Differences in Microbial Communities and Pathogen Survival Between a Covered and Uncovered Anaerobic Lagoon
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Sample Analysis
2.3. Pathogen Detection
2.4. DNA Extraction
2.5. Deep 16S sequencing and Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Wastewater Characteristics
3.2. Pathogen Reduction
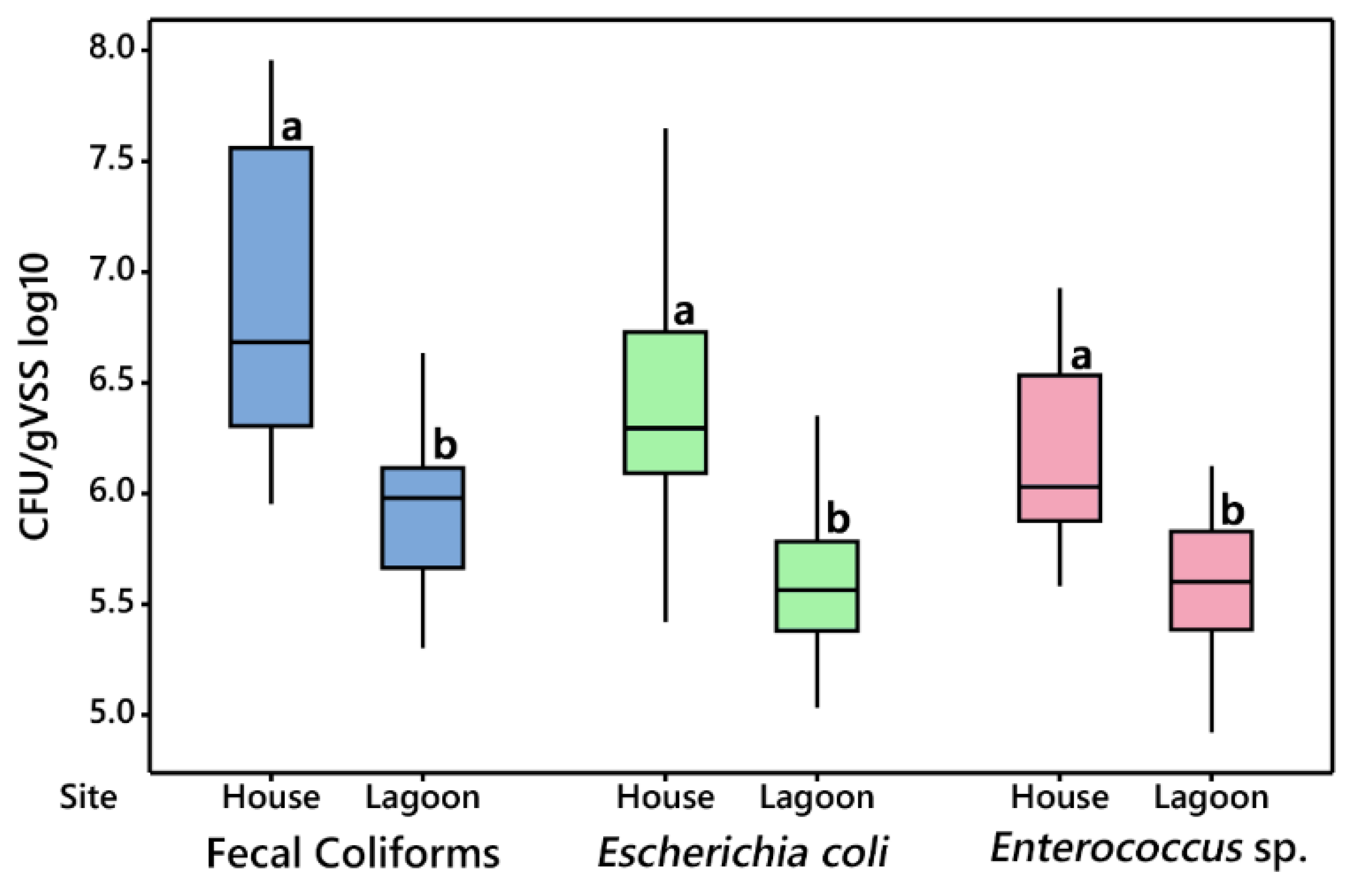
3.2.1. House vs. Lagoon
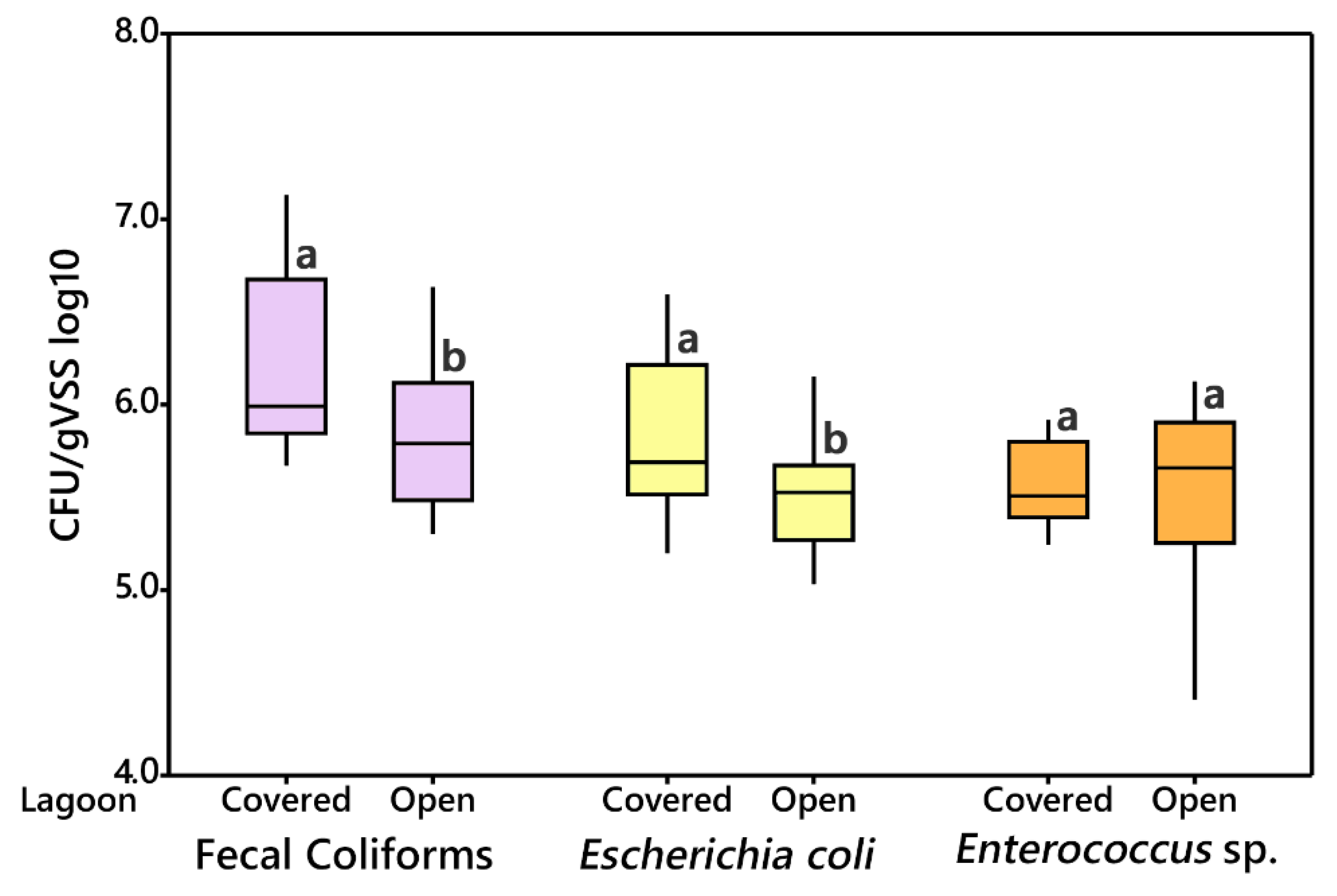
3.2.2. Open vs. Covered Lagoon
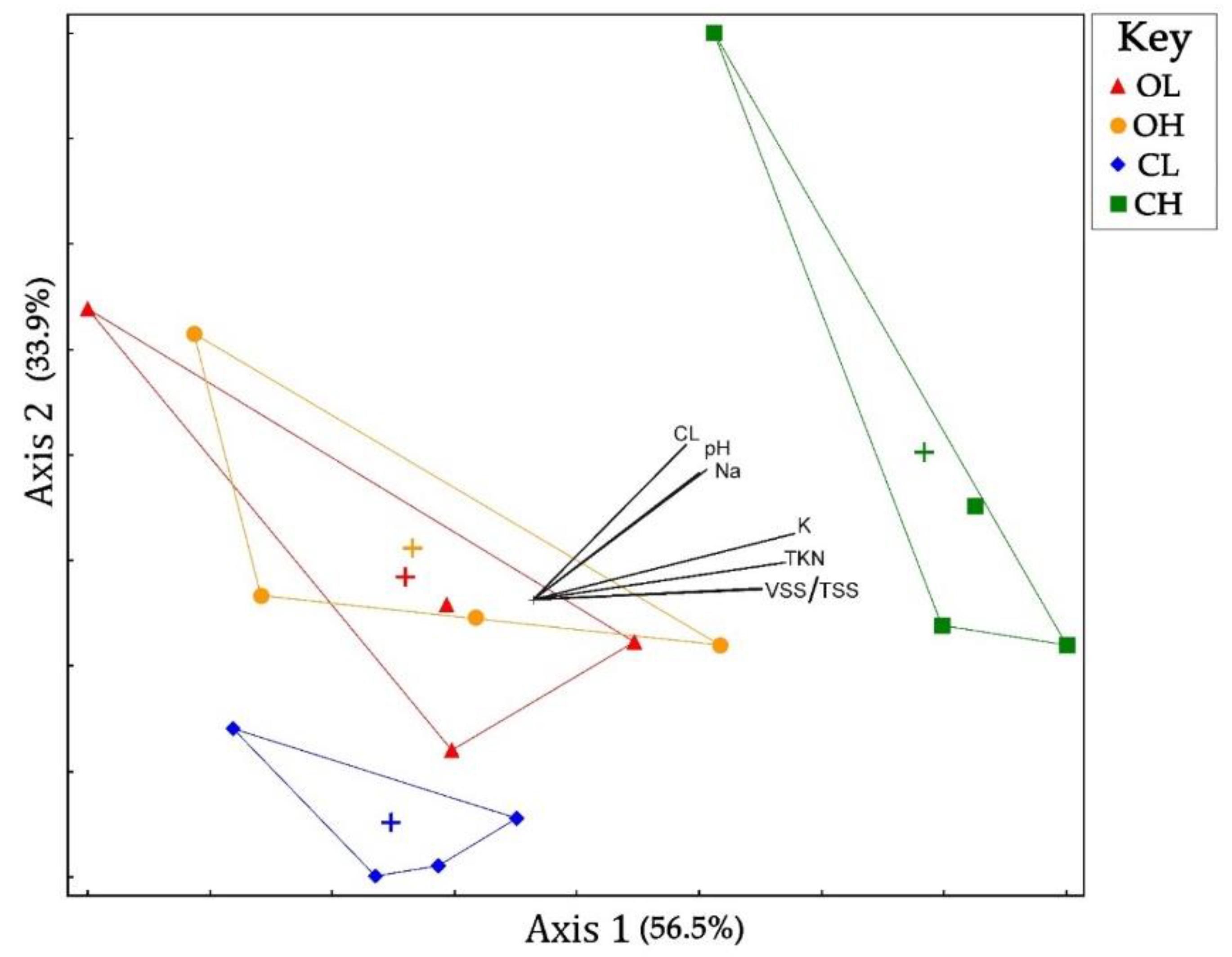
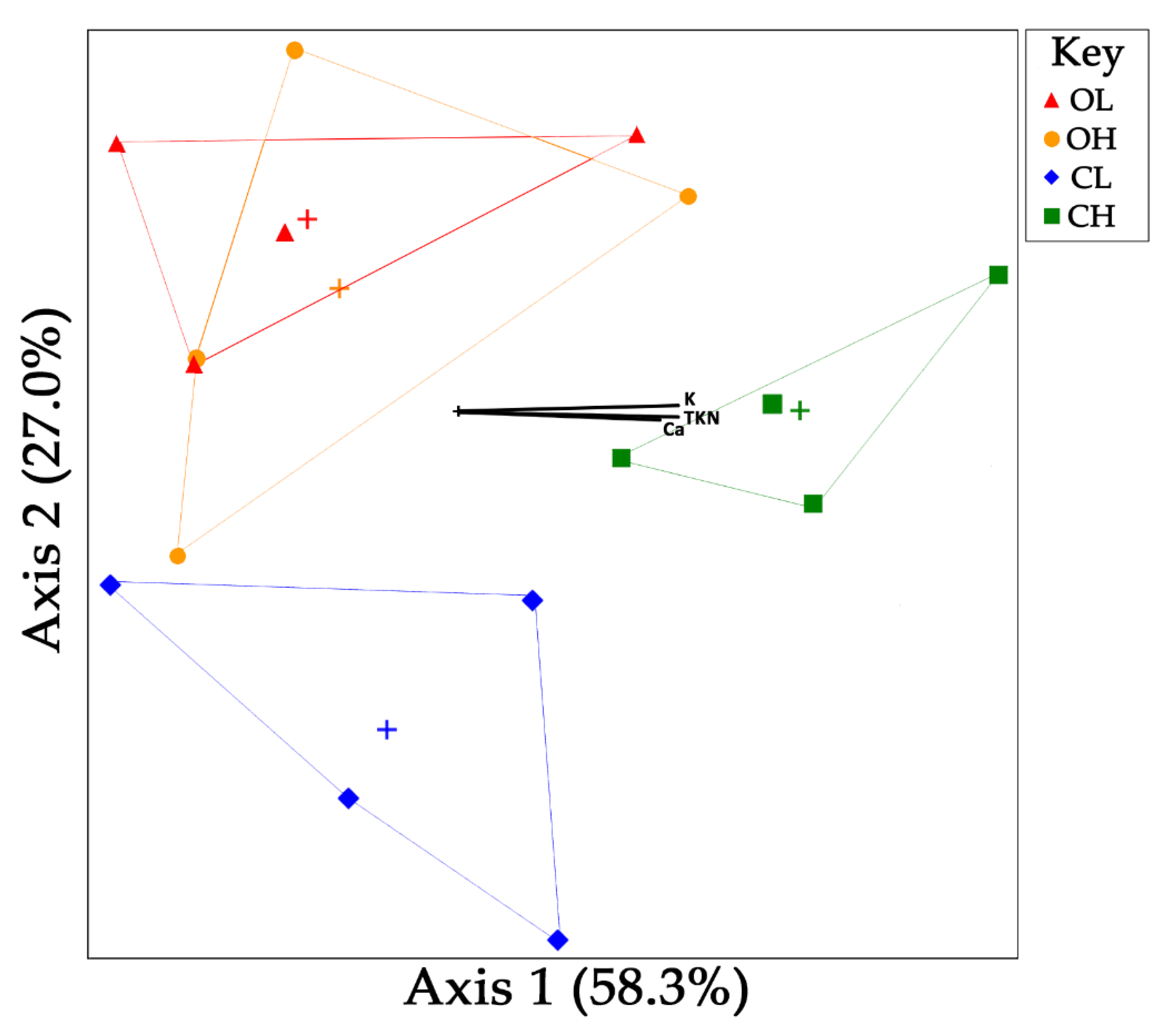
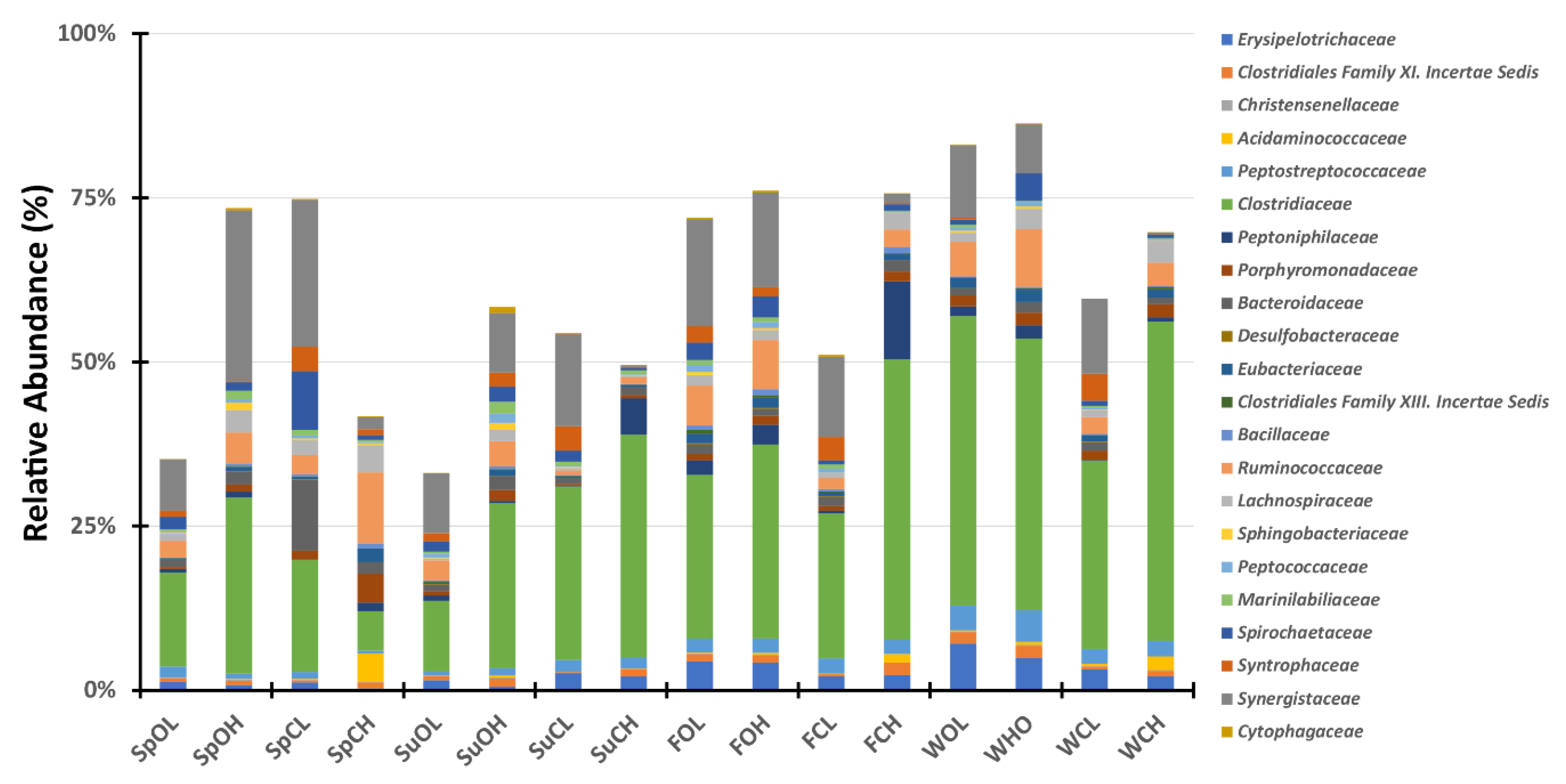
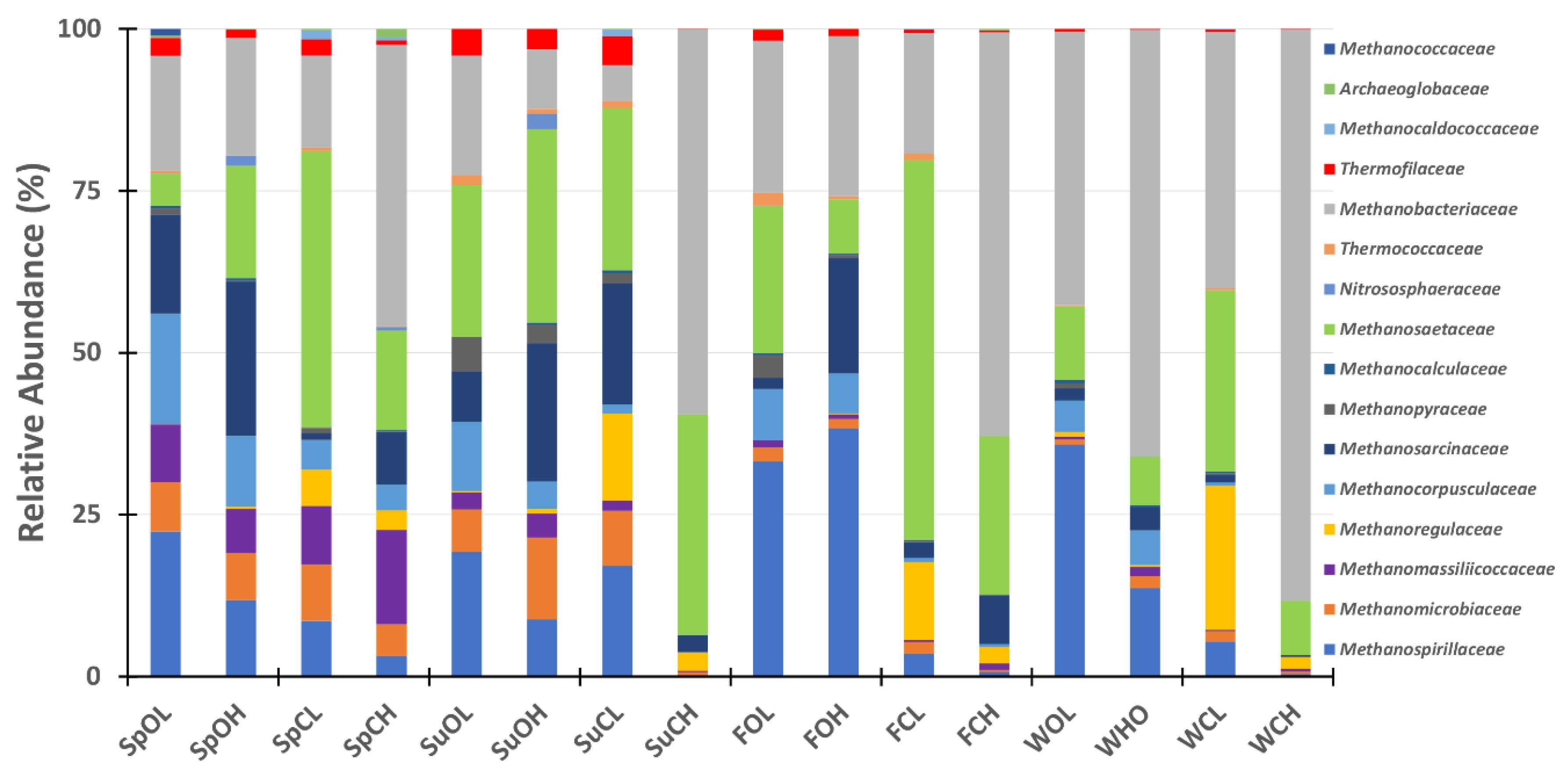
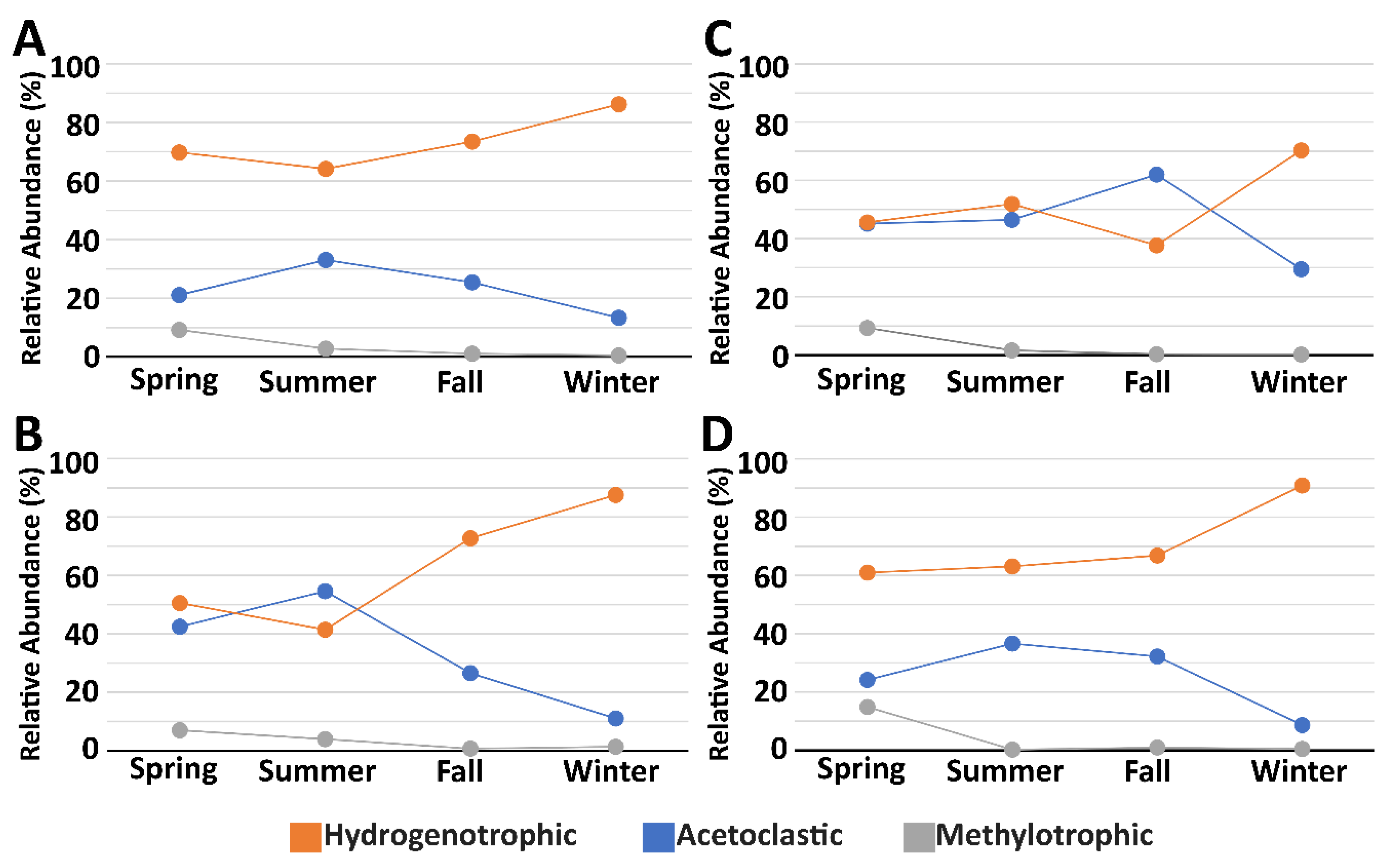
3.3. Microbial Community Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bicudo, J.R.; Goyal, S.M. Pathogens and manure management systems: A review. Environ. Technol. 2003, 24, 115–130. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Wastewater Technology Fact Sheet: Anaerobic Lagoons; USEPA: Washington, DC, USA, 2002. [Google Scholar]
