Nitrogen Mineralization in a Sandy Soil Amended with Treated Low-Phosphorus Broiler Litter
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Sources of Broiler Litter
2.3. Incubations
2.4. Chemical Analysis
2.4.1. Soil and Broiler Litter Material Properties
2.4.2. Gas Analysis
2.5. Data Analysis
3. Results
3.1. Broiler Litter Materials Used in the Soil Incubation
3.2. Emissions of CO2, NH3, and N2O during Soil Incubation
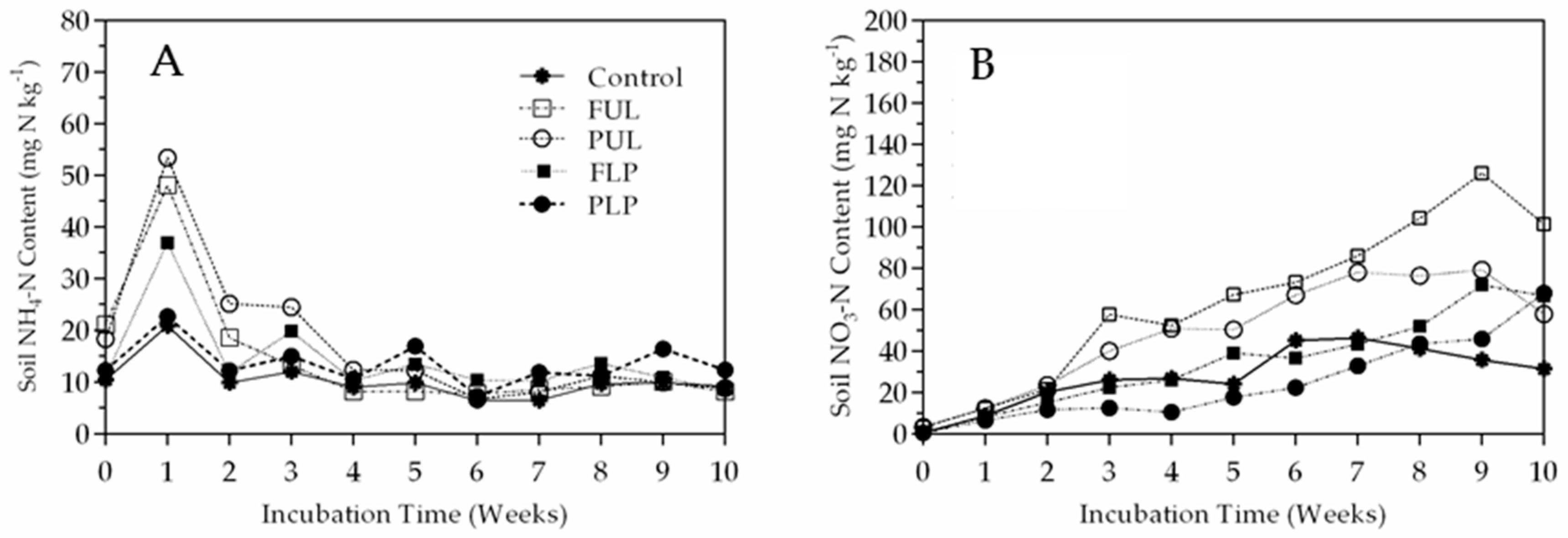
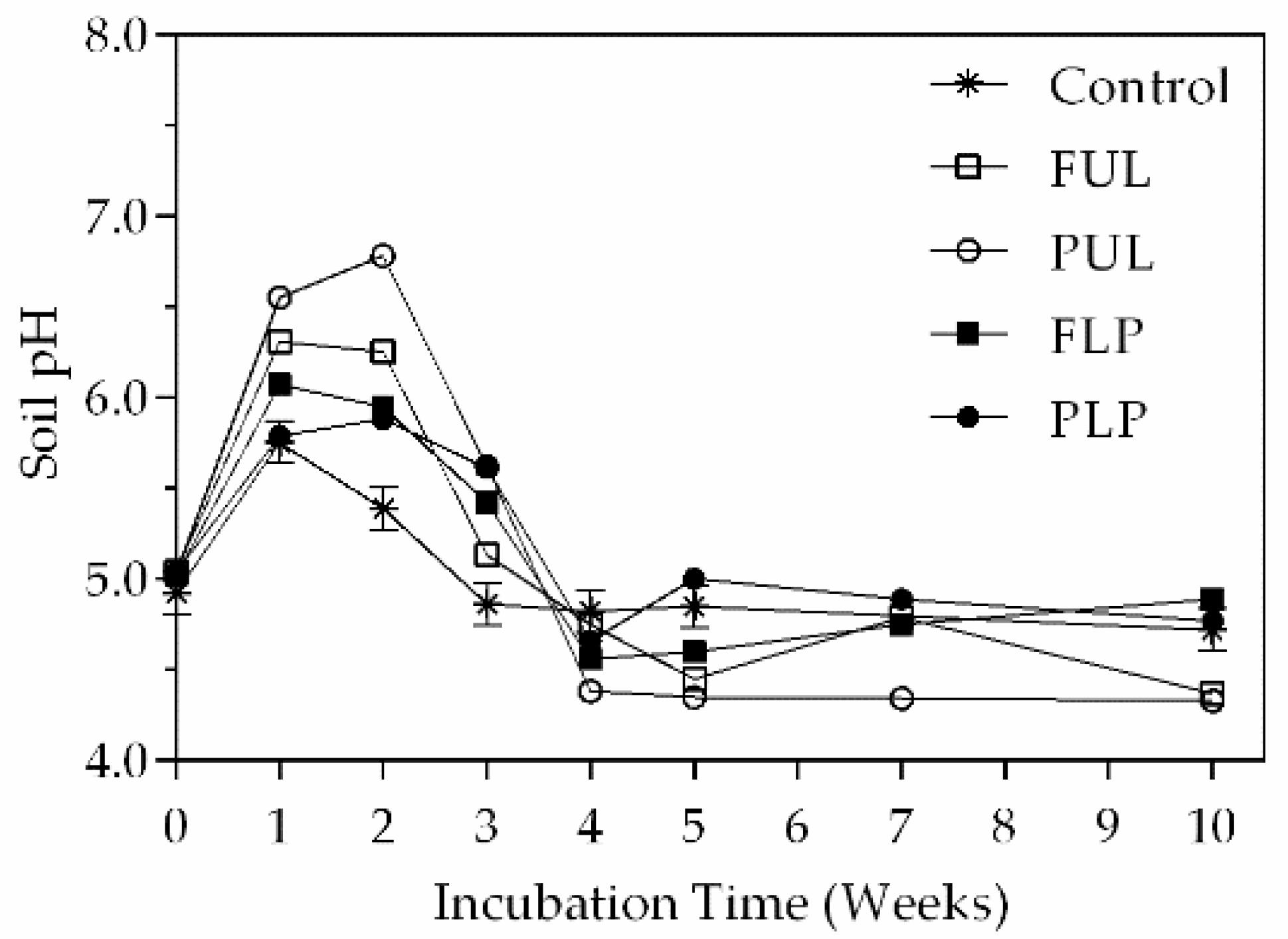
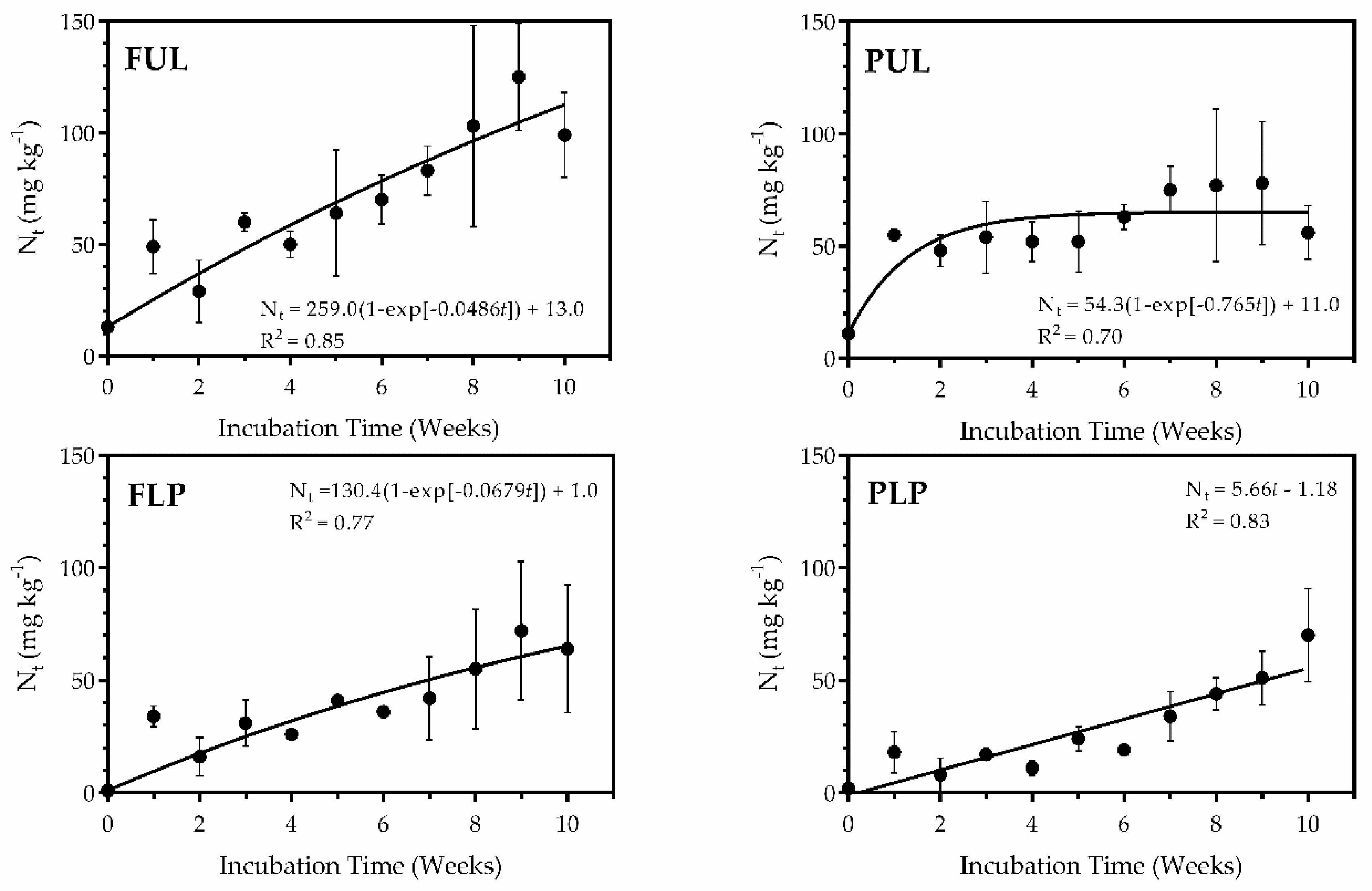
3.3. Nitrogen Mineralization
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and management of poultry litter. World Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef]
- Lin, Y.; Watts, D.B.; van Santen, E.; Cao, G. Influence of poultry litter on crop productivity under different field conditions: A meta-analysis. Agron. J. 2018, 110, 807–818. [Google Scholar] [CrossRef]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.; Capelari, M. Measurement and mitigation of reactive nitrogen species from swine and poultry production. J. Anim. Sci. 2017, 95, 2236–2240. [Google Scholar] [PubMed]
