Effect of Multiple Stresses, Organic Amendment and Compaction, on the Fate and Impact of Isoproturon in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Organic Amendment
2.2. Incubation Procedures
2.2.1. Fate of Isoproturon
2.2.2. Soil Enzyme Activity
2.3. Statistical Analyses
3. Results and Discussion
3.1. Fate of Isoproturon in Soil under Different Stress Conditions: Organic Amendment Addition and Compaction
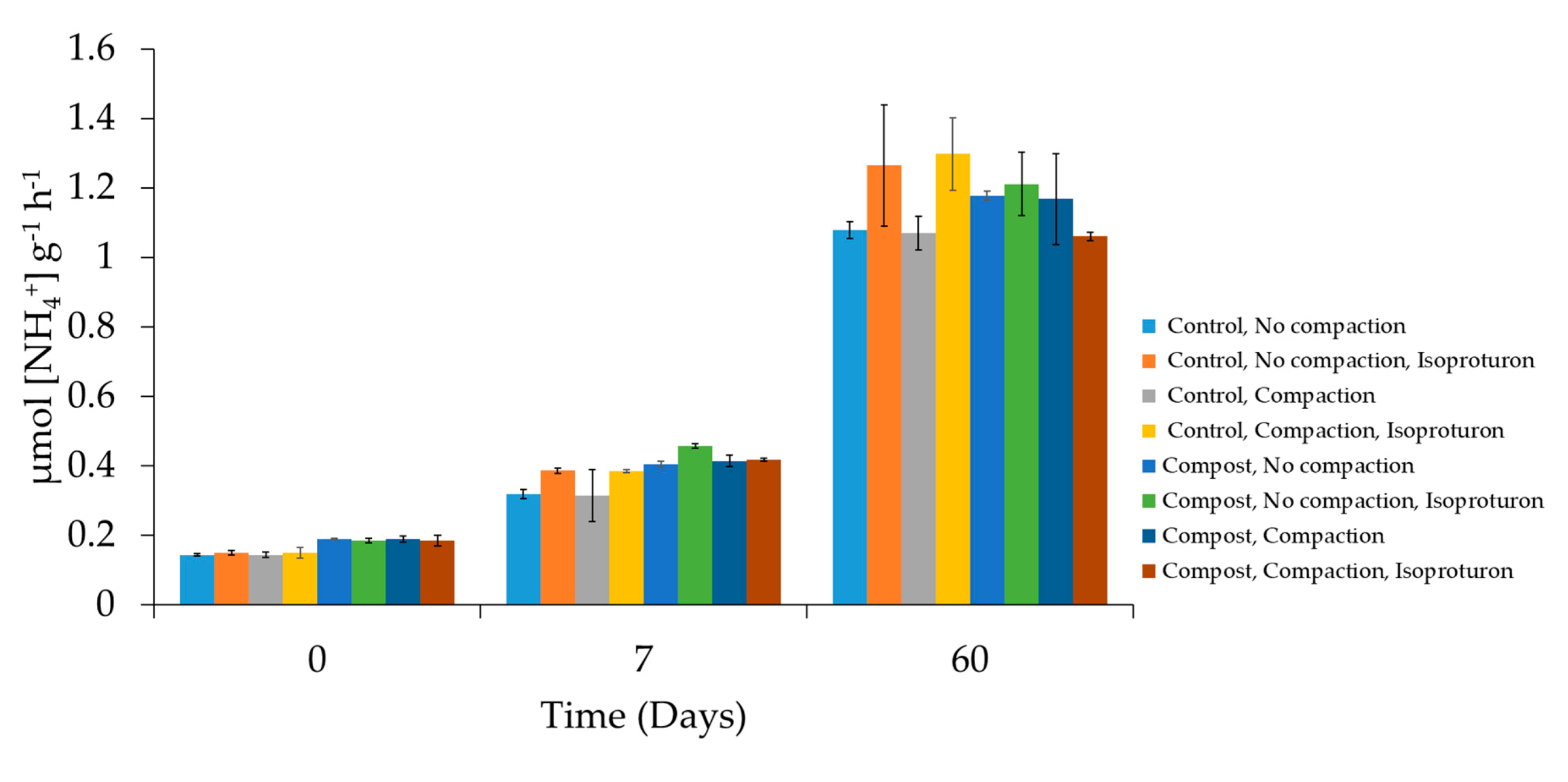
3.2. Soil Enzyme Activity under Different Stress Conditions: Herbicide and Organic Amendment Additions and Compaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barriuso, E.; Calvet, R.; Schiavon, M.; Soulas, G. Les pesticides et les polluants organiques des sols. Transformations et dissipation. Etude Gest. Sols 1996, 3, 279–296. [Google Scholar]
- Directive 2006/0086 (COD). Directive of the European Parliament and of the Council Establishing a Framework for the Protection of Soil and Amending Directive 2004/35/EC; Directive: Brussels, Belgium, 2006; COM (2006) 232 final. [Google Scholar]
- FAO. Voluntary Guidelines for Sustainable Soil Management; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; 26p. [Google Scholar]
- Houot, S.; Pons, M.N.; Pradel, M.; Tibi, A.; Aubry, C.; Augusto, L.; Barbier, R.; Benoit, P.; Brugère, H.; Caillaud, M.A.; et al. Valorisation des Matières Fertilisantes D’origine Résiduaire sur les sols à Usage Agricole ou Forestier, Impacts Agronomiques, Environnementaux, Socio-Economiques; Expertise Scientifique Collective, Rapport; INRA-CNRS-Irstea: Irstea, France, 2014; 930p, Available online: https://www6.paris.inrae.fr/depe/content/download/3819/36314/version/1/file/ESCoMafor+rapport_Avant-propos+et+chap1_oct2014+(2).pdf (accessed on 1 September 2020).
- Vieublé-Gonod, L.; Benoit, P.; Cohen, N.; Houot, S. Spatial and temporal heterogeneity of soil microorganisms and isoproturon degrading activity in a tilled soil amended with urban waste composts. Soil Biol. Biochem. 2009, 41, 2558–2567. [Google Scholar] [CrossRef]
- Pot, V.; Benoit, P.; Etievant, V.; Bernet, N.; Labat, C.; Coquet, Y.; Houot, S. Effects of tillage practice and repeated urban compost application on bromide and isoproturon transport in a loamy Albeluvisol. Eur. J. Soil Sci. 2011, 62, 797–810. [Google Scholar] [CrossRef]
