Effect of the Type of Gas-Permeable Membrane in Ammonia Recovery from Air
Abstract
:1. Introduction
2. Materials and Methods
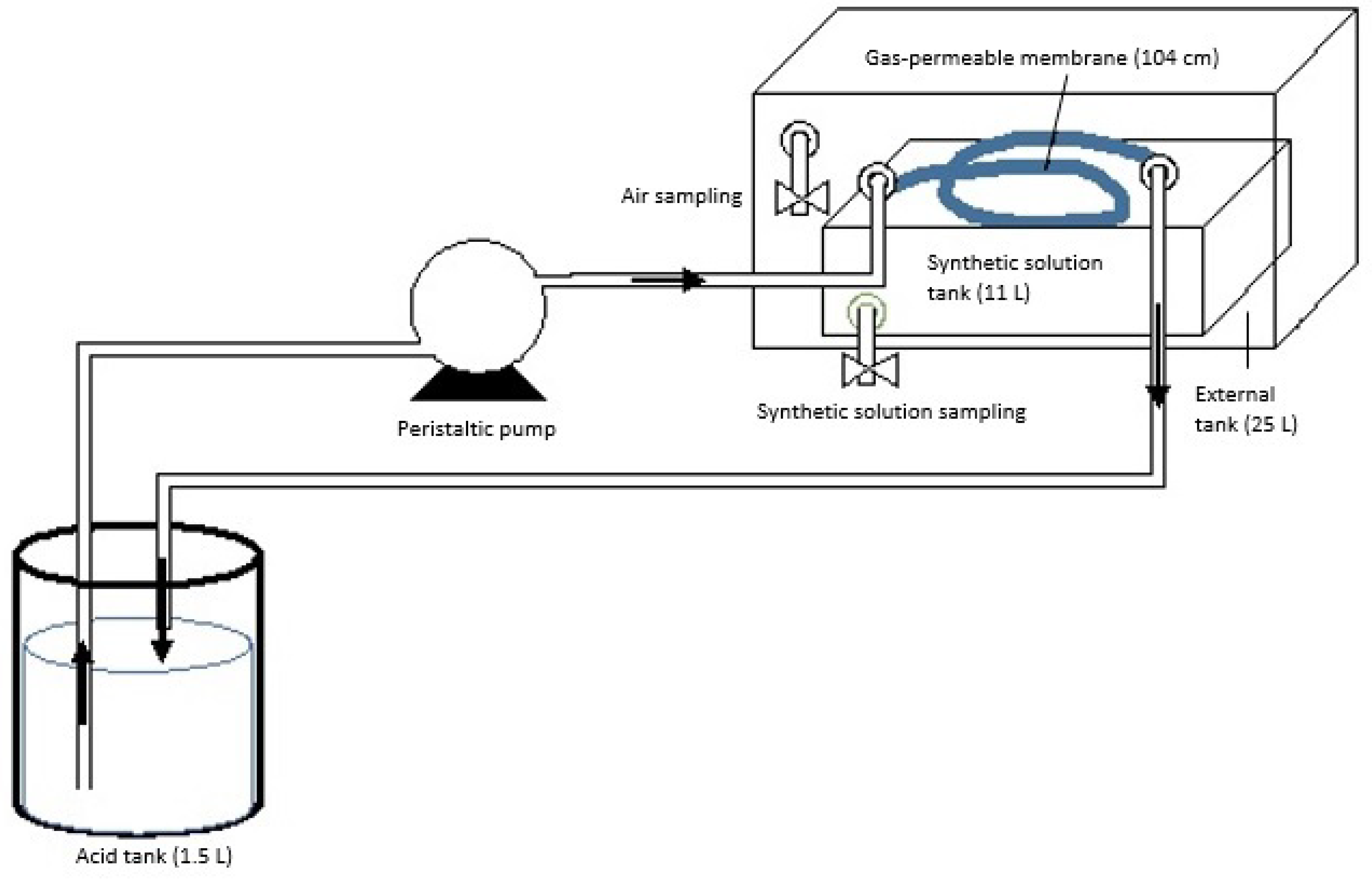
2.1. Experimental Design
2.2. Methodology for Analyses
2.3. Mass Flow Calculation
3. Results and Discussion
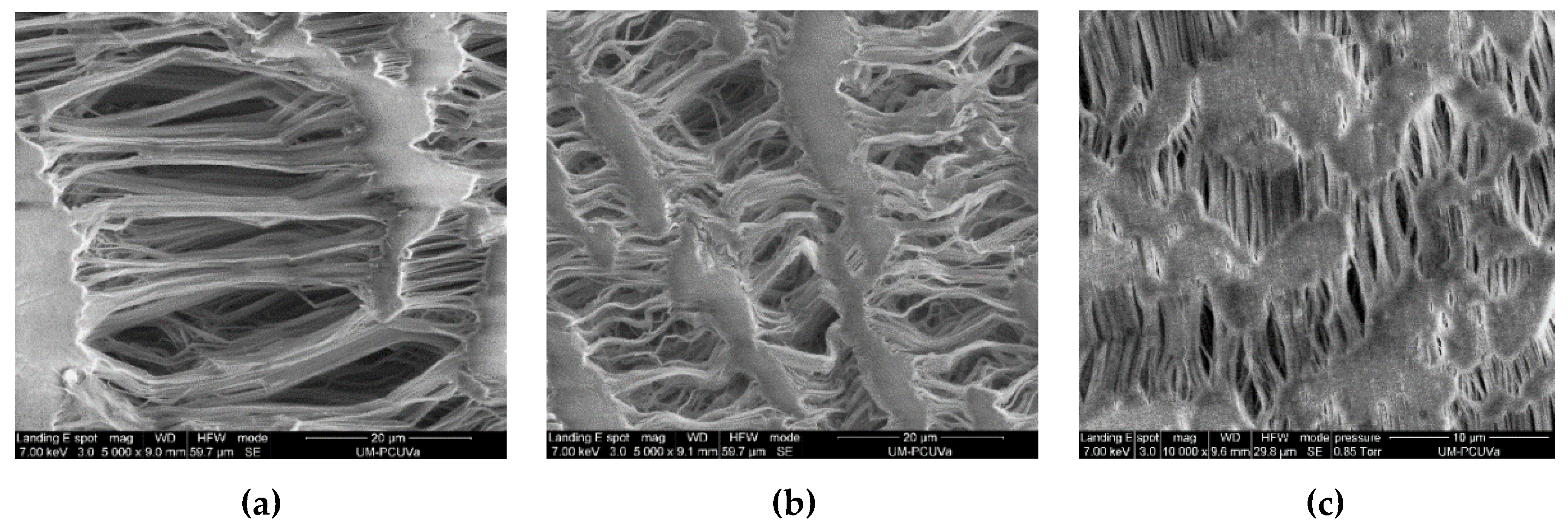
3.1. Characterization of Membranes
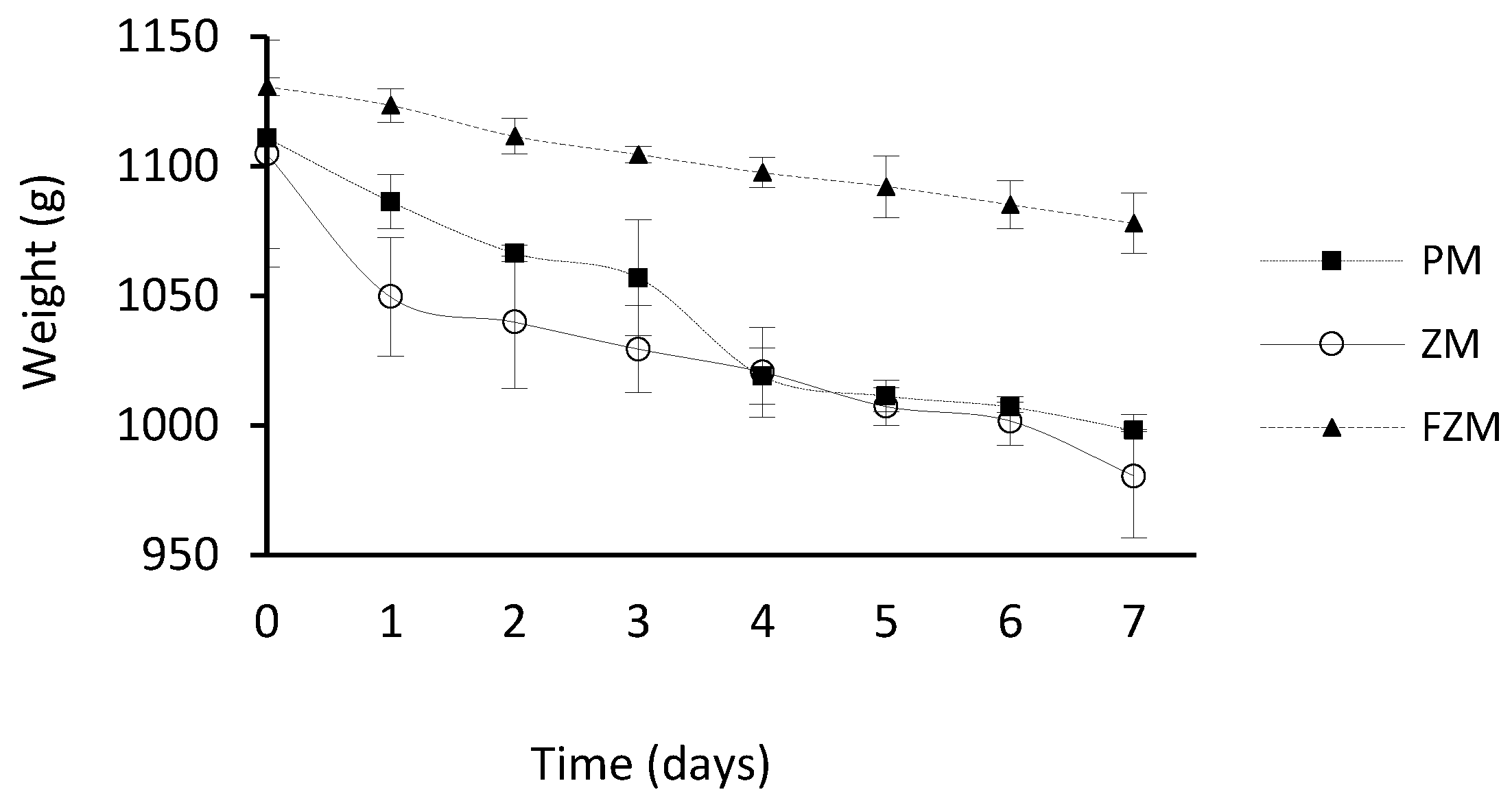