- Huang, T.; Gao, B.; Hu, X.-K.; Lu, X.; Well, R.; Christie, P.; Bakken, L.R.; Ju, X.-T. Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci. Rep. 2014, 4, 3950. [Google Scholar] [CrossRef] [PubMed]
- Sikora, L.J.; Enkiri, N.K. Comparison of phosphorus uptake from poultry litter compost with triple superphosphate in codorus soil. Agron. J. 2005, 97, 668–673. [Google Scholar] [CrossRef]
- Paudel, K.P.; McIntosh, C.S. Country report: Broiler industry and broiler litter-related problems in the southeastern United States. Waste Manag. 2005, 25, 1083–1088. [Google Scholar] [CrossRef]
- Bernhart, M.; Fasina, O.; Fulton, J.; Wood, C. Compaction of poultry litter. Bioresour. Technol. 2010, 101, 234–238. [Google Scholar] [CrossRef]
- Pote, D.; Meisinger, J.J. Effect of poultry litter application method on ammonia volatilization from a conservation tillage system. J. Soil Water Conserv. 2014, 69, 17–25. [Google Scholar] [CrossRef]
- Adeli, A.; McCarty, J.C.; Read, J.J.; Willers, J.L.; Feng, G.; Jenkins, J.N. Subsurface band placement of pelletized poultry litter in cotton. Agron. J. 2016, 108, 1356–1366. [Google Scholar] [CrossRef]