- Filipović, V.; Coquet, Y.; Pot, V.; Houot, S.; Benoit, P. Modeling the effect of soil structure on water flow and isoproturon dynamics in an agricultural field receiving repeated urban waste compost application. Sci. Total Environ. 2014, 499, 546–559. [Google Scholar] [CrossRef][Green Version]
- Filipović, V.; Coquet, Y.; Pot, V.; Houot, S.; Benoit, P. Modeling water and isoproturon dynamics in a heterogeneous soil profile under different urban waste compost applications. Geoderma 2016, 268, 29–40. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Sánchez-Martín, M.S.; Andrades, M.S.; Pérez-Clavijo, M.; Rodríguez-Cruz, M.S. Effect of spent mushroom substrate amendment of vineyard soils on the behavior of fungicides: 1. Adsorption-desorption of penconazole and metalaxyl by soils and subsoils. J. Agric. Food Chem. 2009, 57, 9634–9642. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Sánchez-Martín, M.S.; Andrades, M.S.; Rodríguez-Cruz, M.S. Effect of spent mushroom substrate amendment of vineyard soils on the behavior of fungicides: 2. Mobility of penconazole and metalaxyl in undisturbed soil cores. J. Agric. Food Chem. 2009, 57, 9643–9650. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.S.; Rodríguez-Cruz, M.S. Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J. Agric. Food Chem. 2012, 60, 6936–6945. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Mamy, L. Modeling fungicides mobility in undisturbed vineyard soil cores unamended and amended with spent mushroom substrates. Chemosphere 2015, 134, 408–416. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Mamy, L.; Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Modelling herbicides mobility in amended soils: Calibration and test of PRZM and MACRO. Sci. Total Environ. 2020, 717, 137019. [Google Scholar] [CrossRef]
- Vieublé-Gonod, L.; El Arfaoui, A.; Benoit, P. Impact of spatial distribution of exogenous organic matter on C mineralization and isoproturon fate in soil. Soil Biol. Biochem. 2016, 95, 180–188. [Google Scholar] [CrossRef]
