1. Introduction
The high demand for metals in the fast-growing industrial sector around the world has led to their extensive extraction and production in huge quantities. This phenomenon in turn has exacerbated the problem of severe soil pollution with heavy metals in recent decades.
Soil pollution with various toxic metals, including chromium, occurs in two main ways—natural and anthropogenic. Chromium is present in water, soil, and air in several oxidation states, but the most stable and common forms are Cr(III) and Cr(VI). In water, this element occurs naturally as a result of microorganisms’ interactions with mafic and ultramafic igneous rocks together with geogenic processes [
1]. The total concentration of chromium in water depends on the physicochemical properties of the water basin. Continental dust flux and volcanic ash are natural sources of chromium in the atmosphere. The content of chromium in soil is dependent on soil-forming material, chromium deposition, and the contribution of wastewater containing chromium as well as soil processing technologies [
2].
However, chromium contamination is considered a serious environmental problem mainly due to industrial processes. Chromium compounds are widely applied in machinery, textile industry, steel production, batteries and dyes production, and leather processing. The combustion of oil and coal also contribute to the atmospheric burden of chromium [
1,
3].
Sanitary norms regarding the level of chromium in the environment are established in accordance with national standards and geological conditions (the presence of chromium ores). In European countries (EU), the maximum level of total chromium in wastewater lies between 0.3 and 4.0 mg/L, and that of Cr(VI) should not exceed 0.5 mg/L [
1]. In drinking water, the maximum permissible level of Cr(VI) is 0.05 mg/L.
The prevalence of one of the two valence states in the soil, Cr(III) or Cr(VI), depends on soil pH values, redox potential, and presence of natural reducing agents. It was shown that the dominant Cr(VI) species are anionic forms CrO
42−, HCrO
4−, and Cr
2O
72−. Cr(VI) compounds are characterized by very high solubility and mobility, which makes them inaccessible to natural soil colloids, which could neutralize them, as is the case with Cr(III). As a result, Cr(VI) compounds are extremely toxic to living organisms [
2,
4].
Being highly mobile, Cr(VI) is taken up by plants through carriers of essential ions, such as sulfate transporters. The accumulation of chromium in plants leads to a decrease in germination, growth retardation, inhibition of enzymatic activity in tissues, etc. [
5]. Consequently, chromium uptake into the plants is transferred to the upper links of the food chain, including humans.
When Cr(VI) enters cells it is exposed to cellular reducers, such as glutathione or ascorbic acid. In the process of Cr(VI) reduction, intermediate forms Cr(V) and Cr(IV) are obtained, which are highly reactive and cause the activation of molecular oxygen with the generation of various reactive oxygen species, including hydroxyl radical. This, in turn, reacts with biomolecules, including nucleic acids, inducing various mutations [
3]. This is considered to be the underlying mechanism of the toxicity and carcinogenic effects of hexavalent chromium.
Thus, in order to avoid the risks arising from the presence of Cr(VI) in the soil, chromium undergoes a variety of remediation procedures. In this regard, there are two main approaches—traditional soil remediation by physicochemical methods and bioremediation. The remediation using traditional methods includes soil flushing, landfilling, solidification-stabilization, and chemical reduction of Cr(VI). It is recognized that these methods are hard to achieve, economically disadvantageous, and in most cases cause secondary environmental pollution.
Bioremediation can be performed using a variety of living organisms or biomass derivatives. In most cases, phytoremediation exploits the ability of some plant species or some plant species mixtures adjusted to the habitat conditions to uptake and metabolize heavy metals, in this case chromium [
6,
7]. The disadvantage of this approach lies in the long duration (equivalent to a period of vegetation) of the process. In this respect, microorganisms with a short generation time are more preferable, since they are able to accumulate a large amount of Cr(VI), which subsequently undergo biotransformation/reduction; thus, achieving soil detoxification [
8,
9,
10,
11].
On the other hand, different groups of soil microorganisms possess different degrees of resistance to heavy metals, including chromium, and different abilities to reduce their toxicity. Edaphic cyanobacteria are the least studied in this respect. At the same time, it is known that they can produce a large number of extracellular polymers, such as polysaccharides, glycoproteins, lipopolysaccharides, which have multiple anionic functional groups that can form coordination bonds with Cr(VI) [
12].
In this respect,
Nostoc linckia is a promising edaphic cyanobacterium. It has been shown that it can be used for chromium biosorption from wastewater. In addition to chromium, Nostoc biomass accumulated the amount of iron and zinc that exceeds their concentrations in the control biomass samples by six and eight times, respectively [
13].
Biosorption of metals, including Cr(VI) using dead biomass or biomass derivatives is mainly applicable for aquatic environments and is not considered as a practical method for soil remediation, as further steps are required to complete the metal removal process. Instead, the bioaccumulation process, which is an active process, uses only living organisms that not only concentrate the metal in cells, but also undergo it to subsequent reduction/biotransformation, converting the pollutant to less toxic forms.
