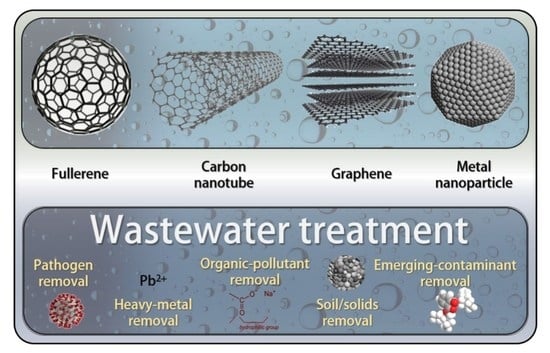
Advances in the Applications of Nanomaterials for Wastewater Treatment
Abstract
1. Introduction
2. Current Wastewater Treatment Technologies
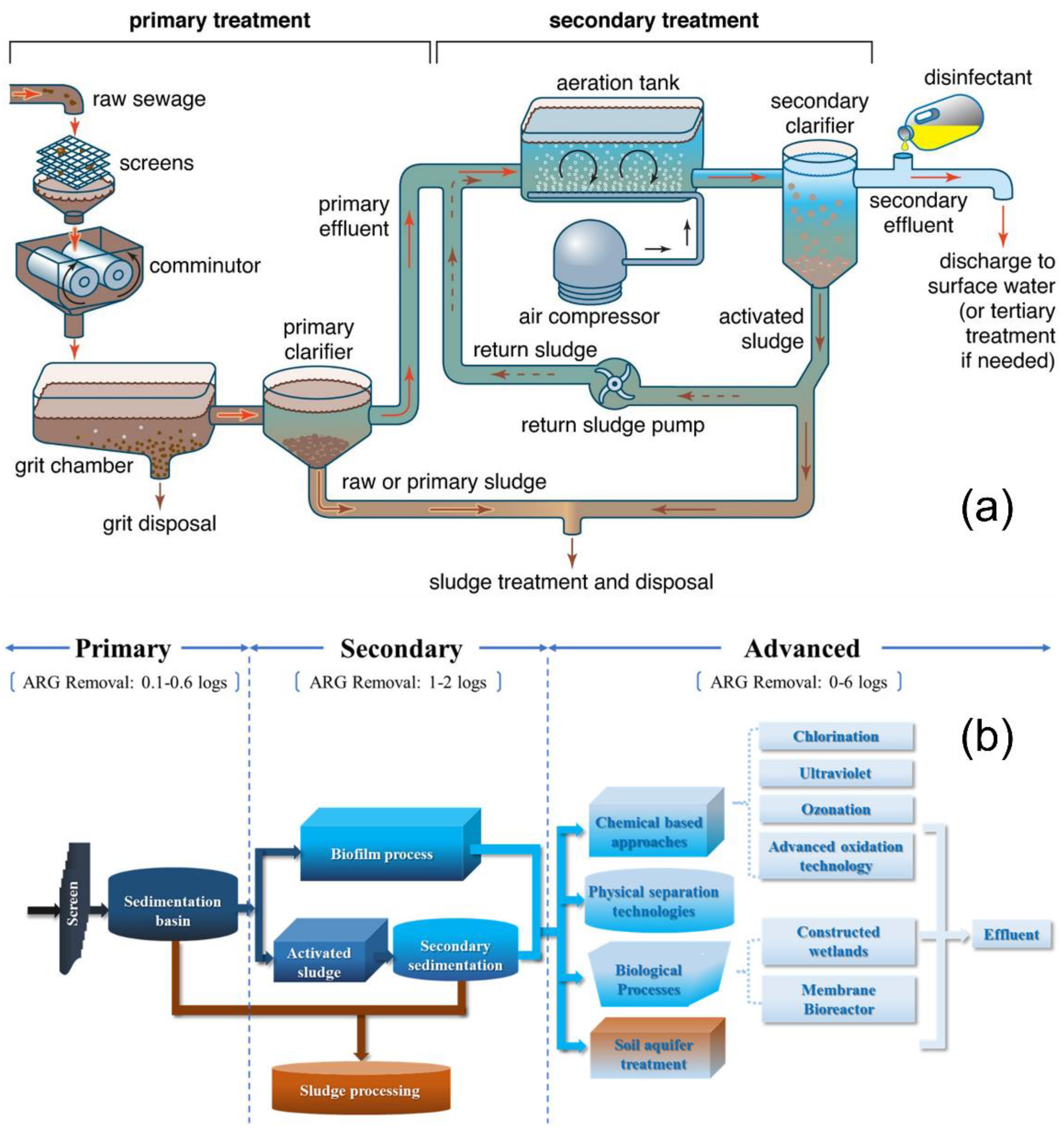
2.1. Preliminary Treatment
2.2. Primary Treatment
2.3. Secondary Treatment
2.4. Tertiary Treatment
2.5. Limitations of Current Treatment Steps
3. The Use of Nanotechnology for Contaminant Removal
4. Types of Nanoparticles for Wastewater Treatment
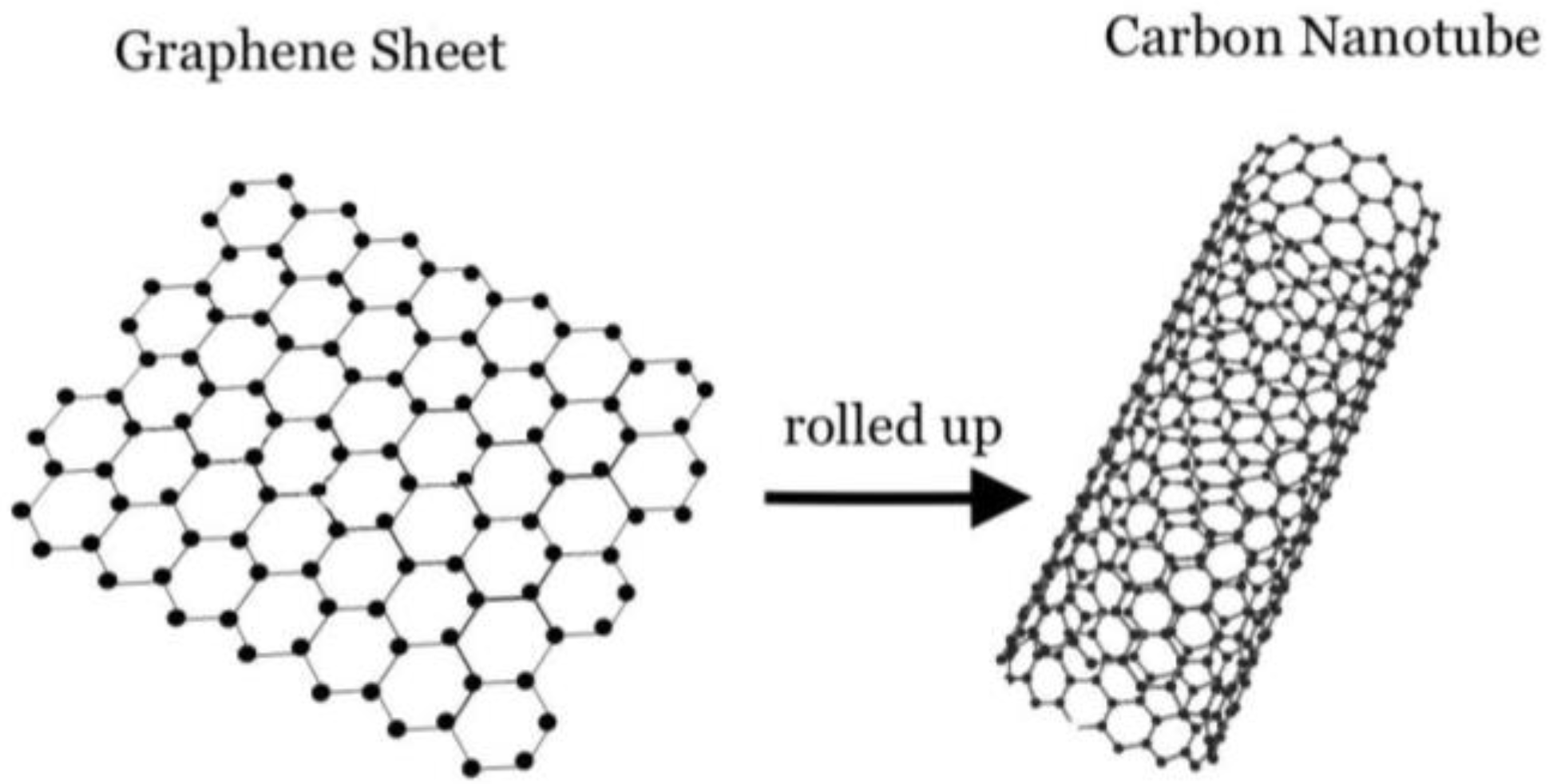
4.1. Carbon Nanotubes
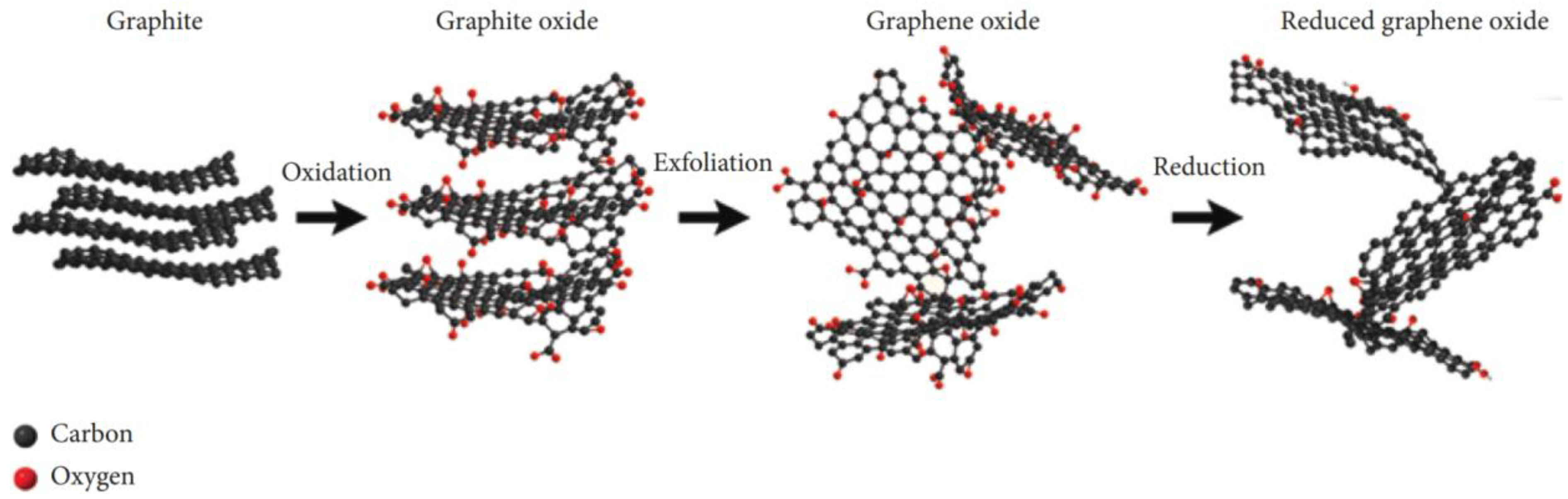
4.2. Graphene-Based Nanoparticles
4.3. Fullerenes
4.4. Silver Nanoparticles
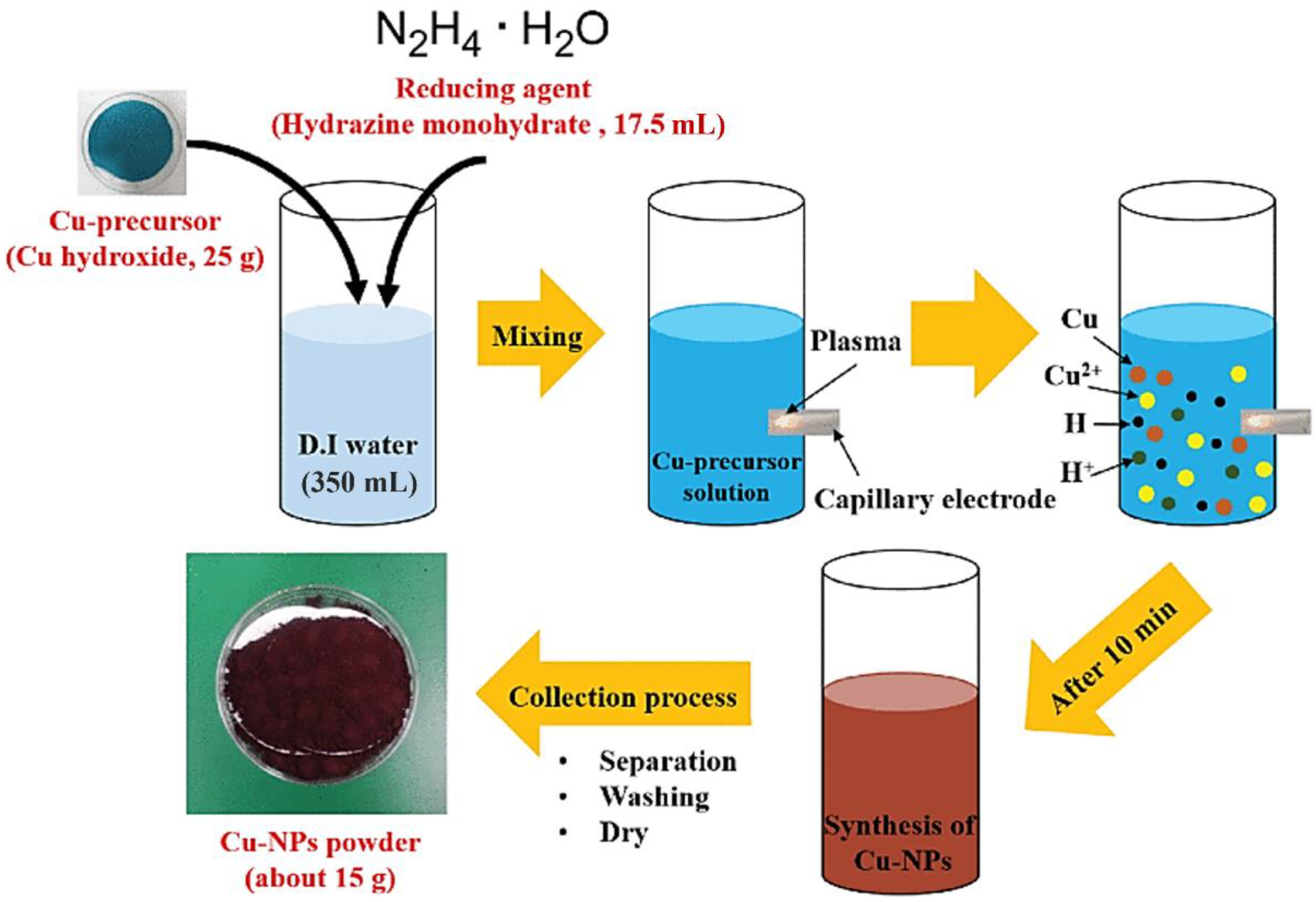
4.5. Copper Nanoparticles
4.6. Iron Nanoparticles
5. Challenges of Nanoparticle Application
5.1. Carbon Nanotubes
5.2. Graphene-Based Nanomaterials
5.3. Fullerenes
5.4. Silver Nanoparticles
5.5. Iron Nanoparticles
5.6. Copper Nanoparticles
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| CEC | Contaminants of emerging concern |
| CFU | Colony forming unit |
| CNT | Carbon nanotube |
| CuO | Copper oxide |
| EDC | Endocrine disrupting chemicals |
| FC | Faecal coliforms |
| FO | Forward osmosis |
| FOG | Fats, oils and grease |
| GBN | Graphene-based nanosheets |
| GO | Graphene oxide |
| ICG | Inert gas condensation |
| MF | Microfiltration |
| MWCNT | Multi-walled carbon nanotube |
| NF | Nanofiltration |
| OCG | Oxygen-containing groups |
| PAH | Polycyclic aromatic hydrocarbons |
| rGO | Reduced graphene oxide |
| RO | Reverse osmosis |
| SBR | Sequencing batch reactor |
| SWCNT | Single-walled carbon nanotube |
| TAOB | Tetra-octyl ammonium bromide |
| TC | Total coliforms |
| TDS | Total dissolved solids |
| UF | Ultrafiltration |
| UV | Ultraviolet |
| WWT | Wastewater treatment |
| WWTP | Wastewater treatment plant |
References
- Diallo, M.; Duncan, J.; Savage, N. Nanotechnology Applications for Clean Water: Solutions for Improving Water Quality; William Andrew: Norwich, NY, USA, 2009; ISBN 0815519737. [Google Scholar]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-based wastewater treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Nations, U. Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 14 September 2022).