- USDA-NRCS. Conservation Practice Standard: Waste Treatment Lagoon (Code 359); USDA-NRCS: Washington, DC, USA, 2017. [Google Scholar]
- VanderZaag, A.; Gordon, R.; Jamieson, R.; Burton, D.; Stratton, G. Permeable synthetic covers for controlling emissions from liquid dairy manure. Appl. Eng. Agric. 2010, 26, 287–297. [Google Scholar] [CrossRef]
- Zahn, J.A.; Tung, A.E.; Roberts, B.A.; Hatfield, J.L. Abatement of ammonia and hydrogen sulfide emissions from a swine lagoon using a polymer biocover. J. Air Waste Manag. 2011, 51, 562–573. [Google Scholar] [CrossRef][Green Version]
- Miner, J.; Humenik, F.; Rice, J.; Rashash, D.; Williams, C.; Robarge, W.; Harris, D.; Sheffield, R. Evaluation of a permeable, 5 cm thick, polyethylene foam lagoon cover. Trans. ASAE 2003, 46, 1421. [Google Scholar] [CrossRef]
- Miller, D.N.; Baumgartner, J.W. Nitrification and denitrification potential associated with semi-permeable swine waste lagoon covers. In Proceedings of the International Symposium on Air Quality and Waste Management for Agriculture, Broomfield, Colorado, 16–19 September 2007; American Society of Agricultural and Biological Engineers: St Joseph, MI, USA, 2007; p. 14. [Google Scholar]
- McLaughlin, M.R.; Brooks, J.P.; Adeli, A. Temporal flux and spatial dynamics of nutrients, fecal indicators, and zoonotic pathogens in anaerobic swine manure lagoon water. Water Res. 2012, 46, 4949–4960. [Google Scholar] [CrossRef] [PubMed]
- Ducey, T.F.; Shriner, A.D.; Hunt, P.G. Nitrification and denitrification gene abundances in swine wastewater anaerobic lagoons. J. Environ. Qual. 2011, 40, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Blunden, J.; Aneja, V.P. Characterizing ammonia and hydrogen sulfide emissions from a swine waste treatment lagoon in North Carolina. Atmos. Environ. 