The bioaccumulation of chromium needs to be studied together with the accumulation of other elements present in the polluted environment since the efficiency of metal removal from mono- and multi-metallic systems is usually different. For instance, cyanobacterium spirulina is more effective in removing Cr(VI) from multi-metallic systems than from monometallic ones [
14].
Moreover, pollution of the environment, including soil, has a discontinuous character, the contact of living microorganisms with pollutants occurs repeatedly, affecting different generations. It is interesting to note microorganisms’ ability to adapt to repeated exposure to contaminants, as well as to monitor the long-term effects of this exposure.
Based on the above, this study aimed to assess the ability of cyanobacterium Nostoc linckia to bioaccumulate chromium along with other metal ions from multi-metallic systems under conditions of repeated pollution and changes in the quality of biomass obtained under these conditions.
2. Materials and Methods
The following salts, K
2CrO
7, CuSO
4, FeCl
3·6H
2O, Ni(NO
3)·6H
2O, and ZnCl
2 (purity ≥ 97%) (Sigma-Aldrich, Darmstadt, Germany) were used for multi-metal batch systems preparation. The chemical composition of multi-metal systems is presented in
Table 1.
The object of the study was cyanobacterial strain Nostoc linckia (Roth) Born et Flah CNM-CB-03, deposited in the National Collection of Nonpathogenic Microorganisms of the Republic of Moldova.
For obtaining inoculum for the first cycle, cyanobacterium was grown in a mineral medium with the following composition: macroelements in g/L—KNO3-0.5, K2HPO4-0.45, NaHCO3-0.05, MgSO4·7H2O-0.1, CaCl2-0.11, and microelements in mg/L—ZnSO4·7H2O-0.05, MnSO4-2.0, H3BO3-0.85, (NH4)6Mo7O24·4H2O-2.25, FeSO4·7H2O-4.0, Co(NO3)2·H2O-0.009, and EDTA-4.75. For experiments, the same medium, but without microelements, was used.
Cultivation was carried out in Erlenmeyer flasks of 1000 mL with a working volume of 700 mL. The following parameters were used: pH of the medium 6.8–7.2, temperature 25–27 °C, light intensity of 37–55 μmol photons m−2 s−1, continuous illumination, and slow periodic shaking. The amount of inoculum was 0.4 g/L.
Metals were added to the culture medium on the sixth day (exponential growth phase) of biomass growth. The cultivation cycle lasted 12 days, the pH of the mixture during one cultivation cycle was in the range of 6.8–7.2. At the end of the cultivation cycle, 100 mL of biomass suspension was used for biochemical tests; 300 mL was used for neutron activation analysis (NAA), and 300 mL was used for subculturing. Each of the 3 portions of biomass was centrifuged to separate the growth medium, then biomass was washed with double-distilled water and used for further experiments. The procedure was repeated two more times. At the end of the bioaccumulation experiments, the biomass was separated from the culture medium by centrifugation. The amount of biomass was determined based on the calibration curve. For biochemical analysis, the samples were standardized to a concentration of 10 mg/mL with distilled water and subjected to a repeated freezing–thawing procedure. For NAA analysis, the biomass was dried at 100 ± 2 °C. The same procedure was performed with control biomass but without the addition of metal ions.
Obtaining extracts. Water and alcohol extracts were obtained by extraction in distilled water and, respectively, in alcohol of 96% in the ratio of 10 mg biomass per 1 mL of extractant. The extraction was performed by biomass stirring at room temperature for 24 h.
2.1. Neutron Activation Analysis
Metal accumulation by
Nostoc linckia biomass was assessed by neutron activation analysis (NAA) at the REGATA facility of the IBR-2 reactor (Dubna, Russia) [
15]. To determine Cr, Fe, Ni, and Zn content samples were irradiated for 3 days at a neutron flux of 3.31 × 10
11 n cm
−2 s
−1, re-packed, and measured twice using HP germanium detectors after 4 and 20 days of decay, respectively. The chromium content in the samples was determined by a
γ-line with the energy of 312.0 keV of isotope
51Cr, iron by a
γ-line with the energy of 1099.25 keV of isotope
59Fe, nickel by a
γ-line with the energy of 810.57 keV of isotope
58Co, and zinc by a
γ-line with the energy of 1115.54 keV of isotope
65Zn. To determine copper content, samples were irradiated for 3 min at a neutron flux of 1.6 × 10
12 n cm
−2 s
−1 and measured directly after irradiation. Copper content in the samples was determined by a
γ-line with the energy of 1039.2 keV of isotope
66Cu. The NAA data processing and calculation of element concentrations were performed using the FLNP JINR software [
16].
2.2. Biochemical Analysis
Protein content in the biomass was determined spectrophotometrically by the Lowry method [
17]. Protein extraction was carried out with 0.1 N NaOH, for which 0.9 mL of 0.1 N NaOH was added to 10 mg biomass for 30 min. Then, to 0.2 mL alkaline protein extract hydrolysate was added 2.0 mL reagent (49 mL of 2% sodium carbonate in 0.1 N sodium hydroxide and 1.0 mL of 0.5% copper sulfate in 1.0% sodium citrate). After 10 min of incubation at room temperature, 0.2 mL of Folin–Ciocalteu reagent (F9252 (Sigma-Aldrich, Darmstadt, Germany) suitable for determination of total protein by Lowry method) diluted with distilled water for four times was added to the reaction mixture. At the end of the incubation time (30 min) the absorbance was measured at 750 nm. Protein content was calculated using a calibration curve for bovine serum albumin.
