Prioritizing Suitable Quality Assurance and Control Standards to Reduce Laboratory Airborne Microfibre Contamination in Sediment Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intralab
2.2. Review of Previous Studies
3. Results
3.1. Number, Composition and Intra-Lab Comparisons
3.2. Study Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, M.; Digka, N.; Anastasopoulou, A.; Tsangaris, C.; Mytilineou, C. Anthropogenic microfibres pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar. Pollut. Bull. 2016, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.; Lusher, A.; Thompson, R.C.; Morley, A. The deposition and accumulation of microplastics in marine sediments and bottom water from the Irish continental shelf. Scient. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, P.L.; Belontz, S.L.; Ryan, K.; Walzak, M.J. Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environ. Sci. Technol. 2020, 54, 818–825. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, H.; Zhou, Q.; Tian, Y.; Chen, T.; Tu, C.; Fu, C.; Luo, Y. Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ. Pollut. 2018, 242, 1557–1565. [Google Scholar] [CrossRef]
- Absher, T.M.; Ferreira, S.L.; Kern, Y.; Ferreira, A.L.; Christo, S.W.; Ando, R.A. Incidence and identification of microfibres in ocean waters in Admiralty Bay, Antarctica. Environ. Sci. Pollut. Res. 2019, 26, 292–298. [Google Scholar] [CrossRef]
- Lenaker, P.L.; Baldwin, A.K.; Corsi, S.R.; Mason, S.A.; Reneau, P.C.; Scott, J.W. Vertical distribution of microplastics in the water column and surficial sediment from the Milwaukee River Basin to Lake Michigan. Environ. Sci. Technol. 2019, 53, 12227–12237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibres in oceanic surface waters: A global characterization. Sci. Advan. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics—The Facts 2015. In An Analysis of European Latest Plastics Production, Demand and Waste Data; Plastic Europe—Association of Plastic Manufacturers: Brussels, Belgium, 2015. [Google Scholar]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.L.; Tonkin, A.; Galloway, T.; Thompson, R.C. Accumulations of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.J.; Venditti, R.A. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plan effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Wat. Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Wat. Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Tot. Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Brahney, J.; Mahowald, N.; Prank, M.; Cornwell, G.; Klimont, Z.; Matsui, H.; Prather, K.A. Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci. USA 2021, 118, e2020719118. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Bergmann, M.; Lutz, B.; Tekman, M.B.; Gutow, L. Citizen scientists reveal: Marine litter pollutes Arctic beaches and affects wild life. Mar. Pollut. Bull. 2017, 125, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Huntington, A.; Corcoran, P.L.; Jantunen, L.; Thaysen, C.; Bernstein, S.; Stern, G.A.; Rochman, C.M. A first assessment of microplastics and other anthropogenic particles in Hudson Bay and the surrounding eastern Canadian Arctic waters of Nunavut. Facets 2020, 5, 432–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M. Importance of atmospheric transport for microplastics deposited in remote areas. Environ. Pollut. 2019, 254, 1–4. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibres, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2016, 221, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Nuelle, M.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J.; Chapman, M.G.; Browne, M.A. Some problems and practicalities in design and interpretation of samples of microplastic waste. Anal. Meth. 2017, 9, 1332–1345. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotox. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Mudoch, A.; Azcue, J.M. Manual of Aquatic Sediment Sampling; Lewis Publishers: Boca Raton, FL, USA, 1995; 240p. [Google Scholar] [CrossRef]
- Gasperi, J.; Dris, R.; Mandin, C.; Tassin, B. First Overview of Microplastics in Indoor and Outdoor Air; HAL archives-ouvertes.fr. 2015. Available online: https://hal.archives-ouvertes.fr/hal-01195546/ (accessed on 3 June 2021).