- Dick, R.P.; Pankhurst, C.E.; Doube, B.M.; Gupta, V.V.S.R. (Eds). Soil Enzyme Activities as Integrative Indicators of Soil Health; Food and Agriculture Organization of the United Nations: Rome, Italy, 1997. [Google Scholar]
- Richard, G.; Boizard, H.; Roger-Estrade, J.; Boiffin, J.; Guérif, J. Field study of soil compaction due to traffic in northern France: Pore space and morphological analysis of the compacted zones. Soil Till Res. 1999, 51, 151–160. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems. A review of the nature, causes and possible solutions. Soil Till Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Wortmann, C.S. Does occasional tillage undo the ecosystem services gained with no-till? A review. Soil Till Res. 2020, 198, 104534. [Google Scholar] [CrossRef]
- Rahman, A.; Burney, B.; Manson, B.E. Effect of soil compaction on phytotoxicity and persistence of soil-applied herbicides. Weed Res. 1978, 18, 93–97. [Google Scholar] [CrossRef]
- Mamy, L.; Vrignaud, P.; Cheviron, N.; Perreau, F.; Belkacem, M.; Brault, A.; Breuil, S.; Delarue, G.; Pétraud, J.P.; Touton, I.; et al. No evidence for effect of soil compaction on the degradation and impact of isoproturon. Environ. Chem. Lett. 2011, 9, 145–150. [Google Scholar] [CrossRef]
- Sørensen, S.R.; Bending, G.D.; Jacobsen, C.S.; Walker, A.; Aamand, J. Microbial degradation of isoproturon and related phenyl urea herbicides in and below agricultural fields. FEMS Microbiol. Ecol. 2003, 45, 1–11. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Karas, P.A.; Nikolaki, S.; Storck, V.; Ferrari, F.; Trevisan, M.; Tsiamis, G.; Martin-Laurent, F.; Karpouzas, D.G. Dissipation and adsorption of isoproturon, tebuconazole, chlorpyrifos and their main transformation products under laboratory and field conditions. Sci. Total Environ. 2016, 569, 86–96. [Google Scholar] [CrossRef]
- GCSD, 2018. General Commission for Sustainable Development. Environment & Agriculture. Key Figures, 2018 Edition. Available online: https://www.statistiques.developpement-durable.gouv.fr/environnement-et-agriculture-les-chiffres-cles-edition-2018 (accessed on 1 September 2020).
- PPDB. Pesticide Properties DataBase; University of Hertfordshire: Hertfordshire, UK; Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm (accessed on 1 September 2020).