- Cooley, H.; Ajami, N.; Ha, M.-L.; Srinivasan, V.; Morrison, J.; Donnelly, K.; Christian-Smith, J. Global water governance in the twenty-first century. In The World’s Water; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–18. [Google Scholar]
- WHO; UNICEF. Progress on Drinking Water and Sanitation: 2012 Update; WHO: Geneva, Switzerland; UNICEF: New York, NY, USA, 2012. [Google Scholar]
- Madhura, L.; Singh, S.; Kanchi, S.; Sabela, M.; Bisetty, K. Inamuddin Nanotechnology-based water quality management for wastewater treatment. Environ. Chem. Lett. 2019, 17, 65–121. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska-Mydlak, A. Nanotechnology in water and wastewater treatment. Graphene–the nanomaterial for next generation of semipermeable membranes. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1515–1579. [Google Scholar] [CrossRef]
- Tlili, I.; Alkanhal, T.A. Nanotechnology for water purification: Electrospun nanofibrous membrane in water and wastewater treatment. J. Water Reuse Desalin. 2019, 9, 232–248. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef]
- Taran, M.; Safaei, M.; Karimi, N.; Almasi, A. Benefits and application of nanotechnology in environmental science: An overview. Biointerface Res. Appl. Chem. 2021, 11, 7860–7870. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, D.; Chen, Q.; Wang, Y.; Sun, M.; Lian, Q.; Shang, J.; Wong, P.K.; He, C.; Xia, D. Nanomaterial-enabled photothermal-based solar water disinfection processes: Fundamentals, recent advances, and mechanisms. J. Hazard. Mater. 2022, 437, 129373. [Google Scholar] [CrossRef]
- Petronella, F.; De Biase, D.; Zaccagnini, F.; Verrina, V.; Lim, S.-I.; Jeong, K.-U.; Miglietta, S.; Petrozza, V.; Scognamiglio, V.; Godman, N.P. Label-free and reusable antibody-functionalized gold nanorod arrays for the rapid detection of Escherichia coli cells in a water dispersion. Environ. Sci. Nano 2022, 9, 3343–3360. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Yasumoto, N.; Imai, J.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Ohtani, B.; Hirai, T. Quantum tunneling injection of hot electrons in Au/TiO2 plasmonic photocatalysts. Nanoscale 2017, 9, 8349–8361. [Google Scholar] [CrossRef]
- Riffat, R. Fundamentals of Wastewater Treatment and Engineering; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Gautam, S.; Saini, G. Use of natural coagulants for industrial wastewater treatment. Glob. J. Environ. Sci. Manag. 2020, 6, 553–578. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Britannica Wastewater Treatment. Available online: https://www.britannica.com/technology/wastewater-treatment/Primary-treatment#/media/1/666611/19281 (accessed on 14 September 2022).
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the art of tertiary treatment technologies for controlling antibiotic resistance in wastewater treatment plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef]
- Dutta, A.; Sarkar, S. Sequencing batch reactor for wastewater treatment: Recent advances. Curr. Pollut. Rep. 2015, 1, 177–190. [Google Scholar] [CrossRef]
- Ge, H.; Batstone, D.J.; Keller, J. Operating aerobic wastewater treatment at very short sludge ages enables treatment and energy recovery through anaerobic sludge digestion. Water Res. 2013, 47, 6546–6557. [Google Scholar] [CrossRef] [PubMed]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Bassin, J.P.; Castro, F.D.; Valério, R.R.; Santiago, E.P.; Lemos, F.R.; Bassin, I.D. The impact of wastewater treatment plants on global climate change. In Water Conservation in the Era of Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 367–410. [Google Scholar]
- Gerba, C.P.; Pepper, I.L. Municipal wastewater treatment. In Environmental and Pollution Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 393–418. [Google Scholar]
- Achokhavatia Tertiary Wastewater Treatment in India. Available online: https://chokhavatia.com/skills/treatment-processes/tertiary-treatment/ (accessed on 14 September 2022).
- Liu, Z.-Q.; Huang, C.; Li, J.-Y.; Yang, J.; Qu, B.; Yang, S.-Q.; Cui, Y.-H.; Yan, Y.; Sun, S.; Wu, X. Activated carbon catalytic ozonation of reverse osmosis concentrate after coagulation pretreatment from coal gasification wastewater reclamation for zero liquid discharge. J. Clean. Prod. 2021, 286, 124951. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel technologies for reverse osmosis concentrate treatment: A review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef]
- de Almeida Lopes, T.S.; Hessler, R.; Bohner, C.; Junior, G.B.A.; de Sena, R.F. Pesticides removal from industrial wastewater by a membrane bioreactor and post-treatment with either activated carbon, reverse osmosis or ozonation. J. Environ. Chem. Eng. 2020, 8, 104538. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Amaeze, N.; Richardson, K.; Mackay, W.; Rateb, M.E.; Yaseen, M. Stabilisation of Ozone in Water for Microbial Disinfection. Environments 2022, 9, 45. [Google Scholar] [CrossRef]
- Epelle, E.I.; Emmerson, A.; Nekrasova, M.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.; Yaseen, M. Microbial Inactivation: Gaseous or Aqueous Ozonation? Ind. Eng. Chem. Res. 2022, 61, 9600–9610. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.; Dutta, J. Applications of nanotechnology in wastewater treatment—A review. J. Nanosci. Nanotechnol. 2014, 14, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; de Lannoy, C.-F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Qi, Y.; Liu, H.; Chen, Y. The Role of Nanomaterials and Nanotechnologies in Wastewater Treatment: A Bibliometric Analysis. Nanoscale Res. Lett. 2018, 13, 233. [Google Scholar] [CrossRef]
