Screening of Pioneer Metallophyte Plant Species with Phytoremediation Potential at a Severely Contaminated Hg and As Mining Site
Abstract
:1. Introduction
- To identify and describe species growing in a paradigmatic mining area affected by As, Hg, and Pb contamination.
- To determine PTEs contents (in soils, roots, and aerial parts) and behavior of most representative plant species.
- To assess the selection of the most suitable combination of plant species to design phytoremediation strategies.
2. Material and Methods
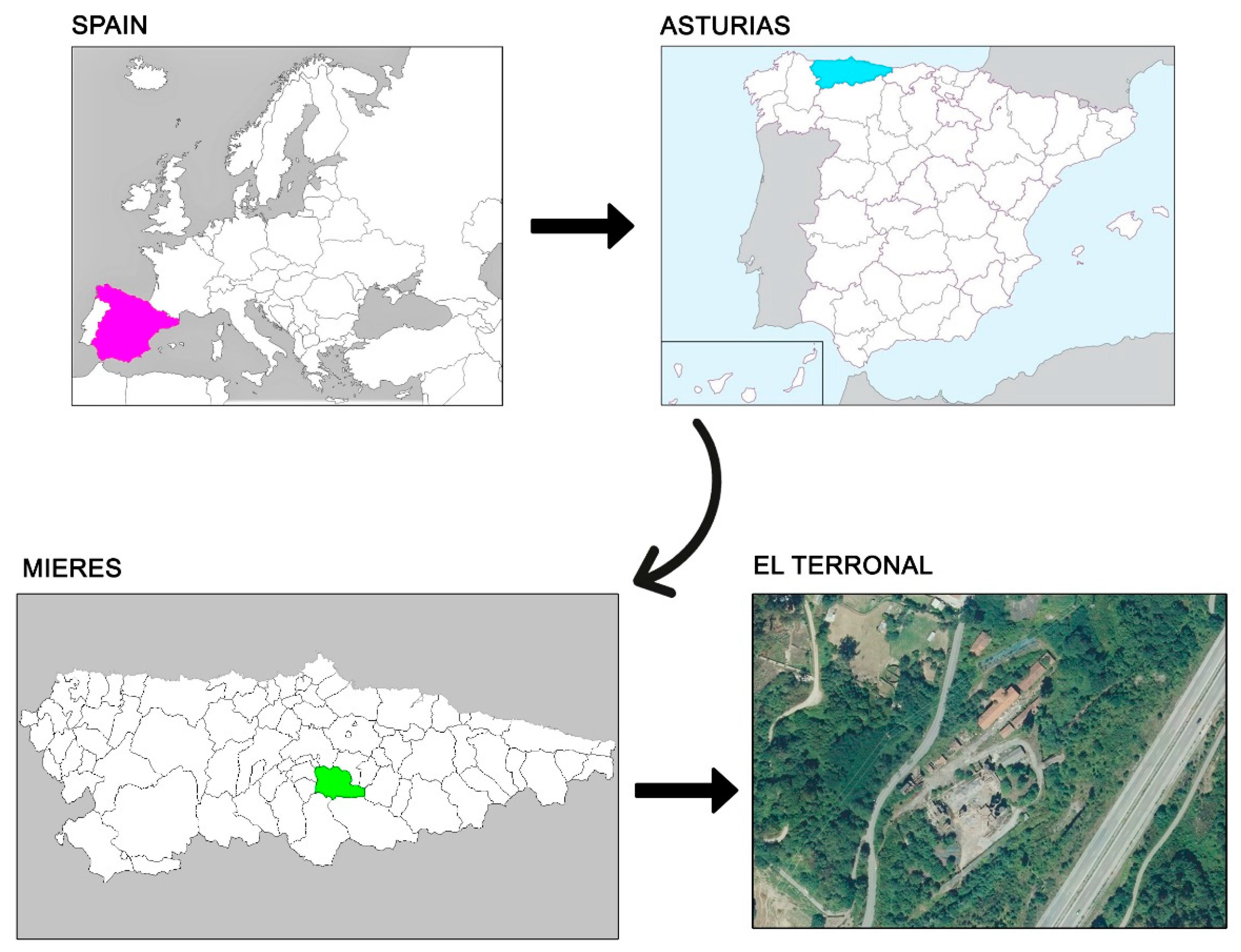
2.1. Site Description
2.2. Plant Classification
2.3. Soil and Plant Sampling
2.4. Soil Analyses
2.5. Plant Analyses
2.6. Data Analysis
Accumulation Factors
3. Results and Discussion
3.1. Description of the Identified Plant Species
3.2. Physicochemical Characterization of Soil Samples
3.3. PTEs in Soil–Plant System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CoI | Coating Index |
| PTEs | Potentially Toxic Elements |
| BCF | Bioaccumulation Factor |
| TF | Translocation Factor |
| MR | Mobility Ratio |
References
- Héctor, C.M.; Rainer, S. The Cartagena—La Union mining district (SE Spain): A review of environmental problems and emerging phytoremediation solutions after fifteen years research. J. Environ. Monit. 2010, 12, 1225–1233. [Google Scholar] [CrossRef]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the cantabrian range, north of spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Millán, R.; Gamarra, R.; Schmid, T.; Sierra, M.J.; Quejido, A.J.; Sánchez, D.M.; Cardona, A.I.; Fernández, M.; Vera, R. Mercury content in vegetation and soils of the Almadén mining area (Spain). Sci. Total Environ. 2006, 368, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.R.; Esquinas, N.; Rodríguez-Valdés, E.; Menéndez-Aguado, J.M.; Sierra, C. Comprehensive waste characterization and organic contamination co-occurrence in a Hg and As mining and metallurgy brownfield. J. Hazard. Mater. 2015, 300, 561–571. [Google Scholar] [CrossRef]
- Mouron, S.A.; Golijow, C.D.; Dulout, F.N. DNA damage by cadmium and arsenic salts assessed by the single cell gel electrophoresis assay. Mutat. Res. 2001, 498, 47–55. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Dam, H. Characterization of metal aerosols in PM10 from urban, industrial, and Asian dust sources. Environ. Monit. Assess. 2010, 160, 289–300. [Google Scholar] [CrossRef]
- Pavlik, M.; Pavlikova, D.; Zemanova, V.; Hnilicka, F.; Urbanova, V.; Szakova, J. Trace elements present in airborne particulate matter—Stressors of plant metabolism. Ecotoxicol. Environ. Saf. 2012, 79, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, J.; Egodawatta, P.; Ayoko, G.A.; Goonetilleke, A. Atmospheric deposition as a source of heavy metals in urban storm water. Atmos. Environ. 2013, 68, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.C.C.; Lo, K.F.A. Effects of Land Use Change on Sediment and Water Yields in Yang Ming Shan National Park, Taiwan. Environments 2015, 2, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, H.; Kay, P.; Slack, R.; Gong, Y.Y. Arsenic species in wheat, raw and cooked rice: Exposure and associated health implications. Sci. Total Environ. 2018, 634, 366–373. [Google Scholar] [CrossRef]
