Abstract
The distribution of As, Cd, Cu and Se among biomolecules of different molecular weight (MW) in the heat-treated cytosolic fraction of livers and gonads of white suckers (WS; Catostomus commersonii) collected in a reference lake and in a lake subject to multi-metal contamination was investigated. Distribution profiles were obtained by separation of the heat-stable protein and peptide (HSP) fractions using size-exclusion high performance-liquid chromatography, coupled online to an inductively coupled plasma mass spectrometer, to quantify the associated metals. Metal-handling strategies did not vary between the reference and exposed fish, with the exception of As. Cadmium and Cu appeared associated with the heat-stable peptides metallothioneins (MTs), indicating their reasonable detoxification and regulation in WS. In contrast, Se and As were not bound to MTs, but rather, to biomolecules of lower MW (<2 kDa). Arsenic was found associated with the same biomolecules in fish from both lakes, but their proportions changed between reference and exposed fish. For future work, the identification of the Se and As binding biomolecules would be of great interest to determine if these metalloids are detoxified or if, conversely, the biomolecules are metal-sensitive and their binding to Se or As represents a threat for the health of these fish.
1. Introduction
Trace element exposure in the environment may lead to accumulation in aquatic organisms and cause toxic effects. Once they interact with biota, trace elements can penetrate into cells and bind to, or interact with, cytosolic molecules and interfere with cell function, leading eventually to deleterious effects [1]. Alternatively, trace elements can be detoxified by binding to molecules designed to sequester them and prevent them from exerting their toxic effects; metallothioneins and metallothionein-like peptides (hereafter referred to as MT and MTLP) are the most common examples of such ligands [2,3]. Therefore, the investigation of subcellular trace element forms within organisms and organs can provide valuable insights into how these elements are handled within living cells, i.e., information on whether they are detoxified or present in biologically sensitive pools, and how they exert their toxicity [4,5].
Different approaches exist to determine the subcellular distribution of trace elements in living organisms and organs. One of the most used approaches involves successive differential centrifugation steps, followed by a heat-treatment that enables the discrimination between cytosolic trace elements associated with heat-denaturable proteins (HDP; e.g., cytosolic enzymes) and those bound to heat-stable proteins or peptides (HSP) such as MT or MTLP. Metallothioneins are low molecular weighted (6–7 kDa), cysteine-rich cytosolic peptides that can complicate various metals by binding to the numerous thiol groups present in its constituent cysteine residues (≈30% of its amino acids). The high proportion of cysteine and also the presence of disulfide bonds confer heat-stability to the peptides. In general, given the important role of MT and MTLP in trace element detoxification and regulation [6,7,8], it is assumed that trace elements present in the HSP fraction are detoxified by binding to these peptides [4].
In a study on juvenile yellow perch (Perca flavescens) collected along an environmental contamination gradient, Giguère et al. [9] observed that Cd and Cu present in fish livers were mainly associated with the HSP fraction, suggesting a detoxification response by the binding of these two metals to MT and/or MTLP. In our recent work, assessing subcellular metal and metalloid partitioning in the liver and gonads of field-collected white suckers (WS) from a reference lake and a contaminated lake, similar observations were reported for Cd and Cu; more than 60% of the total Cd and Cu burdens were associated with the HSP fraction [10]. In the same study, As was also found to be associated with the HSP fraction (close to 50%), whereas the Se burden was associated with both the HSP and HDP fractions, the contribution of the HSP fraction to the total hepatic Se burden being more important in exposed, as compared to reference, fish, suggesting the possible induction of a detoxification response in the fish.
However, although it is generally assumed that trace elements present in the HSP fraction are detoxified by binding to MT and/or MTLP, the subcellular fractionation approach does not provide information about the nature of the biomolecules targeted by trace elements or about their functionality once bound to the trace element. Given the abundant literature about the involvement of MT and MTLP in Cd and Cu handling, it is relatively easy to assign the presence of Cd and Cu in the HSP fraction to their binding to these peptides. However, in the case of less studied elements, such as Se or As, evidence for MT or MTLP involvement in detoxification is not obvious. In the light of the results from our recent work on white suckers [10] and to answer the question “Are the elements present in the HSP fraction truly handled by MT and MTLP?”, the nature of the metal-binding biomolecules in this cytosolic fraction (HSP) was investigated.
The goal of the present study was to assess the distribution of As, Cd, Cu and Se present in the heat-treated cytosolic fraction, HSP, of male and female white sucker liver and gonads (ovaries and testes). To this end, heat-stable cytosolic biomolecules were separated by size-exclusion high performance liquid chromatography and their associated trace elements were determined with an inductively coupled plasma mass spectrometer coupled online to the chromatograph effluent (SEC-ICP-MS). To be able to detect any changes in metal-handling strategies in fish, we collected white suckers at a reference site and in a lake located downstream from a metal mining effluent, where fish were exposed to polymetallic (As, Cd, Cu, Ni, Pb, Se and Zn) contamination. Liver and gonads were the targeted organs: liver as a key organ of metabolism and detoxification; gonads as the reproductive organs.
2. Materials and Methods
2.1. Fish Collection and Selection of Individuals for SEC-ICP-MS Analysis
In summer 2016, mature white suckers, calibrated by length, were collected from a lake located downstream from a metal mining effluent and exhibiting a polymetallic (As, Cd, Cu, Ni, Pb, Se and Zn) contamination (n = 14 females and 6 males) and at a reference site (n = 7 females and 12 males) (for details about the metal exposure gradient, see Urien et al. [10]). Upon capture (using gill nets), white suckers were sacrificed quickly by concussion, and once fish length (±1 mm) and weight (±0.01 g) had been recorded, fish were dissected to collect liver and gonads, and sex was determined (the capture and sampling protocol was approved by the INRS (Institut National de la Recherche Scientifique) animal-care committee). Organs were weighed, placed in 50 mL polypropylene tubes and put on dry ice until the return to the laboratory where they were immediately stored at −80 °C until analysis.
The present study is part of a larger project that is exploring the potential of subcellular distribution methods to identify effects of metals on aquatic organisms and the fish tissues were thus used for many different analyses. Given that the amount of available tissue was limited, we chose to perform SEC-ICP-MS analyses on a limited number of fish. Among all collected fish (39 in total), we aimed to select three fish per lake condition (exposed and reference) and sex (male and female), if possible, that is to say twelve fish in total, to analyze the liver and gonad heat-stable cytosolic fractions (referred as to the HSP fractions hereafter). To select the fish for analysis, we applied the following procedure. Total concentrations of As, Cd, Cu and Se in livers and gonads of the 39 fish were measured (performed by ICP-MS, following an acid digestion—details are provided in Appendix A). Then, for fish from each lake and sex, we determined the median hepatic concentration of the trace elements studied and selected the individuals for which the measured hepatic metal concentration was within 10% of the median for as many trace elements as possible, with a priority given to selenium (most accumulated element in exposed fish compared to reference fish) when more than 3 individuals met the conditions. The final selected individuals were: 2 reference males (RefMale), 4 reference females (RefFem), 3 exposed males (ExpMale) and 3 exposed females (ExpFem), for which hepatic and gonadal HSP fractions were analyzed by SEC-ICP-MS. Selected fish size ranged between 37 and 43 cm. Means of total length of selected fish for each condition are available in the Supplementary Materials (Table S1).