- Corcoran, P.L.; Norris, T.; Ceccanese, T.; Walzak, M.J.; Helm, P.A.; Marvin, C.H. Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ. Pollut. 2015, 204, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.Y.; Corcoran, P.L.; Helm, P.A. Factors influencing microplastic abundances in nearshore, tributary and beach sediments along the Ontario shoreline of Lake Erie. J. Great Lakes Res. 2018, 44, 1002–1009. [Google Scholar] [CrossRef]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmans, A.A. Quality criteria for the analysis of microplastic in biota samples: A critical review. Environ. Sci. Technol. 2018, 52, 10230–10240. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Hazimah, N.; Nor, M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Wat. Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.M.; Renick, V.C.; Foley, M.M.; Steele, C.; Woo, M.; Lusher, A.; Carr, S.; Helm, P.; Box, C.; Cherniak, S.; et al. Sampling and quality assurance and quality control: A guide for scientists investigating the occurrence of microplastics across matrices. Appl. Spectrosc. 2020, 74, 1099–1125. [Google Scholar] [CrossRef] [PubMed]
- Wesch, C.; Elert, A.M.; Wörner, M.; Braun, U.; Klein, R.; Paulus, M. Assuring quality in microplastic monitoring: About the value of clean-air devices as essentials for verified data. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; da Costa, J.P.; Mouneyrac, C.; Duarte, A.C.; Rocha-Santos, T. Contamination issues as a challenge in quality control and quality assurance in microplastics analytics. J. Hazard. Mat. 2021, 403, 123660. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504. [Google Scholar] [CrossRef] [Green Version]
- Saarni, S.; Hartikainen, S.; Meronen, S.; Uurasjärvi, E.; Kalliokoski, M.; Koistinen, A. Sediment trapping—An attempt to monitor temporal variation of microplastic flux rates in aquatic systems. Environ. Pollut. 2021, 274, 116568. [Google Scholar] [CrossRef]
- Bagaev, A.; Mizyuk, A.; Khatmullina, L.; Isachenko, I.; Chubarenko, I. Anthropogenic fibres in the Baltic Sea water column: Field data, laboratory and numerical testing of their motion. Sci. Total Environ. 2017, 599–600, 560–571. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Liu, K.; Wu, T.; Wang, X.; Song, Z.; Zong, C.; Wei, N.; Li, D. Consistent Transport of Terrestrial Microplastics to the Ocean through Atmosphere. Environ. Sci. Technol. 2019, 53, 10612–10619. [Google Scholar] [CrossRef]
- Woodall, L.C.; Gwinnett, C.; Packer, M.; Thompson, R.C.; Robinson, L.F.; Paterson, G.L.J. Using a forensic science approach to minimize environmental contamination and to identify microfibres in marine sediments. Mar. Pollut. Bull. 2015, 95, 40–46. [Google Scholar] [CrossRef]