Carbohydrate content was determined by a spectrophotometric method using an anthrone reagent. For this, 2.5 mL of 0.5% anthrone solution in 66% sulfuric acid was carefully added to 0.25 mL of analyzed sample. The samples were incubated in a boiling water bath for 30 min, after which they were cooled under tap water and exposed in the dark for 30 min. The blue-green solution shows an absorption maximum at 620 nm. The carbohydrate content was calculated using a calibration curve for glucose [
18].
Quantitative determination of lipids was carried out spectrophotometrically using the phospho–vanillin reagent. For this purpose the lipidic extract was prepared by mixing 10 mg of biomass with 1 mL of chloroform and ethanol in the ratio de 9:1 (
v/
v). Extraction was performed at room temperature by continuous stirring for 120 min. The chloroform extract was separated from biomass. After removing the solvent by evaporation, 1 mL of concentrated sulfuric acid was added to the lipid extract. The samples were placed in a water bath for 10 min. Then, 2.9 mL of phospho–vanillin reagent was added to 0.1 mL of lipid hydrolysate. After 30 min, the absorbance was measured at the wavelength of 520 nm. The lipid content was calculated using a calibration curve based on oleic acid [
19].
Total phenolic content was determined using the Folin–Ciocalteu reagent. An amount of 0.3 mL of biomass extract was moxed with 1.5 mL of Folin–Ciocalteu reagent diluted 10 times and 1.2 mL of 7.5% sodium carbonate. The mixture was stirred and incubated at 50 °C for 30 min. After cooling the samples, the absorbance was measured at 760 nm. The total phenolic content was calculated from the calibration curve, and the results were expressed as mg of gallic acid equivalent per g dry weight [
20].
Phycobiliprotein content was calculated on the basis of the formula of Siegelman and Kycia [
21]. The method is based on the determination of the absorbance of water extract, obtained as a result of the procedure of repeated freezing–thawing of standardized Nostoc biomass. The absorbance of the extract was measured at wavelengths 565, 620, and 650 nm, which represent maximum absorption peaks of phycoerythrin (PE), phycocyanin (PC), and allophycocyanin (APC), respectively.
Determination of the products of oxidative degradation of lipids in biomass was carried out by calculating the content of malondialdehyde (MDA) based on the reactive substances of thiobarbituric acid (TBA). An amount of 0.1 mL of biomass (10 mg/mL) was mixed with 3.0 mL of 0.76% solution of TBA in a 20% solution of trichloroacetic acid. The reaction mixture was incubated at 95 °C for 20 min. After cooling the samples, the optical density was determined at wavelengths of 532 nm and 600 nm. The amount of MDA in the samples was calculated using the molar extinction coefficient for the MDA–TBA complex [
22].
Quantitative determination of chlorophyll and β-carotene content was established using 10 mg of Nostoc biomass mixed with 1.0 mL of 96% ethanol. Pigment extraction was performed by continuous stirring at room temperature for 120 min. The ethanolic extract was separated from biomass by centrifugation. The chlorophyll content was determined based on the absorbance at 665 nm and the extinction coefficient 0.8 × 10
5 M
−1·cm
−1, and β-carotene was determined based on the absorbance at 450 nm and the extinction coefficient 1.5 × 10
5 M
−1·cm
−1 [
16].
Antioxidant activity was established using ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay [
23]. The radical cation ABTS
+ is produced from the reaction between ABTS solution and potassium persulfate. For this purpose, a 7 mM ABTS stock solution was prepared in deionized water, to which 2.45 mM potassium persulfate was added in a ratio of 1:1
v/
v. The oxidation of ABTS occurred in the dark at room temperature for at least 12–16 h. The working solution was prepared from the ABTS stock solution and had an absorbance of 0.700 ± 0.020 at 734 nm. The reaction mixture consisted of 0.3 mL of biomass extract and 2.7 mL of ABTS solution. The absorbance of the samples was measured after 6 min. The % of inhibition relative to the absorbance of the ABTS reagent was calculated.
2.3. Statistical Analysis
All experiments were performed in triplicate. The results in all histograms are presented as mean values ± standard deviations. Differences between the values were estimated by Student’s t-tests.