2.2. HSP Fraction Isolation for the Liver and Gonads of Selected Fish
HSP fractions were isolated by applying a subcellular fractionation protocol similar to that employed by Urien et al. [10]. Briefly, tissues (≈0.5 g wet weight) were individually gently homogenized in a fresh ammonium acetate solution (100 mM, pH 7.4) with a motorized Potter-Elvehjem homogenizer equipped with a Teflon pestle (Fisher Scientific, Whitby, ON, Canada) (ratio wet weight tissue: volume of ammonium acetate solution: 1:3 for liver; 1:4 for gonads). About 1 mL of each homogenate was subjected to ultracentrifugation (180,000× g for 60 min at 4 °C) to isolate the cytosol (supernatant) from the rest of the cell contents (pellet). The cytosol was then heated (85 °C, 30 min), cooled (4 °C, 60 min) and subjected to a final ultracentrifugation step (50,000× g for 10 min at 4 °C) to collect heat-stable proteins and peptides present in the final supernatant (HSP fraction). Aliquots from the liver and gonad homogenates, HSP fractions (150 µL) as well as the whole pellets were collected and stored at −80 °C for further metal quantification (see Section 2.4).
2.3. SEC-ICP-MS Analysis of HSP Fractions
Separation of the heat-stable biomolecules contained in the HSP fraction was performed by high performance liquid chromatography (HPLC Spectra system, Thermo Fisher Scientific, Winsford, England, UK) using a size-exclusion column (Superdex peptide 10/300 GL column; SECpep, GE Healthcare Biosciences, Uppsala, Sweden; size separation range 0.1 to 7 kDa). For each sample, 100 μL of sample was run through the column. The separation was achieved using a 100 mM ammonium acetate solution (VWR International, Montreal, QC, Canada), pH 7.4, flow rate of 0.7 mL/min) as the mobile phase (isocratic mode). After passing through the column, the chromatographic effluent was introduced into the HLPC detector for monitoring of the protein and peptide absorbance at 280 and 254 nm, respectively (expressed in absorbance units; mAu). For trace element detection in real time, an ICP-MS (XSERIES 2, Thermo Fisher Scientific, Winsford, England, UK) was directly coupled to the HPLC system; 75As, 111Cd, 65Cu and 82Se were monitored (expressed in number of counts). Each day of analysis, prior to the first HSP sample injection, the column was conditioned with freshly prepared mobile phase for 90 min, and a standard solution containing ethylenediaminetetraacetic acid (EDTA,10 mM) and cysteine (10 mM) and a blank (100 µL aliquot of the mobile phase) were injected to monitor potential metal contamination remaining on the column. During the subsequent analyses, an aliquot of the EDTA-cysteine standard solution was injected between each HSP sample in order (i) to clean the column and prevent memory effects from the previous HSP sample, and (ii) to monitor the constancy of the column performance, by comparing the elution times of the two molecules from one chromatographic run to the next.
The Superdex peptide column was calibrated by running several molecular weight standards through the column under the same condition as the samples (Table 1). The void volume corresponded to an elution time of 11 min.

Table 1.
Retention times (Rt) and molecular weight (MW) of four standards used for calibration of the Superdex peptide column.
2.4. Metal Quantification in Hepatic and Gonadal HSP Fractions
For the determination of heat-stable cytosolic metal concentrations in the liver and gonads of white suckers (Table 2, see Results and Discussion section), aliquots of HSP fractions were digested with 500 µL of HNO3 (70%, v/v, Optima grade; Fisher Scientific) overnight and then heated at 80 °C for 2 h. After first cooling the samples to room temperature, 200 µL of H2O2 (30%, v/v, Optima grade; Fisher Scientific) were added and samples heated and cooled as previously described. To control digestion efficiency, certified reference material (TORT-3, National Research Council of Canada, Ottawa, ON, Canada) was digested with the same procedure. Finally, all samples were diluted with ultrapure water to give a final acid concentration of 10% (v/v) before determination of the metal concentrations by ICP-MS (XSERIES 2, Thermo Fisher Scientific, Winsford, England, UK). TORT 3 recovery (mean ± SD, n = 3) was within the range of certified values (As: 107 ± 2%; Cd: 95 ± 2%; Cu: 93 ± 2%; Se: 105 ± 2%). Digestion blanks did not reveal any appreciable contamination. Metal concentrations in the HSP fraction are expressed as nmol of trace element per gram of organ dry weight (nmol/g dw).

Table 2.
Trace element concentrations (nmol/g dw, mean (standard error)) in hepatic and gonadal HSP (heat-stable proteins or peptides) fractions of white suckers collected in summer 2016 and mean relative contribution of the HSP fraction to the total metal/metalloid burden in liver and both gonad tissues.
2.5. Quality Control
In order to control for potential metal loss or contamination during passage through the HPLC system (column, tubes and injection needle), metal recovery was assessed by comparing metal burdens in the injected HSP (100 µL) and metal burdens in fractions collected after a run with and without the column. For samples from the liver, metal column recoveries were (with and without the column, respectively): As: 60% and 59%; Cd: 98% and 120%; Cu: 101% and 98%; Se: 62% and 84%. For the gonads, measured metal concentrations were close to or below quantification limits, effectively precluding the calculation of column recovery. Note that comparisons between chromatograms of the whole cytosol and the HSP fraction confirmed that the major HSP peaks observed for Cd, Cu, As and Se were already present in the cytosol, i.e., before the heat denaturation step.
2.6. Data Processing
Raw chromatographic data were obtained with PlasmaLab software (Thermo Fisher Scientific, Winsford, England, UK), and then processed using R software (R Foundation for Statistical Computing, Vienna, Austria) [11] for peak area determination, using the trapezoid calculation method, and for chromatogram generation.
For the comparison of the total metal concentrations in the HSP fractions, the non-parametric Wilcoxon–Mann–Whitney test was used (p < 0.05), given the low number of replicates. Comparisons of peak area proportions (in %) were performed by non-parametric tests after arcsine transformation.
3. Results and Discussion
In the SEC-ICP-MS analysis of metal-binding profiles, metal-binding biomolecule pools were operationally defined as MMW (medium molecular weight; >10 kDa; elution time: 0–13 min), LMW (low molecular weight; 10–2 kDa; elution time: 13–24 min) and VLMW (very low molecular weight; <2 kDa; elution time: >24 min). The ultraviolet (UV) absorbance data at 280 and 254 nm were monitored to assess how biomolecules eluted over the entire molecular weight range of the column and to examine overlaps with metal elution peaks. As similar trends were observed among fish with the same sex and exposure conditions, one chromatogram per trace element and fish condition is presented.