2008, 42, 3277–3290. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1998. [Google Scholar]
- ASTM. D4327-11, Test Method for Anions in Water by Chemically Suppressed Ion Chromatography; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM. D6919-09, Test Method for Determination of Dissolved Alkali and Alkaline Earth Cations and Ammonium in Water and Wastewater by Ion Chromatography; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2011, 6, 610–618. [Google Scholar] [CrossRef]
- Viancelli, A.; Kunz, A.; Steinmetz, R.L.; Kich, J.D.; Souza, C.K.; Canal, C.W.; Coldebella, A.; Esteves, P.A.; Barardi, C.R. Performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere 2013, 90, 1539–1544. [Google Scholar] [CrossRef]
- Curtis, T.P.; Mara, D.D.; Silva, S.A. Influence of pH, oxygen, and humic substances on ability of sunlight to damage fecal coliforms in waste stabilization pond water. Appl. Environ. Microbiol. 1992, 58, 1335–1343. [Google Scholar] [PubMed]
- Curtis, T. Bacterial removal in wastewater treatment plants. In Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 477–490. [Google Scholar]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Odey, E.A.; Li, Z.; Zhou, X.; Yan, Y. Optimization of lactic acid fermentation for pathogen inactivation in fecal sludge. Ecotoxicol. Environ. Saf. 2018, 157, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.K.; Pinyon, J.L.; Anantham, S.; Hall, R.M. Commensal Escherichia coli of healthy humans: A reservoir for antibiotic-resistance determinants. J. Med. Microbiol. 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Ducey, T.F.; Hunt, P.G. Microbial community analysis of swine wastewater anaerobic lagoons by next-generation DNA sequencing. Anaerobe 2013, 21, 50–57. [Google Scholar] [CrossRef]