- Bauer, P.J.; Szogi, A.A.; Shumaker, P.D. Fertilizer efficacy of poultry litter ash blended with lime or gypsum as fillers. Environments 2019, 6, 50. [Google Scholar] [CrossRef]
- Marchioro, V.; Steinmetz, R.L.R.; do Amaral, A.C.; Gaspareto, T.C.; Treichel, H.; Kunz, A. Poultry itter solid state anaerobic digestion: Effect of digestate recirculation intervals and substrate/inoculum ratios on process efficiency. Front. Sustain. Food Syst. 2018, 2, 46. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, P.; Savin, M.; Moore, P., Jr. Long-term applications of untreated and alum-treated poultry litter drive soil nitrogen concentrations and associated microbial community dynamics. Biol. Fertil. Soils 2014, 51, 43–55. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B.; Hunt, P.G. Process for Removing and Recovering Phosphorus from Animal Waste. U.S. Patent No. 8,673,046, 18 March 2014. [Google Scholar]
- Szögi, A.A.; Vanotti, M.B.; Hunt, P.G. Phosphorus recovery from poultry litter. Trans. ASABE 2008, 51, 1727–1734. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Cheng, S.C.; Merka, W.C.; Pancorbo, O.C.; Thompson, S.A. Nitrous oxide and carbon dioxide emissions from pelletized in non-pelletized poultry litter incorporated into soil. Plant Soil 1994, 163, 189–196. [Google Scholar] [CrossRef]
- Kim, S.U.; Ruangcharus, C.; Kumar, S.; Lee, H.H.; Park, H.J.; Jung, E.S.; Hong, C.O. Nitrous oxide emission from upland soil amended with different animal manures. Appl. Biol. Chem. 2019, 62, 8. [Google Scholar] [CrossRef]
- Sharpe, R.; Schomberg, H.; Harper, L.; Endale, D.; Jenkins, M.; Franzluebbers, A. Ammonia volatilization from surface-applied poultry litter under conservation tillage management practices. J. Environ. Qual. 2004, 33, 1183–1188. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Bauer, P.J.; Frederick, J.R.; Busscher, W.J.; Novak, J.M.; Fortnum, B.A. Soil sampling for fertilizer recommendations in conservation tillage with paratill subsoiling. Crop Manag. 2008, 7. [Google Scholar] [CrossRef]
- Clemson University. Regulatory Services, Soil Test Rating System; Ag Service Lab, Clemson University: Clemson, SC, USA, 2019; Available online: https://www.clemson.edu/public/regulatory/ag-srvc-lab/soil-testing/pdf/rating-system.pdf (accessed on 13 July 2019).