- Gress, J.; de Oliveira, L.M.; da Silva, E.B.; Lessl, J.M.; Wilson, P.C.; Townsend, T.; Ma, L.Q. Cleaning-induced arsenic mobilization and chromium oxidation from CCA-wood deck: Potential risk to children. Environ. Int. 2015, 82, 35–40. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Arsenic: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Richardson, J.B.; Görres, J.H.; Sizmur, T. Synthesis of earthworm trace metal uptake and bioaccumulation data: Role of soil concentration, earthworm ecophysiology and experimental design. Environ. Pollut. 2020, 262, 114–126. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Bloom, N.S.; Que Hee, S.S. The environmental geochemistry and bioaccessibility of mercury in soils and sediments: A review. Risk Anal. 1997, 17, 557–569. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Blaylock, M.; Kumar, P.B.; Dushenkov, V.; Enslev, B.D.; Chet, I.; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 1995, 13, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Asante-Badu, B.; Kgorutla, L.E.; Li, S.S.; Danso, P.O.; Xue, Z.; Qiang, G. Phytoremediation of organic and inorganic compounds in a natural and an agricultural environment: A review. Appl. Ecol. Environ. Res. 2020, 18, 6875–6904. [Google Scholar] [CrossRef]
- Del Río, M.; Font, R.; Almela, C.; Vélez, D.; Montoro, R.; De Haro Bailón, A. Heavy metals and arsenic uptake by wild vegetation in the guadiamar river area after the toxic spill of the aznalcóllar mine. J. Biotechnol. 2002, 98, 125–137. [Google Scholar] [CrossRef]
- Franchi, E.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci. Total. Environ. 2019, 655, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, K.S.; Kim, J.T.; Kang, D.; Sung, K. Effects of humic acid on phytodegradation of petroleum hydrocarbons in soil simultaneously contaminated with heavy metals. J. Environ. Sci. 2011, 23, 2034–2041. [Google Scholar] [CrossRef]
- Van Der Ent, A.; Baker, A.J.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Van Der Ent, A. A Global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal contaminated soils. In Phytoremediation of Contaminated Soils and Waters, 1st ed.; Terry, N., Bañuelos, G., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 85–107. [Google Scholar]
- Eskander, S.; Saleh, H. Phytoremediation: An overview. In Environmental Science and Engineering, Soil Contamination and Phytoremediation, 1st ed.; Abrol, Y.P., Gurjar, B.R., Eds.; Studium Press: Devon, UK, 2017; Volume 11, pp. 124–161. [Google Scholar]
- Lebrun, M.; Miard, F.; Hattab-Hambli, N.; Bourgerie, S.; Morabito, D. Assisted phytoremediation of a multi-contaminated industrial soil using biochar and garden soil amendments associated with Salix alba or Salix viminalis: Abilities to stabilize As, Pb, and Cu. Water Air Soil Pollut. 2018, 229, 163. [Google Scholar] [CrossRef]