- Sharma, J.; Singh, R. Effect of nutrients supplementation on anaerobic sludge development and activity for treating distillery effluent. Bioresour. Technol. 2001, 79, 203–206. [Google Scholar] [CrossRef]
- Schnürer, A.; Zellner, G.; Svensson, B.H. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol. Ecol. 1999, 29, 249–261. [Google Scholar] [CrossRef]
- Whitehead, T.R.; Cotta, M.A. Characterisation and comparison of microbial populations in swine faeces and manure storage pits by 16S rDNA gene sequence analyses. Anaerobe 2001, 7, 181–187. [Google Scholar] [CrossRef]
- Imhoff, J.F. The Chromatiaceae; Springer: New York, NY, USA, 2006; pp. 846–873. [Google Scholar]
- Sletten, O.; Singer, R.H. Sulfur bacteria in red lagoons. J. Water Pollut. Control Fed. 1971, 43, 2118–2122. [Google Scholar]
- Ghosh, W.; Dam, B. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 2009, 33, 999–1043. [Google Scholar] [CrossRef]
- Kuever, J.; Galushko, A. The Family Desulfomicrobiaceae; Springer: Berlin/Heidelberg, Germany, 2014; pp. 97–102. [Google Scholar]
- Belila, A.; Abbas, B.; Fazaa, I.; Saidi, N.; Snoussi, M.; Hassen, A.; Muyzer, G. Sulfur bacteria in wastewater stabilization ponds periodically affected by the ‘red-water’ phenomenon. Appl. Microbiol. Biotechnol. 2012, 97, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Phan, H.V.; Bustamante, H.; Guo, W.; Ngo, H.H.; Nghiem, L.D. Effects of shearing on biogas production and microbial community structure during anaerobic digestion with recuperative thickening. Bioresour. Technol. 2017, 234, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Smithfield Foods Announces Landmark Investment to Reduce Greenhouse Gas Emissions. Available online: https://www.smithfieldfoods.com/press-room/company-news/smithfield-foods-announces-landmark-investment-to-reduce-greenhouse-gas-emissions (accessed on 14 June 2019).