- Linn, D.M.; Doran, J.W. Effect of water-field pore space on carbon dioxide and nitrous oxide production in tilled and non-tilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Mahimairaja, S.; Bolan, N.S.; Hedley, M.J.; McGregor, A.N. Evaluation of methods of measurement of nitrogen in poultry and animal manure. Nutr. Cycl. Agroecosyst. 1990, 24, 141–148. [Google Scholar] [CrossRef]
- Lahav, O.; Mor, T.; Heber, A.J.; Molchanov, S.; Ramirez, J.C.; Li, C.; Broday, D.M. A new approach for minimizing ammonia emissions from poultry houses. Water Air Soil Pollut. 2008, 191, 183–197. [Google Scholar] [CrossRef]
- Sikora, F.J.; Moore, K.P. Soil Test Methods from the South Eastern United States; SERA-IEG-6; Southern Cooperative Series Bulletin: Lexington, KY, USA, 2014. [Google Scholar]
- Sims, G.K.; Ellsworth, T.R.; Mulvaney, R.L. Microscale determination of organic nitrogen in water and soil extracts. Commun. Soil Sci. Plant Anal. 1995, 26, 303–316. [Google Scholar] [CrossRef]
- Duvekot, C. Analysis of greenhouse gases by gas chromatography. In Agilent Technologies Application Note SI-01741; Agilent Technologies: Santa Clara, CA, USA, 2010. [Google Scholar]
- ASTM. Test method for determination of dissolved alkali and alkaline earth cations and ammonium in water and waste water by iron chromatography. In Annual Book of Standards. VOL 11.02; ASTM Standard D6919-09; American Society for Testing and Materials: Washington, DC, USA, 2009. [Google Scholar]
- Wagner, S.W.; Reicosky, D.C.; Alessi, R.S. Regression models for calculating gas fluxes measured with a closed chamber. Agron. J. 1997, 89, 279–284. [Google Scholar] [CrossRef]
- Venterea, R. Simplified method for quantifying theoretical underestimation of chamber—Based trace gas fluxes. J. Environ. Qual. 2010, 39, 126–135. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T. Sampling Protocols. Chamber-based trace gas flux measurements. In Sampling Protocols; Follet, R.F., Ed.; USDA-ARS Gracenet: Washington, DC, USA, 2010; Chapter 3; pp. 3-1–3-9. [Google Scholar]
- Gezan, S.A.; Carvalho, M. Chapter 10: Analysis of repeated measures for the biological and agricultural sciences. In Applied Statistics in Agricultural, Biological, and Environmental Sciences; Glaz, B., Yeater, K.M., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 279–297. [Google Scholar]
- Hadas, A.; Feigembaum, S.; Feigin, A.; Portnoy, R. Nitrification rates in profiles of differently managed soil types. Soil Sci. Soc. Am. J. 1986, 50, 633–639. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers. An Introduction to Nutrient Management, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Moore, A.D.; Mikkelsen, R.L.; Israel, D.W. Nitrogen mineralization of anaerobic swine lagoon sludge as influenced by seasonal tempertures. Commun. Soil Sci. Plant Anal. 2004, 35, 991–1005. [Google Scholar] [CrossRef]