- Gutiérrez-Claverol, M.; Luque, C. Recursos del Subsuelo de Asturias, 1st ed.; Servicio de Publicaciones: Oviedo, Spain, 1993; 374p. [Google Scholar]
- Dory, A. Le mercure dans les Asturies. Rev. Univ. Mines Metall. 1984, 32, 145–210. [Google Scholar]
- Loredo, J.; Luque, C.; García Iglesias, J. Conditions of formation of mercury deposits from the Cantabrian Zone (Spain). Bull. Mineral. 1988, 111, 393–400. [Google Scholar] [CrossRef]
- Loredo, J.; Ordóñez, A.; Gallego, J.R.; Baldo, C.; García-Iglesias, C. Geochemical characterization of mercury mining spoil heaps in the area of Mieres (Asturias, northern Spain). J. Geochem. Explor. 1999, 67, 377–390. [Google Scholar] [CrossRef]
- González-Fernández, B.; Rodríguez-Valdés, E.; Boente, C.; Menéndez-Casares, E.; Fernández-Braña, A.; Gallego, J.R. Long-term ongoing impact of arsenic contamination on the environmental compartments of a former mining-metallurgy area. Sci. Total Environ. 2018, 610–611, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Castroviejo, S. Flora Ibérica, 1st ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 1986; pp. 1–8. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, D.M.; Moore, D.H.; Valentine, S.M. Flora Europaea; Cambridge University Press: Cambridge, UK, 1964; pp. 1–5. [Google Scholar]
- Fernández Prieto, J.A.; Cires Rodríguez, E.; Bueno Sánchez, A.; Vázquez, V.M.; Nava Fernández, H.S. Catálogo de las Plantas Vasculares de Asturias; Jardín Botánico Atlántico: Gijón, Spain, 2014; Volume 11, pp. 7–267. [Google Scholar]
- Hubbard, C.E. A Guide to Their Structure, Identification, Uses and Distribution in the Bristish Isles; New Edition Penguin Books: London, UK, 1985; 476p. [Google Scholar]
- Ashburner, K.; Mc Allister, H.A. The Genus Betula. A Taxonomic Revision of Birches; Royal Botanical Gardens: Kew, UK, 2013; 431p. [Google Scholar]
- Curtis, J. The Vegetation of Wisconsin. An Ordination of Plant Communities; University of Wisconsin Press: Madison, WI, USA, 1959; 657p. [Google Scholar]
- Finol, H. Nuevos parámetros a considerarse en el análisis estructural de las selvas vírgenes tropicales. Rev. For. Ven. 1971, 13, 29–42. [Google Scholar]
- Mueller-Dumbois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1974; 547p. [Google Scholar]
- Matteucci, S.D.; Colma, A. Metodología Para el Estudio de la Vegetación. Secretaría General de la Organización de Estados Americanos; Programa Regional de Desarrollo Científico y Tecnológico: Washington, DC, USA, 1982; 72p. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of Soil Organic Matter by Weight Loss-On-Ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; SSA Special Publications: Madison, WI, USA, 1996; Volume 46, pp. 21–31. [Google Scholar]
- Klute, A. Nitrogen-Total. In Methods of Soil Analyses; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 595–624. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006; 993p. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods of Soil Analysis; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 383–411. [Google Scholar]