| Season | System | Site | pH | DO | Temp | Total Suspended Soils | Volatile Suspended Solids | Total Kjeldahl Nitrogen |
|---|---|---|---|---|---|---|---|---|
| mg L−1 | °C | mg L−1 | ||||||
| Spring | Open | Lagoon | 8.11 a1 | 0.60 bcde | 20.5 i | 189 e | 166 d | 630 gh |
| House | 7.24 d | 0.96 a | 21.5 h | 3200 e | 3125 d | 1741 e | ||
| Cover | Lagoon | 6.92 h | 0.66 bcd | 20.6 i | 293 e | 289 d | 906 fg | |
| House | 7.33 def | 0.86 ab | 26.1 c | 6275 de | 5975 cd | 2445 d | ||
| Summer | Open | Lagoon | 7.86 b | 0.92 a | 27.3 b | 149 e | 124 d | 276 i |
| House | 7.24 efg | 0.25 gh | 28.3 a | 15,600 d | 13,900 c | 1850 e | ||
| Cover | Lagoon | 7.16 fg | 0.53 def | 27.0 b | 410 e | 285 d | 985 f | |
| House | 6.67 i | 0.23 h | 27.5 b | 100,100 a | 89,600 a | 4823 a | ||
| Fall | Open | Lagoon | 7.72 bc | 0.40 efgh | 20.9 i | 276 e | 237 d | 399 hi |
| House | 7.35 def | 0.33 fgh | 22.2 k | 325 e | 265 d | 546 hi | ||
| Cover | Lagoon | 7.28 efg | 0.35 fgh | 21.9 gh | 483 e | 410 d | 1110 f | |
| House | 7.04 gh | 0.40 defgh | 24.1 e | 51,400 b | 44,850 b | 3848 b | ||
| Winter | Open | Lagoon | 7.76 bc | 0.55 cdef | 23.3 f | 467 e | 413 d | 588 ghi |
| House | 7.36 def | 0.81 abc | 25.6 d | 1927 e | 1755 d | 1088 f | ||
| Cover | Lagoon | 7.41 de | 0.53 def | 19.1 j | 427 e | 373 d | 1033 f | |
| House | 7.13 fgh | 0.53 cdefg | 25.5 d | 41,375 c | 37,250 b | 3410 c |
| Season | System | Site | Cl | NH4-N | PO4-P | SO4-S | Ca | K | Mg | Na |
|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Open | Lagoon | 491.8 f2 | 490.0 h | 18.7 efg | 41.3 c | 74.5 g† | 720.1 fgh | 29.6 f | 254.4 e |
| House | 750.8 c | 1182.2 c | 33.3 cdef | 8.4 d | 123.6 bcd | 983.8 c | 34.9 cdef | 389.8 b | ||
| Cover | Lagoon | 444.6 g | 842.4 de | 94.0 b | 5.4 d | 113.7 cd | 658.5 ghi | 57.2 cd | 214.9 f | |
| House | 918.3 a | 1420.0 b | 47.5 cd | 63.8 b | 82.3 efg | 1363.9 b | 43.7 cdef | 431.2 a | ||
| Summer | Open | Lagoon | 407.5 h | 204.1 j | 7.9 g | 40.0 c | 48.4 h | 598.4 i | 35.9 def | 195.4 f |
| House | 543.0 e | 639.8 g | 10.5 fg | 9.3 d | 74.4 g | 831.1 de | 51.6 cdef | 259.9 e | ||
| Cover | Lagoon | 440.7 gh | 807.7 ef | 8.4 g | 6.5 d | 26.1 i | 644.1 hi | 33.8 ef | 206.4 f | |
| House | 665.8 d | 754.2 f | 25.5 cdefg | 2.9 d | 191.3 a | 1038.9 c | 60.2 cd | 345.1 c | ||
| Fall | Open | Lagoon | 423.6 gh | 312.5 i | 16.5 fg | 39.0 c | 65.2 gh | 666.3 ghi | 46.9 cdef | 203.6 f |
| House | 489.7 f | 426.3 h | 21.5 defg | 32.7 c | 85.9 efg | 758.6 ef | 52.9 cdef | 239.0 e | ||
| Cover | Lagoon | 430.0 gh | 885.1 d | 39.5 cde | 4.0 d | 103.0 de | 708.8 fgh | 55.9 cde | 198.0 f | |
| House | 828.3 b | 1580.4 a | 100.8 b | 118.6 a | 198.6 a | 1562.5 a | 104.2 b | 424.9 a | ||
| Winter | Open | Lagoon | 424.8 gh | 509.2 h | 17.8 efg | 2.8 d | 77.8 fg | 690.4 fgh | 44.6 cdef | 200.3 f |
| House | 579.7 e | 886.2 de | 46.3 cd | BDL3 | 101.4 def | 883.7 d | 62.2 c | 293.4 d | ||
| Cover | Lagoon | 433.3 gh | 898.0 de | 46.7 cd | 2.2 d | 134.8 bc | 736.9 fg | 54.8 cde | 203.9 f | |
| House | 777.2 c | 1512.3 ab | 217.9 a | 46.5 c | 140.9 b | 1627.7 a | 290.5 a | 422.7 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducey, T.F.; Rashash, D.M.C.; Szogi, A.A. Differences in Microbial Communities and Pathogen Survival Between a Covered and Uncovered Anaerobic Lagoon. Environments 2019, 6, 91. https://doi.org/10.3390/environments6080091
Ducey TF, Rashash DMC, Szogi AA. Differences in Microbial Communities and Pathogen Survival Between a Covered and Uncovered Anaerobic Lagoon. Environments. 2019; 6(8):91. https://doi.org/10.3390/environments6080091
Chicago/Turabian StyleDucey, Thomas F., Diana M. C. Rashash, and Ariel A. Szogi. 2019. "Differences in Microbial Communities and Pathogen Survival Between a Covered and Uncovered Anaerobic Lagoon" Environments 6, no. 8: 91. https://doi.org/10.3390/environments6080091
APA StyleDucey, T. F., Rashash, D. M. C., & Szogi, A. A. (2019). Differences in Microbial Communities and Pathogen Survival Between a Covered and Uncovered Anaerobic Lagoon. Environments, 6(8), 91. https://doi.org/10.3390/environments6080091