- Sharpley, A.; Slaton, N.; Tabler, T.; VanDevender, K.; Daniels, M.; Jones, F.; Daniels, T. Nutrient Analysis of Poultry Litter; University of Arkansas System, Agriculture and Natural Resources, Research and Extension: Little Rock, AR, USA, 2009. [Google Scholar]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Velthoh, G.L.; Mondini, C.; Sinicco, T.; Van Groeningen, J.W. Nitrous oxide and carbon dioxide emissions during initial decomposition of animal by-products applied as fertilizers to soils. Geoderma 2010, 157, 235–242. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Chiang, S.C.; Merka, W.C.; Pancorbo, O.C.; Thompson, S.A. Pelletizing and soil water effects on gaseous emissions from surface-applied poultry litter. Soil Sci. Soc. Am. J. 1994, 58, 807–811. [Google Scholar] [CrossRef]
- Hayakawa, A.; Akiyama, H.; Sudo, S.; Yagi, K. N2O and NO emissions from an Andisol field as influenced by pelleted poultry manure. Soil Biol. Biochem. 2009, 41, 521–529. [Google Scholar] [CrossRef]
- Cook, K.L.; Rothrock, M.J.; Eiteman, M.A.; Lovanh, N.; Sistani, K. Evaluation of nitrogen retention and microbial populations in poultry litter treated with chemical, biological or adsorbent aamendments. J. Environ. Manag. 2011, 92, 1760–1766. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Cook, K.L.; Warren, J.G.; Sistani, K. Microbial mineralization of organic nitrogen forms in poultry litter. J. Environ. Qual. 2010, 39, 1848–1857. [Google Scholar] [CrossRef]
- Burt, C.D.; Cabrera, M.L.; Rothrock, M.J.; Kissel, D.E. Flue-gas desulfurization effects on urea-degrading bacteria and ammonia volatilization from broiler litter. Poult. Sci. 2017, 6, 2676–2683. [Google Scholar] [CrossRef]
- Nahm, K.H. Factors influencing nitrogen mineralization during poultry litter composting and calculations for available nitrogen. World Poult. Sci. J. 2005, 61, 238–255. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Cavagnaro, T.R.; Ngo, H.T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil amended with high and low C/N residue–Influence of interval between residue additions. Soil Biol. Biochem. 2016, 95, 189–197. [Google Scholar] [CrossRef]
| Depth cm | Cg kg−1 | Nmg kg−1 | NH4-N mg kg−1 | NO3-N mg kg−1 | P mg kg−1 | K mg kg−1 | CEC cmol kg−1 | pH |
|---|---|---|---|---|---|---|---|---|
| 0–7.5 | 10.9 | 891 | 10.4 | 0.54 | 33 | 71 | 4.2 | 5.3 |
| 7.5–15 | - | - | - | - | 17 | 46 | 3.2 | 5.3 |
| Broiler Litter Material | C g kg−1 | N g kg−1 | P g kg−1 | NH4-N mg kg−1 | NO3-N mg kg−1 | C:N Ratio | N:P Ratio | pH |
|---|---|---|---|---|---|---|---|---|
| FUL | 434 | 41.2 | 11.1 | 4891 | 1281 | 10.5 | 3.7 | 7.95 |
| PUL | 434 | 40.9 | 15.1 | 3618 | 1335 | 10.6 | 2.7 | 7.95 |
| FLP | 479 | 26.4 | 6.2 | 159 | BD 1 | 18.1 | 4.3 | 6.31 |
| PLP | 499 | 24.8 | 4.2 | 333 | 20 | 20.10 | 5.9 | 5.36 |
| N Source | CO2 | NH3 | N2O | |||
|---|---|---|---|---|---|---|