- Dehaut, A.; Hermabessiere, L.; Duflos, G. Current frontiers and recommendations for the study of microplastics in seafood. TrAC-Trends Anal. Chem. 2019, 116, 346–359. [Google Scholar] [CrossRef]
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef]
- Gomiero, A.; Øysæd, K.B.; Agustsson, T.; van Hoytema, N.; van Thiel, T.; Grati, F. First record of characterization, concentration and distribution of microplastics in coastal sediments of an urban fjord in south west Norway using a thermal degradation method. Chemosphere 2019, 227, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Hengstmann, E.; Fischer, E.K. Nile red staining in microplastic analysis—Proposal for a reliable and fast identification approach for large microplastics. Environ. Monit. Assess. 2019, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Simon-Sánchez, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef]
- Zobkov, M.; Esiukova, E. Microplastics in Baltic bottom sediments: Quantification procedures and first results. Mar. Pollut. Bull. 2017, 114, 724–732. [Google Scholar] [CrossRef]
- Wessel, C.C.; Lockridge, G.R.; Battiste, D.; Cebrian, J. Abundance and characteristics of microplastics in beach sediments: Insights into microplastic accumulation in northern Gulf of Mexico estuaries. Mar. Pollut. Bull. 2016, 109, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- Fastelli, P.; Blaskovic, A.; Bernardi, G.; Romeo, T.; Cizmek, H.; Andaloro, F.; Russo, G.F.; Guerranti, C.; Renzi, M. Plastic litter in sediments from a marine area likely to become protected (Aeolian Archipelago’s islands, Tyrrhenian sea). Mar. Pollut. Bull. 2016, 113, 526–529. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Nel, H.A.; Dalu, T.; Wasserman, R.J. Sinks and sources: Assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci. Total Environ. 2018, 612, 950–956. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A.; Carney, B.; Ariese, F.; Velzen, M.; et al. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Matsuguma, Y.; Takada, H.; Kumata, H.; Kanke, H.; Sakurai, S.; Suzuki, T.; Itoh, M.; Okazaki, Y.; Boonyatumanond, R.; Zakaria, M.P.; et al. Microplastics in sediment cores from Asia and Africa as indicators of temporal trends in plastic pollution. Arch. Environ. Contam. Toxicol. 2017, 73, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A. Microplastic pollution in St. Lawrence River sediments. Can. J. Fisher. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Jansen, C.R. Microplastics are taken up by mussels (Mytilus Edulis) and lugworms (Arenicola Marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Stolte, A.; Forster, S.; Gerdts, G.; Schubert, H. Microplastic concentrations in beach sediments along the German Baltic coast. Mar. Pollut. Bull. 2015, 99, 216–229. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P. Microplastic fibres in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Laglbauer, B.J.L.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, T.; Glassom, D.; Smit, A.J. Plastic pollution in five urban estuaries of KwaZulu-Natal, South Africa. Mar. Pollut. Bull. 2015, 101, 473–480. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Gago, J.; Otero, V.; Sobral, P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Crew, A.; Gregory-Eaves, I.; Ricciardi, A. Distribution, abundance, and diversity of microplastics in the upper St. Lawrence River. Environ. Pollut. 2020, 260, 113994. [Google Scholar] [CrossRef] [PubMed]
- Minor, E.C.; Lin, R.; Burrows, A.; Cooney, E.M.; Grosshuesch, S.; Lafrancois, B. An analysis of microlitter and microplastics from Lake Superior beach sand and surface-water. Sci. Total Environ. 2020, 744, 140824. [Google Scholar] [CrossRef]
- De Villiers, S. Quantification of microfibre levels in South Africa’s beach sediments, and evaluation of spatial and temporal variability from 2016 to 2017. Mar. Pollut. Bull. 2018, 135, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Käppler, A.; Biniasch, O.; Feldens, P.; Stollberg, N.; Lange, X.; Fischer, D.; Eichhorn, K.J.; Pollehne, F.; Oberbeckmann, S.; et al. Tracing microplastics in aquatic environments based on sediment analogies. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; Trigui El Menif, N. Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Athey, S.N.; Adams, J.K.; Erdle, L.M.; Jantunen, L.M.; Helm, P.A.; Finkelstein, S.A.; Diamond, M.L. The widespread environmental footprint of indigo denim microfibers from blue jeans. Environ. Sci. Technol. Lett. 2020, 7, 840–847. [Google Scholar] [CrossRef]
- Lenaker, P.L.; Corsi, S.R.; Mason, S.A. Spatial distribution of microplastics in surficial benthic sediment of Lake Michigan and Lake Erie. Environ. Sci. Technol. 2021, 55, 373–384. [Google Scholar] [CrossRef]
- Pastorino, P.; Pizzul, E.; Bertoli, M.; Anselmi, S.; Kušće, M.; Menconi, V.; Prearo, M.; Renzi, M. First insights into plastic and microplastic occurrence in biotic and abiotic compartments, and snow from a high-mountain lake (Carnic Alps). Chemosphere 2021, 265, 129121. [Google Scholar] [CrossRef]
