Solvent-Based Elimination of Organic Matter from Marine-Collected Plastics
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
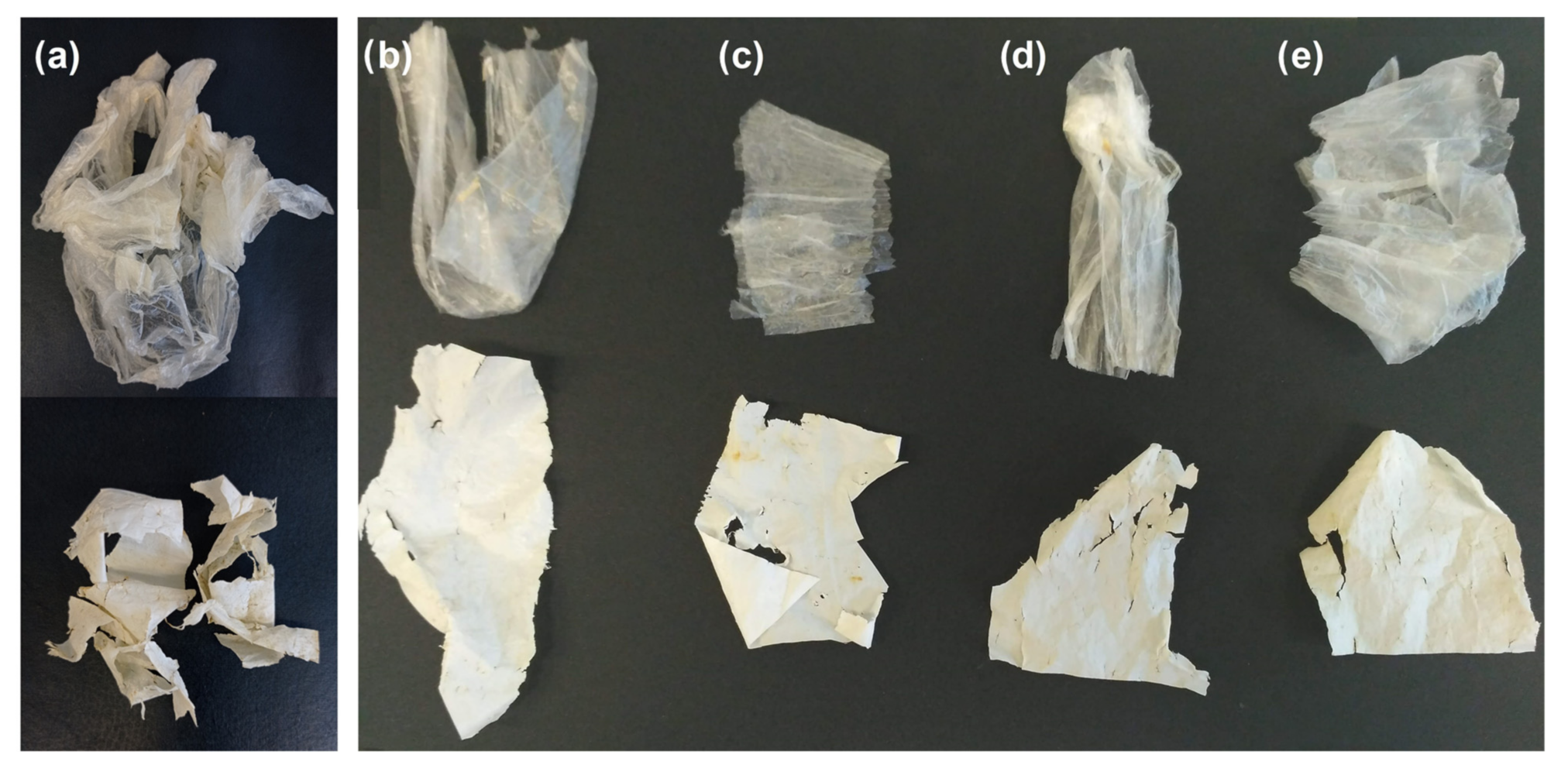
3.1. Visual Observation

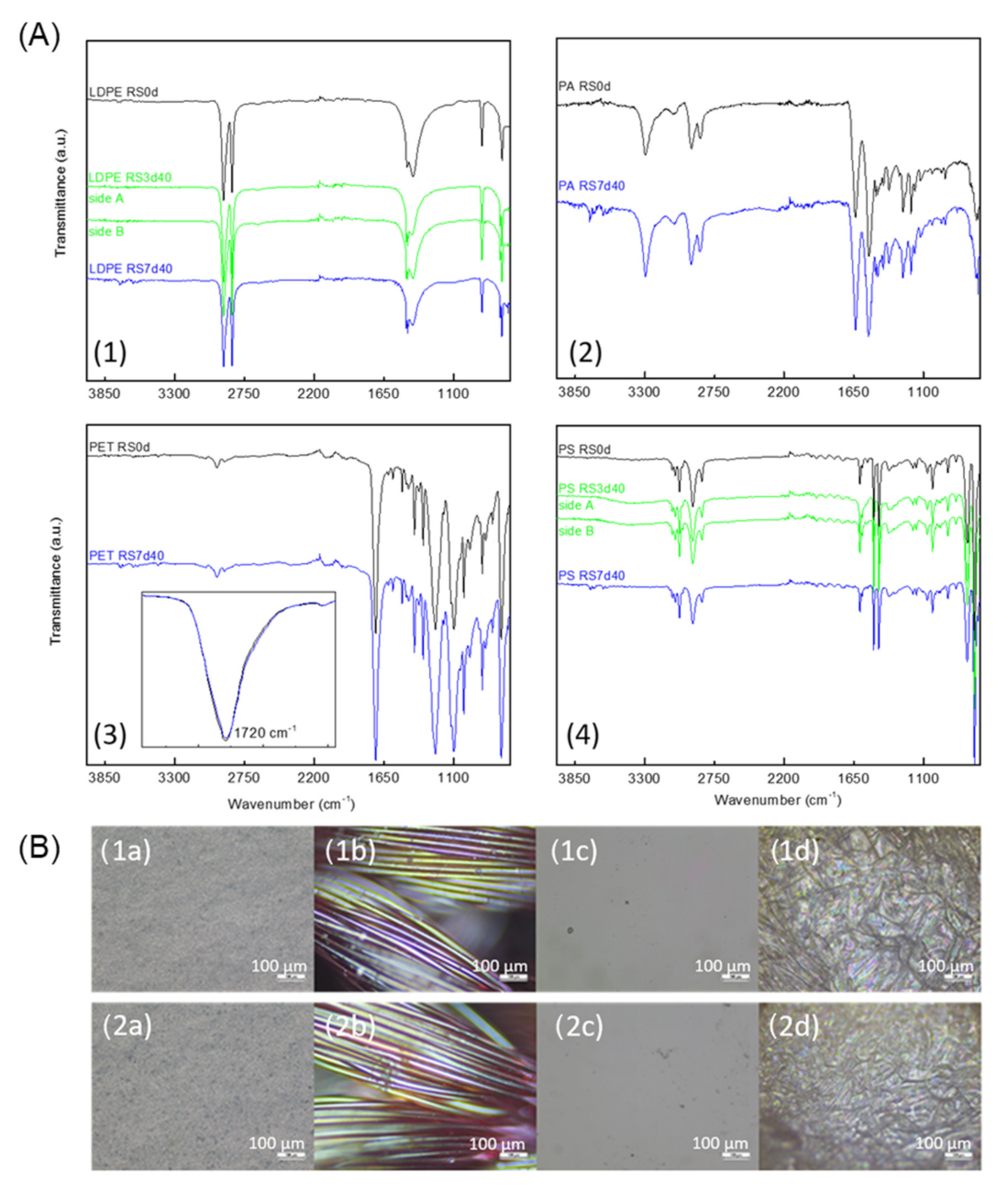
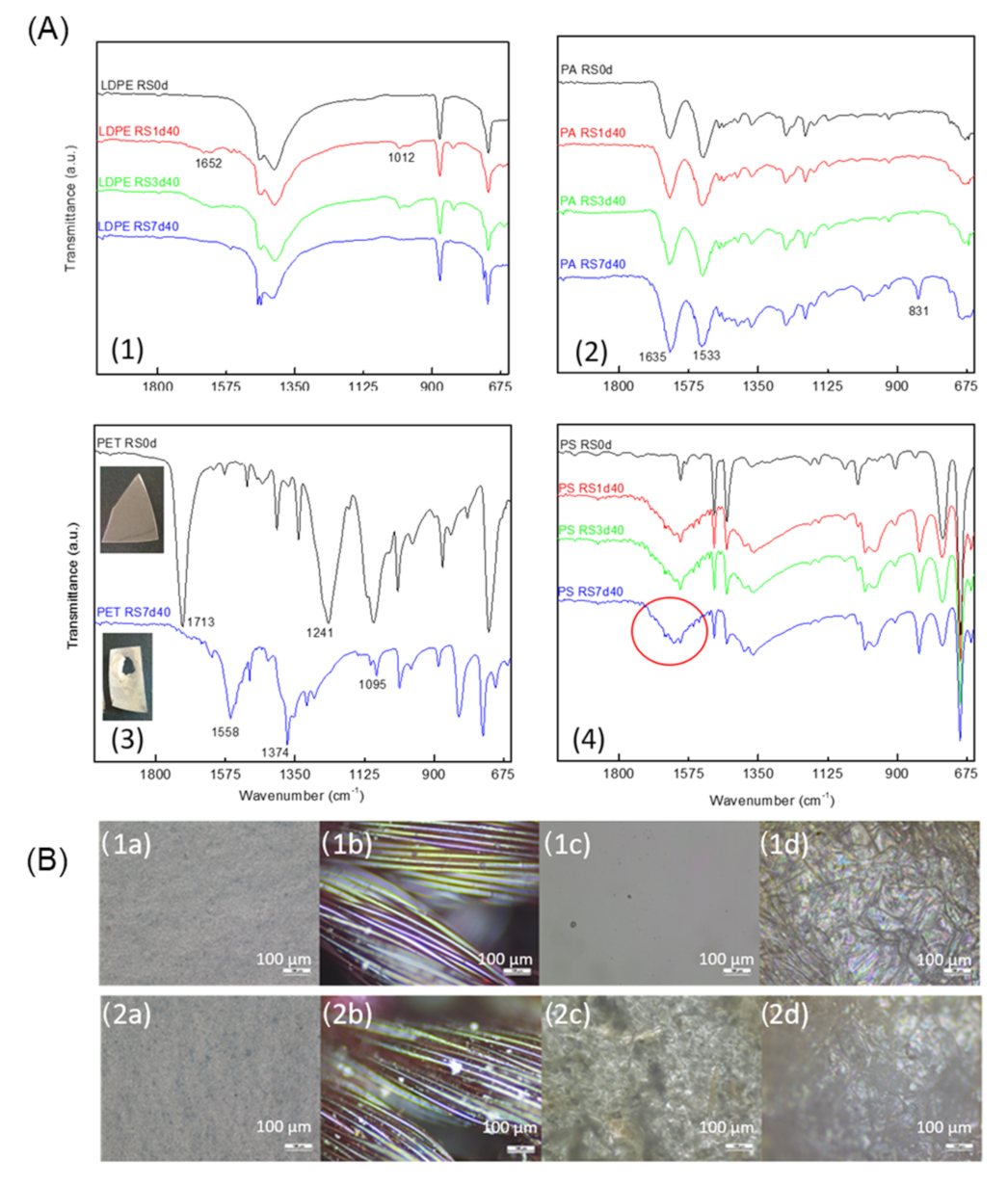
3.2. FTIR and Optical Microscopy Analysis of RS Samples
3.2.1. Samples Tested with H2O2 at 15% (v/v)
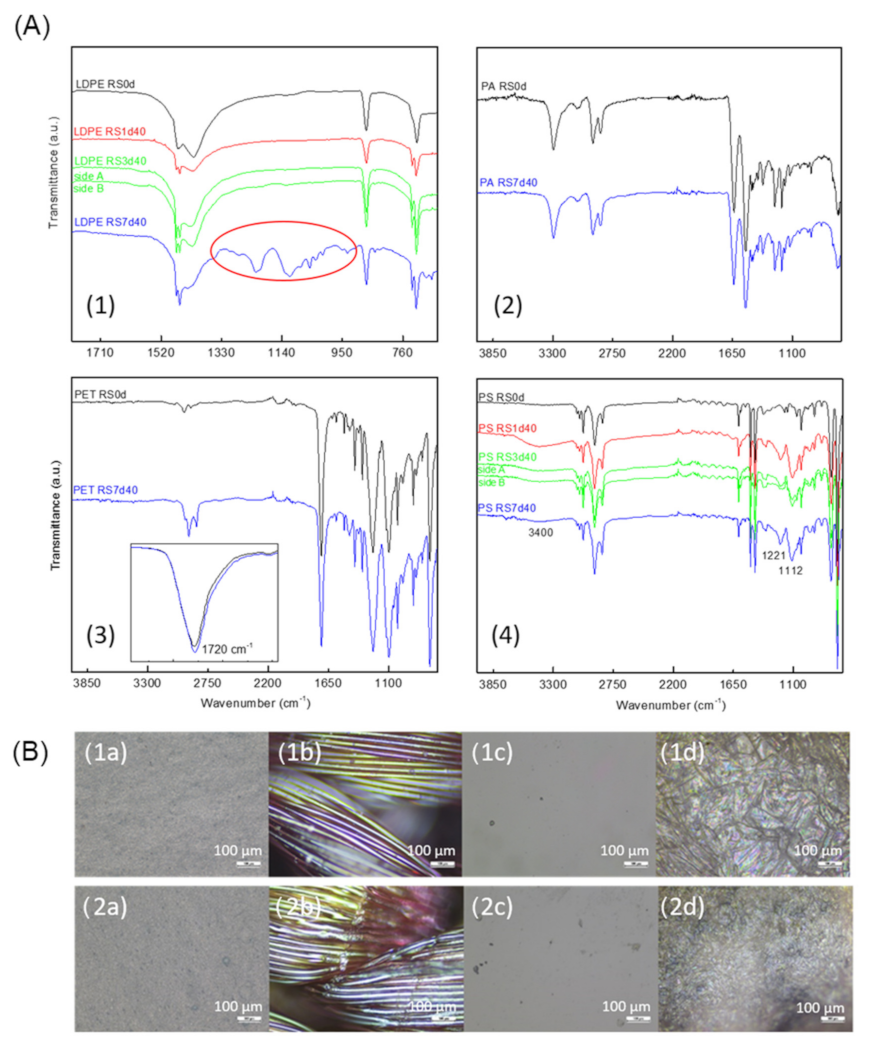
3.2.2. Samples Tested with Enzymatic Detergent at 100% (v/v)
3.2.3. Samples Tested with KOH at 20% and 10% (v/v)
3.3. Discussion of Main Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menchaca, I.; Zorita, I.; Rodríguez-Ezpeleta, N.; Erauskin, C.; Erauskin, E.; Liria, P.; Mendibil, I.; Santesteban, M.; Urtizberea, I. Guide for the evaluation of biofouling formation in the marine environment. Revista de Investigación Marina AZTI-Tecnalia 2014, 21, 89–99. [Google Scholar]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Environmental Deterioration of Biodegradable, Oxo-biodegradable, Compostable, and Conventional Plastic Carrier Bags in the Sea, Soil, and Open-Air Over a 3-Year Period. Environ. Sci. Technol. 2019, 53, 4775–4783. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Gerdts, G.M. Methodology used for the detection and identification of microplastics—A critical appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 201–227. [Google Scholar] [CrossRef] [Green Version]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago, J.; Ana, F.; Pedrotti, M.L.; Caetano, M.; Frias, J. Standardised Protocol for Monitoring Microplastics in Seawater; JPI-Oceans BASEMAN Project: Bremerhaven, Germany, 2018. [Google Scholar]
- Reguera, P.; Viñas, L.; Gago, J. Microplastics in wild mussels (Mytilus sp.) from the north coast of Spain: Comparison between digestive treatments. Sci. Mar. 2019, 83, 4. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [Green Version]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [Green Version]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding microplastics in soils: A review of analytical methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, S.; Piehl, S.; Gilfedder, B.S.; Löder, M.G.J.; Krutzke, J.; Wilhelm, L.; Laforsch, C. Occurence of Microplastics in the Hyporheic Zone of Rivers. Sci. Rep. 2019, 9, 15256. [Google Scholar] [CrossRef] [Green Version]
- O’Brine, T.; Thompson, R.C. Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull. 2010, 60, 2279–2283. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Degradation of common polymer ropes in a sublittoral marine environment. Mar. Pollut. Bull. 2017, 118, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Kedzierski, M.; D’Almeida, M.; Magueresse, A.; Le Grand, A.; Duval, H.; César, G.; Sire, O.; Bruzaud, S.; Le Tilly, V. Threat of plastic ageing in marine environment. Adsorption/desorption of micropollutants. Mar. Pollut. Bull. 2018, 127, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Mendoza, A.; Osa, J.L.; Basurko, O.C.; Rubio, A.; Santos, M.; Gago, J.; Galgani, F.; Peña-Rodríguez, C. Microplastics in the Bay of Biscay: An overview. Mar. Pollut. Bull. 2020, 153, 110996. [Google Scholar] [CrossRef]
- Esmaeili, A.; Pourbabaee, A.A.; Alikhani, H.A.; Shabani, F.; Esmaeili, E. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. PLoS ONE 2013, 8, e71720. [Google Scholar] [CrossRef] [Green Version]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chércoles Asensio, R.; San Andrés Moya, M.; de la Roja, J.M.; Gómez, M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Ghosh, E.; Mukherjee, P.; Gajendiran, A. Microbial Degradation of Low Density Polyethylene. Environ. Prog. Sustain. Energy 2016, 36, 147–154. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2017; Volume 78. [Google Scholar] [CrossRef]