| g C kg−1 | % 1 | mg N kg−1 | % 1 | mg N kg−1 | % 1 | |
| FUL | 1.2a 2 | 61a | 21.5ab | 24ab | 3.0b | 3.3b |
| PUL | 1.1a | 56a | 31.0a | 35a | 6.8a | 7.5a |
| FLP | 1.5a | 38a | 5.1b | 5.7b | 1.7b | 1.9b |
| PLP | 2.1a | 36a | 3.7b | 4.1b | 0.4b | 0.5b |
| LSD0.05 | 1.2 | 34 | 25 | 28 | 3.1 | 3.5 |
| Source | NH4-N | NO3-N | |||
|---|---|---|---|---|---|
| df | F-value | p-value 1 | F-value | p-value | |
| Trt | 4 | 8.7 | 0.0028 * | 19.4 | <0.0001 * |
| Week | 10 | 83.4 | <0.0001 * | 22.1 | <0.0001 * |
| Trt*Week | 40 | 8.2 | <0.0001 * | 1.7 | 0.0309 * |
| Combined Trt effect per week | |||||
| Time (Week) | df | SEM | p-value | SEM | p-value |
| 0 | 4 | 14.4 | 0.0008 * | 1.80 | 0.9995 |
| 1 | 4 | 36.5 | <0.0001 * | 9.1 | 0.9739 |
| 2 | 4 | 17.5 | <0.0001 * | 17.3 | 0.6836 |
| 3 | 4 | 16.9 | 0.0001 * | 31.9 | 0.0056 * |
| 4 | 4 | 10.3 | 0.7326 | 33.4 | 0.0052 * |
| 5 | 4 | 12.1 | 0.0393 | 39.8 | 0.0011 * |
| 6 | 4 | 7.8 | 0.7217 | 48.9 | 0.0005 * |
| 7 | 4 | 9.0 | 0.4754 | 57.3 | <0.0001 * |
| 8 | 4 | 11.2 | 0.6946 | 63.5 | <0.0001 * |
| 9 | 4 | 11.5 | 0.1612 | 71.9 | <0.0001 * |
| 10 | 4 | 9.5 | 0.6677 | 65.2 | <0.0001 * |
| N Source | a 1 | K | [NO3]0 | Kmax | R 2 |
|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 wk−1 | ||||
| FUL | 119 | 0.0043 | 11.3 | 15.2 | 0.93 3 |
| PUL | 73 | 0.0107 | 6.4 | 14.3 | 0.93 * |
| FLP | 87 | 0.0043 | 7.2 | 8.2 | 0.95 * |
| PLP | -- 2 | -- | -- | 5.8 | 0.88 * |
| Control | -- | -- | -- | 3.5 | 0.64 * |
| Time (Week) | FUL 1 | PUL | FLP | PLP |
|---|---|---|---|---|
| 0 | 15a 2 | 12a | 1c | 2d |
| 1 | 55a | 61a | 38b | 21c |
| 2 | 32b | 53a | 18bc | 9c |
| 3 | 68a | 60a | 35b | 19b |
| 4 | 56a | 58a | 29b | 12c |
| 5 | 72a | 58ab | 46ab | 27b |
| 6 | 78a | 70a | 40b | 21c |
| 7 | 87a | 84a | 47b | 38b |
| 8 | 87a | 86a | 60a | 49a |
| 9 | 127a | 87ab | 80ab | 57b |
| 10 | 99a | 78a | 72a | 63a |
| Mean | 71 | 64 | 42 | 29 |
| Standard deviation | 31 | 21 | 23 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szogi, A.A.; Shumaker, P.D.; Ro, K.S.; Sigua, G.C. Nitrogen Mineralization in a Sandy Soil Amended with Treated Low-Phosphorus Broiler Litter. Environments 2019, 6, 96. https://doi.org/10.3390/environments6080096
Szogi AA, Shumaker PD, Ro KS, Sigua GC. Nitrogen Mineralization in a Sandy Soil Amended with Treated Low-Phosphorus Broiler Litter. Environments. 2019; 6(8):96. https://doi.org/10.3390/environments6080096
Chicago/Turabian StyleSzogi, Ariel A., Paul D. Shumaker, Kyoung S. Ro, and Gilbert C. Sigua. 2019. "Nitrogen Mineralization in a Sandy Soil Amended with Treated Low-Phosphorus Broiler Litter" Environments 6, no. 8: 96. https://doi.org/10.3390/environments6080096
APA StyleSzogi, A. A., Shumaker, P. D., Ro, K. S., & Sigua, G. C. (2019). Nitrogen Mineralization in a Sandy Soil Amended with Treated Low-Phosphorus Broiler Litter. Environments, 6(8), 96. https://doi.org/10.3390/environments6080096








