Aeration to Improve Biogas Production by Recalcitrant Feedstock
Abstract
:1. Introduction
2. Materials and Methods
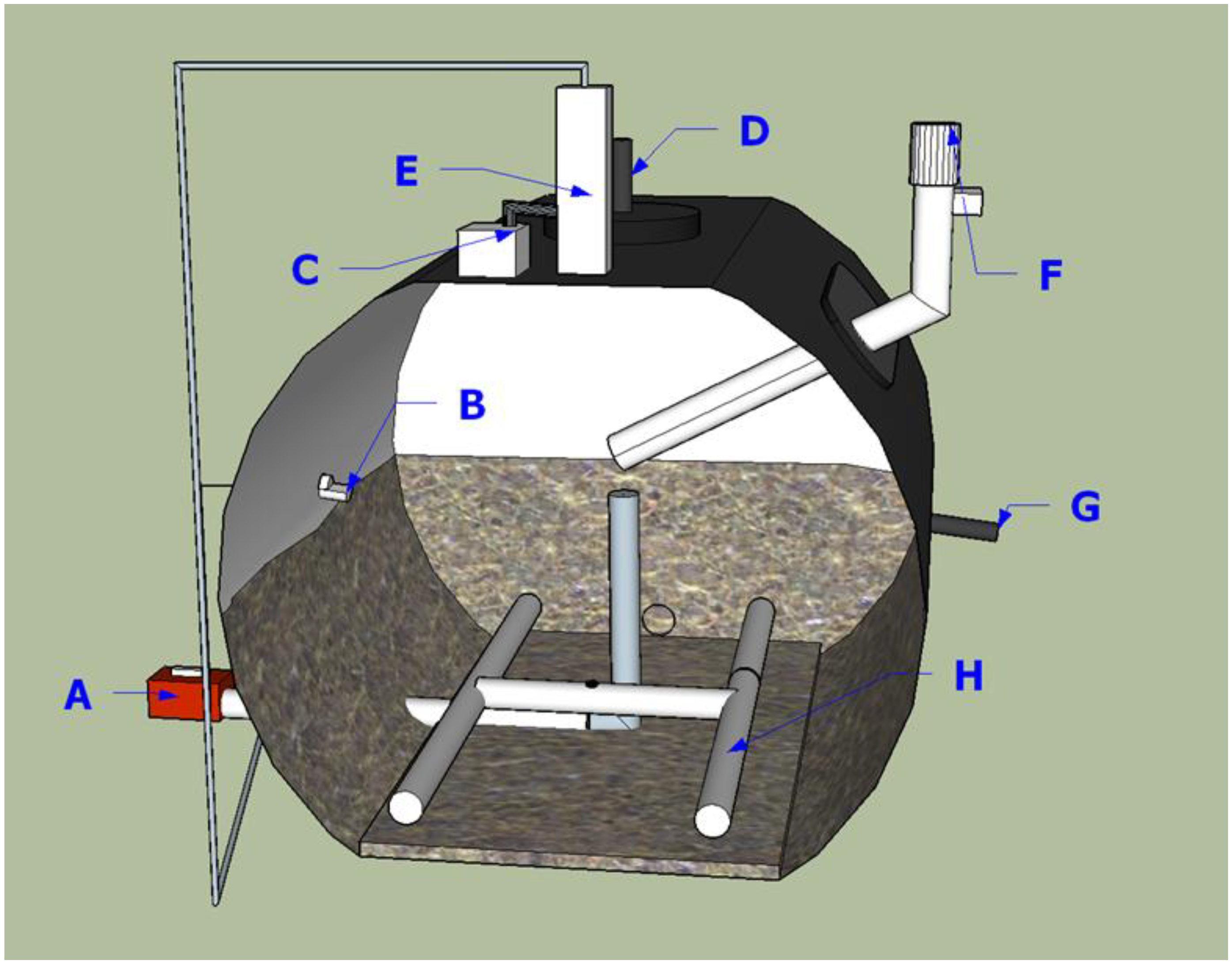
2.1. Digester Descriptions
2.2. Digester Operation
2.3. Analyses
3. Results and Discussion
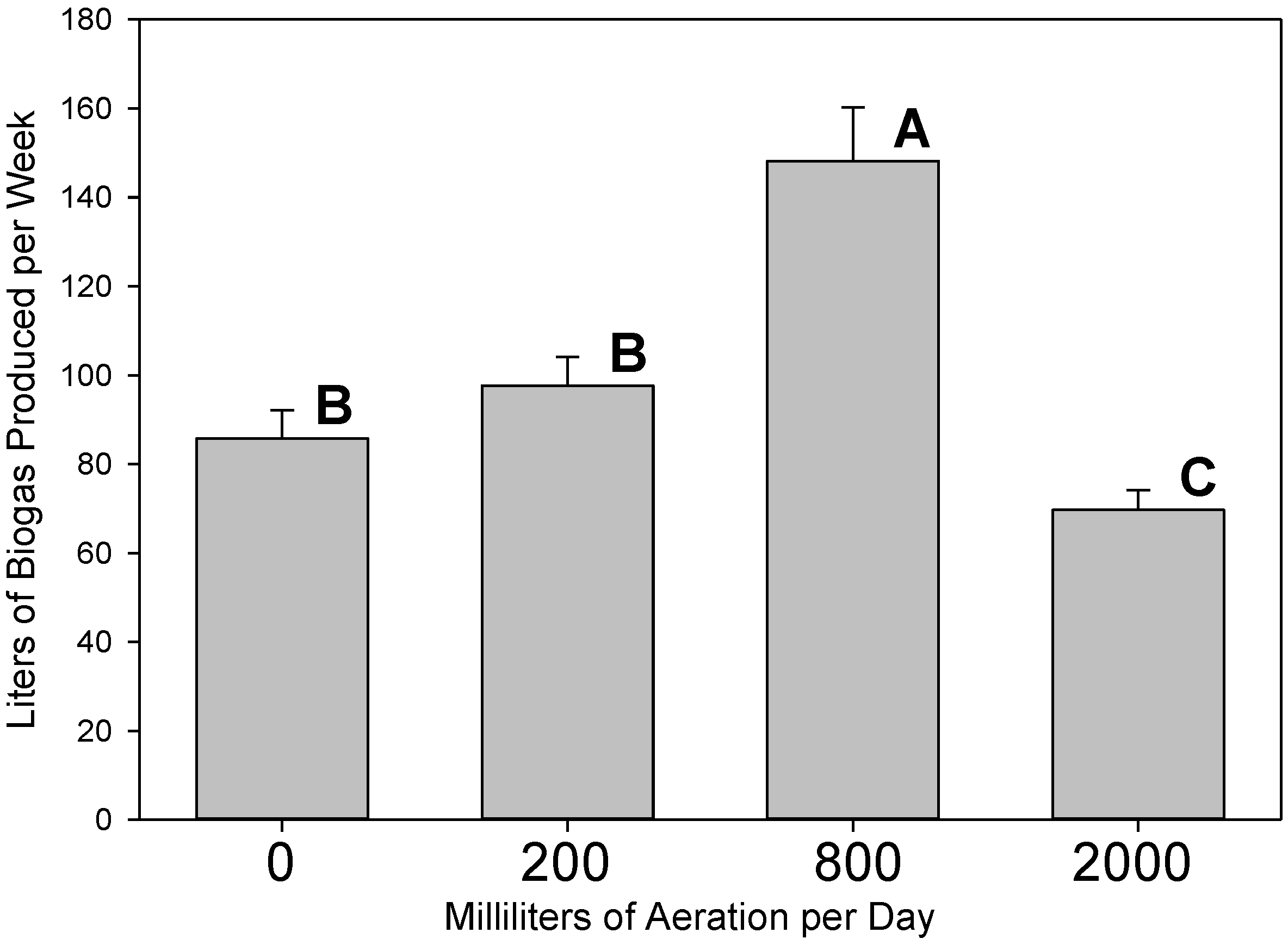
3.1. Gas Production
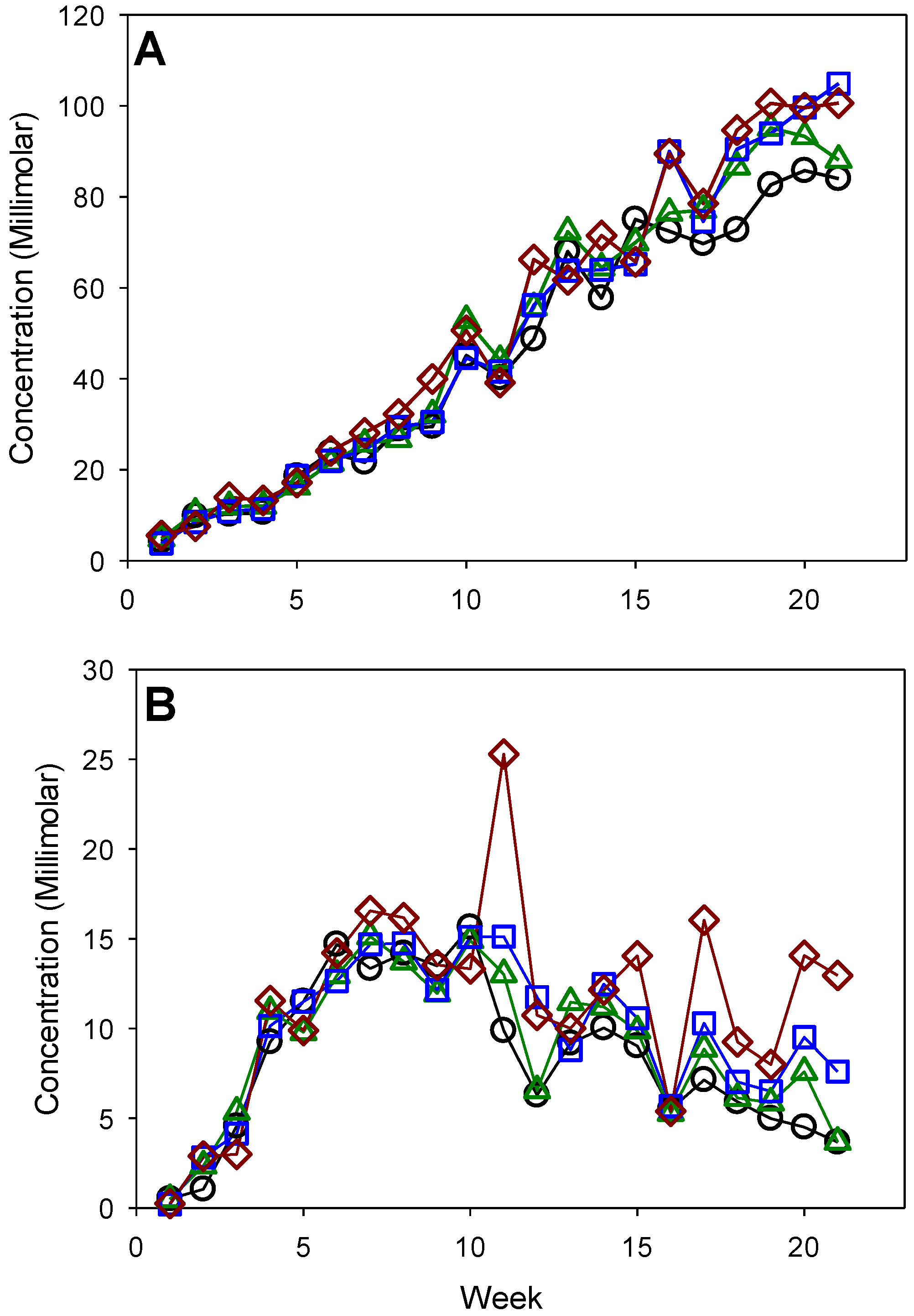
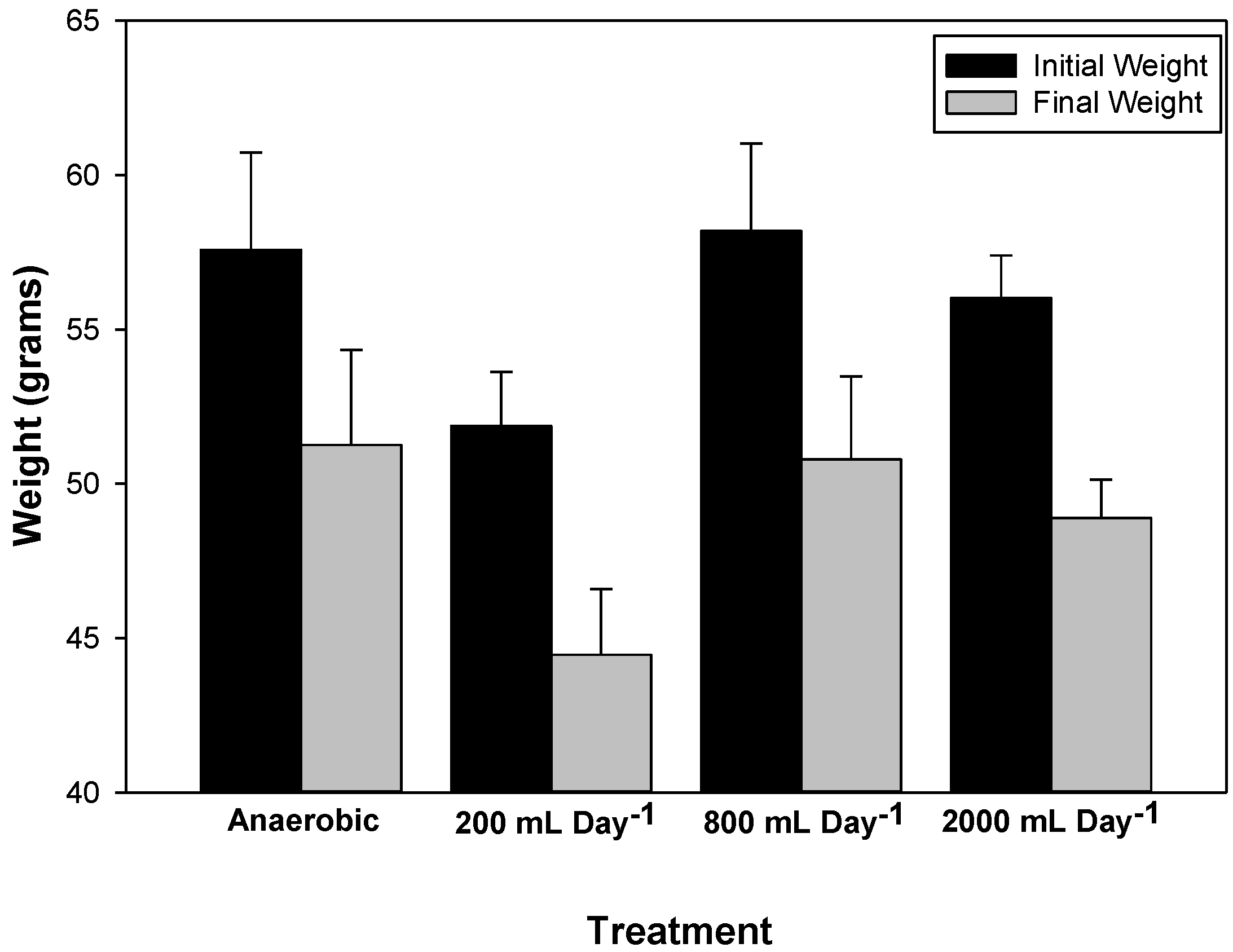
3.2. Waste Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Agricultural Statistics Service. Available online: https://www.usda.gov/sites/default/files/documents/nass-poultry-stats-factsheet.pdf. (accessed on 8 March 2019).
- Stephenson, A.H.; McCaskey, T.A.; Ruffin, B.G. A survey of broiler litter composition and potential value as a nutrient resource. Biol. Wastes 1990, 34, 1–9. [Google Scholar] [CrossRef]
- Kibet, L.C.; Allen, A.; Kleinman, P.J.; Feyereisen, G.W.; Church, C. Phosphorus runoff losses from subsurface-applied poultry litter on coastal plain soils. J. Environ. Qual. 2011, 40, 412–420. [Google Scholar] [CrossRef]
- Kirk, T.K.; Farrell, R.L. Enzymatic “combustion”: The microbial degradation of lignin. Ann. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef] [PubMed]