- Sánchez Mora, J.J. Comportamiento Térmico y Mecánico Del Poli (Etilén Tereftalato) (PET) Modificado Con Resinas Poliméricas Basadas en Bisfenol-A. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2003. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
| Aquarium Samples(AQ) | Raw Marine Plastic Samples (MS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test Parameters | Testing period (day) | 1 | 3 | 7 | 97 | 1 | 3 | 7 | 97 |
| Test temperature (° C) | 40 | 40 | 40 | RT | 40 | 40 | 40 | RT | |
| Solvents | H2O2 (15% v/v) | 3 | 3 | 4 | 4 | 3 | 3 | 3 | 3 |
| Ethanol (96% v/v) | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | |
| Enzymatic detergent (100% v/v) | 1 | 2 | 3 | 3 | 2 | 2 | 2–3 | 2–3 | |
| KOH (20% v/v) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| KOH (10% v/v) | 2 | 2 | 2 | 2 | - | - | - | - | |
| Solvent | H2O2 (15% v/v) | Ethanol (96% v/v) | Enzymatic Detergent (100% v/v) | KOH (20% v/v) | KOH (10% v/v) |
|---|---|---|---|---|---|
| Period Material | RS0d/RS1d40/RS3d40/RS7d40 | RS0d/RS1d40/RS3d40/RS7d40 | RS0d/RS1d40/RS3d40/RS7d40 | RS0d/RS1d40/RS3d40/RS7d40 | RS0d/RS1dRT/RS3dRT/RS7dRT |