3.2. Variation of the Weight of the Acidic Solution
3.3. Process pH in the N Capturing Acidic Solution and N Emitting Synthetic Solution
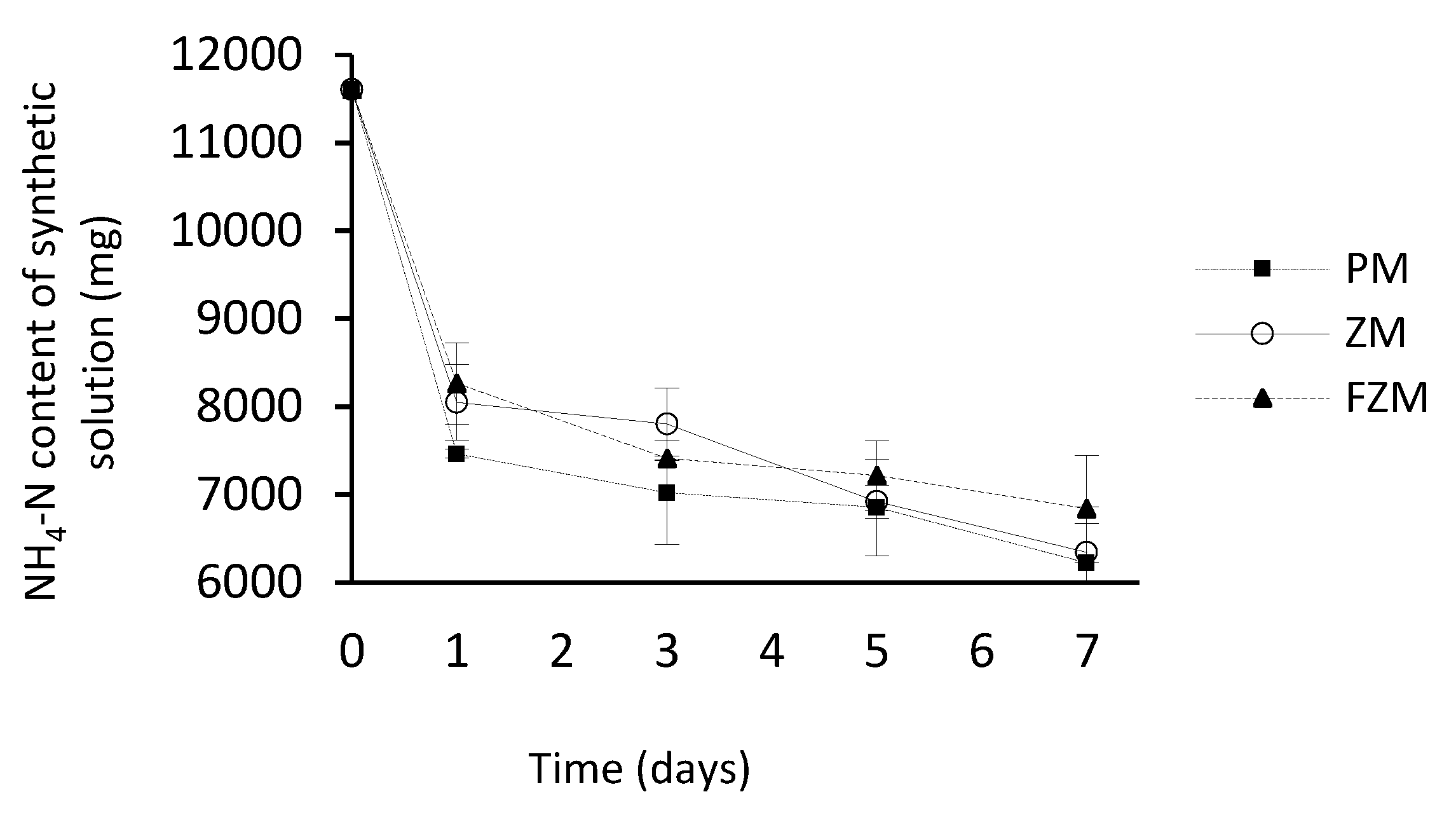
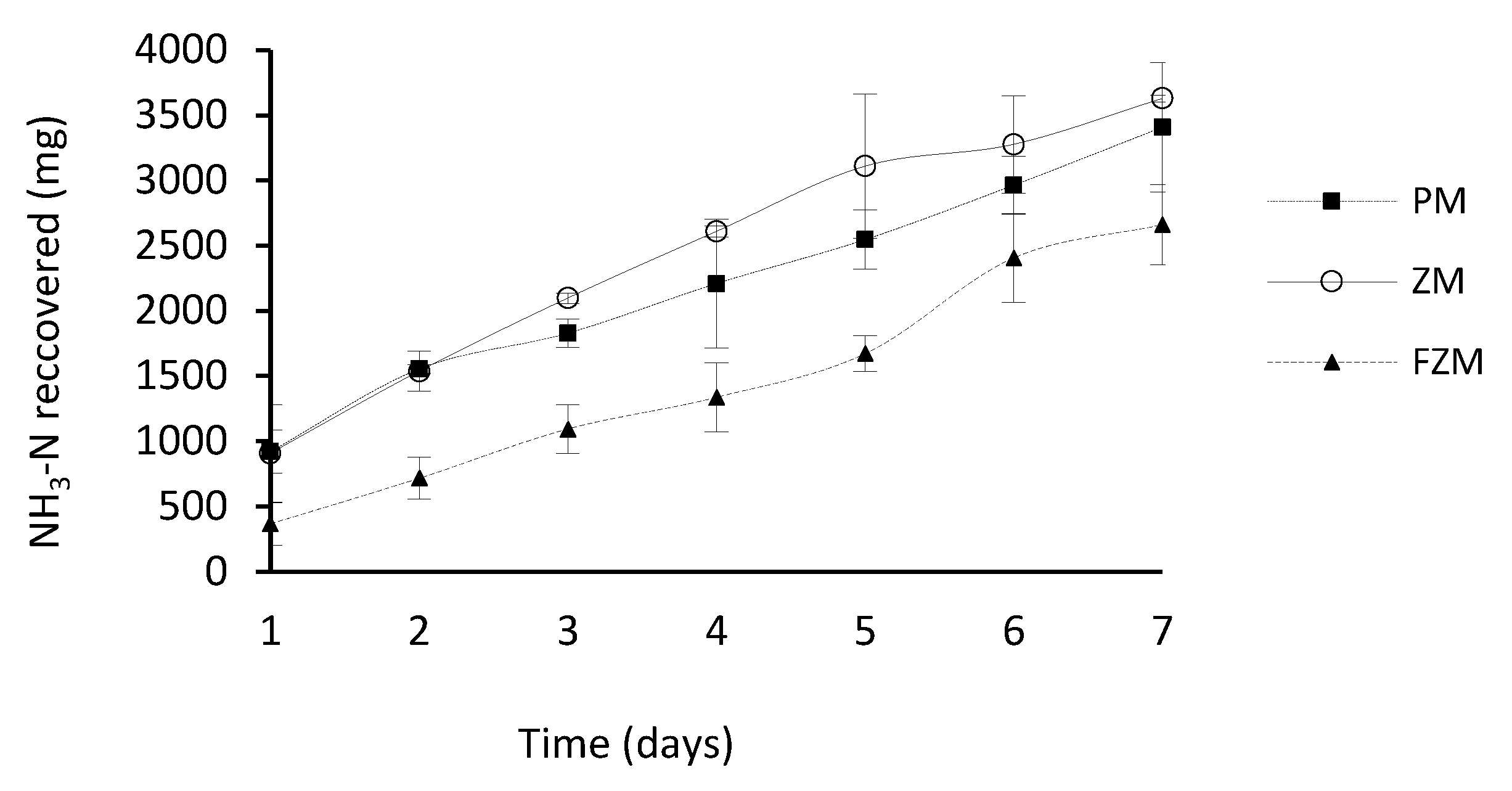
3.4. Effect of the Type of Membrane on Ammonia Capture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beusen, A.; Bouwman, A.; Heuberger, P.; Van Drecht, G.; Van Der Hoek, K. Bottom-up uncertainty estimates of global ammonia emissions from global agricultural production systems. Atmos. Environ. 2008, 42, 6067–6077. [Google Scholar] [CrossRef]
- Erisman, J.W.; Bleeker, A.; Hensen, A.; Vermeulen, A. Agricultural air quality in Europe and the future perspectives. Atmos. Environ. 2008, 42, 3209–3217. [Google Scholar] [CrossRef] [Green Version]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing? Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.F.; Van Vuuren, D.P.; Derwent, R.G.; Posch, M. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut. 2002, 141, 349–382. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). European Union Emission Inventory Report 1990–2016 under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP); ISNN: Cambridge, UK, 2018; pp. 1977–8449.
- MAPAMA (Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente). Inventario de Emisiones de España Emisiones de Contaminantes en el Marco de la Directiva de Techos Nacionales de Emisión Serie 1990–2015; MAPAMA: Madrid, Spain, 2017. Available online: http://www.mapama.gob.es/es/calidad-y-evaluacion-ambiental/temas/sistema-espanol-de-inventario-sei-/documentoresumeninventariotechosespana-serie1990-2015_tcm30-378885.pdf (accessed on 5 March 2019).