- Swartjes, F.A.; Van der, A.A. Measures to reduce pesticides leaching into groundwater-based drinking water resources: An appeal to national and local governments, water boards and farmers. Sci. Total Environ. 2020, 699, 134186. [Google Scholar] [CrossRef]
- Alletto, L.; Coquet, Y.; Benoit, P.; Bergheaud, V. Effects of temperature and water content on degradation of isoproturon in three soil profiles. Chemosphere 2006, 64, 1053–1061. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.S.; Jones, J.E.; Bending, G.D. Field-scale study of the variability in pesticide biodegradation with soil depth and its relationship with soil characteristics. Soil Biol. Biochem. 2006, 38, 2910–2918. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Paris-Palacios, S.; Couderchet, M.; Vernet, G. Effects of the herbicide isoproturon on survival, growth rate, and protein content of mature earthworms (Lumbricus terrestris L.) and its fate in the soil. Appl. Soil Ecol. 2003, 23, 69–77. [Google Scholar] [CrossRef]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance isoproturon. EFSA J. 2015, 13, 4206. [Google Scholar] [CrossRef]
- Lebrun, J.D.; Trinsoutro-Gattin, I.; Vinceslas-Akpa, M.; Bailleul, C.; Brault, A.; Mougin, C.; Laval, K. Assessing impacts of copper on soil enzyme activities in regard to their natural spatiotemporal variation under long-term different land uses. Soil Biol. Biochem. 2012, 49, 150–156. [Google Scholar] [CrossRef]
- Kucharski, J.; Baćmaga, M.; Wyszkowska, J. Dehydrogenase activity as an indicator of soil contamination with herbicides. Ecol. Chem. Eng. A 2009, 16, 253–261. [Google Scholar]
- Lu, Y.C.; Zhang, S.; Miao, S.S.; Jiang, C.; Huang, M.T.; Liu, Y.; Yang, H. Enhanced degradation of herbicide isoproturon in wheat rhizosphere by salicylic acid. J. Agric. Food Chem. 2015, 63, 92–103. [Google Scholar] [CrossRef]
- Nowak, A.; Nowak, J.; Klodka, D.; Przybulewska, K.; Telesinski, A.; Szopa, E. Changes in the microflora and biological activity of the soil during the degradation of isoproturon. J. Plant. Dis. Protect. 2004, 19, 1003–1016. [Google Scholar]
- Karas, P.A.; Baguelin CPertile, G.; Papadopoulou, E.S.; Nikolaki, S.; Storck, V.; Ferrari, F.; Trevisan, M.; Ferrarini, A.; Fornasier, F.; Vasileiadis, S.; et al. Assessment of the impact of three pesticides on microbial dynamics and functions in a lab-to-field experimental approach. Sci. Total Environ. 2018, 637, 636–646. [Google Scholar] [CrossRef]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of pesticides on soil enzymes: A review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Défossez, P.; Richard, G.; Boizard, H.; O’Sullivan, M.F. Modeling change in soil compaction due to agricultural traffic as a function of soil water content. Geoderma 2003, 116, 89–105. [Google Scholar] [CrossRef]
- De Santiago-Martín, A.; Cheviron, N.; Quintana, J.R.; González, C.; Lafuente, A.L.; Mougin, C. Metal contamination disturbs biochemical and microbial properties of calcareous agricultural soils of the Mediterranean area. Arch. Environ. Contam. Toxicol. 2013, 64, 388–398. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: http://cran.r-project.org/ (accessed on 1 September 2020).
- Barriuso, E.; Benoit, P.; Dubus, I.G. Formation of pesticide nonextractable (bound) residues in soil: Magnitude, controlling factors and reversibility. Environ. Sci. Technol. 2008, 42, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, E.; Houot, S.; Serra-Witling, C. Influence of compost addition to soil on the behavior of herbicides. Pestic. Sci. 1997, 49. [Google Scholar]
- Coquet, Y.; Barriuso, E. Spatial variability of pesticide adsorption within the topsoil of a small agricultural catchment. Agronomie 2002, 22, 389–398. [Google Scholar] [CrossRef]
- Perrin-Ganier, C.; Schiavon, F.; Morel, J.L.; Schiavon, M. Effect of sludge-amendment or nutrient addition on the biodegradation of the herbicide isoproturon in soil. Chemosphere 2001, 44, 887–892. [Google Scholar] [CrossRef]
- Sadet-Bourgeteau, S.; Houot, S.; Dequiedt, S.; Nowak, V.; Tardy, V.; Terrat, S.; Montenach, D.; Mercier, V.; Karimi, B.; Chemidlin Prévost-Bouré, N.; et al. Lasting effect of repeated application of organic waste products on microbial communities in arable soils. Appl. Soil Ecol. 2018, 125, 278–287. [Google Scholar] [CrossRef]