3.1. Biomolecule UV Profiles of White Sucker Liver and Gonads
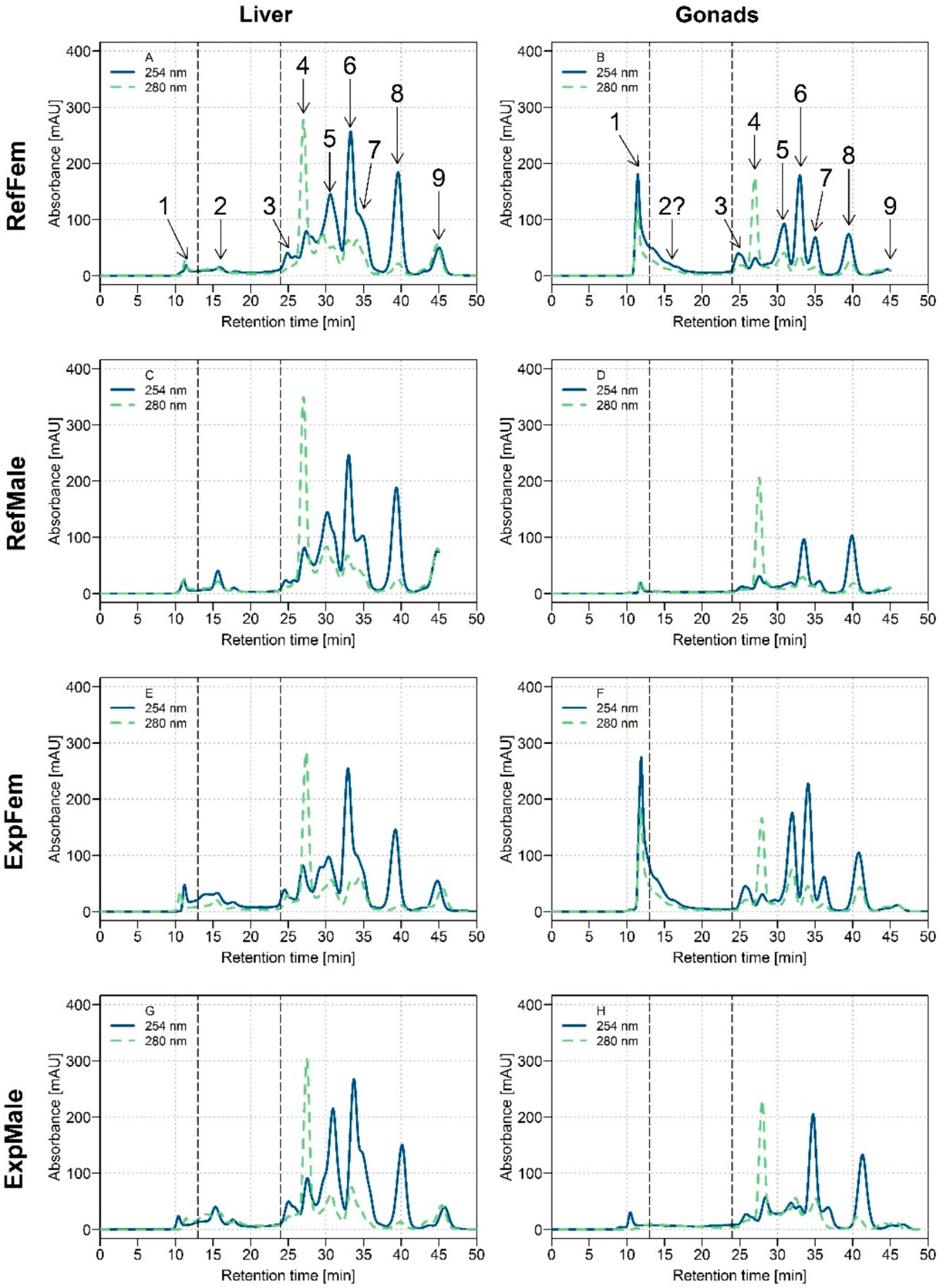
Chromatograms presenting the UV absorbance data at 254 and 280 nm for the HSP fractions of white sucker liver and gonads are presented in Figure 1. Results showed that it was possible to detect nine pools of biomolecules (peaks #1 to #9; a peak is considered as a potential pool of biomolecules because co-elution of many biomolecules of similar molecular weight may occur and will not be necessarily detected individually with the column). The majority of the peaks were observed in the liver and in both gonad tissues (ovaries and testes), but with different relative intensities. Note that peak #2 (elution time: 15.8 min) presumably corresponds to metallothionein (See Table 1). Note too that the tissue:buffer ratios for the liver and gonads applied for the homogenization step were different (more diluted for gonads than for liver; see Materials and Methods section), and thus it was anticipated that lower peak intensities would be observed for the gonad chromatograms than for the liver chromatograms.
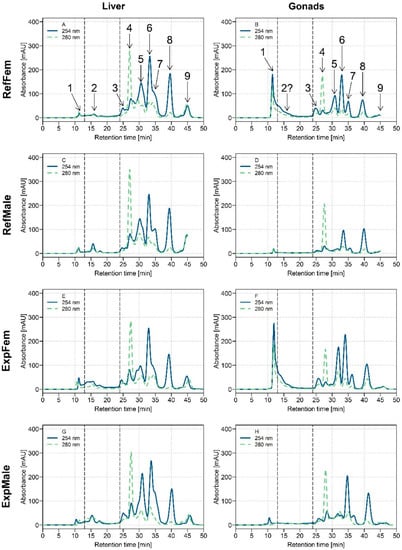
Figure 1.
Size-exclusion chromatograms with ultraviolet (UV) detection at 254 nm (blue plain line) and 280 nm (olive dotted line) in the heat-treated cytosolic (HSP) fraction of white suckers for male and female livers and gonads. Vertical dotted lines delimit the three operationally defined molecular weight biomolecule pools: medium molecular weight (MMW) (0–13 min; >10 kDa), low molecular weight (LMW) (13–24 min; 10–2 kDa) and very low molecular weight (VLMW) (>24 min; <2 kDa). (A) Liver of reference females (RefFem); (B) Gonads of reference females (RefFem); (C) Liver of reference males (RefMale); (D) Gonads of reference males (RefMale); (E) Liver of exposed females (ExpFem); (F) Gonads of exposed females (ExpFem); (G) Liver of exposed males (ExpMale); (H) Gonads of exposed males (ExpMale).
In the liver, respective profiles at 254 and 280 nm were similar between both sexes and exposure conditions (Figure 1, chromatograms A, C, E, G). At 254 nm, the majority of the peaks observed were eluted after 24 min (from peak #3), indicative of the presence of VLMW biomolecules (<2 kDa), presumably peptides. At 280 nm, a major peak was observed at 26.8 min (peak #4).
Biomolecule profiles were different between male and female gonads (Figure 1B–D,F–H), but were similar between reference and exposed fish (Figure 1B–F,D–H). At 254 nm, the peak eluting directly after the void volume (peak #1; elution time: 11 min), and corresponding to biomolecule pools >10 kDa, was much more intense in ovaries than in testes (ovaries; 200–300 mAu, testes; ≤10 mAu), regardless of exposure conditions. This result suggests either that the same biomolecules are present in both organs but in higher amounts in female gonads, and/or that additional and different biomolecules of high molecular weight (and sensitive to UV detection) are present in female gonads, but not in male gonads. Peaks eluting at 30 and 35 min (peaks #5 and #7, respectively) were also more intense in female gonads than in male gonads. Note that in testes, peak #2 was not observable/detected in chromatograms (Figure 1D–H) probably because of the low intensity of the signal. Peak #2 was also difficult to distinguish in female gonads because of the tailing of the elution peak observed at 11 min.
Hepatic and gonadal biomolecule profiles were different, especially with respect to the MMW biomolecule pool, peak #1, which was much more intense in female gonads than in the liver (Figure 1A,B,E,F). Otherwise, the biomolecule profile for female gonads resembled that of the liver (at 254 and 280 nm). Compared to the liver, for which three VLMW biomolecule pools (peaks #5, 6 and 8) were relatively dominant in the HSP fraction, only two of them were dominant in male gonads (#6 and 8).