3. Results
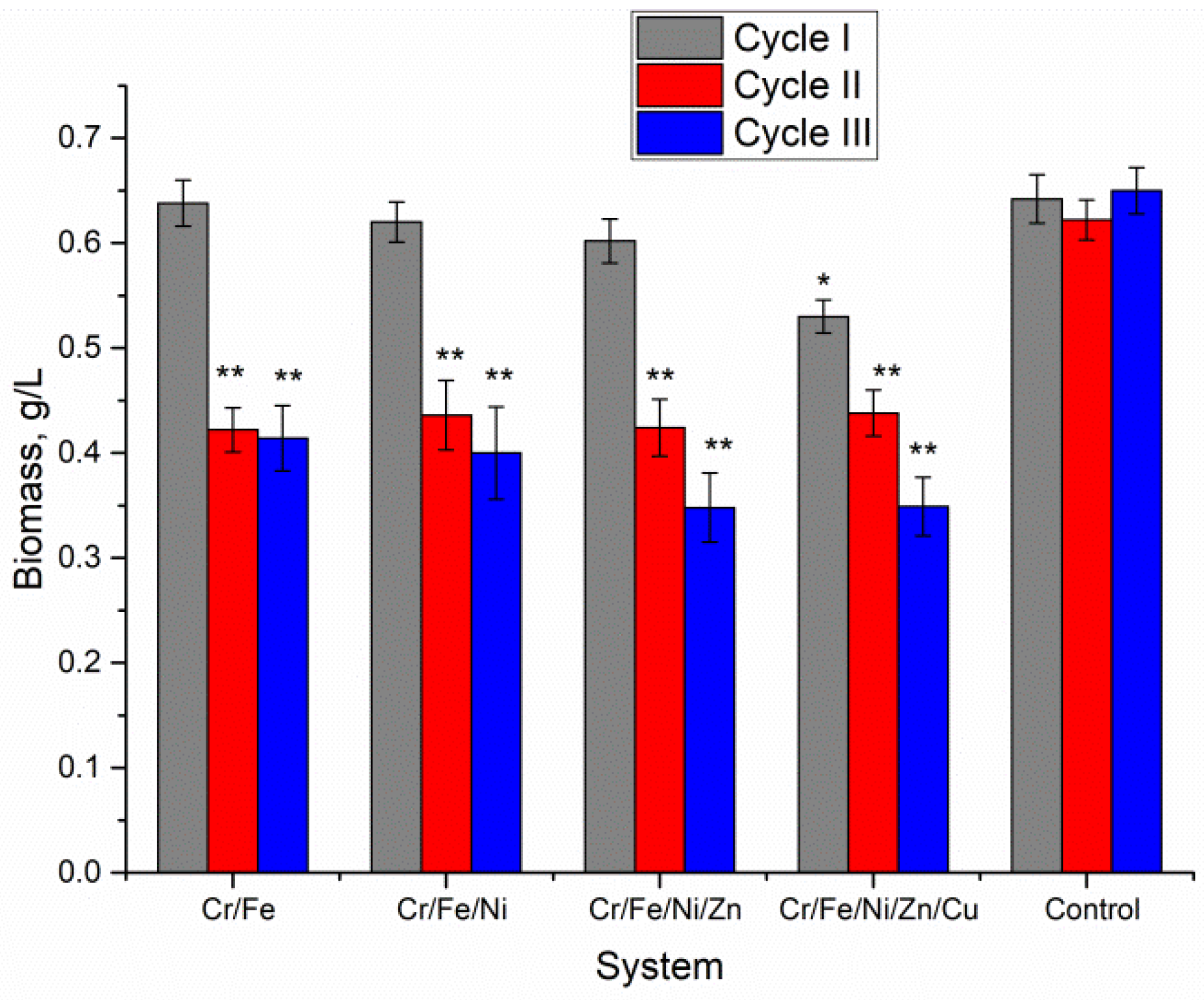
3.1. The Biomass of Nostoc in Three Cultivation Cycles
Biomass accumulation is one of the main parameters which allow assessment of the level of pollutants toxicity for microorganisms. For this reason, the amount of Nostoc biomass accumulated during each of the three vital cycles was monitored (
Figure 1). The first vital cycle in all studied polymetallic systems was characterized by a high level of biomass productivity. In the Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn systems, the amount of biomass did not differ significantly from the control. In the Cr/Fe/Ni/Zn/Cu system, the amount of Nostoc biomass accumulated at the end of the first cycle was 16.5% lower compared with the control sample (
p = 0.00138). In the second cycle of Nostoc cultivation, the amount of biomass was rather uniform but significantly lower than in the control (on average by 32–35%,
p < 0.001). The Nostoc culture was exposed to metals during the first cultivation cycle; in the second cycle it showed a more pronounced sensitivity, expressed in a decrease in productivity by about one third compared with the normal level, regardless of the composition of the multi-metallic system. The third cultivation cycle for Cr/Fe and Cr/Fe/Ni systems ended with the same biomass level as in the second one. In the other two systems, Cr/Fe/Ni/Zn and Cr/Fe/Ni/Zn/Cu, the amount of biomass continued to decrease. For these two systems, the biomass productivity at the end of the third cycle decreased compared with the second cycle by 18% and 20%, respectively. In these two systems, biomass decreased about two times at the end of the third cultivation cycle compared with the control.