- Yin, Z.; Cui, C.; Chen, H.; Duoni; Yu, X.; Qian, W. The Application of Carbon Nanotube/Graphene-Based Nanomaterials in Wastewater Treatment. Small 2020, 16, 1902301. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Lin, K.S.; Ke, W.J.; Hsieh, C.T.; Chiang, C.L.; Tzou, D.Y.; Liu, S.T. The antimicrobial properties of silver nanoparticles in bacillus subtilis are mediated by released Ag+ ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef]
- Hadei, M.; Mesdaghinia, A.; Nabizadeh, R.; Mahvi, A.H.; Rabbani, S.; Naddafi, K. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. Environ. Sci. Pollut. Res. 2021, 28, 13055–13071. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C. Fabrication and implementation of carbon nanotubes for piezoresistive-sensing applications: A review. J. Sci. Adv. Mater. Devices 2021, 7, 100416. [Google Scholar] [CrossRef]
- Moaseri, E.; Karimi, M.; Maghrebi, M.; Baniadam, M. Fabrication of multi-walled carbon nanotube–carbon fiber hybrid material via electrophoretic deposition followed by pyrolysis process. Compos. Part A Appl. Sci. Manuf. 2014, 60, 8–14. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R. A review on nanomaterial dispersion, microstructure, and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J. Nanomater. 2013, 2013, 80. [Google Scholar] [CrossRef]
- Öner, D.; Ghosh, M.; Bové, H.; Moisse, M.; Boeckx, B.; Duca, R.C.; Poels, K.; Luyts, K.; Putzeys, E.; Van Landuydt, K.; et al. Differences in MWCNT- and SWCNT-induced DNA methylation alterations in association with the nuclear deposition. Part. Fibre Toxicol. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Endo, M.; Iijima, S.; Dresselhaus, M.S. Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 0080426824. [Google Scholar]
- Das, R. Carbon nanotube in water treatment. In Nanohybrid Catalyst Based on Carbon Nanotube; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–54. [Google Scholar]
- Kalra, A.; Garde, S.; Hummer, G. Osmotic water transport through carbon nanotube membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Zafariyan, M.; Igwegbe, C.A.; Onyechi, K.K.; Ighalo, J.O. Adsorption of acid blue 92 dye from aqueous solutions by single-walled carbon nanotubes: Isothermal, kinetic, and thermodynamic studies. Environ. Process. 2021, 8, 869–888. [Google Scholar] [CrossRef]
- Dutta, A.K.; Ghorai, U.K.; Chattopadhyay, K.K.; Banerjee, D. Removal of textile dyes by carbon nanotubes: A comparison between adsorption and UV assisted photocatalysis. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 6–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Du, Q.; Pi, X.; Wang, Y. Adsorption of Methylene Blue from Aqueous Solution Using Gelatin-Based Carboxylic Acid-Functionalized Carbon Nanotubes@ Metal–Organic Framework Composite Beads. Nanomaterials 2022, 12, 2533. [Google Scholar] [CrossRef]
- Essa, W.K.; Yasin, S.A.; Abdullah, A.H.; Thalji, M.R.; Saeed, I.A.; Assiri, M.A.; Chong, K.F.; Ali, G.A.M. Taguchi L25 (54) Approach for Methylene Blue Removal by Polyethylene Terephthalate Nanofiber-Multi-Walled Carbon Nanotube Composite. Water 2022, 14, 1242. [Google Scholar] [CrossRef]
- Akha, N.Z.; Salehi, S.; Anbia, M. Removal of arsenic by metal organic framework/chitosan/carbon nanocomposites: Modeling, optimization, and adsorption studies. Int. J. Biol. Macromol. 2022, 208, 794–808. [Google Scholar] [CrossRef]
- Mazumder, M.A.J.; Chowdhury, I.R.; Chowdhury, S.; Al-Ahmed, A. Removal of Pb2+ from water using the carbon nanotube-g-poly [(sodium methacrylate)-co-2-(methacryloyloxy) ethyl acetoacetate]: Experimental investigation and modeling. Environ. Sci. Pollut. Res. 2022, 29, 54432–54447. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Sadeghalvad, B.; Ghasemi, I.; Ebrahimi, H.; Rezaeian, I. Fabrication of polyethersulfone/functionalized MWCNTs nanocomposite and investigation its efficiency as an adsorbent of Pb (II) ions. Arab. J. Sci. Eng. 2021, 46, 6259–6273. [Google Scholar] [CrossRef]
- Wang, H.; Shang, H.; Sun, X.; Hou, L.; Wen, M.; Qiao, Y. Preparation of thermo-sensitive surface ion-imprinted polymers based on multi-walled carbon nanotube composites for selective adsorption of lead (II) ion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124139. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, Y.; Huang, X.; Tang, S.; Chen, H.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Biological self-assembled hyphae/starch porous carbon composites for removal of organic pollutants from water. Chem. Eng. J. 2022, 450, 138264. [Google Scholar] [CrossRef]
- Salam, M.A. Preparation and characterization of chitin/magnetite/multiwalled carbon nanotubes magnetic nanocomposite for toxic hexavalent chromium removal from solution. J. Mol. Liq. 2017, 233, 197–202. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z. Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 2017, 171, 502–511. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, B.; Wang, F.; Xu, N. Effect of carbon nanotubes on Cd (II) adsorption by sediments. Chem. Eng. J. 2015, 264, 645–653. [Google Scholar] [CrossRef]