- EU (European Union). Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the Reduction of National Emissions of Certain Atmospheric Pollutants, Amending Directive 2003/35/EC and Repealing Directive 2001/81/EC; EU: Brussels, Belgium, 2016. [Google Scholar]
- Cho, M.S.; Ko, H.J.; Kim, D.; Kim, K.Y. On-site application of air cleaner emitting plasma ion to reduce airborne contaminants in pig building. Atmos. Environ. 2012, 63, 276–281. [Google Scholar] [CrossRef]
- Koerkamp, P.W.G.; Metz, J.H.M.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.; Hartung, J.; Seedorf, J.; et al. Concentration and emission of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Schauberger, G.; Piringer, M.; Heber, A.J. Odour emission scenarios for fattening pigs as input for dispersion models: A step from an annual mean Value to time series. Agric. Ecosyst. Environ. 2014, 193, 108–116. [Google Scholar] [CrossRef]
- García-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A. Systems and Methods for Reducing Ammonia Emissions from Liquid Effluents and for Recovering Ammonia. U.S. Patent 9,005,333 B1, 29 October 2015. [Google Scholar]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J.L. Liquid fertilizer production by ammonia recovery from treated ammonia-rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; He, D.; Waite, T.D. Capacitive Membrane Stripping for Ammonia Recovery (CapAmm) from Dilute Wastewaters. Environ. Sci. Technol. 2018, 5, 43–49. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A. Use of Gas-Permeable Membranes for the Removal and Recovery of Ammonia from High Strength Livestock Wastewater. Proc. Water Environ. Fed. 2011, 2011, 659–667. [Google Scholar] [CrossRef]
- Nelson, N.O.; Mikkelsen, R.L.; Hesterberg, D.L. Struvite formation to remove phosphorus from anaerobic swine lagoon effluent. In Animal, Agricultural and Food Processing Wastes: Proceedings of the Eighth International Symposium; Moore, J.A., Ed.; American Society of Agricultural Engineers: St. Joseph, MI, USA, 2000; pp. 18–26. [Google Scholar]
- Zarebska, A.; Romero-Nieto, D.; Christensen, K.V.; Fjerbæk Søtoft, L.; Norddahl, B. Ammonium fertilizers production from manure: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B.; Rothrock, M.J. Gaseous ammonia removal system. US Patent 8,906,332 B2, 9 December 2014. [Google Scholar]