3.2. Bioaccumulation of Chromium and Other Metals in the Biomass
The bioaccumulation of chromium in Nostoc biomass in all systems during all cycles was at a quite high level ranging from 1190 to 3300 μg/g dry biomass, taking into account that the content of chromium detected in the control biomass was 3.4 μg/g (
Figure 2). For all four systems, the amount of chromium taken up by Nostoc biomass in the second cultivation cycle was 11–26% higher than in the first cycle. In the third cycle, the systems differed in this respect. Thus, in three systems, Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn, chromium accumulation capacity was reduced in comparison with the second cycle and was comparable with that in the first cycle, while in the Cr/Fe/Ni/Zn/Cu system, chromium uptake did not differ from the second cycle. Among all analyzed systems, the greatest uptake of chromium was noticed in the Cr/Fe/Ni/Zn system, its accumulation was 29–178% higher in comparison with other analyzed variants (systems and cycles). The maximum level of chromium accumulation in Nostoc biomass was 3300 µg/g, and it was reached at the end of the second cultivation cycle in the Cr/Fe/Ni/Zn system. Chromium concentration in this variant was almost 1000 times higher than in the control biomass and indicates the ability of excessive accumulation of Cr(VI) by Nostoc biomass.
Iron was the second element present in all analyzed systems. The amount of iron accumulated in Nostoc biomass grown in multi-metallic systems was 3060–7100 μg/g, which was significantly higher compared with control, where its level reached the value of 820 μg/g (
Figure 2). Iron uptake increased from one cultivation cycle to another in all systems, and within each cycle this parameter was rather uniform. Thus, in the first cycle of cultivation, iron uptake in different systems ranged from 3060–3340 μg/g without any statistically significant differences between the variants of the system. The second cycle of Nostoc growth ended with an accumulation of iron by 49–89% higher than in the first cycle of cultivation (
p < 0.001). In the third cycle, iron uptake increased in three of the analyzed systems, Cr/Fe/Ni, Cr/Fe/Ni/Zn, and Cr/Fe/Ni/Zn/Cu; the accumulated amount was 20.3–40.5% higher than in the second cycle (
p < 0.005). No significant differences were found in the Cr/Fe system between iron uptake in the second and third cycles. The amount of iron taken up by Nostoc biomass grown on multi-metallic systems was 3.7–8.6 times higher than in control biomass, while the ability to uptake this metal was less pronounced compared with that of chromium.
Nickel was not detected in control Nostoc biomass, and in biomass grown on three multi-metallic systems containing this element it was 348–1110 μg/g (
Figure 2). As in the case of iron, nickel uptake increased over the cycles. Thus, compared with the first growth cycle, nickel uptake in the second cycle was 37–88% higher (
p < 0.001), and in the third cycle it was 13–30% higher than in the second cycle. As for chromium and iron, the Cr/Fe/Ni/Zn system turned out to be the most efficient in terms of Ni uptake, where the maximum level of Ni accumulation in Nostoc biomass reached 980 μg/g in the second cultivation cycle and 1110 μg/g in the third cycle, while the difference between them was statistically insignificant.
Zinc was present in two analyzed systems, and its amount taken up by Nostoc biomass was 312–850 µg/g (
Figure 2). Zinc uptake also increased in biomass over the cycles. Thus, in comparison with the first cycle for Cr/Fe/Ni/Zn and Cr/Fe/Ni/Zn/Cu systems, in the second cycle, zinc uptake increased by 28% and 55%, respectively, and in the third—by 27% and 75%, respectively, compared with the second cycle. Taking into account that the control biomass contained 69 ± 2.9 μg/g of zinc, Nostoc biomass after three cultivation cycles on multi-metallic zinc-containing systems accumulated 9–12 times more zinc in comparison with control.
The amount of copper in the control biomass was 32 ± 0.38 μg/g, and in biomass obtained on a multi-metallic system containing copper—439–740 μg/g, which was 13.7–23.1 times higher than in control (
Figure 2). In the first cycle of cultivation, copper uptake by Nostoc biomass was 439 μg/g, and in the second cycle metal uptake increased by 50.3% compared with the first cycle. There was no statistical difference between copper uptake in second and third cycles.
3.3. The Biochemical Composition of Nostoc Biomass
The biochemical composition of Nostoc biomass undergoes various changes depending on the analyzed system and the cultivation cycle. The amount of proteins decreased after the first cycle of cultivation in all four systems (
Figure 3). In addition, if in the Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn systems this decrease was moderate, up to 12% (
p < 0.005), then in the Cr/Fe/Ni/Zn/Cu system the reduction in the amount of proteins was by 29% compared with control. In the second cycle of cultivation, protein content in Nostoc biomass was at the level recorded in the first cycle, without significant differences. Instead, differences between the systems were observed in the third cultivation cycle, when two opposite situations were observed: in the Cr/Fe and Cr/Fe/Ni/Zn/Cu systems the protein content decreased by 27% and 44%, respectively, compared with control, while in the Cr/Fe/Ni and Cr/Fe/Ni/Zn systems this parameter attained the control level (in the Cr/Fe/Ni system), or increased by 18% (in the Cr/Fe/Ni/Zn system) in respect to the control (
p < 0.005). It should be noted that the most serious changes in protein content occurred in the Cr/Fe/Ni/Zn/Cu system, where the biomass in all three cultivation cycles had a consistently low protein level (by 29–44% compared with the control).