- Tessier, A.; Campbell, P.G.; Bisson, M. Sequential extraction procedure for speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Zhang, X.; Zhang, S.; Xu, X.; Li, T.; Gong, G.; Jia, Y. Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J. Hazard. Mater. 2010, 180, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Bech, J.; Duran, P.; Roca, N.; Poma, W.; Sánchez, I.; Roca-Pérez, L.; Boluda, R.; Barceló, J.; Poschenrieder, C. Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. Spontaneous species from mine spoils in Peru and their potential use in phytoremediation. J. Geochem. Explor. 2012, 123, 109–113. [Google Scholar] [CrossRef]
- Díaz, T.E.; Fernández, J. Biogeografía de Asturias: Bases Para su Actualización. In Proceedings of the I Congreso de Estudios Asturianos, Oviedo, Spain, 10–13 May 2006. [Google Scholar]
- Rivas-Martínez, S.; Penas, A.; Díaz, T.E. Bioclimatic and Biogeographic Maps of Europe; Universidad de León: Leon, Spain, 2004. [Google Scholar]
- Cunningham, S.D.; Ow, D.W. Promises and prospects of phytoremediation. Plant Physiol. 1996, 110, 715–719. [Google Scholar] [CrossRef]
- Ligenfelter, D.D.; Hartwig, N.L. Introduction to Weeds and Herbicides; Pennsylvania State University, Publication Distribution Center: State College, PA, USA, 2007. [Google Scholar]
- BOPA Generic Reference Levels for Heavy Metals in Soils from Principality of Asturias, Spain. Available online: http://sede.612asturias.es/bopa/2014/04/21/2014e06617.pdf (accessed on 25 March 2021).
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; 413p. [Google Scholar]
- Gisbert, C.; Almela, C.; Vélez, D.; López-Moya, J.R.; de Haro, A.; Serrano, R.; Montoro, R.; Navarro-Aviñó, J. Identification of As accumulation plant species growing on highly contaminated soils. Int. J. Phytoremediat. 2008, 10, 185–196. [Google Scholar] [CrossRef]
- Sharples, J.M.; Meharg, A.A.; Chambers, S.M.; Cairney, J.W.G. Symbiotic solution to arsenic contamination. Nature 2000, 404, 951–952. [Google Scholar] [CrossRef]
- Pichtel, J.; Salt, C.A. Vegetative growth and trace metal accumulation on metalliferous wastes. J. Environ. Qual. 1998, 27, 618–624. [Google Scholar] [CrossRef]
- Pichtel, J.; Kuroiwa, K.; Sawyerr, H.T. Distribution of Pb, Cd and Ba in soils and plants of two contaminated sites. Environ. Pollut. 2000, 110, 171–178. [Google Scholar] [CrossRef]
- Rasmussen, G.; Olsen, R.A. Sorption and biological removal of creosote-contaminants from groundwater in soil/sand vegetated with orchard grass (Dactylis glomerata). Adv. Environ. Res. 2004, 8, 313–327. [Google Scholar] [CrossRef]
- Salas-Luévano, M.A.; Mauricio-Castillo, J.A.; González-Rivera, M.L.; Vega-Carrillo, H.R.; Salas-Muñoz, S. Accumulation and phytostabilization of As, Pb and Cd in plants growing inside mine tailings reforested in Zacatecas, Mexico. Environ. Earth. Sci. 2021, 76, 806. [Google Scholar] [CrossRef]
- Bech, J.; Roca, N.; Tume, P.; Ramos-Miras, J.; Gil, C.; Boluda, R. Screening for new accumulator plants in potential hazards elements contaminated soil surrounding Peruvian mine tailings. Catena 2016, 136, 66–73. [Google Scholar] [CrossRef]