- Li, Y.-H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon N. Y. 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Rodríguez, C.; Leiva, E. Enhanced heavy metal removal from acid mine drainage wastewater using double-oxidized multiwalled carbon nanotubes. Molecules 2019, 25, 111. [Google Scholar] [CrossRef]
- DaNa Graphene. Available online: https://nanopartikel.info/en/knowledge/materials/graphene/ (accessed on 14 September 2022).
- Jayakaran, P.; Nirmala, G.S.; Govindarajan, L. Qualitative and Quantitative Analysis of Graphene-Based Adsorbents in Wastewater Treatment. Int. J. Chem. Eng. 2019, 2019, 9872502. [Google Scholar] [CrossRef]
- Jayakaran, P.; Nirmala, G.; Lakshmanarao, G. Synthesis and Analysis of Graphene Nano Composite. Compos. Mater. 2019, 2, 43. [Google Scholar]
- Lee, C.; Chang, H.; Jang, H.D. Synthesis of Porous Graphene Balls by the Activation and Aerosol Process for Supercapacitors Application. Part. Aerosol Res. 2019, 15, 183–190. [Google Scholar]
- Huang, T.; Yan, M.; He, K.; Huang, Z.; Zeng, G.; Chen, A.; Peng, M.; Li, H.; Yuan, L.; Chen, G. Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci. 2019, 543, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave assisted synthesis of xanthan gum-cl-poly (acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Hossain, M.D.F.; Akther, N.; Zhou, Y. Recent advancements in graphene adsorbents for wastewater treatment: Current status and challenges. Chin. Chem. Lett. 2020, 31, 2525–2538. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Chen, X.; Zhao, Q.; Ghaffar, A.; Chen, B. Application of graphene-based materials in water purification: From the nanoscale to specific devices. Environ. Sci. Nano 2018, 5, 1264–1297. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Elgoud, E.M.; Emam, S.; Aly, H.F. Superior adsorption performance of citrate modified graphene oxide as nano material for removal organic and inorganic pollutants from aqueous solution. Sci. Rep. 2022, 12, 9204. [Google Scholar] [CrossRef]
- Farooq, N.; Shanableh, A.; Qureshi, A.M.; Jabeen, S.; ur Rehman, A. Synthesis and characterization of clay graphene oxide iron oxide (clay/GO/Fe2O3)-nanocomposite for adsorptive removal of methylene blue dye from wastewater. Inorg. Chem. Commun. 2022, 145, 109956. [Google Scholar] [CrossRef]
- Kim, N.; Cha, B.; Yea, Y.; Njaramba, L.K.; Vigneshwaran, S.; Elanchezhiyan, S.S.D.; Park, C.M. Effective sequestration of tetracycline and ciprofloxacin from aqueous solutions by Al-based metal organic framework and reduced graphene oxide immobilized alginate biosorbents. Chem. Eng. J. 2022, 450, 138068. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A.; Abdelfattah, A.M. Engineering nanocomposite of graphene quantum dots/carbon foam/alginate/zinc oxide beads for efficacious removal of lead and methylene. J. Ind. Eng. Chem. 2022, 115, 365–377. [Google Scholar] [CrossRef]
- Basha, I.K.; El-Monaem, A.; Eman, M.; Khalifa, R.E.; Omer, A.M.; Eltaweil, A.S. Sulfonated graphene oxide impregnated cellulose acetate floated beads for adsorption of methylene blue dye: Optimization using response surface methodology. Sci. Rep. 2022, 12, 9339. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.H.; Azqhandi, M.H.A.; Ghalami-Choobar, B. Synthesis, characterization, and application of graphene oxide/layered double hydroxide/poly acrylic acid nanocomposite (LDH-rGO-PAA NC) for tetracycline removal: A comprehensive chemometric study. Chemosphere 2022, 308, 136007. [Google Scholar] [CrossRef] [PubMed]
- Ebratkhahan, M.; Zarei, M.; Arsalani, N. Facile synthesis and preparation of graphite/chitosan/graphene quantum dots nanocomposite cathode for electrochemical removal of tetracycline from aqueous solution. Sep. Purif. Technol. 2022, 299, 121663. [Google Scholar] [CrossRef]
- Tishbi, P.; Mosayebi, M.; Salehi, Z.; Fatemi, S.; Faegh, E. Synthesizing magnetic graphene oxide nanomaterial (GO-Fe3O4) and kinetic modelling of methylene blue adsorption from water. Can. J. Chem. Eng. 2022, 100, 3321–3334. [Google Scholar] [CrossRef]
- Politaeva, N.; Yakovlev, A.; Yakovleva, E.; Chelysheva, V.; Tarantseva, K.; Efremova, S.; Mukhametova, L.; Ilyashenko, S. Graphene Oxide-Chitosan Composites for Water Treatment from Copper Cations. Water 2022, 14, 1430. [Google Scholar] [CrossRef]
- Khah, M.H.; Jamshidi, P.; Shemirani, F. Applicability of an eco-friendly deep eutectic solvent loaded onto magnetic graphene oxide to preconcentrate trace amount of indigotin blue dye. J. Mol. Liq. 2021, 342, 117346. [Google Scholar] [CrossRef]
- Nundy, S.; Ghosh, A.; Nath, R.; Paul, A.; Tahir, A.A.; Mallick, T.K. Reduced graphene oxide (rGO) aerogel: Efficient adsorbent for the elimination of antimony (III) and (V) from wastewater. J. Hazard. Mater. 2021, 420, 126554. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials. Energy Storage-Wiley 2019, 159, 781–801. [Google Scholar] [CrossRef]
- Jani, M.; Arcos-Pareja, J.A.; Ni, M. Engineered Zero-Dimensional Fullerene/Carbon Dots-Polymer Based Nanocomposite Membranes for Wastewater Treatment. Molecules 2020, 25, 4934. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.; Alonso, J.A.; Méndez, F. Synthesis of fullerenes. J. Phys. Org. Chem. 2013, 26, 526–539. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R.; Lorents, D.C. Solubility of C60 in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Scrivens, W.A.; Tour, J.M.; Creek, K.E.; Pirisi, L. Synthesis of 14C-Labeled C60, Its Suspension in Water, and Its Uptake by Human Keratinocytes. J. Am. Chem. Soc. 1994, 116, 4517–4518. [Google Scholar] [CrossRef]