At 280 nm and for all tissues, the same peak #4 was observed at 26.8 min. Note too that peak #7 observed in the gonad tissues (Figure 1B,D,F,H) had a better resolution than the one observed in the liver (Figure 1A,C,E,F), an observation that may be indicative of different biomolecules.
Overall, UV absorbance data showed distinctive biomolecule profiles for each organ tissue studied (i.e., liver, ovaries and testes) but no influence of the fish exposure on peak intensities. Since liver, ovaries and testes are very different organs in terms of composition, and also functions (even in terms of reproductive functions between ovaries and testes), the different biomolecule profiles observed are likely a result of this specificity.
3.2. Distribution of Trace Elements in Hepatic and Gonadal HSP Fractions of White Suckers ([M]HSP)
3.2.1. Copper (Cu)
In the present study, [Cu]HSP in reference and exposed fish fell in a narrow range with maximum to minimum ratios of mean concentrations in the liver and gonads lower or equal to two (Table 2). Note also that [Cu]HSP in all organs studied were different and decreased in the following order: liver > ovaries > testes. This could be explained by the different functions played by these organs: (i) liver and gonads, the liver being a key organ in detoxification and storage, whereas gonads are dedicated to reproduction, and (ii) ovaries and testes, ovaries being dedicated to egg production and constrained to accumulate many nutrients, including Cu, for further maternal transfer, which is not the case for testes. Comparisons between total [Cu] and [Cu]HSP indicate that the contribution of the HSP fraction to the total metal burden was above 60%; only for the testes was this contribution lower (≈20%). Similar relative contributions were previously reported in Urien et al. [10].
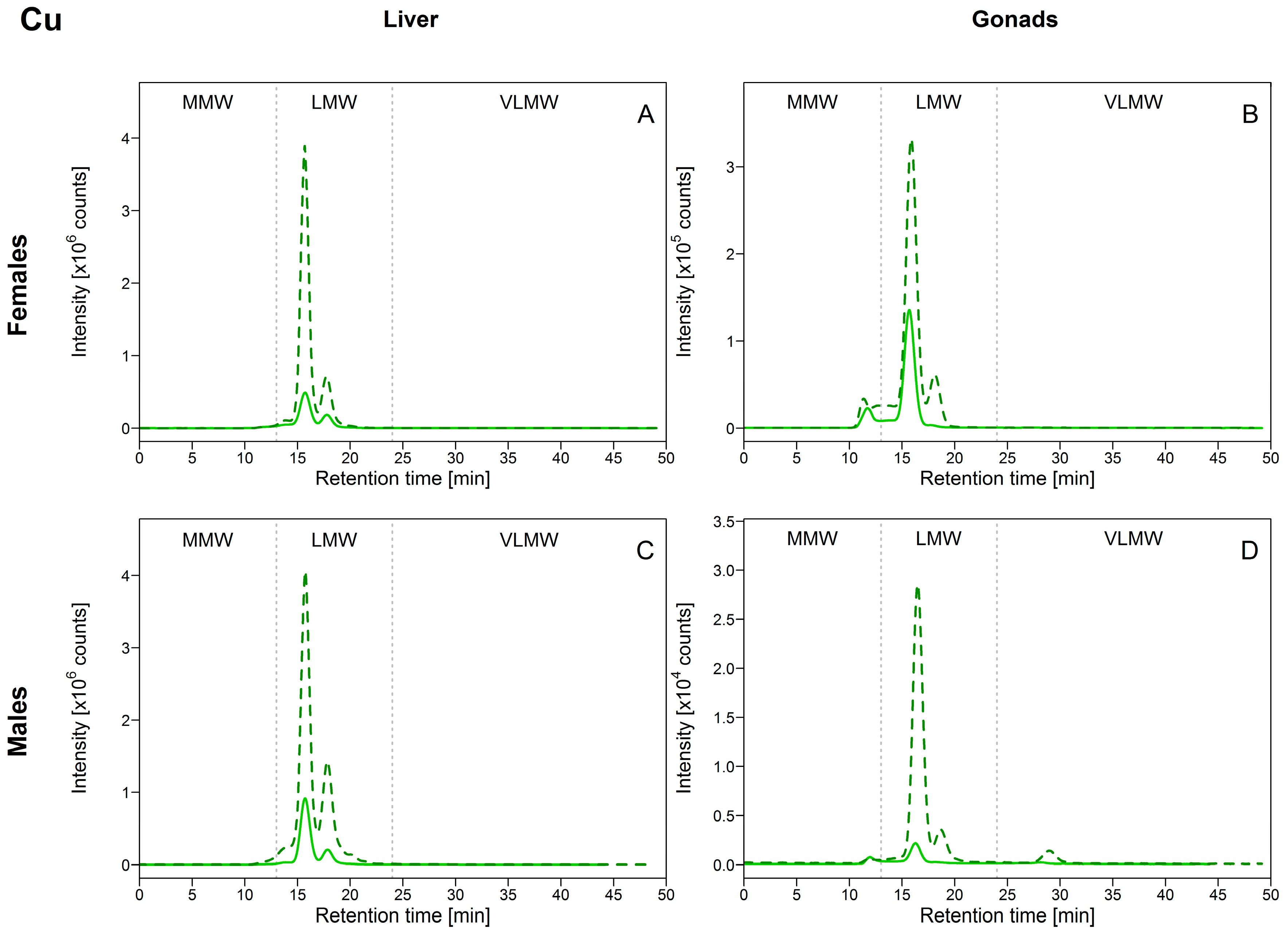
As depicted in Figure 2A,C, for the liver, Cu was eluted exclusively within the LMW biomolecule pool, with a major Cu-binding biomolecule eluting at 15.8 min, coinciding with the elution time of the MT-2 standard (see Table 1), suggesting that Cu was associated with MTs. Copper distribution in the gonads resembled that observed in the liver, with the majority of Cu eluting within the LMW biomolecule pool, but also a peak eluting within the MMW biomolecule pool (elution time = 11 min) in female gonads, and a peak in both the MMW and VLMW biomolecule pools (elution times = 11 min and 29 min) in male gonads (Figure 2B,D). In all tissues, peak areas of Cu in the LMW biomolecule pool increased as [Cu]HSP increased (see Figure S1 in Supplementary Materials). In comparison, Cu bound to MMW biomolecules in the gonads responded modestly to increasing [Cu]HSP. Overall, these observations suggest that biomolecules present in LMW pool in both the liver and gonads play a central role in handling Cu, the latter presumably being associated with MTs and MTLPs. Metallothioneins and metallothionein-like peptides and proteins are biomolecules of low molecular weight, rich in cysteine, with a high affinity for “soft” metals such as Cu and Cd. They are known to be involved in the regulation and storage of Cu (and detoxification of Cd) [3]. In 1991, Klaverkamp et al. [12] reported that in white suckers collected from a metal-contaminated environment, half of the hepatic Cu burden was bound to MT. Our results showed that Cu distribution in white suckers agreed with previous studies investigating Cu-binding biomolecules in the liver of freshwater fish collected in metal-impacted areas, where most of Cu was present at the same elution time as the elution time for MT (European eels (Anguilla anguilla) [13,14]; European chub and Vardar chub (Squalius cephalus and S. vardarensis) [15,16]; gibel carp (Carassius auratus gibelio) [17]). Comparable interpretations were made in field-collected juvenile yellow perch (Perca flavescens), although the major Cu peak did not elute at exactly the same elution time as was observed for the MT standard [18]; many isoforms of MTs co-exist and may explain this difference [17]. In this vein, the presence of Cu associated with a second biomolecule at 17.5 min in our study could be explained by the possible existence MT isoforms of slightly different MW from the MT commonly tested as a standard.
