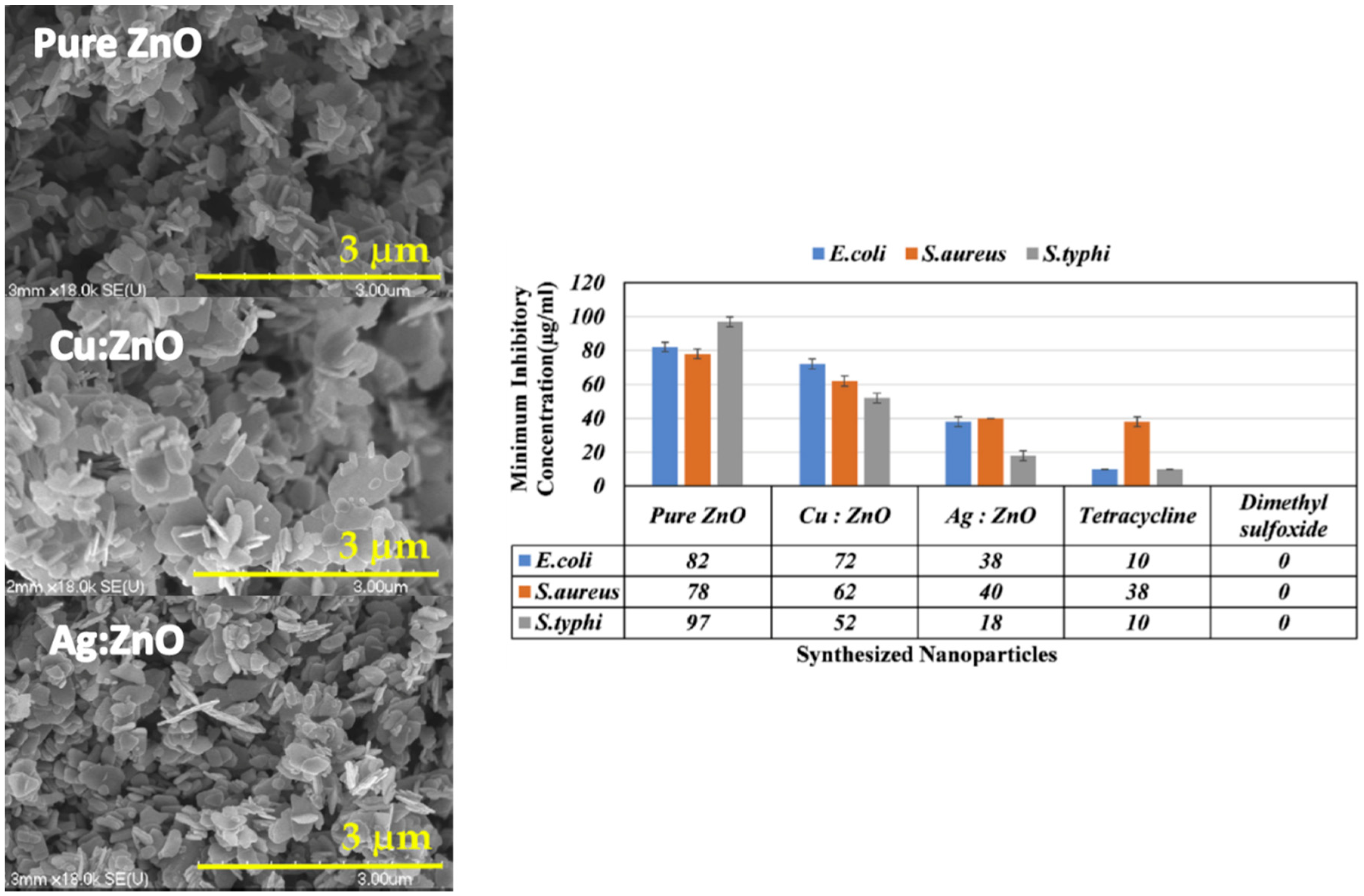
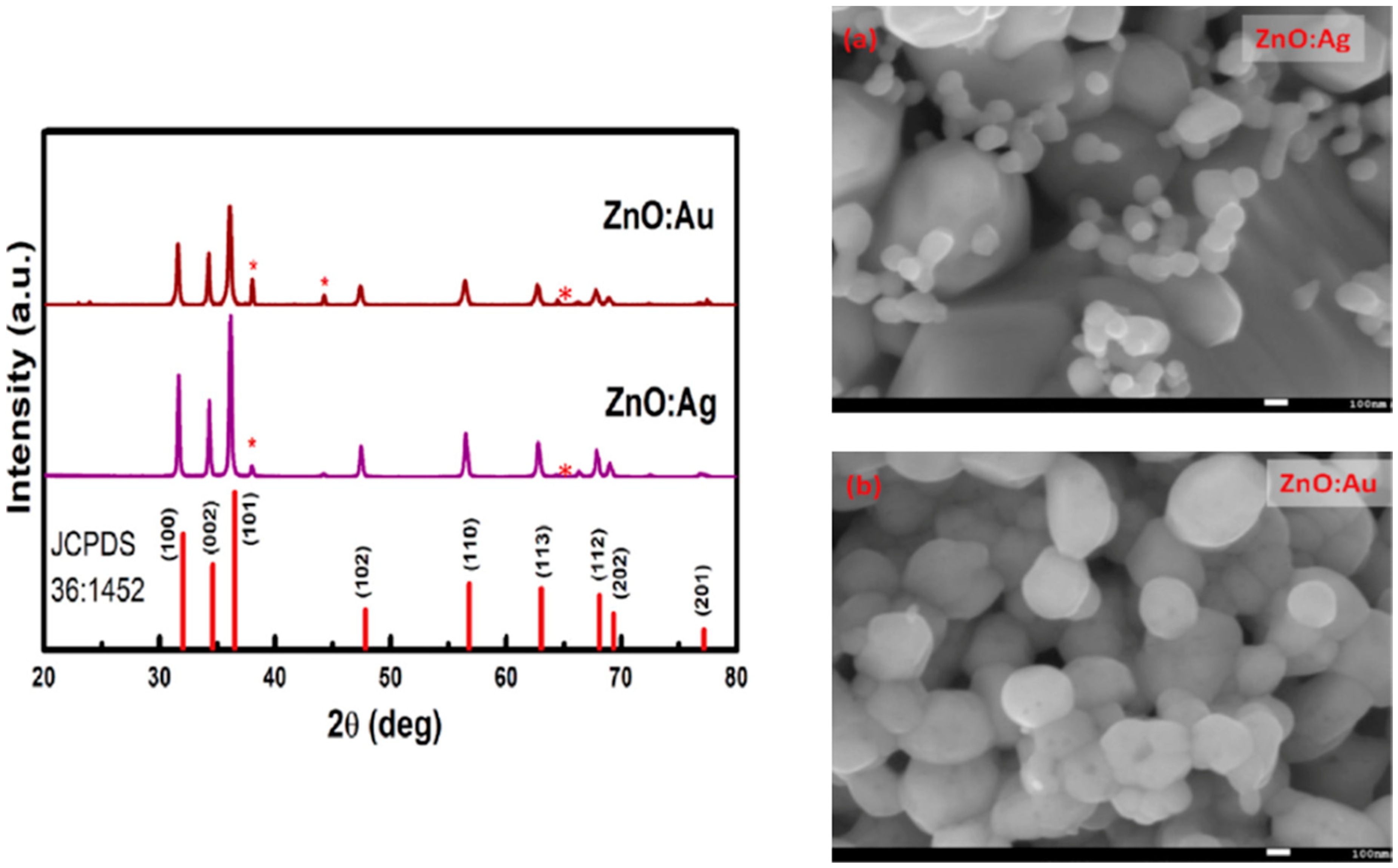
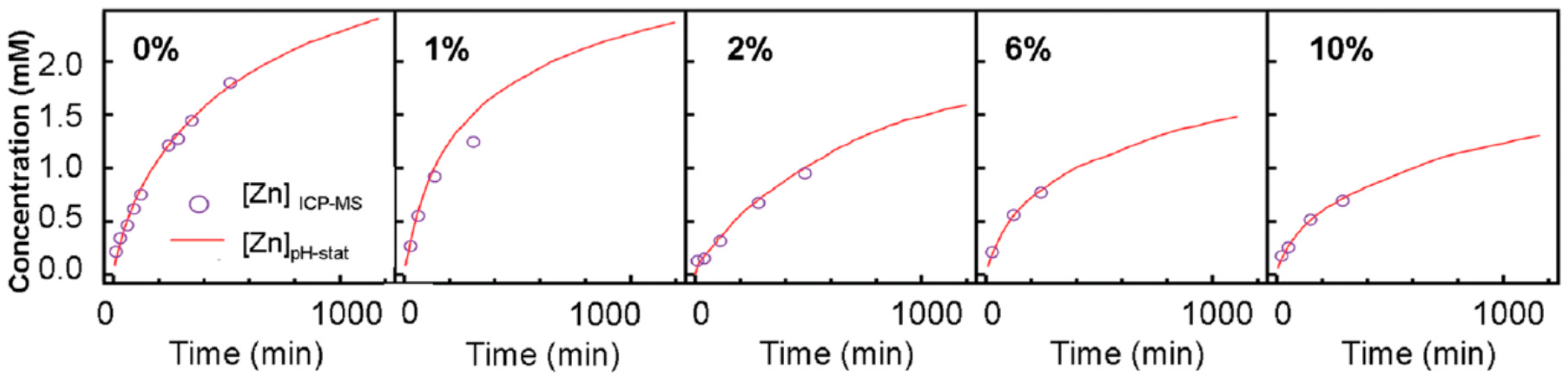
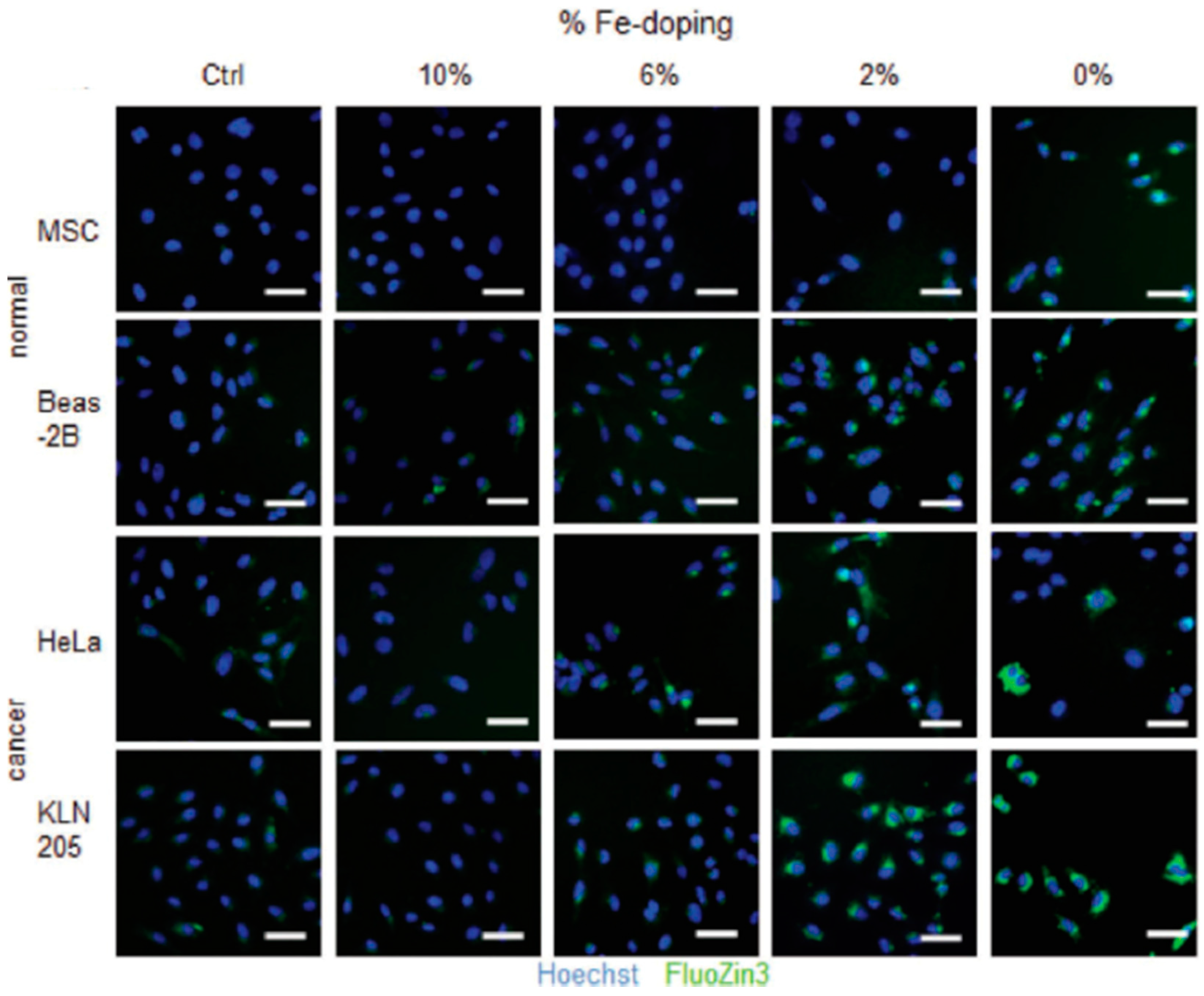
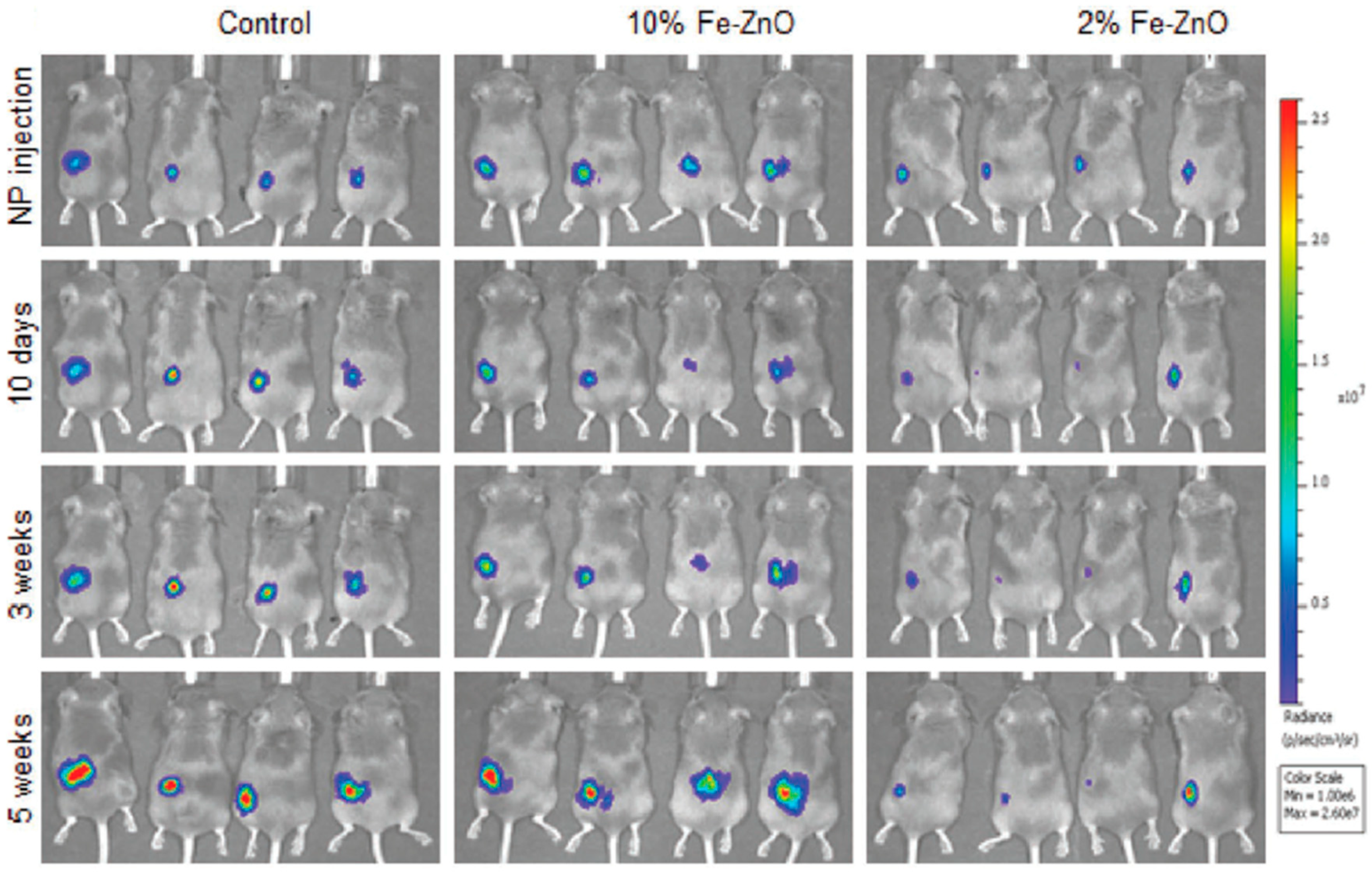
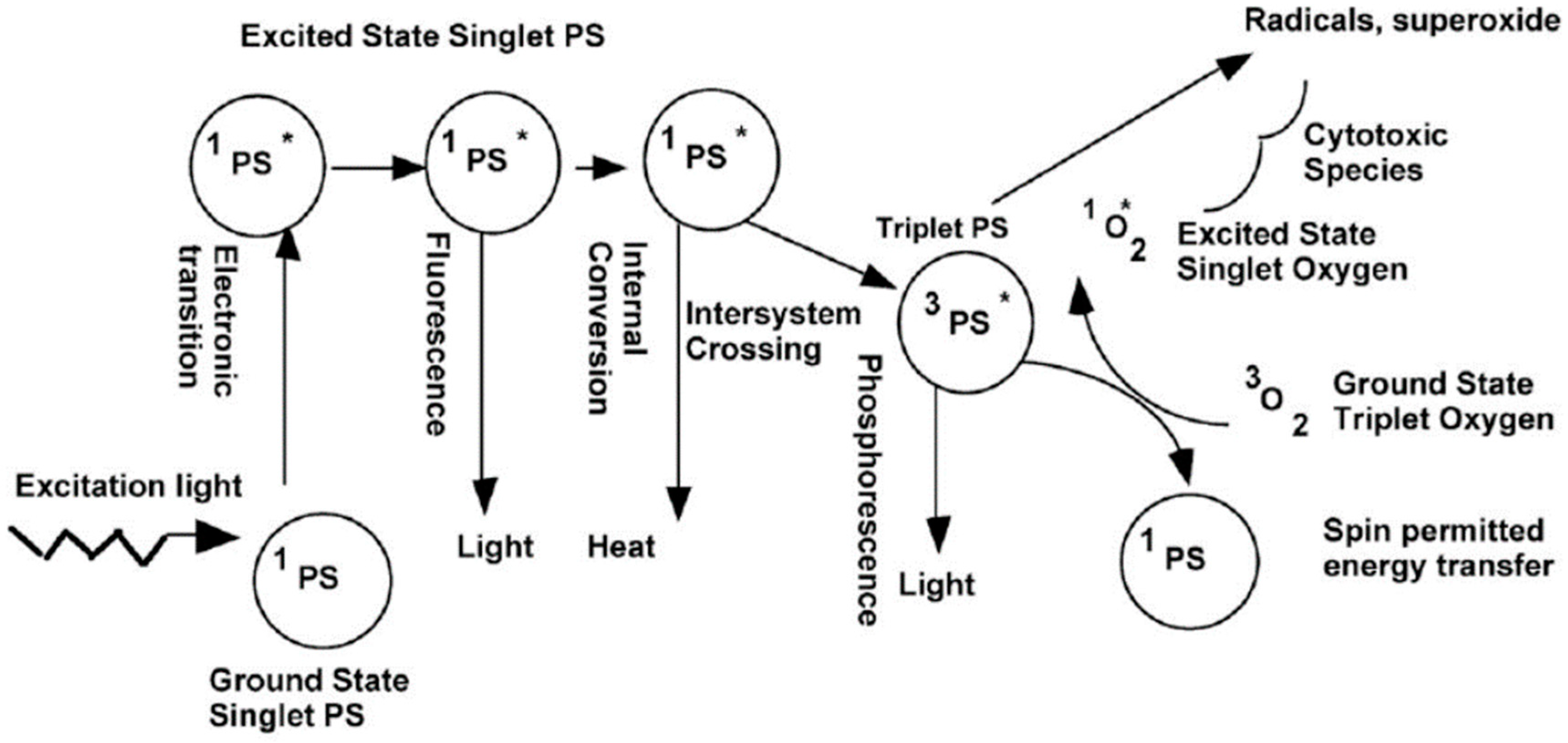
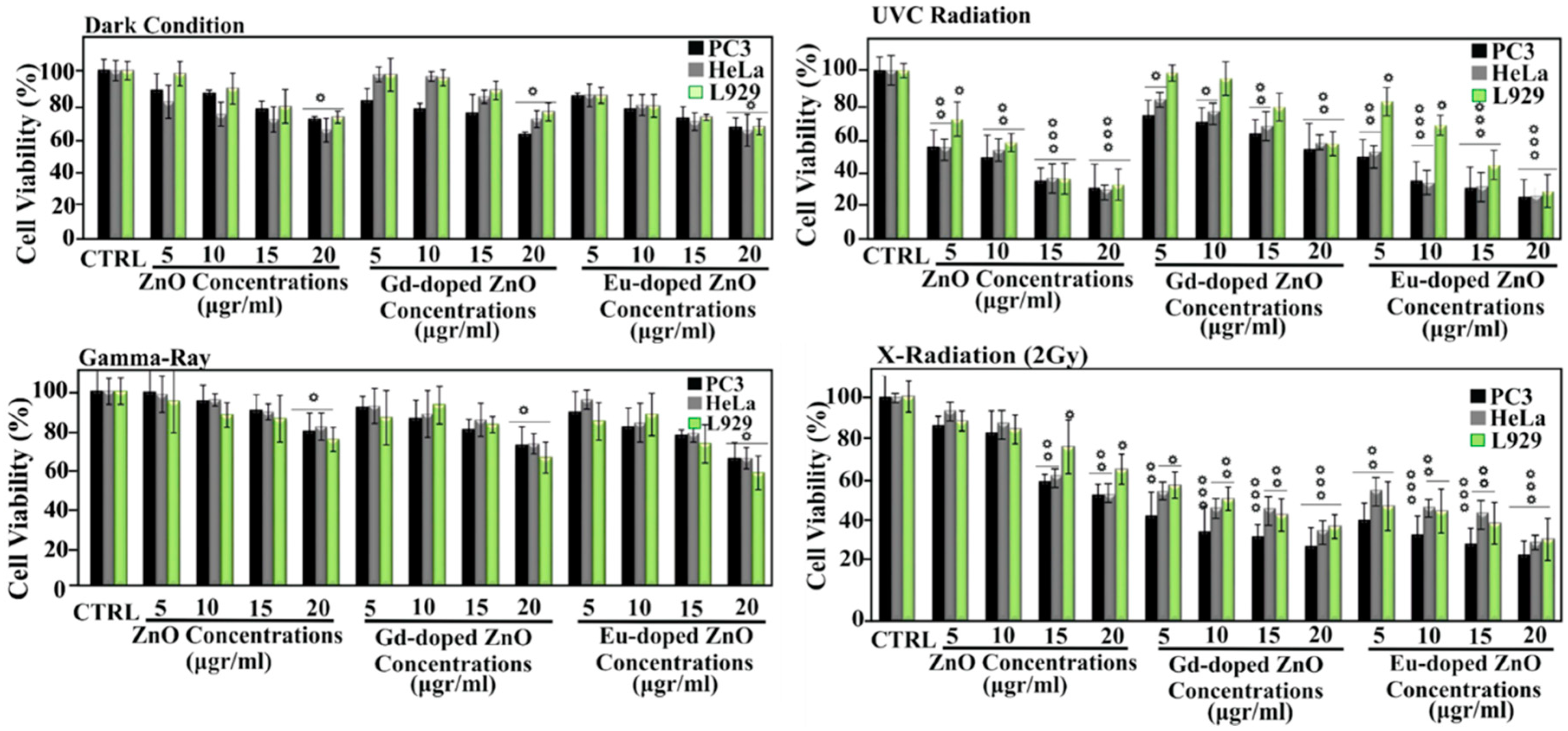
| Ref. | Dopant Element | Doping Level | (Ox. State) Ionic Radius [pm] | a | aD-a01 | c | cD-c02 | Unit Cell Volume |
|---|---|---|---|---|---|---|---|---|
| [170] | Bulk ZnO | - | (+2) 74 | 3.2500 Å | 5.2047 Å | - | 47.609 Å3 | |