A decrease in the amount of carbohydrates in Nostoc biomass by 16% (
p < 0.005) was recorded only in the first cultivation cycle in the Cr/Fe/Ni/Zn system (
Figure 3). In other cases, the amount of carbohydrates was at the level of control or exceeded it by up to 55%. In the Cr/Fe system, during first and third cycles, the highest amount of carbohydrates was accumulated in Nostoc biomass, reaching 66% of the dry biomass. In the Cr/Fe/Ni/Zn/Cu system during cycles I and III, a significant amount of carbohydrates was synthesized, which exceeded the level of the control by 29.5% and 20.4%, respectively.
The amount of lipids practically did not change upon contact with media containing heavy metals, remaining at the level of 3.0–3.5% of dry biomass (
Figure 3). Malondialdehyde, one of the end products of oxidative lipid degradation, was found at an adequate level in Nostoc biomass in the range from 95.5 to 126.4 nmol/g biomass (
Figure 3). In all cultivation cycles, values significantly exceeding the content in control biomass were recorded in the Cr/Fe/Ni/Zn system. The level of malondialdehyde in the third cycle in the Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn systems was 15.8–29.7% higher than in the control sample.
Pigments are cellular components, which usually respond quickly to the action of stress factors. Nostoc biomass contains three groups of pigments: phycobiliproteins, chlorophyll, and carotenoid pigments. The change in their content in biomass in multi-metallic systems is shown in
Figure 4.
The most pronounced change in the content of phycobiliproteins occurred during the first two cycles of Nostoc cultivation in multi-metallic systems. At the end of these two cycles, in all studied systems, the content of total phycobiliproteins was 15.2–62.4% lower than in the control. In the third cultivation cycle, two different situations were observed. Thus, in multi-metallic systems, Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn, the amount of phycobiliproteins increased significantly, exceeding the characteristic control level by 23.7–46.3%. On the contrary, in the Cr/Fe/Ni/Zn/Cu system, it decreased both in comparison with the control as well as cycles I and II. Thus, at the end of cycle III, Nostoc biomass in the system with copper contained 53.1% less phycobiliproteins than in the control biomass. This system produced biomass with a low content of phycobiliproteins, although in cycle II there was a tendency to improve the consistency of this parameter. In contrast, in the third cycle of cultivation, the amount of phycobiliproteins again showed a significant decrease in comparison with both the control and the second cycle. The most interesting situation was observed in the Cr/Fe/Ni/Zn system, where at the end of first cycle the lowest content of phycobiliproteins in Nostoc biomass was recorded (with a reduction of over 60% compared with control). Then, in the second cycle, an increase in this parameter compared with cycle I was noticed, but it was not able to reach the control values and remained 17.3% lower compared with the control. In the third cycle, phycobiliprotein pigments almost doubled their content in biomass in comparison with the second cycle. This was the maximum value of the parameter within the experiment (46.3% more than in the control sample).
The amount of chlorophyll in Nostoc biomass during cycles I and II was at the control level in Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn/Cu systems. However, in the Cr/Fe/Ni/Zn system, it was 33.3% and 47.8%, respectively, higher than in the control (p < 0.005). At the end of the third cultivation cycle, the amount of chlorophyll in the biomass obtained in Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn systems was 1.7–2.0 times higher than in the control biomass. Only in one Cr/Fe/Ni/Zn/Cu system did the chlorophyll content in Nostoc biomass not change during three cultivation cycles compared with control.
For β-carotene, the same pattern was observed. In Cr/Fe and Cr/Fe/Ni systems, carotene content in the biomass in the first two cultivation cycles as well as in the Cr/Fe/Ni/Zn system in the first cycle was at the level of control. The biomass in cycle II in the system containing copper contained 21.8% more β-carotene in comparison with the control (p < 0.01). In the third cycle of Nostoc growth, an increase in the amount of β-carotene in the three studied systems Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn by 15.4%, 33.5%, and 58.5%, respectively, compared with control took place. In the Cr/Fe/Ni/Zn/Cu system, a decrease in the content of β-carotene by 14.5% and 26.3%, respectively, in cycles II and III (p < 0.005) was noticed.
3.4. The Antioxidant Activity of Nostoc Biomass
The antioxidant activity of Nostoc biomass obtained in various multi-metallic systems has also changed (
Figure 5). The antioxidant activity of aqueous extracts from Nostoc biomass grown in Cr/Fe and Cr/Fe/Ni systems was lower than in the control in all three cultivation cycles (
Figure 5). In Cr/Fe system, a significant decrease in antioxidant activity was noted. In cycles I and II, the capacity to reduce ABTS
•+ radical cation was lower than in the control by 50.5% and 53.9%, respectively, and in the cycle III—by 19.7% (
p < 0.005). The biomass obtained in the first cycle in the Cr/Fe/Ni system exhibited an antioxidant activity (water extract) 36.4% lower than the control. In the next two cycles, this parameter increased but did not reach the control level. In Cr/Fe/Ni/Zn system, the biomass after the first two cycles had the antioxidant activity of water-soluble components of about 33% lower compared with the control, while the antioxidant activity of biomass after the third cycle was equal to the control biomass. The Cr/Fe/Ni/Zn/Cu system in first and third cycles produced Nostoc biomass, whose activity of water-soluble components was 16.6% and 46.8%, respectively, lower than in the control, and in the second cycle the value of this parameter was at the level of the control.