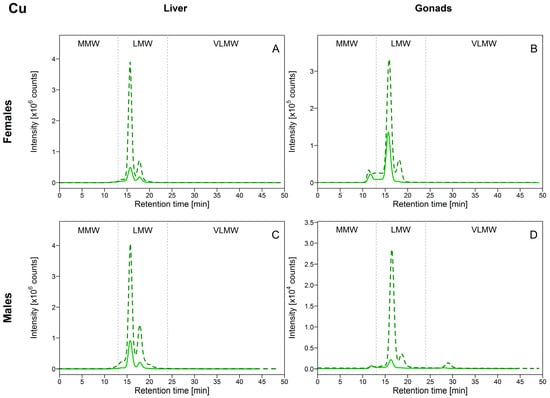
Figure 2.
Size-exclusion chromatograms of Cu-binding biomolecules in hepatic and gonadal HSP (heat-stable proteins or peptides) fractions of white suckers from a reference (full line) and metal-impacted lake (dotted line). Vertical dotted lines delimit the three operationally defined molecular weight biomolecule pools: MMW (0–13 min; >10 kDa), LMW (13–24 min; 10–2 kDa) and VLMW (>24 min; <2 kDa). (A) Female liver; (B) Female gonads; (C) Male liver; (D) Male gonads.
3.2.2. Cadmium (Cd)
In the present study, hepatic and gonadal [Cd]HSP in exposed fish were significantly higher compared to reference fish, with concentrations differences that reached a factor of ten (Table 2). As was the case for Cu, levels of Cd in the liver were greater than those found in the gonads. The relative contribution from the HSP fraction to the total Cd burden in livers was above 60%, as was observed for Cu. For Cd in gonads, the proportion bound in HSP fraction is higher in females (approximately 26% in RefFem, and 37% in ExpFem) than in males (about 15% in both RefMal and ExpMal), suggesting better protection in females (also corroborated by the maximum to minimum ratios of mean Cd concentrations being higher in testes than in ovaries). In our previous study assessing trace element hepatic and gonadal subcellular distributions in white suckers collected in the same lakes, similar trends were reported; in particular, we reported a shift of Cd from the metal-sensitive cytosolic fraction in ovaries of reference fish to the HSP fraction in exposed fish, suggesting that Cd detoxification became more effective above a threshold tissue concentration [10].
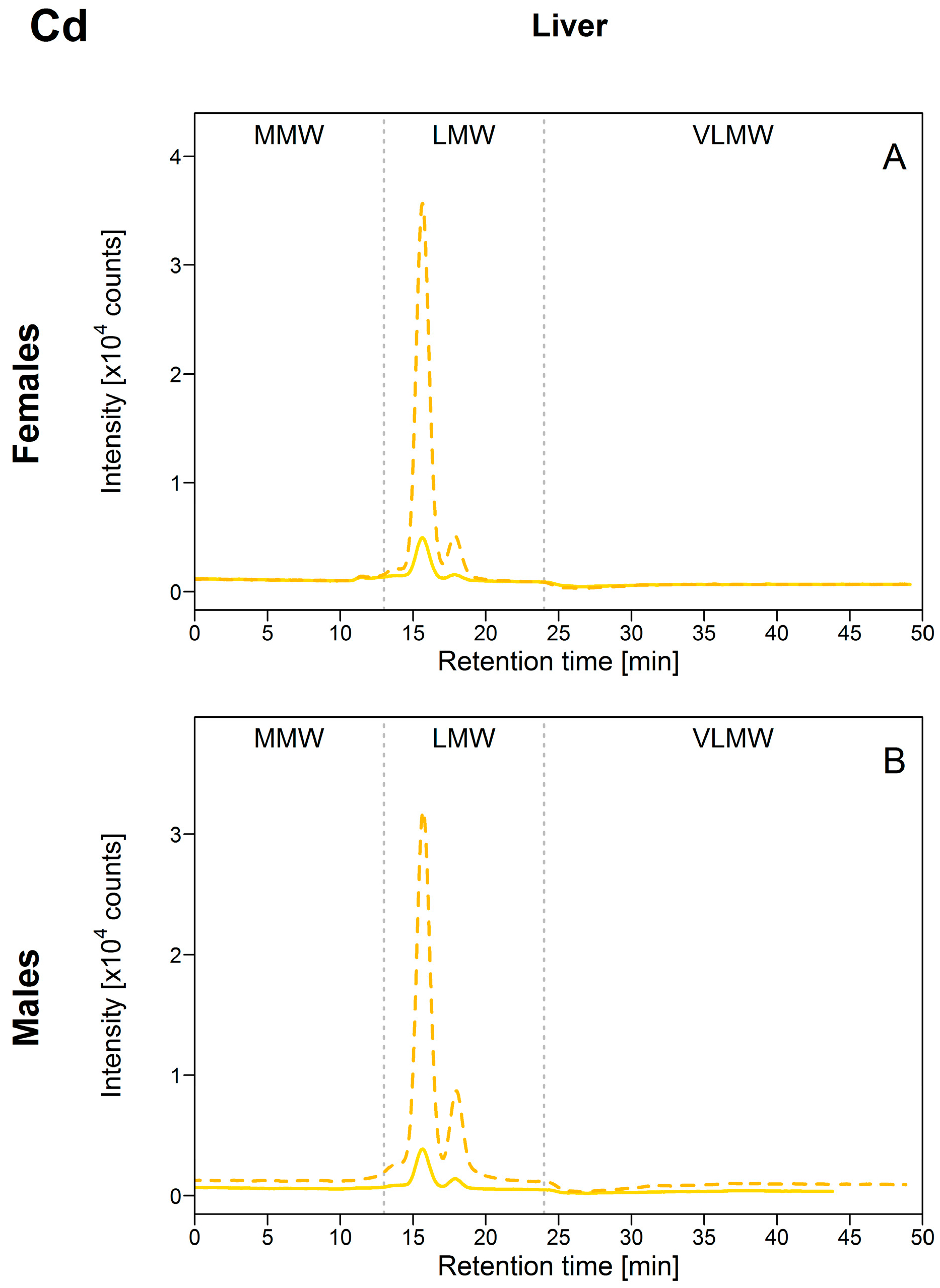
As was observed for Cu, all the hepatic Cd was found in the LMW biomolecule pool, with a major peak at an elution time of 15.8 min (corresponding to the elution time of the MT standard) and a second at 17.8 min (Figure 3). Unfortunately, [Cd]HSP in both gonad tissues were too low compared to the background signal for Cd, and the SEC data could not be interpreted for the ovaries and testes. However, results for individual fish for which clear CdHSP peaks were detected indicated that Cd was present in the LMW biomolecule pool and the peaks detected appeared at the same elution times as those observed in the liver (data not shown).