- Leschine, S.B. Cellulose degradation in anaerobic environments. Annu. Rev. Microbiol. 1995, 49, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Donoso-Bravo, A.; Fdz-Polanco, M. Effect of microaerobic conditions on the degradation kinetics of cellulose. Bioresour. Technol. 2011, 102, 10139–10142. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Kougias, P.G.; Vasileiou, S.A.; Lyberatos, G.; Angelelidaki, I. Effect of micro-aeration and inoculum type on the biodegradation of lignocellulosic substrate. Bioresour. Technol. 2017, 225, 246–253. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour. Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-F.; Wang, F.; Shi, X.-S.; Guo, R.-B. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem. Eng. J. 2016, 287, 523–528. [Google Scholar] [CrossRef]
- Zhu, M.; Lü, F.; Hao, L.-P.; He, P.-J.; Shao, L.-M. Regulating the hydrolysis of organic wastes by micro-aeration and effluent recirculation. Waste Manag. 2009, 29, 2042–2050. [Google Scholar] [CrossRef]
- Lim, J.W.; Wang, J.-Y. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013, 33, 813–819. [Google Scholar] [CrossRef]
- Xu, S.; Selvan, A.; Wong, J.W.C. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014, 34, 363–369. [Google Scholar] [CrossRef]
- Malovanyy, M.; Shandrovych, V.; Malovanyy, A.; Polyuzhyn, I. Comparative analysis of the effectiveness of regulation of aeration depending on the quantitative characteristics of treated sewage water. J. Chem. 2016, 2016, 6874806. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Wastewater, 20th ed.; APHA; AWWA; WPCF: Washington, DC, USA, 1998. [Google Scholar]
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch equation: Its history and limitations. J. Chem. Educ. 2001, 78, 1499–1503. [Google Scholar] [CrossRef]
- Howard, P.H.; Meylan, W.H. Handbook of Physical Properties of Organic Chemicals, 1st ed.; Chemical Rubber Corporation Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Jacinthe, P.A.; Groffman, P.M. Silicone rubber sampler to measure dissolved gases in saturated soils and waters. Soil Biol. Biochem. 2001, 33, 907–912. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Cook, K.L.; Lovanh, N. Recirculating swine waste through a silicone membrane in an aerobic chamber improves biogas quality and wastewater malodors. Trans. ASABE 2014, 55, 1929–1937. [Google Scholar] [CrossRef]
- Nuguyen, P.H.L.; Kuruparan, P.; Visvanathan, C. Anaerobic digestion of municipal solid waste as a treatment prior to landfill. Bioresour. Technol. 2007, 98, 380–387. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jenicek, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef]