The antioxidant activity of ethanol extracts from Nostoc biomass also changed depending on the system and the cultivation cycle (
Figure 5). Both decrease and increase in this parameter were observed. In the Cr/Fe system, a decrease in antioxidant activity was observed in the first two cycles (by about 30%,
p < 0.001). The highest increase in antioxidant activity by 22.9–48.2% in comparison with the control was obtained for Nostoc biomass in the third cultivation cycle, in three analyzed systems Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn. In the Cr/Fe/Ni/Zn/Cu system, the decrease in antioxidant activity of ethanol extracts with respect to control was noticed.
The content of phenolic compounds in Nostoc biomass, which largely exhibit antioxidant activity, also changed in the presence of heavy metals in the systems (
Figure 5). The amount of phenols in the biomass of the first cycle, regardless of the system, decreased by 18.5–24.4% compared with the control (
p < 0.001). In the second cycle, with the exception of the Cr/Fe/Ni/Zn system, where there was a slight decrease in the content of phenolic compounds (by 16.4%,
p < 0.01), the values of this parameter in other systems were almost on the level of control biomass. In cycle III, biomass grown in Cr/Fe and Cr/Fe/Ni systems contained an amount of phenols very close to that in the control, while in the Cr/Fe/Ni/Zn system this parameter was 23.6% higher than in the control (
p < 0.001), and in the Cr/Fe/Ni/Zn/Cu system it was 44.7% lower than in the control (
p < 0.001).
4. Discussion
The main objective of this study was to assess the ability of cyanobacterium Nostoc linkia to accumulate Cr(VI) along with other metal ions during three successive cultivation cycles.
Currently, the ability of various bacterial species to accumulate Cr(VI) is being intensively studied. It is known that bacteria,
Bacillus pumilus,
Bacillus circulans,
Bacillus megaterium,
Bacillus sphaericus,
Exiguobacterium aurantiacum, Pseudomonas synxantha, and
Pseudomonas brenneri, depending on the experimental conditions, accumulate from 23% to 90% of Cr(VI) present in the medium [
24,
25,
26,
27].
Cyanobacteria
Chroococcus sp.,
Limnococcus sp.,
Limnococcus ceylanica, and
Gleocapsa sp. also possess a very high bioaccumulation efficiency toward metal ions [
28,
29,
30]. Several species
Limnococcus sp.,
Nostoc muscorum, and
Synechococcus sp. have been appreciated for their ability of high and rapid bioaccumulation of heavy metals, others, such as
Synechococcus sp.—for the ability to accumulate a wide variety of metals.
Among cyanobacteria of the genus
Nostoc, the most studied in this regard is
Nostoc muscorum [
31,
32], while there are few research papers devoted to
Nostoc linckia. It is rather important to obtain new knowledge about the ability of cyanobacterium
N. linckia to accumulate metals, including Cr(VI).
In present research, the bioaccumulation of Cr(VI) from multi-metallic systems was studied—a situation that is closer to real conditions, since in most cases the contamination of certain areas occurs due to the presence of several pollutants. Among the four studied systems, in Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn/Cu, the rate of chromium uptake by Nostoc was very similar and varied with the cultivation cycle. Thus, in these three systems, chromium recovery in the first cycle was 35.8–40.2% of the initial level of metal; in the second cycle—27.2–32.7%, and in the third cycle—19.7–27.1%. In the Cr/Fe/Ni/Zn system, the bioaccumulation capacity of Nostoc biomass was significantly higher and amounted to 63.1%, 56.0%, and 34.8% of Cr(VI), which corresponds to three cultivation cycles.
It was shown that the uptake of chromium by Nostoc biomass can reach values that are 1000 times higher than the normal level of this element in biomass. Comparing the ability of spirulina and Nostoc to accumulate Cr(VI) under similar conditions it can be seen that spirulina accumulate 20 times less metal compared with Nostoc—from 62 to 182 µg/g biomass [
18].
As a minor element for cyanobacterium, chromium was most likely absorbed through nonspecific mechanisms, and the ability to excessively accumulate this metal is an obvious feature of this strain. The peculiarity observed in this experiment was that the metal content relative to biomass was higher in the second cultivation cycle, regardless of the system. However, the bioaccumulation capacity of biomass in the second cycle was lower due to a decrease in the amount of produced biomass. It can be assumed that Nostoc culture, which has not been previously exposed to the toxic effects of heavy metals (the first cultivation cycle), has a higher degree of resistance to the action of exogenous stress, while repeated stress (in the second and third cycles) leads to a significant diminution of resistance. As a result, cell proliferation was limited, which led to a decrease in productivity and biomass accumulation.