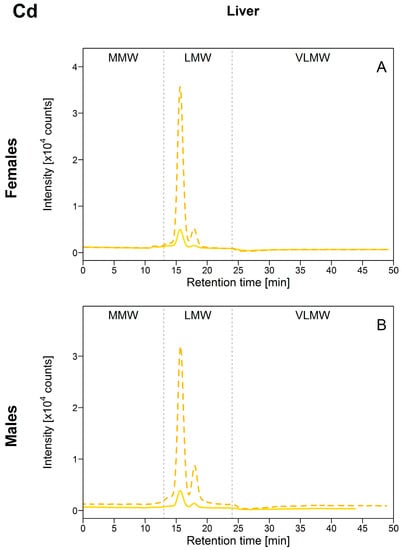
Figure 3.
Size-exclusion chromatograms of Cd-binding biomolecules in hepatic HSP fractions of white suckers from a reference (full line) and metal-impacted lake (dotted line). Vertical dotted lines delimit the three operationally defined molecular weight biomolecule pools: MMW (0–13 min; >10 kDa), LMW (13–24 min; 10–2 kDa) and VLMW (>24 min; <2 kDa). (A) Female liver; (B) Male liver.
As hepatic [Cd]HSP increased, peak areas in the LMW pool also increased and no changes in the Cd-binding biomolecule profiles between reference and exposed fish were observed (Figure 3; and Figure S2, Supplementary Materials), indicating that, as expected and similar to Cu, Cd was probably bound to MT and MTLP. The corollary of these results is that the Cd present in the HSP fraction was efficiently detoxified. Cadmium is an element with no known function in fish, and in the literature, it is widely reported that MT is a major player it its detoxification [8]. In a recent study of juvenile yellow perch, Caron et al. [18] reported hepatic Cd partitioning within the HSP fraction that was comparable to our results (Cd-binding biomolecule found in the LMW pool), but only one peak, eluting close to the elution time of a standard corresponding to a MW of 9.3 kDa, was detected. Co-elution with Cu was also reported, and the authors speculated that the metal-binding ligand was probably MT. Cadmium presence in the cytosol and its probable binding to MT were previously reported for the liver and gills of Vardar and European chubs collected in metal mining-impacted rivers [15,16,19]. Increased Cd associated with hepatic MT was also reported in field-collected European eels (Anguilla anguilla) and gibel carp (Carassius auratus gibelio) [14,17].
3.2.3. Arsenic (As)
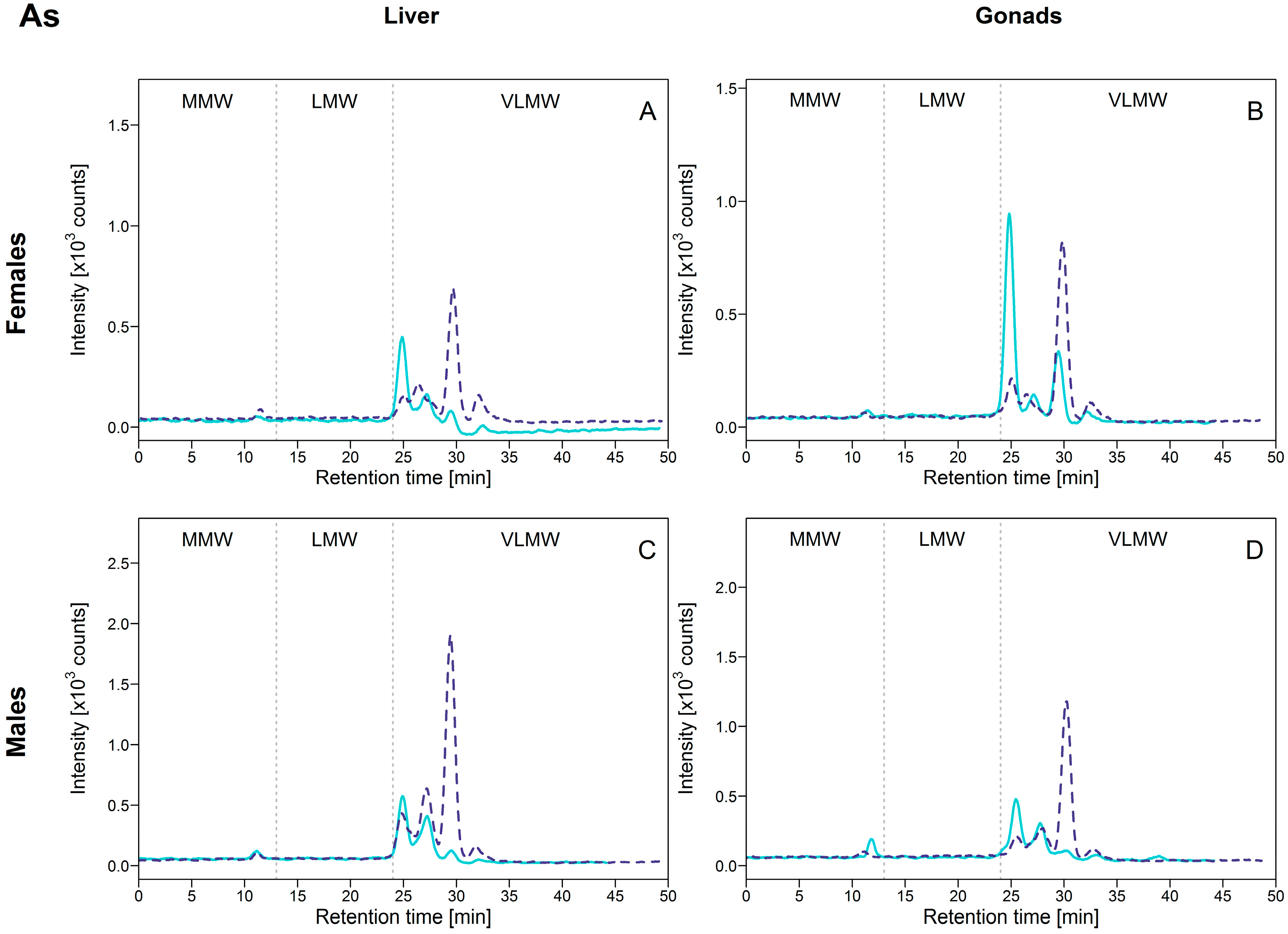
For As, modest [As]HSP differences between exposed and reference fish were observed, with maximum/minimum mean concentration ratios lower than 2.5. Concentrations were of a similar order of magnitude in all organs. The relative contribution from the HSP fraction to the total As burden was relatively stable within an organ (about 35% in female liver and testes, and about 53% in female gonads) except for the male livers where proportion increased from 29% to 45% between reference and exposed fish.
Arsenic distribution in the HSP fraction was very similar among liver, ovaries and testes: As was predominantly present within the VLMW biomolecule pool, with three major biomolecule complexes eluting at 25, 26.9 and 29.4 min (Figure 4). Interestingly, for all organs, different relative intensities were clearly observed between exposed and reference fish for these three As-binding biomolecules; in reference fish, the peak at 25 min was dominant (Figure 4; full lines), whereas in exposed fish the peak eluting at 29.4 min was dominant (Figure 4, dotted lines). The peak eluting at 29.4 min responded the most as total [As]HSP increased (except for the ovaries, for with no significant differences between reference and exposed fish were observed) (Figure S3, Supplementary Materials). Note that a peak was also observed at approximately 32 min, but it was of negligible importance compared to the other 3 peaks.