- Abel, S.M.; Primpke, S.; Int-Veen, I.; Brandt, A.; Gerdts, G. Systematic identification of microplastics in abyssal and hadal sediments of the Kuril Kamchatka trench. Environ. Pollut. 2021, 269, 116095. [Google Scholar] [CrossRef] [PubMed]
- Maghsodian, Z.; Sanati, A.M.; Ramavandi, B.; Ghasemi, A.; Sorial, G.A. Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere 2021, 264, 128543. [Google Scholar] [CrossRef]
- Gerolin, C.R.; Pupim, F.N.; Sawakuchi, A.O.; Grohmann, C.H.; Labuto, G.; Semensatto, D. Microplastics in sediments from Amazon rivers, Brazil. Sci. Total Environ. 2020, 749, 141604. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Martellini, T.; Pogojeva, M.; Slobodnik, J. Microplastics in the Black Sea sediments. Sci. Total Environ. 2021, 760, 143898. [Google Scholar] [CrossRef]
- Díaz-Jaramillo, M.; Islas, M.S.; Gonzalez, M. Spatial distribution patterns and identification of microplastics on intertidal sediments from urban and semi-natural SW Atlantic estuaries. Environ. Pollut. 2021, 273, 116398. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- De Wael, K.; Lepot, L.; Lunstroot, K.; Gason, F. Evaluation of the shedding potential of textile materials. Sci. Justice 2010, 50, 192–194. [Google Scholar] [CrossRef] [PubMed]

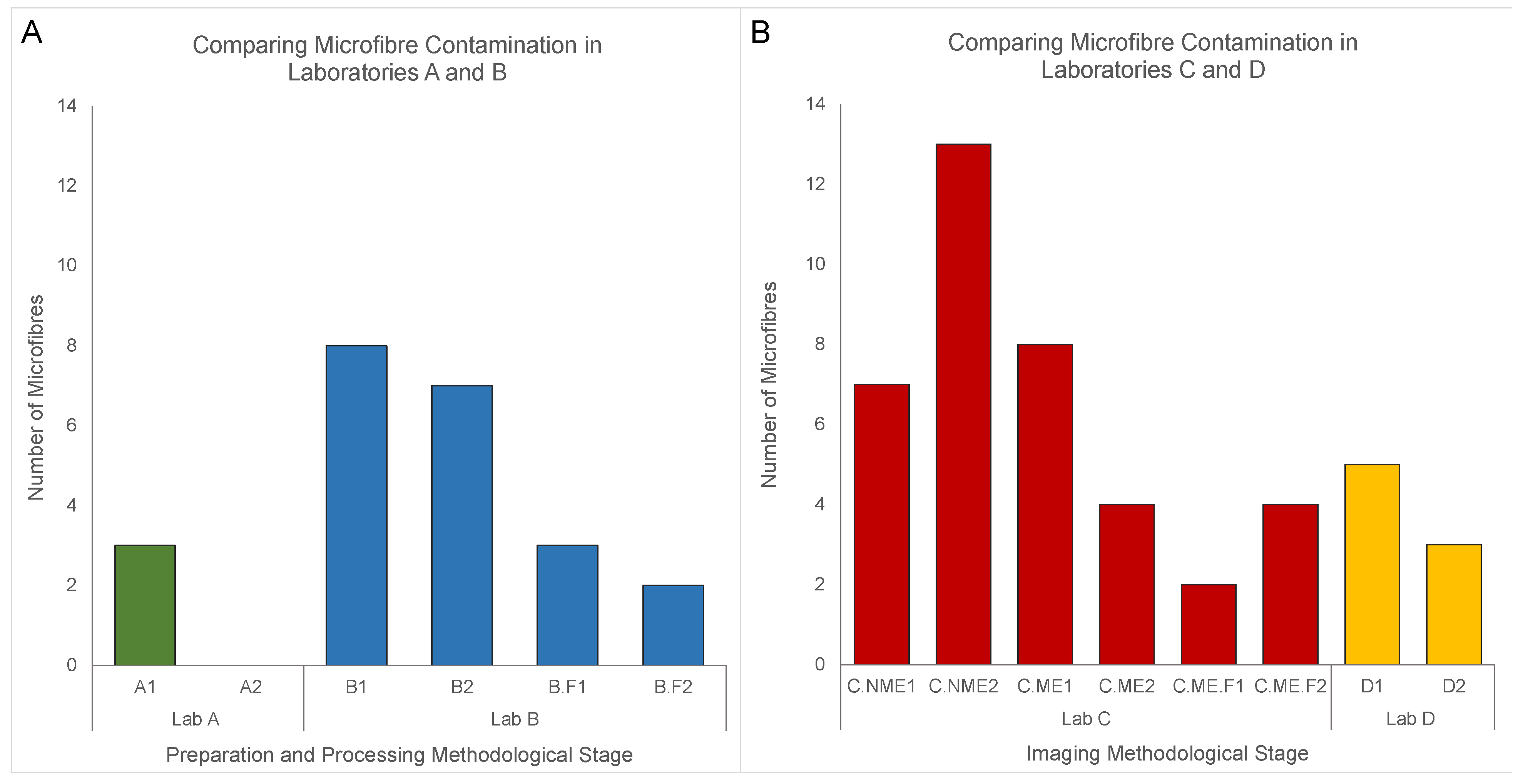
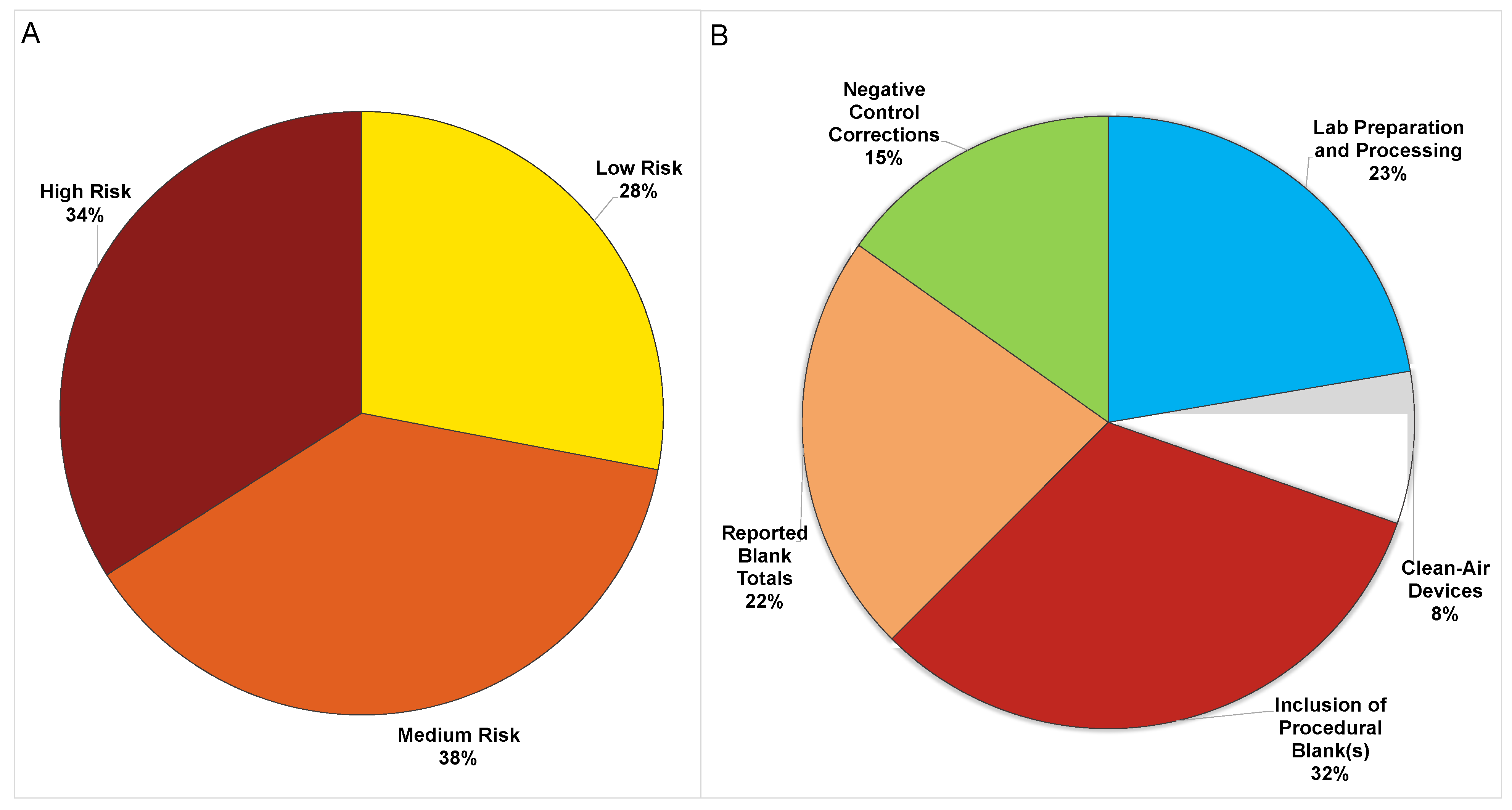
| Laboratory Space | Methodological Stage and Purpose | Exposure Time | Precautionary Measures | Blank | Blue | Black | Pink | Total |
|---|---|---|---|---|---|---|---|---|
| Lab A | Preparation and Processing Sediment-microplastic density separation using a magnetic stirrer, glass separatory funnels, polycarbonate sieve for rinsing, drying oven | 1 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Glassware covered with aluminum foil | A1 A2 | 2 0 | 1 0 | 0 0 | 3 0 |
| Lab B | Preparation and Processing Sediment-microplastic density separation using a magnetic stirrer, glass separatory funnels, polycarbonate sieve for rinsing, drying oven | 1 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Glassware covered with aluminum foil | B1 | 4 | 2 | 2 | 8 |
| 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Glassware covered with aluminum foil | B2 | 4 | 2 | 1 | 7 | |||
| 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Glassware covered with aluminum foil 4. HEPA air purifier 5. Glass-fibre filter | B.F1 1 | 1 | 1 | 1 | 3 | |||
| 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Glassware covered with aluminum foil 4. HEPA air purifier 5. Glass-fibre filter | B.F2 1 | 1 | 0 | 1 | 2 | |||
| Lab C | Imaging Visual examination, characterization and collection of anthropogenic particles using a Nikon SMZ1500 microscope | 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use | C.NME1 2 | 3 | 3 | 1 | 7 |