- Otones, V.; Álvarez-Ayuso, E.; García-Sánchez, A.; Santa Regina, I.; Murciego, A. Arsenic distribution in soils and plants of an arsenic impacted former mining area. Environ. Pollut. 2011, 159, 2637–2647. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Natural attenuation for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komat, K.M.; Tu, C.; Zhang, W.; Cai, Y. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, L. A plant species (Trifolium repens) with strong enrichment ability for mercury. Ecol. Eng. 2014, 70, 349–350. [Google Scholar] [CrossRef]
- Brooks, R.R. Plants that hyperaccumulate heavy metals. In Plants and The Chemical Elements: Biochemistry Uptake, Tolerance and Toxicity, 1st ed.; Farago, M.E., Ed.; Wiley-VCH: Weinheim, Germany, 1994; pp. 88–105. [Google Scholar]

| Coating Index Categories | Description |
|---|---|
| 1 | Quite abundant individuals but of weak coverage. Covering from 1% to 10% (Medium coating = 5%) |
| 2 | Very abundant individuals that cover at least 1/20 of the surface. Covering from 10% to 25% (Medium coating = 17.5) |
| 3 | Individuals of variable number, but who cover from ¼ to ½ of the surface. Covering from 25% to 50%. (Medium coating = 37.5%) |
| 4 | Individuals of variable number, but that cover of ½ to ¾ of the surface. Coating from 50% to 75%. (Medium coating = 62.5%) |
| Identified Species | Botanical Family | Coating Index (CoI) |
|---|---|---|
| Agrostis tenuis L. | Poaceae | 4 |
| Betula celtiberica Rothm. & Vasc. | Betulaceae | 4 |
| Calluna vulgaris L. Hull | Ericaceae | 4 |
| Dactylis glomerata L. | Poaceae | 4 |
| Plantago lanceolata L. | Plantaginaceae | 4 |
| Salix atrocinerea Brot. | Salicaceae | 4 |
| Trifolium repens L. | Fabaceae | 4 |
| Agrostis capillaris L. | Poaceae | 3 |
| Cornus sanguinea L. | Cornaceae | 3 |
| Lolium perenne L. | Poaceae | 3 |
| Lotus hispidus Desf. ex DC. | Fabaceae | 3 |
| Medicago lupulina L. | Fabaceae | 3 |
| Pastinaca sativa L. subsp. sylvestris (Mill.) Rouy & Camus | Apiaceae | 3 |
| Piptatherum miliaceum L. Coss. | Poaceae | 3 |
| Sonchus asper L. Hill | Asteraceae | 3 |
| Sonchus oleraceus L. | Asteraceae | 3 |
| Holcus lanatus L. | Poaceae | 3 |
| Hypericum pulchrum L. | Hypericaceae | 3 |
| Cirsium vulgare L. Scop. | Asteraceae | 2 |
| Conyza canadensis L. Cronquist | Asteraceae | 2 |
| Desmazeria rigida L. Tutin (= Catapodium rigidum) | Poaceae | 2 |
| Lolium perenne L. | Poaceae | 2 |
| Lotus corniculatus L. | Fabaceae | 2 |
| Poa annua L. | Poaceae | 2 |
| Prunella vulgaris L. | Lamiaceae | 2 |
| Pteridium aquilinum L. Kuhn | Dennstaedtiaceae | 2 |
| Rubus gr. fruticosus L. | Rosaceae | 2 |
| Sagina apetala Ard. | Caryophyllaceae | 2 |
| Stellaria media L. | Caryophyllaceae | 2 |
| Trifolium dubium Sibth. | Fabaceae | 2 |
| Verbena officinalis L. | Verbenaceae | 2 |
| Arabis glabra L. Bernh. | Brassicaceae | 1 |
| Blechnum spicant L. Roth | Blechnaceae | 1 |
| Festuca nigrescens Lam. | Poaceae | 1 |
| Hedera Helix L. | Araliaceae | 1 |