| [116] | La | 5% mol | (+3) 103 | 3.2497 Å | −1.3 × 10−3 Å | 5.2058 Å | −3.0 × 10−3 Å | 47.610 Å3 |
| [171] | Ce | 1% mol | (+3) 101 | 3.2503 Å | −1.6 × 10−3 Å | 5.2058 Å | −6.2 × 10−3 Å | 47.629 Å3 |
| [172] | Nd | 5% mol | (+2) 129, (+3) 98 | 3.2495 Å | 12.9 × 10−3 Å | 5.2058 Å | 20.7 × 10−3 Å | 47.605 Å3 |
| [150] | Eu | 5% mol | (+2) 117, (+3) 95 | 3.251 Å | 2.0 × 10−3 Å | 5.209 Å | 4.0 × 10−3 Å | 47.693 Å3 |
| [157] | Gd | 5% mol | (+3) 93 | 3.2735 Å | 20.6 × 10−3 Å | 5.2128 Å | 2.6 × 10−3 Å | 48.375 Å3 |
| [173] | V | 5% mol | (+2) 79, (+3) 64, (+4) 58, (+5) 54 | 3.2522 Å | 0.5 × 10−3 Å | 5.2075 Å | −1.0 × 10−3 Å | 47.699 Å3 |
| [126] | Mn | 5% at. | (+2) 81, (+3) 72 (+4) 67, (+7) 60 | 3.2520 Å | 2.2 × 10−3 Å | 5.2093 Å | 3.0 × 10−3 Å | 47.710 Å3 |
| [174] | Fe | 5.09% mol | (+2) 75, (+3) 69 | 3.2536 Å | 2.3 × 10−3 Å | 5.2093 Å | 11.1 × 10−3 Å | 47.757 Å3 |
| [175] | Co | 5% mol | (+2) 79, (+3) 68 | 3.2503 Å | −2.0 × 10−3 Å | 5.2059 Å | −0.8 × 10−3 Å | 47.629 Å3 |