| 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use | C.NME2 2 | 7 | 5 | 1 | 13 | ||
| 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Microscope enclosure | C.ME1 3 | 4 | 2 | 2 | 8 | ||
| 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Microscope enclosure | C.ME2 3 | 2 | 1 | 1 | 4 | ||
| 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Microscope enclosure 4. HEPA air purifier 5. Glass-fibre filter | C.ME.F1 4 | 2 | 0 | 0 | 2 | ||
| 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use 3. Microscope enclosure 4. HEPA air purifier 5. Glass-fibre filter | C.ME.F2 4 | 3 | 1 | 0 | 4 | ||
| Lab D | Imaging Visual examination, characterization and collection of anthropogenic particles using a Nikon SMZ1500 microscope | 2 h | 1. Cotton laboratory coats worn during preparation and processing 2. Equipment rinsed and wiped down before and after use | D1 D2 | 4 2 | 1 1 | 0 0 | 5 3 |
| Study # in Ref. List | Type | Lab Prep. and Process | Clean-Air Devices | Inclusion of Blanks | Reported Blank Totals | Negative Control Corrections | Total (Max = 5) | Risk Scale |
|---|---|---|---|---|---|---|---|---|
| [47] | Sed. | 0 | 0 | 1 | 0 | 1 | 2 | Medium |
| [48] | Sed. | 0 | 0 | 1 | 0 | 0 | 1 | High |
| [49] | Sed., Wat. | 1 | 1 | 1 | 1 | 0 | 4 | Low |
| [50] | Sed. | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [51] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [52] | Sed. | 0 | 0 | 1 | 1 | 0 | 2 | Medium |
| [5] | Sed., Wat. | 1 | 0 | 1 | 0 | 0 | 2 | Medium |
| [53] | Sed. | 0 | 0 | 1 | 1 | 1 | 3 | Medium |
| [54] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [55] | Sed., Biota | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [56] | Sed., Wat.,Biota | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [57] | Sed. | 1 | 0 | 1 | 1 | 0 | 3 | Medium |
| [33] | Sed. | 1 | 0 | 1 | 1 | 0 | 3 | Medium |
| [58] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [4] | Sed. | 1 | 0 | 1 | 1 | 0 | 3 | Medium |
| [59] | Sed., Wat. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [60] | Sed. | 1 | 1 | 1 | 0 | 0 | 3 | Medium |
| [27] | Sed. | 1 | 0 | 1 | 1 | 0 | 3 | Medium |
| [61] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [62] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [63] | Sed. | 0 | 0 | 1 | 1 | 1 | 3 | Medium |
| [64] | Sed., Wat.,Biota | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [65] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [66] | Sed. | 0 | 0 | 1 | 1 | 0 | 2 | Medium |