- Shestak, C.J.; Busse, M.D. Compaction alters physical but not biological indices of soil health. Soil Sci. Soc. Am. J. 2005, 69, 236–246. [Google Scholar] [CrossRef]
- Gregory, A.S.; Watts, C.W.; Whalley, W.R.; Kuan, H.L.; Griffiths, B.S.; Hallett, P.D.; Whitmore, A.P. Physical resilience of soil to field compaction and the interactions with plant growth and microbial community structure. Eur. J. Soil Sci. 2007, 58, 1221–1232. [Google Scholar] [CrossRef]
- Tejada, M.; García-Martínez, A.M.; Gómez, I.; Parrado, J. Application of MCPA herbicide on soils amended with biostimulants: Short-time effects on soil biological properties. Chemosphere 2010, 80, 1088–1094. [Google Scholar] [CrossRef]
- Yan, H.; Wang, D.; Dong, B.; Tang, F.; Wang, B.; Fang, H.; Yu, Y. Dissipation of carbendazim and chloramphenicol alone and in combination and their effects on soil fungal:bacterial ratios and soil enzyme activities. Chemosphere 2011, 84, 634–641. [Google Scholar] [CrossRef]
- Baćmaga, M.; Kucharski, J.; Wyszkowska, J.; Borowik, A.; Tomkiel, M. Responses of microorganisms and enzymes to soil contamination with metazachlor. Environ. Earth Sci. 2014, 72, 2251–2262. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Hernández, T.; García, C. The impacts of organic amendments: Do they confer stability against drought on the soil microbial community? Soil Biol. Biochem. 2017, 113, 173–183. [Google Scholar] [CrossRef]
- Han, L.; Liu, Y.; Fang, K.; Zhang, X.; Liu, T.; Wang, F.; Wang, X. Dissipation of chlorothalonil in the presence of chlortetracycline and ciprofloxacin and their combined effects on soil enzyme activity. Environ. Sci. Pollut. Res. 2020, 27, 13662–13669. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.A.; Hernandez, T.; Garcia, C.; Ayuso, M. Enzymatic activities in an arid soil amended with urban organic wastes: Laboratory experiment. Biores. Technol. 1998, 64, 131–138. [Google Scholar] [CrossRef]
- Castillo, J.M.; Beguet, J.; Martin-Laurent, F.; Romero, E. Multidisciplinary assessment of pesticide mitigation in soil amendedwith vermicomposted agroindustrial wastes. J. Hazard. Mat. 2016, 304, 379–387. [Google Scholar] [CrossRef]
- Mahía, J.; Martín, A.; Carballas, T.; Díaz-Raviña, M. Atrazine degradation and enzyme activities in an agricultural soil under two tillage systems. Sci. Total Environ. 2007, 378, 187–194. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.X.; Kabzems, R. Soil compaction and forest floor removal reduced microbial biomass and enzyme activities in a boreal aspen forest soil. Biol. Fertil. Soils 2008, 44, 471–479. [Google Scholar] [CrossRef]
- Romero, E.; Fernández-Bayo, J.; Castillo Díaz, J.M.; Nogales, R. Enzyme activities and diuron persistence in soil amended with vermicompost derived from spent grape marc and treated with urea. Appl. Soil Ecol. 2010, 44, 198–204. [Google Scholar] [CrossRef]
- Tejada, M.; Morillo, E.; Gómez, I.; Madrid, F.; Undabeytia, T. Effect of controlled release formulations of diuron and alachlorherbicides on the biochemical activity of agricultural soils. J. Hazard. Mat. 2017, 322, 334–347. [Google Scholar] [CrossRef]
- Šantric, L.; Radivojevic, L.; Gajic-Umiljendic, J.; Saric-Krsmanovic, M.; Ðurovic-Pejcev, R. The effects of nicosulfuron and glyphosate on microbial activity of different soils. Plant. Dahina 2018, v36, e018159989. [Google Scholar] [CrossRef]
- Qian HHu, B.; Wang, Z.; Xu, X.; Hong, T. Effects of validamycin on some enzymatic activities in soil. Environ. Monit. Assess. 2007, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z.; Kozdrój, J. Responses of indigenous microorganisms to a fungicidal mixture of mancozeb and dimethomorph added to sandy soils. Int. Biodet. Biodeg. 2010, 64, 316–323. [Google Scholar] [CrossRef]
- Crecchio, C.; Curci, M.; Pizzigallo, M.D.R.; Riccuiti, P.; Ruggiero, P. Effects of municipal solid waste compost amendments on soil enzyme activities and bacterial genetic diversity. Soil Biol. Biochem. 2004, 36, 1595–1605. [Google Scholar] [CrossRef]
 Control (unamended), Compaction;
Control (unamended), Compaction;  Compost amended, No compaction;
Compost amended, No compaction;  Compost amended, Compaction;
Compost amended, Compaction;  Control (unamended), No compaction.