| [176] | Cu | 5% at | (+1) 91, (+2) 87 | 3.2494 Å | −0.2 × 10−3 Å | 5.2054 Å | −0.4 × 10−3 Å | 47.598 Å3 |
| [152] | Ag | 5% mol | (+1) 129, (+2) 108 | 3.2579 Å | 3.6 × 10−3 Å | 5.2220 Å | 3.7 × 10−3 Å | 48.000 Å3 |
| [144] | Li | 5% at | (+1) 90 | 3.225 Å | −30 × 10−3 Å | 5.162Å | −50 × 10−3 Å | 46.495 Å3 |
| [138] | Mg | 5% mol | (+2) 86 | 3.2585 Å | −3.6 × 10−3 Å | 5.2181 Å | −7.5 × 10−3 Å | 47.982 Å3 |
| Dopant Element | Dopant Precursors | Doping Level | Solvent | Particles Dimensions | Ref. |
|---|---|---|---|---|---|
| La | LaCl3⋅7H2O | 5% mol | H2O a | 123 nm 1 | [116] |
| Ce | CeCl3⋅7H2O | 1% mol | H2O a | 20–30 nm 1 | [171] |
| Ce(NO3)2⋅6H2O | 0.1–5% mol | H2O b | 70–85 nm 1 | [112] | |
| Nd | Nd(NO3)3⋅6H2O | 5% mol | H2O a | 101 nm 1 | [172] |
| Sm | Sm(NO3)3⋅6H2O | 1–4% mol | H2O a | 35 nm 1 | [193] |
| Eu | EuCl3⋅6H2O | 5% mol | H2O a | 79 nm 1 | [150] |
| Eu(NO3)3⋅5H2O | 5% mol | CH3OH a | 9 nm 2 | [164] | |
| Gd | Gd(NO3)3⋅6H2O | 5% mol | CH3OH a | 9 nm 2 | |
| Gd(CH3CO2)3 | 2–30% mol | CH3CH2OH | 4 nm 2,3 | [163] | |