- Zhou, W.; Imai, T.; Ukita, M.; Li, F.; Yuasa, A. Effect of limited aeration on the anaerobic treatment of evaporator condensate from a sulfite paper mill. Chemosphere 2007, 66, 924–929. [Google Scholar] [CrossRef]
- Kristjansson, J.K.; Sch6nheit, P.; Thauer, R.K. Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria: An explanation for the apparent inhibition of methanogenesis by sulfate. Arch. Microbiol. 1982, 131, 278–282. [Google Scholar] [CrossRef]
- Da Silva, R.R.; Pesezzi, R.; Souto, T.B. Exploring the bioprospecting and biotechnological potential of white-rot and anaerobic Neocallimastigomycota fungi: Peptidases, esterases, and lignocellulolytic enzymes. Appl. Microbiol. Biotechnol. 2017, 101, 3089–3101. [Google Scholar] [CrossRef]
- Marvin-Sikkema, F.D.; Richardson, A.J.; Stewart, C.S.; Gorrschal, J.C.; Prins, R.A. Influence of Hydrogen-consuming bacteria on cellulose degradation by anaerobic fungi. Appl. Environ. Microbiol. 1990, 56, 3793–3797. [Google Scholar]
| Milliliters of Aeration Per Day | ||||
| 0 | 200 | 800 | 2000 | |
| pH | 6.99 (0.09) a | 6.98 (0.09) a | 6.94 (0.08) a | 6.93 (0.08) a |
| Biogas Concentration (µg/L) | ||||
| CO2 | 755,000 (21,300) a | 740,000 (17,700) a | 718,000 (17,800) a | 683,000 (16,500) a |
| CH4 | 318,000 (14,300) a | 331,000 (24,600) a | 314,000 (17,300) a | 298,000 (9650) a |