Control (unamended), No compaction.
 Control (unamended), Compaction;
Control (unamended), Compaction;  Compost amended, No compaction;
Compost amended, No compaction;  Compost amended, Compaction;
Compost amended, Compaction;  Control (unamended), No compaction.
Control (unamended), No compaction.
 Mineralised;
Mineralised;  CaCl2 extractable;
CaCl2 extractable;  CH3OH extractable;
CH3OH extractable;  Non-extractable (bound) residues.
Non-extractable (bound) residues.
 Mineralised;
Mineralised;  CaCl2 extractable;
CaCl2 extractable;  CH3OH extractable;
CH3OH extractable;  Non-extractable (bound) residues.
Non-extractable (bound) residues.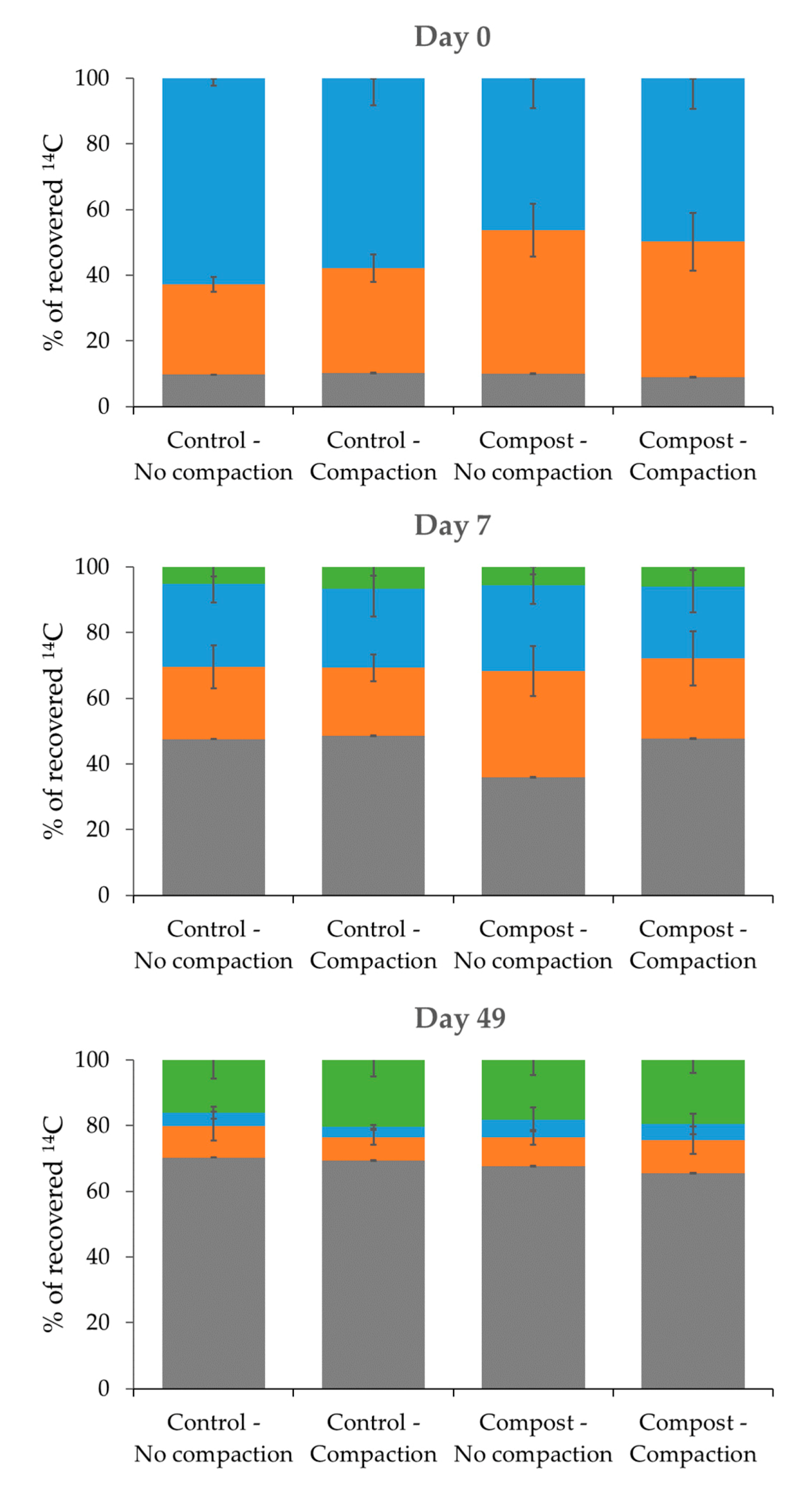
 Isoproturon;
Isoproturon;  Monodemethyl-isoproturon;
Monodemethyl-isoproturon;  Other metabolites.