- Chien, S.H.; Gearhart, M.M.; Villagarcía, S. Comparison of Ammonium Sulfate with Other Nitrogen and Sulfur Fertilizers in Increasing Crop Production and Minimizing Environmental Impact: A Review. Soil Sci. 2011, 176, 327–335. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Hao, X.; Zhang, J.; Feng, X.; Zhang, H. A novel process for preparing expanded Polytetrafluoroethylene (ePTFE) micro-porous membrane through ePTFE/ePTFE co-stretching technique. J. Mater. Sci. 2007, 42, 2081–2085. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using flat gas permeable membranes. Waste Manage. 2013, 33, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using gas permeable membranes. Trans. ASABE 2010, 53, 1267–1275. [Google Scholar] [CrossRef]
- Majd, A.M.S.; Mukhtar, S.; Kunz, A. Application of Diluted Sulfuric Acid for Manure Ammonia Extraction Using a Gas-Permeable Membrane; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012. [Google Scholar] [CrossRef]
- Majd, A.M.S.; Mukhtar, S. Ammonia Recovery Enhancement Using a Tubular Gas-Permeable Membrane System in Laboratory and Field-Scale Studies. Am. Soc. Agric. Biol. Eng. 2013, 56, 1951–1958. [Google Scholar] [CrossRef]
- Blet, V.; Pons, M.N.; Greffe, J.L. Separation of ammonia with a gas-permeable tubular membrane. Anal. Chim. Acta 1989, 219, 309–311. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous Ammonia Equilibrium Calculations: Effect of pH and Temperature. J. Fish. Res. Board Can. 1975, 32, 4. [Google Scholar] [CrossRef]
- Lahav, O.; Mor, T.; Heber, A.J.; Molchanov, S.; Ramirez, J.C.; Li, C.; Broday, D.M. A new approach for minimizing ammonia emissions from poultry houses. Water Air Soil Pollut. 2008, 191, 183–197. [Google Scholar] [CrossRef]
- Dube, P.J.; Vanotti, M.B.; Szogi, A.A.; Garcia-González, M.C. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Venkataraman, K.; Choate, W.T.; Torre, E.R.; Husung, R.D.; Batchu, H.R. Characterization studies of ceramic membranes. A novel technique using a Coulter Porometer. J. Membr. Sci. 1988, 39, 259. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide, Ver. 9.2; SAS Inst.: Cary, NC, USA, 2008. [Google Scholar]
- Perfilov, V.; Fila, V.; Sanchez Marcano, J. A general predictive model for sweeping gas membrane distillation. Desalination 2018, 443, 285–306. [Google Scholar] [CrossRef]
- Schneider, I.M.; Marison, W.; Stockar, U. Principles of an efficient new method for the removal of ammonia from animal cell cultures using hydrophobic membranes. Enzym. Microb. Technol. 1994, 16, 957–963. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Fillingham, M.; VanderZaag, A.C.; Singh, J.; Burtt, S.; Crolla, A.; Kinsley, C.; MacDonald, J.D. Characterizing the performance of gas-permeable membranes as an ammonia recovery strategy from anaerobically digested dairy manure. Membranes 2017, 7, 59. [Google Scholar] [CrossRef]
| Membrane Characteristics | ZM | FZM | PM | PM | ZM4 |
|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | ||||
| Length (cm) | 104.0 | 70.0 | 104.0 | 46.3 | 100.0 |
| Outer diameter (mm) | 8.6 | 3.0 | 8.6 | 8.6 | 4.1 |