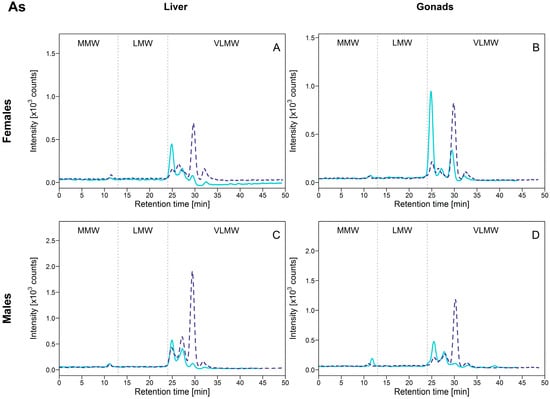
Figure 4.
Size-exclusion chromatograms of As-binding biomolecules in hepatic and gonadal HSP fractions of white suckers from a reference (full line) and metal-impacted lake (dotted line). Vertical dotted lines delimit the three operationally defined molecular weight biomolecule pools: MMW (0–13 min; >10 kDa), LMW (13–24 min; 10–2 kDa) and VLMW (>24 min; <2 kDa). (A) Female liver; (B) Female gonads; (C) Male liver; (D) Male gonads.
These results indicate first that As was associated with biomolecules of very low MW (<2 kDa), but not with MT and MTLP. In contrast, the induction of MT by As was reported in rodents and in some aquatic species exposed to inorganic arsenic (AsIII and AsV) in the laboratory [20,21,22,23]. Note however that these experiments were performed in the laboratory at higher exposure As concentrations than those found in the environment. Arsenicals (as trivalent arsenic species) are known to have high affinity for sulfhydryl groups and to be able to bind to cysteine in proteins and peptides, including MT and MTLP [24], which appears not to be the case in our study.
Secondly, given that the [As]HSP difference between exposed and reference fish was not marked and sometimes absent (no significant differences for female gonads, for example; see Table 2), we speculate that As exposure itself may not be responsible for induction of specific As-binding biomolecules but rather that As binds to biomolecules that are naturally present in the cytosol or that were induced by a combination of other environmental factors, such as the exposure to other trace elements. In a recent laboratory study, dietary supplementation of rainbow trout (Oncorhynchus mykiss) with selenomethionine combined with As exposure resulted in increased As retention and oxidative stress in different organs such as the liver [25]. The induction of some biomolecules to cope with other contaminants may afford the opportunity for As to bind to these other molecules.
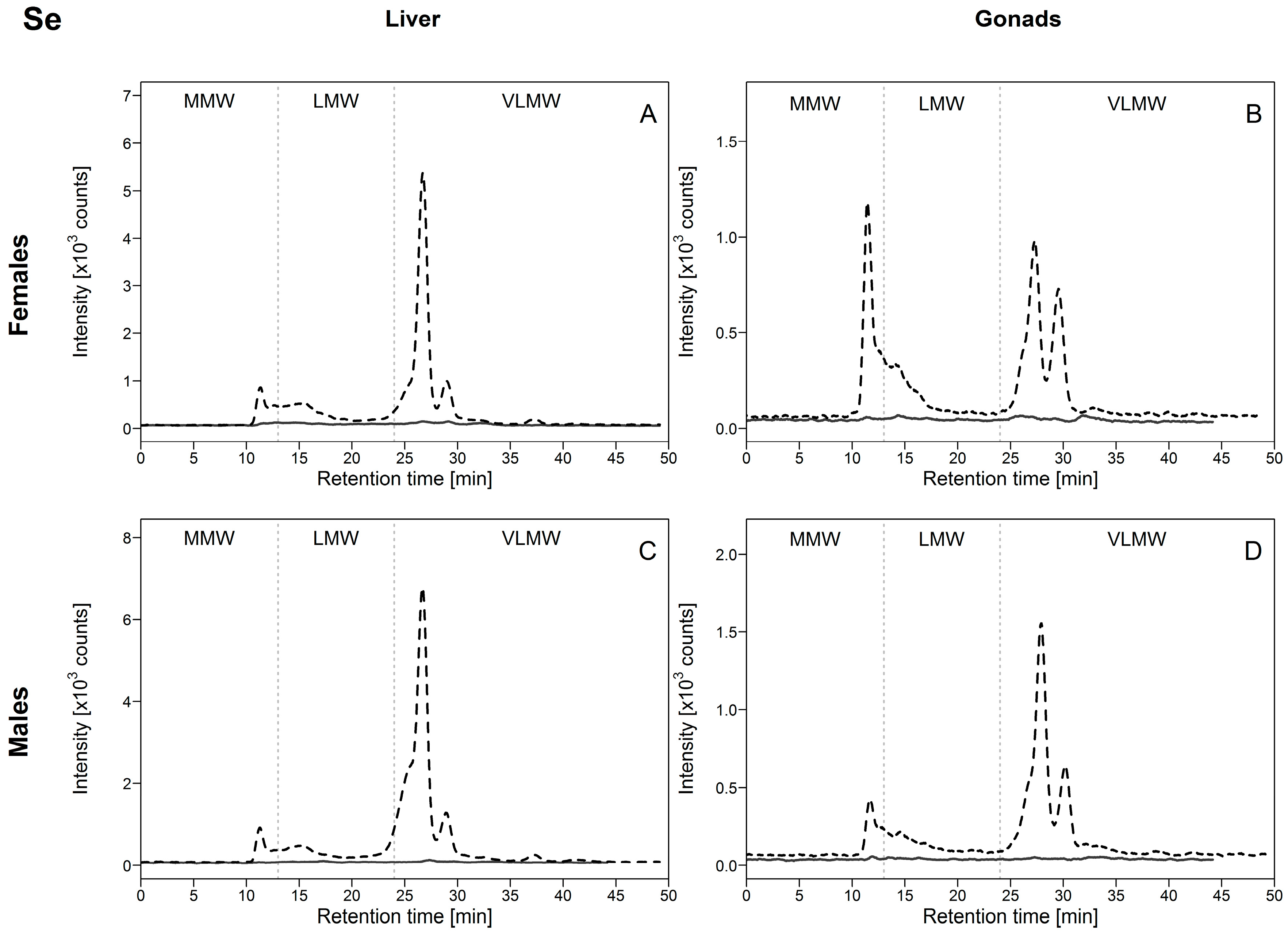
3.2.4. Selenium (Se)
Selenium concentrations in the HSP fractions markedly increased in exposed compared to reference fish, with maximum to minimum mean concentration ratios ranging from 7 to 15 (Table 2). In both male and female livers, the contribution from the HSP fraction to the total Se burden reached a maximum of 14%. In contrast, in both gonad tissues (ovaries and testes) of reference fish, this proportion was about 20% but decreased to around 10% in exposed fish, with a more marked drop in ovaries compared to testes, which corroborates our previous observations in white suckers from same sampling areas as in the present study [10]. For reference and exposed fish, [Se]HSP were comparable among organs, with the exception of male gonadal concentrations being consistently lower, likely due to the different functions assigned to ovaries and testes.