| Melilotus albus Medik. | Fabaceae | 1 |
| Rubus ulmifolius Schott | Rosaceae | 1 |
| Verbascum virgatum Stokes | Scrophulariaceae | 1 |
| Vulpia bromoides L. Gray | Poaceae | 1 |
| Soil Parameter | Units | Average | Std. Deviation |
|---|---|---|---|
| pH | 1:2.5 H2O | 7.67 | 0.82 |
| C.E 1 | dS m−1 | 0.01 | 0.001 |
| Sand | % | 89.49 | 4.50 |
| Silt | % | 6.36 | 3.99 |
| Clay | % | 5.45 | 1.66 |
| O.M 2 | % | 6.25 | 0.78 |
| C | % | 9.43 | 1.03 |
| N (total) | % | 0.17 | 0.07 |
| C/N 3 | - | 58.30 | 14.11 |
| Fe | mg kg−1 | 8.33 | 3.01 |
| PM3 4 | mg kg−1 | 1.70 | 0.61 |
| Ex Ca | cmol(+)kg−1 | 17.07 | 0.65 |
| Ex Mg | cmol(+)kg−1 | 1.55 | 0.07 |
| Ex K | cmol(+)kg−1 | 1.83 | 0.16 |
| Ex Na | cmol(+)kg−1 | 1.60 | 0.21 |
| E.C.E.C 5 | cmol(+)kg−1 | 22.35 | 1.60 |
| Specie | Element | Concentration (mg·kg−1 = ppm) | ||
|---|---|---|---|---|
| Soil | Aerial Parts | Roots | ||
| Agrostis tenuis | As | 197 | 3 | 23 |
| Hg | 131 | 5 | 18 | |
| Betula celtiberica | As | 107 | 9 | 15 |
| Hg | 238 | 2 | 8 | |
| Calluna vulgaris | As | 24,600 | 571 | 1270 |
| Pb | 105 | 11 | 10 | |
| Dactylis glomerata | As | 180 | 28 | 11 |
| Hg | 260 | 5 | 9 | |
| Plantago lanceolata | As | 177 | 12 | 26 |
| Hg | 132 | 9 | 16 | |
| Trifolium repens | As | 142 | 6 | 15 |
| Hg | 222 | 3 | 8 | |
| Salix atrocinerea | As | 112 | 6 | 12 |
| Hg | 229 | 2 | 5 | |
| Specie | BCF | TF | MR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Hg | Pb | As | Hg | Pb | As | Hg | Pb | |
| A. tenuis | 0.12 | 0.14 | - | 0.13 | 0.27 | 0.01 | 0.04 | ||
| B. celtiberica | 0.14 | 0.03 | - | 0.59 | 0.27 | 0.08 | 0.01 | ||
| C. vulgaris | 0.05 | - | 0.09 | 0.45 | - | 1.13 | 0.02 | - | 0.11 |
| D. glomerata | 0.06 | 0.03 | - | 2.54 | 0.54 | - | 0.15 | 0.02 | - |
| P. lanceolata | 0.15 | 0.12 | - | 0.45 | 0.55 | - | 0.07 | 0.06 | - |
| T. repens | 0.10 | 0.04 | - | 0.45 | 0.38 | - | 0.05 | 0.01 | |
| S. atrocinerea | 0.11 | 0.02 | 0.45 | 0.38 | - | 0.05 | 0.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matanzas, N.; Afif, E.; Díaz, T.E.; Gallego, J.L.R. Screening of Pioneer Metallophyte Plant Species with Phytoremediation Potential at a Severely Contaminated Hg and As Mining Site. Environments 2021, 8, 63. https://doi.org/10.3390/environments8070063
Matanzas N, Afif E, Díaz TE, Gallego JLR. Screening of Pioneer Metallophyte Plant Species with Phytoremediation Potential at a Severely Contaminated Hg and As Mining Site. Environments. 2021; 8(7):63. https://doi.org/10.3390/environments8070063
Chicago/Turabian StyleMatanzas, Nora, Elías Afif, Tomás Emilio Díaz, and José Luis R. Gallego. 2021. "Screening of Pioneer Metallophyte Plant Species with Phytoremediation Potential at a Severely Contaminated Hg and As Mining Site" Environments 8, no. 7: 63. https://doi.org/10.3390/environments8070063
APA StyleMatanzas, N., Afif, E., Díaz, T. E., & Gallego, J. L. R. (2021). Screening of Pioneer Metallophyte Plant Species with Phytoremediation Potential at a Severely Contaminated Hg and As Mining Site. Environments, 8(7), 63. https://doi.org/10.3390/environments8070063