Iron, Ni, Zn, and Cu in the studied systems were taken up during the cultivation of Nostoc biomass over the cycles. For instance, iron uptake from the solution in the first cycle was 59–78% and it was accumulated almost completely in the next two cycles. Nickel uptake constituted 43.2–62.6% in the first cycle and 49.7–83.1% in the next two cycles, being in ascent from one cycle to the next in all systems containing this element.
Zinc was present in two systems: Cr/Fe/Ni/Zn and Cr/Fe/Ni/Zn/Cu. In the first system, the percentage of metal recovery by Nostoc biomass was at the same level in three cycles and amounted to 37.1–39.8% of the initial zinc content. In the Cr/Fe/Zn/Ni/Cu system, zinc uptake increased from 25.8% in the first cycle to 54.5% in the third. Copper uptake was 46.5–57.8%, and its maximum amount was accumulated in the second cycle of Nostoc cultivation.
Thus, Nostoc linkia can be considered as a good accumulator of chromium as well as other metals in multi-metallic systems. The resistance of cyanobacterium to these metals was also confirmed by the quality of biomass, which at the first cycle of cultivation in the presence of metals did not change or changed insignificantly. This allows us to appreciate the protection systems of Nostoc linkia as effective.
The repeated contact of cyanobacterium with pollutants induced several changes in Nostoc biomass, which most likely aimed at diminishing the negative effects of heavy metals, especially Cr(VI). In particular, in the third cycle, there was a significant increase in the antioxidant activity of the ethanol-soluble components, β-carotene, chlorophyll, and phycobiliproteins in Cr/Fe, Cr/Fe/Ni, and Cr/Fe/Ni/Zn systems. A significant presence of antioxidant components can annihilate the harmful action of reactive oxygen species, which can be generated as a result of the interaction of heavy metals with Nostoc cells. Pigments, in addition to having direct antioxidant activity, absorb the light energy needed for photosynthesis. As a result, in systems where Nostoc biomass had an increased level of pigments, the amount of lipids, carbohydrates, and proteins was at an adequate level. The increase in the amount of chlorophyll and its derivatives, carotene and phycobiliproteins, in the biomass of cyanobacteria and microalgae exposed to stress caused by the presence of chromium, as well as other metals, was observed by other researchers, and is in line with our results [
29,
33].
However, it was obvious that the described changes cannot completely remove the negative effects of heavy metals. Thus, a reduced amount of biomass in cycles II and III and an increase in the amount of malondialdehyde, a recognized marker of oxidative stress, denoted a moderate level of stress, but did not seriously affect the quality of Nostoc biomass.
Nostoc linckia is a filamentous nitrogen-fixing cyanobacterium which populates both water and soil. In soil, different species of Nostoc can be a part of the biological soil crust, or they can be free-living [
34,
35]. The live cyanobacterial cells are used as a simple, low-cost and efficient bio-fertilizer that can improve the physicochemical properties of the soil by enriching it with carbon, nitrogen, and available phosphorus [
36].
Based on the foregoing, there are two ways in which Nostoc biomass can be used for soil bioremediation: introduction of culture in soil by spraying and application of Nostoc culture immobilized on a neutral carrier. In the first case, it is necessary to ensure humidity for the development of the Nostoc colonies, which will ensure the remediation of the soil at the surface and at a depth of 1–5 cm. In conditions of low humidity, Nostoc forms easily removable crusts, which can be collected or, when they remain on the ground with an increase in humidity their vital activity resumes (which corresponds to cycle II and III of the experiment). In the second case, the technology of the use of immobilized microorganisms in soil conditions can be applied. It is obvious that for application in practice, additional research is required, and remediation technologies will be applicable for bioremediation of limited sites. Nevertheless, the high bioaccumulation capacity toward metal ions, as well as the specific growth with surface crust formation and the resistance to drought make this cyanobacterium an important candidate for the development of bioremediation technologies of soils contaminated with heavy metals, especially Cr(VI).
5. Conclusions
The culture of Nostoc linckia, which was not previously subjected to heavy metal stress, has demonstrated resistance to polymetallic systems and a high bioaccumulation capacity for Cr(VI) and other metal ions.
The ability to bioaccumulate Cr(VI) from the contaminated medium by cyanobacterium Nostoc linckia still remained high over three generations, while the uptake of Fe, Ni, Cu, and Zn in biomass increased from generation to generation.
The repeated action of metals led to a state of moderate stress, expressed in a decrease in the amount of biomass and the accumulation of malondialdehyde. At the same time, the quality of biomass remained unaltered.
Maintaining the quality of Nostoc biomass under stress conditions caused by the presence of metals is ensured by an increase in the content of compounds with antioxidant action.
Due to its high bioaccumulation capacity and a specific growth pattern with the formation of crusts on soil surface, edaphic cyanobacterium Nostoc linckia is an important candidate for bioremediation of soil contaminated with Cr in combination with other metals. In this regard, further research in real soil conditions is necessary.