- Moussa, F.; Trivin, F.; Céolin, R.; Hadchouel, M. Fullerene Science and Technology Early effects of C 60 Administration in Swiss Mice: A Preliminary Account for in vivo C60 Toxicity. Fuller. Sci. Technol. 1996, 4, 21–29. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Fortner, J.D.; Sayes, C.M.; Colvin, V.L.; Hughes, J.B. Bacterial cell association and antimicrobial activity of a C60 water suspension. Environ. Toxicol. Chem. 2005, 24, 2757–2762. [Google Scholar] [CrossRef]
- Bai, W.; Krishna, V.; Wang, J.; Moudgil, B.; Koopman, B. Enhancement of nano titanium dioxide photocatalysis in transparent coatings by polyhydroxy fullerene. Appl. Catal. B Environ. 2012, 125, 128–135. [Google Scholar] [CrossRef]
- Nyberg, L.; Turco, R.F.; Nies, L. Assessing the impact of nanomaterials on anaerobic microbial communities. Environ. Sci. Technol. 2008, 42, 1938–1943. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Oguri, I.; Yamakoshi, Y.N.; Miyata, N. Novel harmful effects of [60] fullerene on mouse embryos in vitro and in vivo. FEBS Lett. 1996, 393, 139–145. [Google Scholar] [CrossRef]
- Lima, J.D.M.; Gomes, D.S.; Frazão, N.F.; Soares, D.J.B.; Sarmento, R.G. Glyphosate adsorption on C6 0 fullerene in aqueous medium for water reservoir depollution. J. Mol. Model. 2020, 26, 110. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.; Saad, H.A.; Atta, A.A.; Alsawat, M.; Hegab, M.S.; Altalhi, T.A.; Refat, M.S. An environmentally friendly method for removing Hg(II), Pb(II), Cd(II) and Sn(II) heavy metals from wastewater using novel metal–carbon-based composites. Crystals 2021, 11, 882. [Google Scholar] [CrossRef]
- Qi, K.; Selvaraj, R.; Al Fahdi, T.; Al-Kindy, S.; Kim, Y.; Wang, G.-C.; Tai, C.-W.; Sillanpää, M. Enhanced photocatalytic activity of anatase-TiO2 nanoparticles by fullerene modification: A theoretical and experimental study. Appl. Surf. Sci. 2016, 387, 750–758. [Google Scholar] [CrossRef]
- Park, Y.; Singh, N.J.; Kim, K.S.; Tachikawa, T.; Majima, T.; Choi, W. Fullerol–titania charge-transfer-mediated photocatalysis working under visible light. Chem. Eur. J. 2009, 15, 10843–10850. [Google Scholar] [CrossRef]
- WHO. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 14 September 2022).
- Raffi, M.; Rumaiz, A.K.; Hasan, M.M.; Shah, S.I. Studies of the growth parameters for silver nanoparticle synthesis by inert gas condensation. J. Mater. Res. 2007, 22, 3378–3384. [Google Scholar] [CrossRef]
- Bordoni, A.V.; Zalduendo, M.M.; Escobar, A.; Amenitsch, H.; Moya, S.E.; Angelomé, P.C. Phosphonate mesoporous hybrid thin films: Synthesis of organophosphosilane by thiol-ene click chemistry and applications in formation and stabilization of silver nanoparticles. Microporous Mesoporous Mater. 2020, 295, 109958. [Google Scholar] [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Pietrzak, K.; Gutarowska, B.; Machnowski, W.; Mikołajczyk, U. Antimicrobial properties of silver nanoparticles misting on cotton fabrics. Text. Res. J. 2016, 86, 812–822. [Google Scholar] [CrossRef]
- Morsi, R.E.; Alsabagh, A.M.; Nasr, S.A.; Zaki, M.M. Multifunctional nanocomposites of chitosan, silver nanoparticles, copper nanoparticles and carbon nanotubes for water treatment: Antimicrobial characteristics. Int. J. Biol. Macromol. 2017, 97, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Nagaraja, B.M.; Shashikala, V.; Padmasri, A.H.; Madhavendra, S.S.; Raju, B.D.; Rao, K.S.R. Highly efficient Ag/C catalyst prepared by electro-chemical deposition method in controlling microorganisms in water. J. Mol. Catal. A Chem. 2004, 223, 313–319. [Google Scholar] [CrossRef]
- Daniel, S.; Malathi, S.; Balasubramanian, S.; Sivakumar, M.; Sironmani, T.A. Multifunctional silver, copper and zero valent iron metallic nanoparticles for wastewater treatment. In Application of Nanotechnology in Water; Wiley: New York, NY, USA, 2014; pp. 435–457. [Google Scholar]
- Madeła, M. Impact of silver nanoparticles on wastewater treatment in the SBR. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2019; Volume 86, p. 27. [Google Scholar]
- Madeła, M. Effect of copper nanoparticles on biological wastewater treatment. Desalin. Water Treat. 2020, 199, 493–498. [Google Scholar] [CrossRef]
- Najafpoor, A.; Norouzian-Ostad, R.; Alidadi, H.; Rohani-Bastami, T.; Davoudi, M.; Barjasteh-Askari, F.; Zanganeh, J. Effect of magnetic nanoparticles and silver-loaded magnetic nanoparticles on advanced wastewater treatment and disinfection. J. Mol. Liq. 2020, 303, 112640. [Google Scholar] [CrossRef]
- Ganguly, K.; Dutta, S.D.; Patel, D.K.; Lim, K.-T. Silver nanoparticles for wastewater treatment. In Aquananotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 385–401. [Google Scholar]
- Bryaskova, R.; Georgieva, N.; Pencheva, D.; Todorova, Z.; Lazarova, N.; Kantardjiev, T. Synthesis and characterization of hybrid materials with embedded silver nanoparticles and their application as antimicrobial matrices for waste water purification. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 114–119. [Google Scholar] [CrossRef]