| LDPE |  |  |  |  |  |
| PA |  |  |  |  |  |
| PET |  |  |  |  |  |
| PS |  | 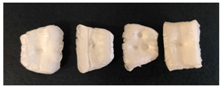 |  | 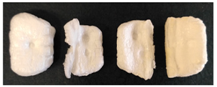 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, A.; Kortaberria, G.; Marzo, F.F.; Mayor, U.; Basurko, O.C.; Peña-Rodriguez, C. Solvent-Based Elimination of Organic Matter from Marine-Collected Plastics. Environments 2021, 8, 68. https://doi.org/10.3390/environments8070068
Mendoza A, Kortaberria G, Marzo FF, Mayor U, Basurko OC, Peña-Rodriguez C. Solvent-Based Elimination of Organic Matter from Marine-Collected Plastics. Environments. 2021; 8(7):68. https://doi.org/10.3390/environments8070068
Chicago/Turabian StyleMendoza, Amaia, Galder Kortaberria, Florencio F. Marzo, Ugo Mayor, Oihane C. Basurko, and Cristina Peña-Rodriguez. 2021. "Solvent-Based Elimination of Organic Matter from Marine-Collected Plastics" Environments 8, no. 7: 68. https://doi.org/10.3390/environments8070068
APA StyleMendoza, A., Kortaberria, G., Marzo, F. F., Mayor, U., Basurko, O. C., & Peña-Rodriguez, C. (2021). Solvent-Based Elimination of Organic Matter from Marine-Collected Plastics. Environments, 8(7), 68. https://doi.org/10.3390/environments8070068