Other metabolites.
 Isoproturon;
Isoproturon;  Monodemethyl-isoproturon;
Monodemethyl-isoproturon;  Other metabolites.
Other metabolites.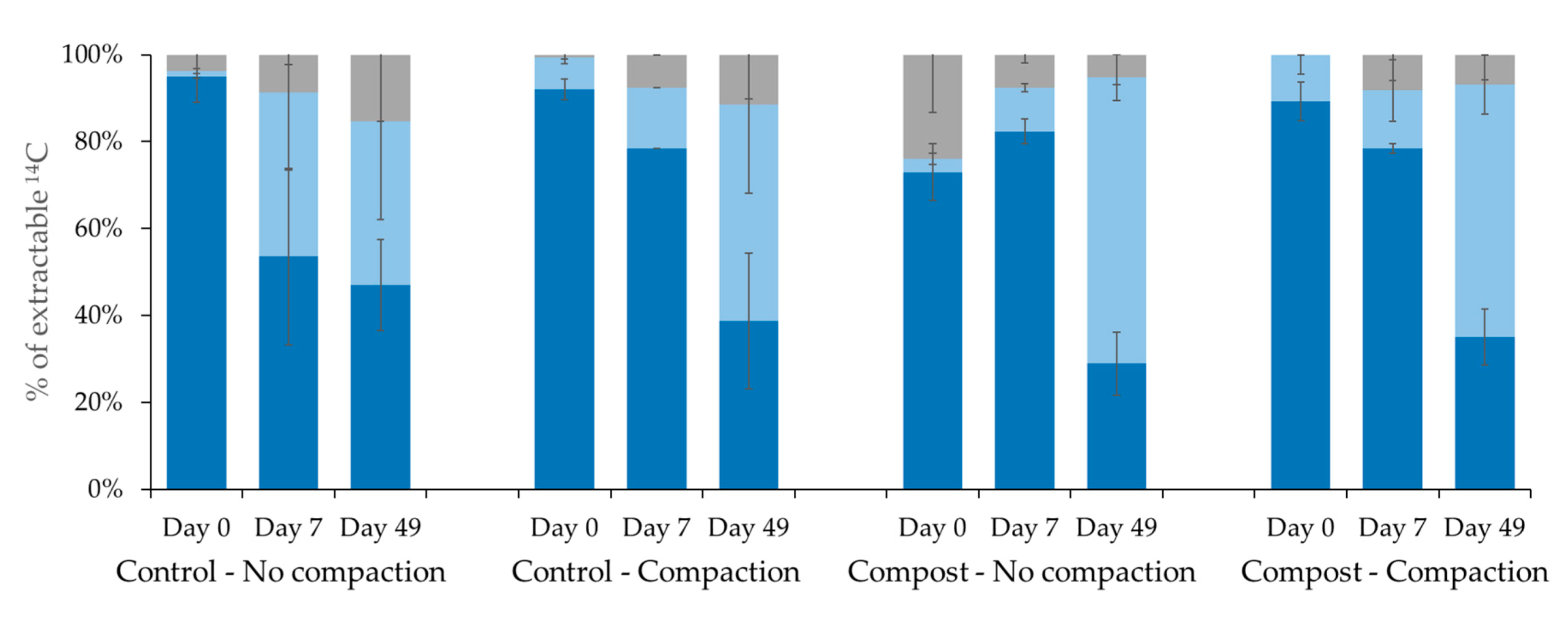
| Structural Formula | Molecular Mass (g mol−1) | Water Solubility (mg L−1) | Log Kow | Vapor Pressure (mPa) |
|---|---|---|---|---|
 | 206.28 | 70.2 | 2.5 | 5.5 × 10−3 |
| Soil | Bulk Density ρ (g cm−3) | Porosity P (-) | Pore Volume Vp (cm3) | Water Content Vw (cm3) | Degree of Saturation s (%) |
|---|---|---|---|---|---|
| Control, No compaction | 1.16 ± 0.14 | 0.56 ± 0.05 | 22.0 ± 2.0 | 8.5 ± 0.9 | 39.3 ± 7.5 |
| Control, Compaction | 1.52 ± 0.14 | 0.43 ± 0.05 | 13.4 ± 1.7 | 8.8 ± 0.8 | 67.3 ± 13.2 |
| Compost, No compaction | 1.08 ± 0.13 | 0.59 ± 0.05 | 23.2 ± 1.9 | 8.2 ± 0.9 | 35.9 ± 7.3 |
| Compost, Compaction | 1.41 ± 0.25 | 0.47 ± 0.09 | 14.7 ± 2.9 | 8.5 ± 1.4 | 62.3 ± 23.9 |
| Conditions of Soil Incubation | Urease Activity | |
|---|---|---|
| Effect of compost (no isoproturon) | No compaction (compost/no compost) Compaction (compost/no compost) | s s |
| Effect of compaction (no isoproturon) | No compost (compaction/no compaction) Compost (compaction/no compaction) | ns ns |
| Effect of isoproturon | No compost, no compaction (isoproturon/no isoproturon) No compost, compaction (isoproturon/no isoproturon) | s s |
| Compost, no compaction (isoproturon/no isoproturon) Compost, compaction (isoproturon/no isoproturon) | ns ns | |