- Bhargava, S.; Uma, V. Rapid extraction of Cu (II) heavy metal from industrial waste water by using silver nanoparticles anchored with novel Schiff base. Sep. Sci. Technol. 2019, 54, 1182–1193. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Sivaranjani, S.; Prasath, V.A.; Pandiselvam, R.; Kothakota, A.; Mousavi Khaneghah, A. Recent advances in applications of ozone in the cereal industry. LWT 2021, 146, 111412. [Google Scholar] [CrossRef]
- Huh, J.Y.; Kim, K.; Ma, S.H.; Choi, E.H.; Hong, Y.C. One-Pot Synthesis of Copper Nanoparticles Using Underwater Plasma. IEEE Trans. Plasma Sci. 2019, 47, 1690–1694. [Google Scholar] [CrossRef]
- Khare, P.; Sharma, A.; Verma, N. Synthesis of phenolic precursor-based porous carbon beads in situ dispersed with copper–silver bimetal nanoparticles for antibacterial applications. J. Colloid Interface Sci. 2014, 418, 216–224. [Google Scholar] [CrossRef]
- Allaker, R.P.; Memarzadeh, K. Nanoparticles and the control of oral infections. Int. J. Antimicrob. Agents 2014, 43, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Chen, Y.; Li, M.; Liu, K.; Li, X. Influence of copper nanoparticles on the physical-chemical properties of activated sludge. PLoS ONE 2014, 9, e0092871. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Zhu, X.; Zheng, X.; Feng, L. Long-term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ. Sci. Technol. 2012, 46, 12452–12458. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Chen, Y.; Liu, Y.; Zhang, H.; Xue, G. Performance of wastewater biological phosphorus removal under long-term exposure to CuNPs: Adapting toxicity via microbial community structure adjustment. RSC Adv. 2015, 5, 61094–61102. [Google Scholar] [CrossRef]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, A.L.K.; Tao, Y.; Shan, D.; Ting, K.E.; Yin, X.J. Synthesis and characterization of iron oxide nanoparticles and applications in the removal of heavy metals from industrial wastewater. Int. J. Photoenergy 2012, 2012, 608298. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef]
- Sawafta, R.; Shahwan, T. A comparative study of the removal of methylene blue by iron nanoparticles from water and water-ethanol solutions. J. Mol. Liq. 2019, 273, 274–281. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Sun, M.; Han, X.; Chen, S. Synthesis and photocatalytic activity of nano-cobalt ferrite catalyst for the photo-degradation various dyes under simulated sunlight irradiation. Mater. Sci. Semicond. Process. 2019, 91, 367–376. [Google Scholar] [CrossRef]
- Abbas, M.; Rao, B.P.; Reddy, V.; Kim, C. Fe3O4/TiO2 core/shell nanocubes: Single-batch surfactantless synthesis, characterization and efficient catalysts for methylene blue degradation. Ceram. Int. 2014, 40, 11177–11186. [Google Scholar] [CrossRef]
- Feng, X.; Guo, H.; Patel, K.; Zhou, H.; Lou, X. High performance, recoverable Fe3O4ZnO nanoparticles for enhanced photocatalytic degradation of phenol. Chem. Eng. J. 2014, 244, 327–334. [Google Scholar] [CrossRef]
- Abou Taleb, M.F. Adsorption and photocatalytic degradation of 2-CP in wastewater onto CS/CoFe2O4 nanocomposite synthesized using gamma radiation. Carbohydr. Polym. 2014, 114, 65–72. [Google Scholar] [CrossRef]
- Gautam, S.; Shandilya, P.; Priya, B.; Singh, V.P.; Raizada, P.; Rai, R.; Valente, M.A.; Singh, P. Superparamagnetic MnFe2O4 dispersed over graphitic carbon sand composite and bentonite as magnetically recoverable photocatalyst for antibiotic mineralization. Sep. Purif. Technol. 2017, 172, 498–511. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Chu, W. Heterogeneous catalytic ozonation of phenacetin in water using magnetic spinel ferrite as catalyst: Comparison of surface property and efficiency. J. Mol. Catal. A Chem. 2015, 396, 164–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Donovan, A.R.; Adams, C.D.; Ma, Y.; Stephan, C.; Eichholz, T.; Shi, H. Fate of nanoparticles during alum and ferric coagulation monitored using single particle ICP-MS. Chemosphere 2018, 195, 531–541. [Google Scholar] [CrossRef]
- Tuccillo, M.E.; Boyd, G.R.; Sandvig, A.; Shatkin, J.A.; Dionysiou, D.D. Nanotechnology what are the Challenges and Emerging Benefits for Water Treatment? Opflow 2012, 38, 10–13. [Google Scholar] [CrossRef]
- Dong, J. Signaling pathways implicated in carbon nanotube-induced lung inflammation. Front. Immunol. 2020, 11, 3250. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Type 2 immune mechanisms in carbon nanotube-induced lung fibrosis. Front. Immunol. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.F.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 3, 230–243. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Acuña, V.; Gutierrez, M.; Sanchís, J.; Santos, L.H.M.L.M.; Rodriguez-Mozaz, S.; Farré, M.; Barceló, D.; Sabater, S. Fullerenes influence the toxicity of organic micro-contaminants to river biofilms. Front. Microbiol. 2018, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. Silver in health care: Antimicrobial effects and safety in use. Biofunctional Text. Ski. 2006, 33, 17–34. [Google Scholar]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Świdwińska-Gajewska, A.; Czerczak, S. Nanosrebro-szkodliwe skutki działania biologicznego [Nanosilver-harmful effects of biological activity]. Med. Pracy 2014, 65, 831–845. [Google Scholar]