| Wastewater Concentration (Millimolar) | ||||
| HCO3− | 49.8 (6.0) b | 53.8 (6.6) a | 54.5 (7.2) a | 57.2 (7.1) a |
| sCO2 | 9.1 (0.9) a | 9.7 (0.8) a | 10.5 (0.7) a | 12.4 (1.0) a |
| sCH4 | 21.1 (0.5) a | 21.7 (0.5) a | 23.5 (0.7) a | 26.2 (0.5) a |
| Wastewater Concentration (mg/L) | ||||
| Chemical oxygen demand | 3240 (194) a | 2720 (120) b | 2760 (98) b | 2420 (96) b |
| Total suspended solids | 287 (29) a | 276 (24) a | 241 (27) a | 262 (28) a |
| NH4+ | 153 (83) a | 162 (65) a | 150 (65) a | 154 (68) a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loughrin, J.; Lovanh, N. Aeration to Improve Biogas Production by Recalcitrant Feedstock. Environments 2019, 6, 44. https://doi.org/10.3390/environments6040044
Loughrin J, Lovanh N. Aeration to Improve Biogas Production by Recalcitrant Feedstock. Environments. 2019; 6(4):44. https://doi.org/10.3390/environments6040044
Chicago/Turabian StyleLoughrin, John, and Nanh Lovanh. 2019. "Aeration to Improve Biogas Production by Recalcitrant Feedstock" Environments 6, no. 4: 44. https://doi.org/10.3390/environments6040044
APA StyleLoughrin, J., & Lovanh, N. (2019). Aeration to Improve Biogas Production by Recalcitrant Feedstock. Environments, 6(4), 44. https://doi.org/10.3390/environments6040044