| [67] | Sed., Biota | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [68] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [69] | Sed., Wat. | 1 | 0 | 1 | 0 | 0 | 2 | Medium |
| [70] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [71] | Sed. | 1 | 0 | 1 | 0 | 0 | 2 | Medium |
| [2] | Sed., Wat.,Biota | 1 | 0 | 1 | 1 | 0 | 3 | Medium |
| [12] | Sed., Sewage | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [6] | Sed. | 1 | 0 | 1 | 0 | 1 | 3 | Medium |
| [32] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [72] | Sed., Wat. | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| [73] | Sed., Wat. | 0 | 0 | 1 | 1 | 0 | 2 | Medium |
| [22] | Sed. | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| [74] | Sed. | 0 | 0 | 1 | 1 | 0 | 2 | Medium |
| [75] | Sed. | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [76] | Sed. | 0 | 0 | 1 | 0 | 0 | 1 | High |
| [23] | Sed., Wat., Biota, Snow | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| [77] | Sed., Biota, WWTP Effl. | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| [9] | Sed., Wat. | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [78] | Sed. | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [79] | Sed., Wat., Biota, Snow | 0 | 1 | 1 | 1 | 0 | 3 | Medium |
| [80] | Sed. | 1 | 1 | 1 | 0 | 1 | 4 | Low |
| [81] | Sed., Biota | 0 | 0 | 1 | 0 | 0 | 1 | High |
| [82] | Sed. | 0 | 0 | 0 | 0 | 0 | 0 | High |
| [83] | Sed. | 1 | 1 | 1 | 0 | 0 | 3 | Medium |
| [84] | Sed. | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| [39] | Sed. | 1 | 0 | 1 | 1 | 1 | 4 | Low |
| Average | 0.50 | 0.18 | 0.72 | 0.50 | 0.34 | 2.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belontz, S.L.; Corcoran, P.L. Prioritizing Suitable Quality Assurance and Control Standards to Reduce Laboratory Airborne Microfibre Contamination in Sediment Samples. Environments 2021, 8, 89. https://doi.org/10.3390/environments8090089
Belontz SL, Corcoran PL. Prioritizing Suitable Quality Assurance and Control Standards to Reduce Laboratory Airborne Microfibre Contamination in Sediment Samples. Environments. 2021; 8(9):89. https://doi.org/10.3390/environments8090089
Chicago/Turabian StyleBelontz, Sara L., and Patricia L. Corcoran. 2021. "Prioritizing Suitable Quality Assurance and Control Standards to Reduce Laboratory Airborne Microfibre Contamination in Sediment Samples" Environments 8, no. 9: 89. https://doi.org/10.3390/environments8090089
APA StyleBelontz, S. L., & Corcoran, P. L. (2021). Prioritizing Suitable Quality Assurance and Control Standards to Reduce Laboratory Airborne Microfibre Contamination in Sediment Samples. Environments, 8(9), 89. https://doi.org/10.3390/environments8090089