| Effect of compaction on the impact of isoproturon | No compost (compaction/no compaction) Compost (compaction/no compaction) | ns ns |
| Effect of compost on the impact of isoproturon | No compaction (compost/no compost) Compaction (compost/no compost) | ns ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamy, L.; Mougin, C.; Benoit, P.; Houot, S.; Brault, A.; Cheviron, N.; Delarue, G.; Dumeny, V.; Vieublé-Gonod, L. Effect of Multiple Stresses, Organic Amendment and Compaction, on the Fate and Impact of Isoproturon in Soil. Environments 2020, 7, 79. https://doi.org/10.3390/environments7100079
Mamy L, Mougin C, Benoit P, Houot S, Brault A, Cheviron N, Delarue G, Dumeny V, Vieublé-Gonod L. Effect of Multiple Stresses, Organic Amendment and Compaction, on the Fate and Impact of Isoproturon in Soil. Environments. 2020; 7(10):79. https://doi.org/10.3390/environments7100079
Chicago/Turabian StyleMamy, Laure, Christian Mougin, Pierre Benoit, Sabine Houot, Agathe Brault, Nathalie Cheviron, Ghislaine Delarue, Valérie Dumeny, and Laure Vieublé-Gonod. 2020. "Effect of Multiple Stresses, Organic Amendment and Compaction, on the Fate and Impact of Isoproturon in Soil" Environments 7, no. 10: 79. https://doi.org/10.3390/environments7100079
APA StyleMamy, L., Mougin, C., Benoit, P., Houot, S., Brault, A., Cheviron, N., Delarue, G., Dumeny, V., & Vieublé-Gonod, L. (2020). Effect of Multiple Stresses, Organic Amendment and Compaction, on the Fate and Impact of Isoproturon in Soil. Environments, 7(10), 79. https://doi.org/10.3390/environments7100079