| Width of the wall (mm) | 0.8 | 1.0 | 1.2 | 1.2 | 0.6 |
| Average pore size length (µm) * | 27.6 ± 8.3 | 5.8 ± 0.8 | 14.7 ± 2.3 | 14.7 ± 2.3 | - |
| Average pore size width (µm) * | 7.6 ± 0.9 | 0.7 ± 0.1 | 5.5 ± 0.6 | 5.5 ± 0.6 | - |
| Polymer density (g/cm3) | 0.45 | 1.09 | 0.95 | 0.95 | 0.95 |
| Absorption surface (cm2) | 282.3 | 66.0 | 282.3 | 125.7 | 125.7 |
| Type of Membrane | N° pores (pores/m2) | Porosity (%) | Water permeability (L·min−1) | Air permeability (L·min−1·cm2) | MFP (µm) |
|---|---|---|---|---|---|
| ZM | 1.2·× 1011 ± 4.1·× 1010 | 21.8 ± 3.2 | 2.5·× 10−7 ± 6.8·× 10−9 | 10–25–40 a | 1.7 ± 0.1 |
| PM | 5.2·× 1010 ± 1.4·× 1010 | 5.6 ± 0.9 | 1.3·×10−7 ± 2.0·×10−8 | 2–5–10 a | 1.2 ± 0.1 |
| Type of Membrane | e-PTFE Density (g cm−3) | i.d. 1 (mm) | Acidic Solution Velocity 2 (cm min−1) | Surface Area (cm2) | NH3–N Mass Removed 3 (mg) | NH3–N Mass Recovered (mg) | N flux (mg N·cm−2·d−1) |
|---|---|---|---|---|---|---|---|
| PM | 0.95 | 6.2 | 69 | 282.3 | 5381 a 4 | 3407 a | 1.7 b |
| ZM | 0.45 | 7.0 | 54 | 282.3 | 5260 a | 3628 a | 1.8 b |
| FZM | 1.09 | 1.0 | 2654 | 66.0 | 4764 a | 2661 b | 5.8 a |
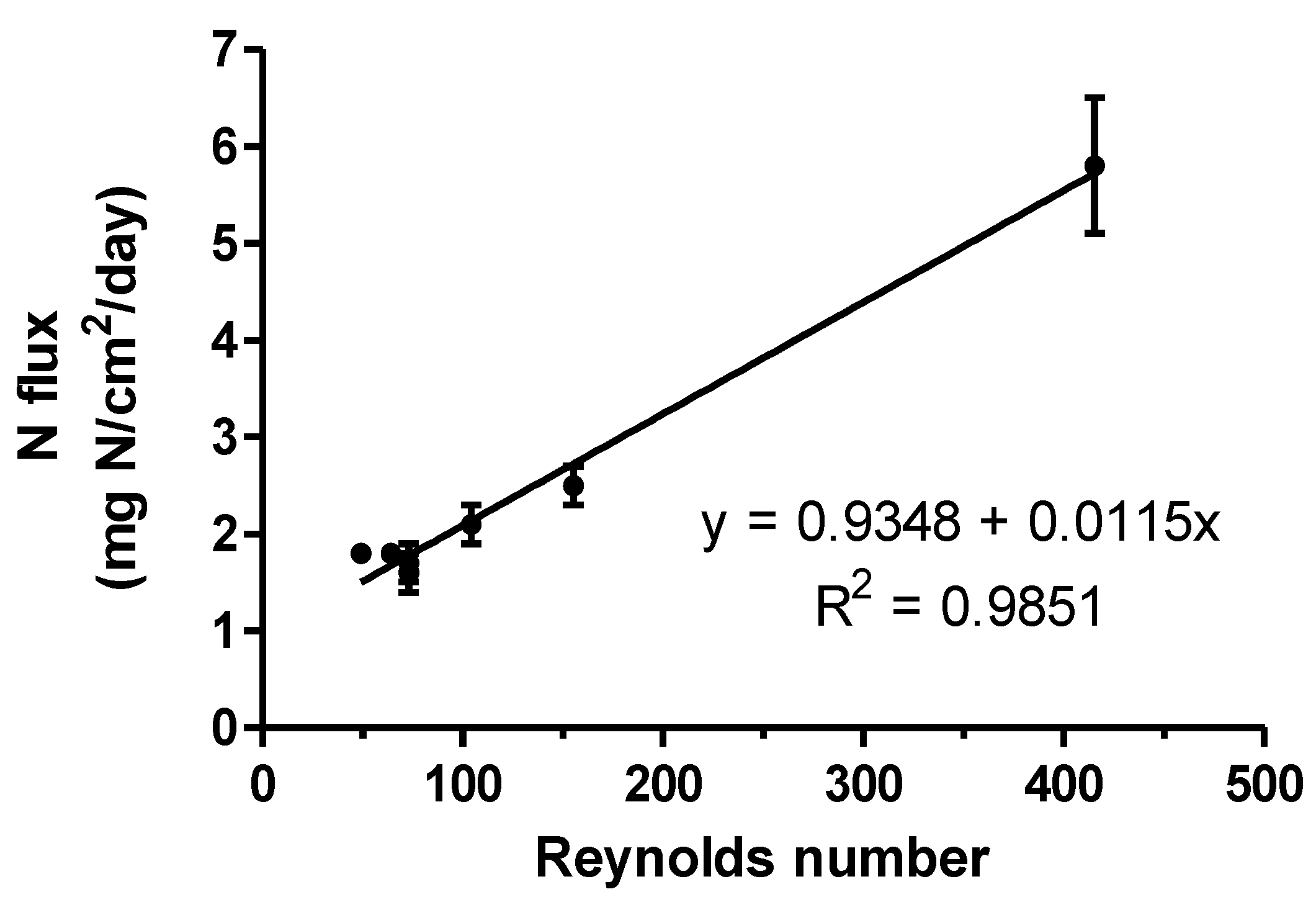
| Type of Membrane | e-PTFE Density (g cm3) | i.d. 1 (mm) | Surface Area (cm2) | Acidic Solution Flow Rate (L h−1) | Acidic Solution Velocity 2 (cm/min) | Reynolds Number 3 | NH3–N Mass Recovered (mg) | N Flux (mg N·cm−2·d−1) |
|---|---|---|---|---|---|---|---|---|
| PM | 0.95 | 6.2 | 125.7 | 0.83 | 46 | 49 | 3162 bc 4 | 1.8 bc |
| PM | 0.95 | 6.2 | 125.7 | 1.25 | 69 | 73 | 2780 c | 1.6 c |
| ZM4 | 0.95 | 2.9 | 125.7 | 0.83 | 210 | 104 | 3686 ab | 2.1 b |
| ZM4 | 0.95 | 2.9 | 125.7 | 1.25 | 315 | 155 | 4444 a | 2.5 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Conde-Cid, D.; Vanotti, M.B. Effect of the Type of Gas-Permeable Membrane in Ammonia Recovery from Air. Environments 2019, 6, 70. https://doi.org/10.3390/environments6060070
Soto-Herranz M, Sánchez-Báscones M, Antolín-Rodríguez JM, Conde-Cid D, Vanotti MB. Effect of the Type of Gas-Permeable Membrane in Ammonia Recovery from Air. Environments. 2019; 6(6):70. https://doi.org/10.3390/environments6060070
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, Juan Manuel Antolín-Rodríguez, Diego Conde-Cid, and Matias B. Vanotti. 2019. "Effect of the Type of Gas-Permeable Membrane in Ammonia Recovery from Air" Environments 6, no. 6: 70. https://doi.org/10.3390/environments6060070
APA StyleSoto-Herranz, M., Sánchez-Báscones, M., Antolín-Rodríguez, J. M., Conde-Cid, D., & Vanotti, M. B. (2019). Effect of the Type of Gas-Permeable Membrane in Ammonia Recovery from Air. Environments, 6(6), 70. https://doi.org/10.3390/environments6060070