- Fiorati, A.; Bellingeri, A.; Punta, C.; Corsi, I.; Venditti, I. Silver nanoparticles for water pollution monitoring and treatments: Ecosafety challenge and cellulose-based hybrids solution. Polymers 2020, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Favela-Camacho, S.E.; Samaniego-Benítez, E.J.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. An overview of nanomaterials for water and wastewater treatment. Adv. Mater. Sci. Eng. 2016, 2016, 4964828. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone application in different industries: A review of recent developments. Chem. Eng. J. 2022, 454, 140188. [Google Scholar] [CrossRef]
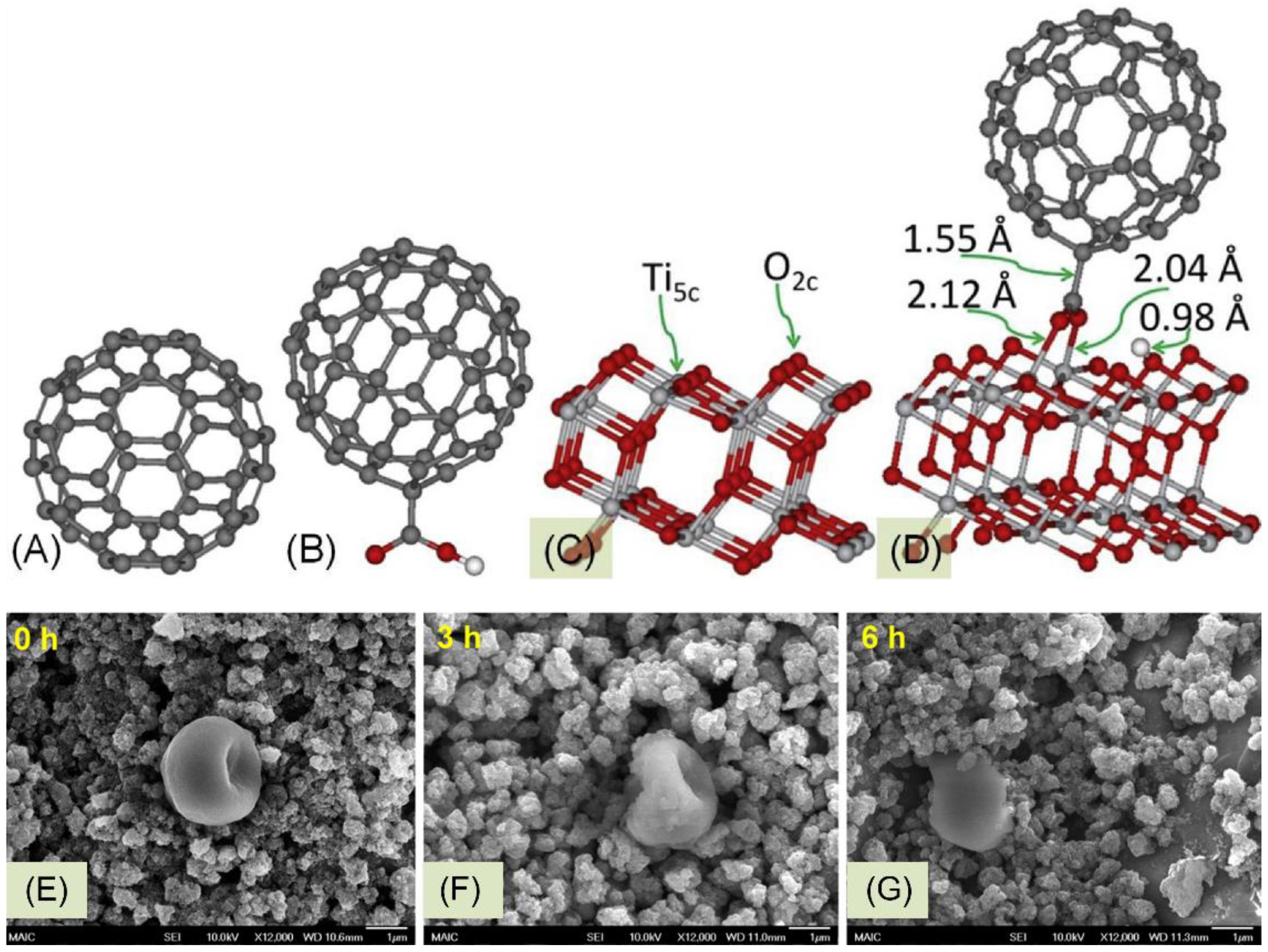
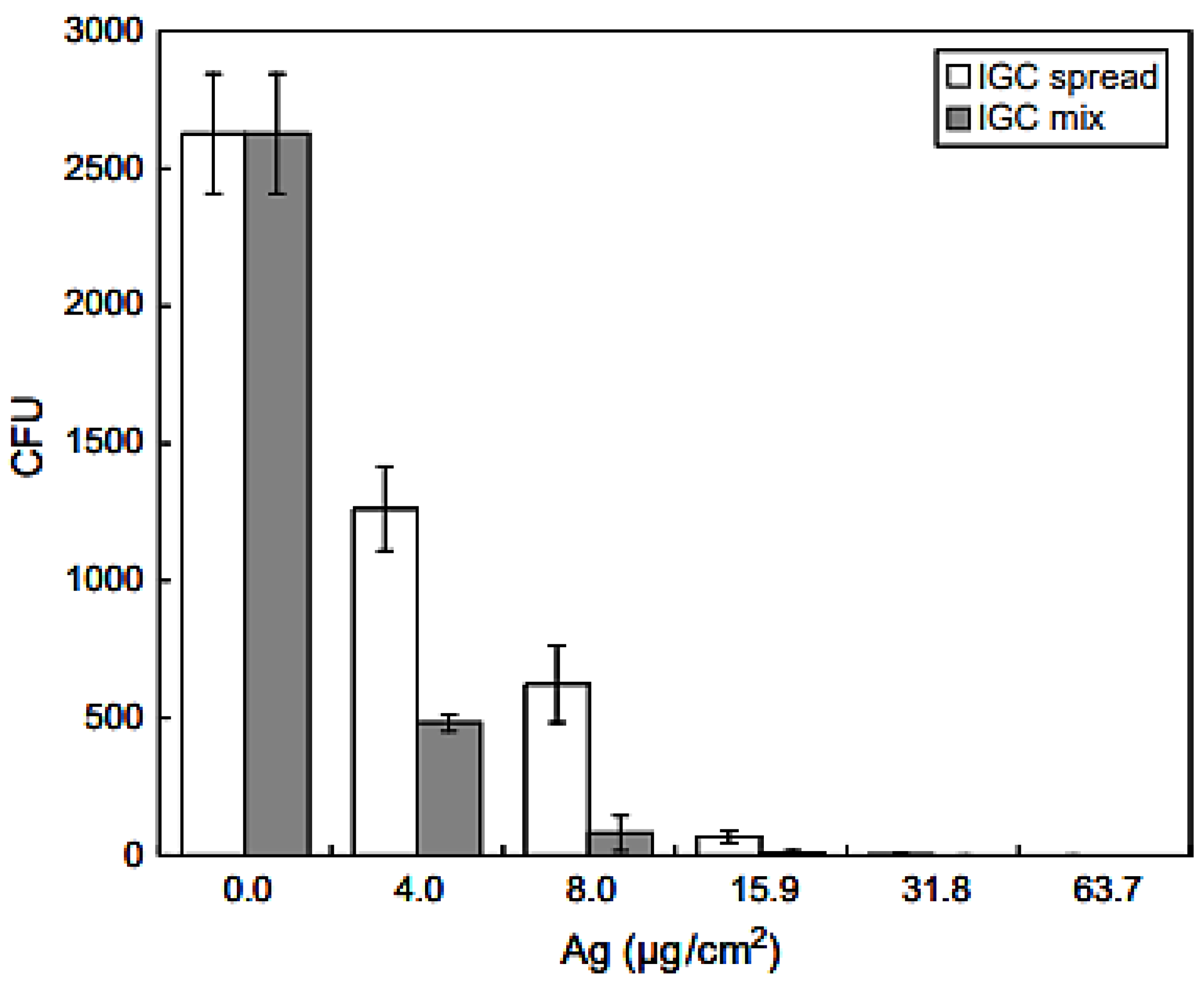
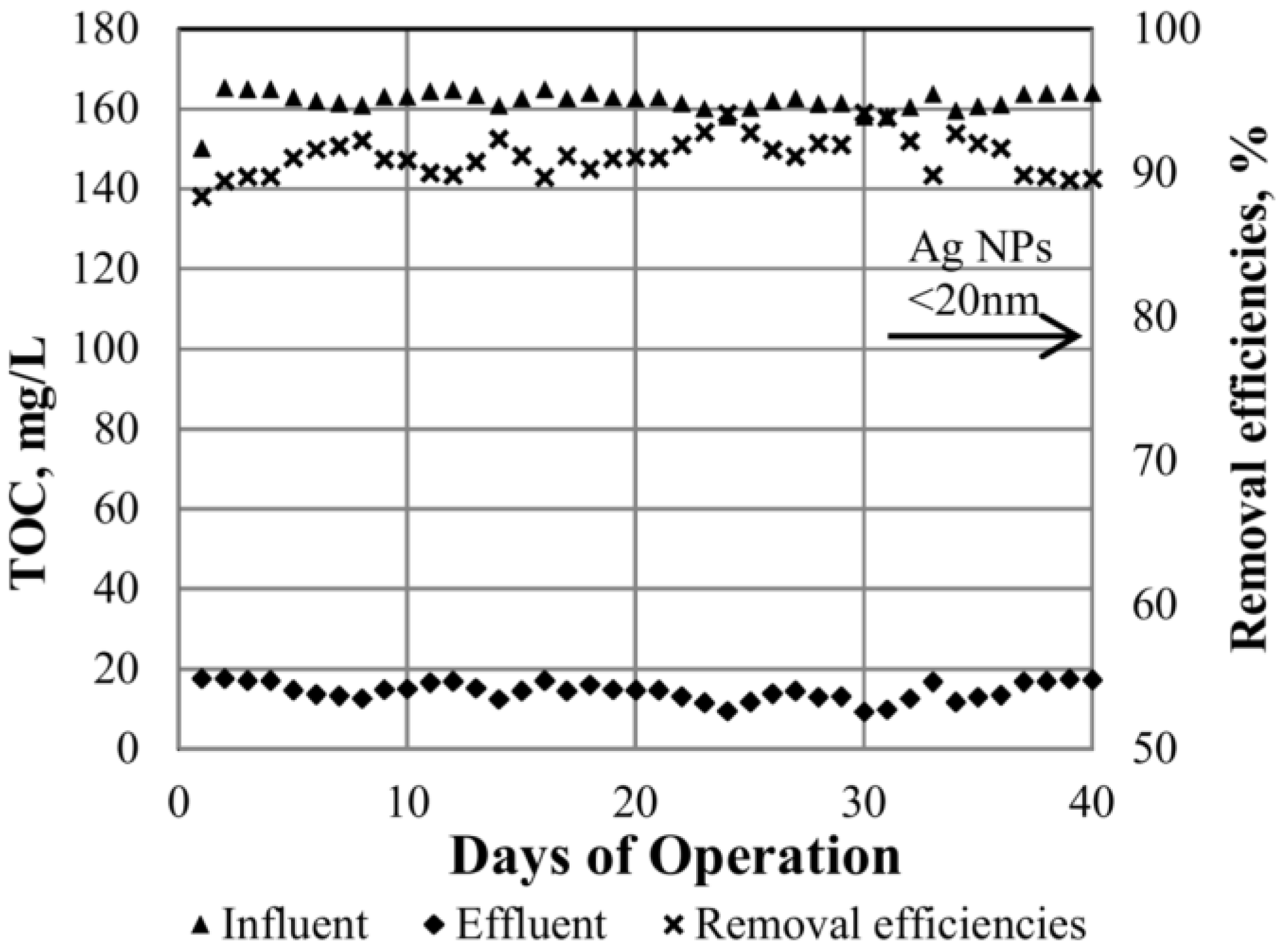
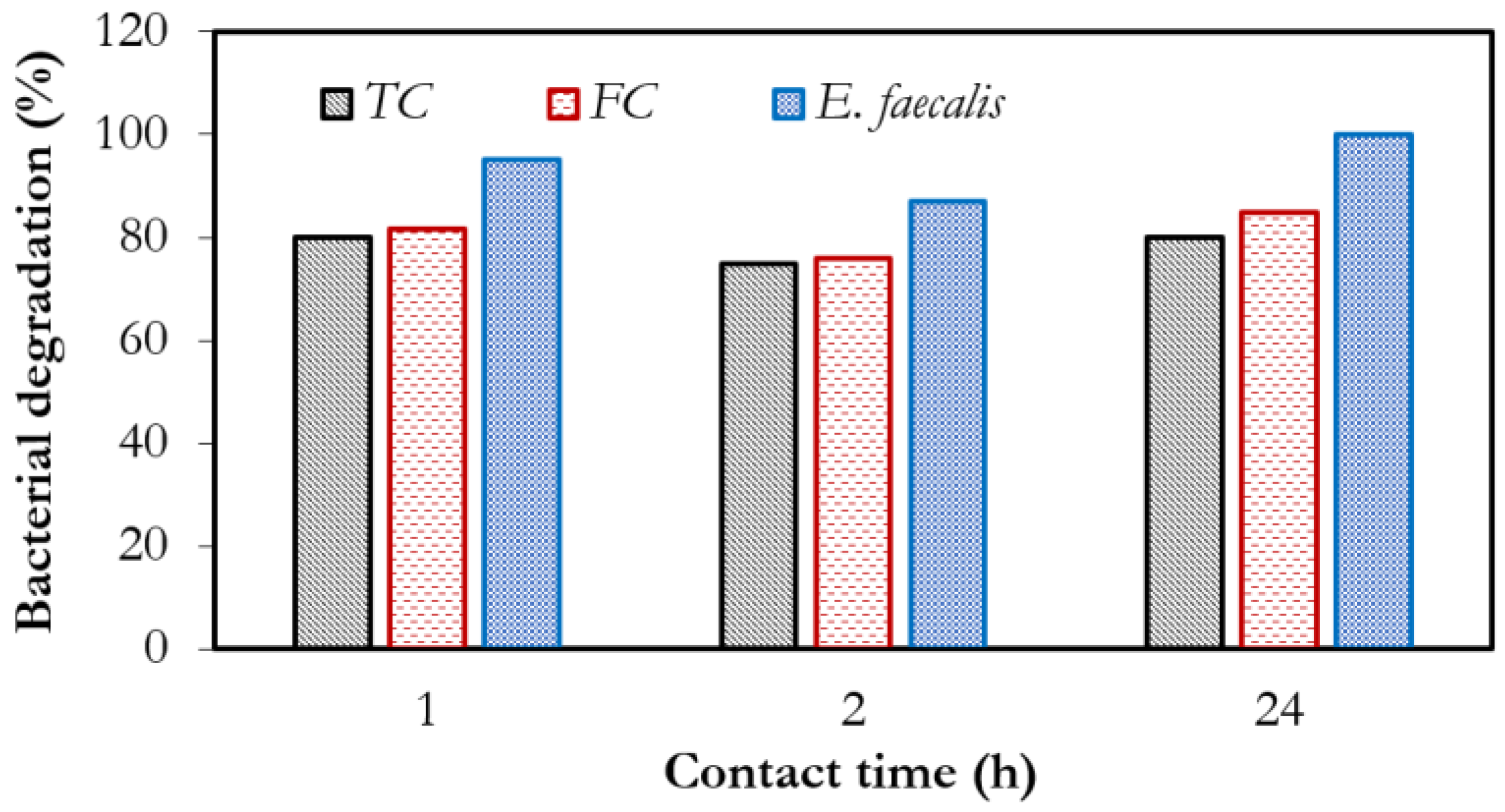

| Property | SWCNT | MWCNT |
|---|---|---|
| Bulk synthesis | Difficult | Easy |
| Graphene layer | Single | Multiple |
| Purity | Poor | High |
| Thermal conductivity (W/(m K)) | 6000 | 2000 |
| Specific gravity (g/cm3) | 0.8 | 1.8 |
| Electrical conductivity (S/cm) | 102–106 | 103–105 |
| Electron mobility (cm2/(Vs)) | ~105 | 104–105 |
| Thermal stability in air (°C) | >600 | >600 |
| Adsorbents | pH | Contact Time (min) | Adsorbent Dosage (g) | Target Pollutant | Adsorption Capacity (mg/g) | Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Chitin/magnetite/MWCNT | 2.0 | 45 | 0.05 | Cr(VI) | 11.3 | Chemisorption | [56] |
| Zero-valent iron/MWCNT | 8.0 | 60 | 4 * | Arsenate | 250 | Complexation mechanism | [57] |
| Zero-valent iron/MWCNT | 7.0 | 90 | 4 * | Arsenite | 200 | Complexation mechanism | [57] |
| CNT-sediments | 10.0 | 300 | 10 ** | Cd(II) | 1.482 | Physisorption | [58] |
| PES/1% MWCNTs-NH2 | 7.0 | 10 | 0.1 | Pb(II) | 272 | Chemisorption | [53] |
| Ion-imprinted polymers/MWCNT | 6.0 | 15 | 0.02 | Pb(II) | 83.20 | Chemisorption | [54] |
| MWCNT | 6.0 | - | 0.3 | Pb(II) | 97.08 | Physisorption | [59] |
| MWCNT | 6.0 | - | 0.3 | Cu(II) | 24.49 | Physisorption | [59] |
| MWCNT | 11.0 | - | 0.3 | Cd(II) | 10.86 | Physisorption | [59] |
| Oxidized-MWCNTs | 5.5 | 120 | 0.02 | Cu(II) | 14.00 | Chemisorption | [60] |
| Zn-BDC@CT/GO | 4.0 | 20 | 0.01 | Arsenic | 128.20 | Chemisorption | [51] |
| F-CNTs@MOF@Gel | 9.0 | 2000 | 0.02 | Methyelene blue | 106.50 | Physisorption | [49] |
| SWCNT | 3 | 75 | 0.12 | Acid Blue 92 | 86.91 | - | [47] |
| PET-NF-MWCNT | 8 | 120 | 0.008 | Methylene blue | 7.047 | Chemisorption | [50] |
| Adsorbent | pH | Contact Time (min) | Adsorbent Dose (g) | Initial Concentration (mg/L) | Target Pollutant | Maximum Adsorption Capacity (mg/g) | No. of Reuse | Ref. |
|---|---|---|---|---|---|---|---|---|
| GO-Citrate | 7 | 5 | 0.006 | 50 | MB | 222.22 | 5 | [70] |
| GO-Citrate | 6 | 1 | 0.0024 | 150 | Cu(II) | 270.27 | 5 | [70] |
| Clay/GO/Fe2O3 | 11 | - | 0.100 | 1 | MB | 19.99 | - | [71] |
| Alginate@MOF-rGO | 7 | 720 | 0.001 | 10 | Tetracycline | 43.76 | 6 | [72] |
| Alginate@MOF-rGO | 7 | 720 | 0.001 | 10 | Ciprofloxacin | 40.76 | 6 | [72] |
| ZnO/C-foam/GQDs/Alginate | 6 | 30 | 0.001 | 5 | MB | 92.048 | 5 | [73] |
| ZnO/C-foam/GQDs/Alginate | 6 | 30 | 0.001 | 5 | Pb(II) | 135.624 | 5 | [73] |
| SGO/cellulose acetate | 6 | 30 | 0.005 | 300 | MB | 239.8 | 6 | [74] |
| LDH/rGO/PAA-NC | 6.3 | 18.50 | 0.02 | 110 | Tetracycline | 887.5 | 5 | [75] |
| G/CS/GQD | 5 | 150 | - | 30 | Tetracycline | - | 8 | [76] |
| GO-Fe3O4 | 6 | 5 | 0.01 | 350 | MB | 212.54 | 5 | [77] |
| GO-Chitosan | 7 | 20 | 0.002 | 10 | Cu(II) | 58.5 | - | [78] |
| UT-mGO | 3 | 15 | 0.01 | 0.1 | Indigotin blue dye | - | - | [79] |
| rGO aerogel | 6 | 120 | 0.001 | 300 | Antimony (II) and (V) | 168.58 and 206.72 | 10 | [80] |
| Bacteria/pH | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|
| CuO (4) | CuO-TOAB (3) | CuO (4) | CuO-TOAB (3) | CuO (4) | CuO-TOAB (3) | |
| TC | 88% | 97% | 87% | 96% | 86% | 94% |
| FC | 89% | 95% | 88% | 92% | 86% | 90% |
| E. faecalis | 92% | 98.5% | 91% | 98% | 89% | 97% |
| Nanoparticle | Pathogen |
|---|---|
| Silver | Klebsiella pneumoniae, Bacillus anthracis, Bacillus subtilis, Staphylococcus aureus, Acinetobacter baylyi, E. coli, Candida albicans, Salmonella Typhimurium, Salmonella epidermidis, P. aeruginosa, P. vulgaris, methicillin sensitive S. aureus. |
| Copper | Micrococcus luteus, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Aspergillus flavus, Aspergillus niger and Candida albicans. |
| Iron Oxide | Staphylococcus aureus, Shigella flexneri, Bacillus licheniformis, Bacillus brevis, Vibrio cholerae, Pseudomonas aeruginosa, Streptococcus aureus, Staphylococcus epidermidis, Bacillus subtilis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. https://doi.org/10.3390/environments9110141
Epelle EI, Okoye PU, Roddy S, Gunes B, Okolie JA. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments. 2022; 9(11):141. https://doi.org/10.3390/environments9110141
Chicago/Turabian StyleEpelle, Emmanuel I., Patrick U. Okoye, Siobhan Roddy, Burcu Gunes, and Jude A. Okolie. 2022. "Advances in the Applications of Nanomaterials for Wastewater Treatment" Environments 9, no. 11: 141. https://doi.org/10.3390/environments9110141
APA StyleEpelle, E. I., Okoye, P. U., Roddy, S., Gunes, B., & Okolie, J. A. (2022). Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments, 9(11), 141. https://doi.org/10.3390/environments9110141