| V | NH4VO3 | 1% mol | H2O a | 47 nm 2 | [194] |
| Mn | Mn(NO3)2 | 0.5–3% mol | CH3CH2OH | 50–120 nm 2 | [183] |
| MnCl2⋅4H2O | 1–5% mol | CH3OH | 100 nm 2 | [126] | |
| Fe | Fe(SO4)⋅7H2O | 3–7% mol | H2O a | 15–35 nm 2 | [195] |
| FeCl3 | 1–10% mol | H2O a | 9–15 nm 2 | [162] | |
| Fe(NO3)3 | 2–6% mol | H2O a | ~250 nm 4 | [196] | |
| Co | Fe(NO3)2⋅6H2O | 1–10% mol | H2O | 25–50 nm 2 | [184] |
| CoCl2 | 5–10% mol | H2O a | Various morphologies | [175] | |
| Ni | NiCl2⋅6H2O | 3% mol | CH3CH2OH a | 25–40 nm 1 | [159] |
| Cu | CuCl2⋅2H2O | 0.5–30 at.% | H2O b | ~250 nm 4 | [176] |
| Ag | AgNO3 | 5% mol | H2O a | 80 nm × 350 nm 5 | [152] |
| Li | Li(CH3CO2)3⋅2H2O | 3–5 at.% | TREG (C6H14O4) | ~250 nm 4 | [144] |
| Mg | Mg(NO3)2⋅6H2O | 5% mol | H2O a | 62 nm | [166] |
| Al | Al(NO3)3⋅9H2O | 15% mol | H2O a | ~60 nm 1 | [187] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. https://doi.org/10.3390/app10155194
Carofiglio M, Barui S, Cauda V, Laurenti M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Applied Sciences. 2020; 10(15):5194. https://doi.org/10.3390/app10155194
Chicago/Turabian StyleCarofiglio, Marco, Sugata Barui, Valentina Cauda, and Marco Laurenti. 2020. "Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine" Applied Sciences 10, no. 15: 5194. https://doi.org/10.3390/app10155194
APA StyleCarofiglio, M., Barui, S., Cauda, V., & Laurenti, M. (2020). Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Applied Sciences, 10(15), 5194. https://doi.org/10.3390/app10155194