Selenium is an essential nutrient mainly incorporated covalently into selenoproteins in the form of seleno-amino acids, i.e., the genetically encoded amino-acid selenocysteine and the amino-acid selenomethionine that non-specifically replaces methionine [26]. These selenoproteins are required for normal biochemical processes such as those catalyzed by glutathione peroxidases and thioredoxin reductase [27,28,29]. However, Se is an element with a relatively narrow concentration range between the threshold for physiological requirements and the onset of toxicity, e.g., teratogenic effects on aquatic biota inhabiting Se-contaminated environments [30,31,32]. It is possible that excess Se incorporated into proteins may affect their functions and play a role in Se toxicity.
Regarding Se distribution in the HSP fraction of white suckers, we observed that in reference fish, no peaks were significantly above the background signal (Figure 5, full lines), whereas in exposed fish several peaks were detected (Figure 5, dotted lines). In the liver and male gonads, similar Se-binding biomolecule profiles were observed, with Se mainly found in the VLMW biomolecule pool (Figure 5A,C,D). A major peak eluted at 26.8 min (representing 80% of the total peak area; Figure S4, Supplementary Materials) with a second peak at 29 min, followed by a negligible but observable peak at 37.4 min. Selenium was also detected in the MMW pool but the peak area represented less than 10% of total peak areas (Figure S4, Supplementary Materials). Selenium was also present in the hepatic LMW pool (<10% of the total peak area), but no elution peaks were clearly distinguished. These results indicate that Se present in the heat-stable cytosolic HSP fraction of white sucker livers and testes was mainly associated with biomolecules of molecular weight <2 kDa. No co-elution peaks with arsenic were observed, indicating that distinct biomolecules are involved in the handling of these two metalloids.
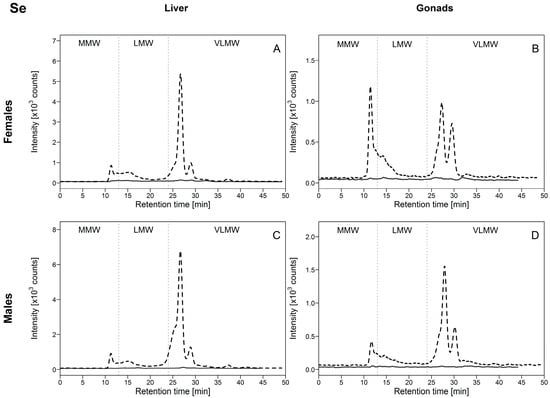
Figure 5.
Size-exclusion chromatograms of Se-binding biomolecules in hepatic and gonadal HSP fractions of white suckers from a reference (full line) and metal-impacted lake (dotted line). Vertical dotted lines delimit the three operationally defined molecular weight biomolecule pools: MMW (0–13 min; >10 kDa), LMW (13–24 min; 10–2 kDa) and VLMW (>24 min; <2 kDa). (A) Female liver; (B) Female gonads; (C) Male liver; (D) Male gonads.
In ovaries, the same elution peaks were observed but with different dominance; the Se-binding biomolecules were more equally distributed between the VLMW and MMW pools (Figure 5B). The major peak eluted at 11 min (representing about 50% of the total peak area; Figure S4, Supplementary Materials), possibly a pool of many different biomolecules >10 kDa, ahead of the peak eluting at 26.8 min (30% of the total peak area; Figure S4, Supplementary Materials). Although no clear peaks were detected in the ovarian LMW pool, a modest proportion of Se seems to be associated with biomolecules in this pool. The difficulty in distinguishing potential elution peak(s) in LMW pool could be explained by their masking by the tailing of the elution peak at 11 min. Moreover, since selenomethionine can be incorporated non-specifically into proteins, many biomolecules can contain Se. Importantly, the overall results do not show any evidence of an association of Se with MT and MTLP, in contrast to the observations for Cd and Cu.
Selenium-containing biomolecules (selenoproteins, selenometabolites, etc.) naturally exist in biota and research into the speciation of protein-bound Se has increased with the rapid development of analytical chemistry techniques. Although more than 20 selenoproteins have been identified in mammals, little is known about aquatic organisms and many seleno-compounds are yet unidentified [33,34]. Recently, a low MW Se-containing compound, selenoneine (an ergothioneine selenium analogue, i.e., with sulfur replaced by selenium, C9H14N3O2Se, MW = 275.2 g/mol and 553 g/mol for the autoxidized dimer [35]), apparently involved in oxidative stress defense, was identified as the predominant form of organic Se in the blood and other organs (e.g., hepatopancreas, heart, white muscle) of bluefin Tuna (Thunnus orientalis) [35], as well as in sea turtle liver (Eretmochelys imbricata and Chelonia mydas) [36]. Overall, these results highlight the need to determine the nature of the Se-containing biomolecules in the HSP fraction to better understand Se ecotoxicity.
4. Conclusions
Implications Regarding How the HSP Fraction Should Be Interpreted in Future Studies of Subcellular Metal Partitioning
Knowledge of metal subcellular partitioning, within fish or for specific fish organs, yields information about trace element behavior once they are internalized into cells, and it may afford better estimates of the risk of metal toxicity than does the current approach of using total tissue metal accumulation [37]. The partitioning of metals among subcellular fractions can be determined by successive differential centrifugation and heat-denaturation steps, leading to the separation of operational subcellular fractions, where the heat-stable cytosolic proteins and peptides fraction (HSP) is generally considered as a detoxified fraction in which metals have been handled in order to limit their toxic effects [4].
In the present study, the presence of biomolecules involved in the handling of both essential (Cu and Se) and nonessential (Cd and As) trace elements in the hepatic and gonadal HSP fraction of field-collected white suckers has been demonstrated. Overall results showed that metal handling strategies in the HSP fraction were specific to a given trace element but were generally similar among organs. The handling of Se in female gonads constituted an exception to this generalization: the Se distribution was different from that in the liver and testes with almost half of the Se found in the HSP fraction associated with biomolecules of MMW, whereas in the liver and testes more than 70% of Se was associated with biomolecules of MW lower than 2 kDa. Trace element distributions were also similar between reference and exposed fish, except in the case of As, where a change in As-binding biomolecule proportions was observed.
Previous studies exploring trace element partitioning within cells have tended to interpret the presence of trace elements in the HSP fraction as elements complexed by MT or MTLP, reflecting the regulation of essential elements and the detoxification of non-essential elements. The present study supports this generalization for Cd and Cu. However, our results showed no evidence for an association of As or Se with MT, raising questions about the nature of the metalloid-containing molecules present in the HSP fractions and also about the role of these molecules, i.e., are they involved in As/Se detoxification or are they metalloid-sensitive biomolecules? Future work focusing on the identification of these biomolecules is needed to clarify the distinction between “detoxified” or “sensitive” fractions for As and Se. In the same vein, studies using the online SEC-ICP-MS approach have previously demonstrated that other elements such as Ni or Tl do not elute with MT [18,38]. Overall, these results highlight the fact that, at this stage of our knowledge, one should be careful when interpreting the presence of certain trace elements in the HSP fraction as evidence of their detoxification. The results also demonstrate the contribution of the SEC-ICP-MS approach as a relevant and complementary approach to subcellular partitioning studies, as a tool to improve our capacity to monitor the risk for aquatic organisms of trace element contamination.
Supplementary Materials
The following materials are available online at http://www.mdpi.com/2076-3298/5/9/102/s1. Table S1: Summary of the total lengths of fish selected for the SEC-ICP-MS analyses. Figure S1: Peak area proportions among MW pools for Cu, and peak areas normalized by organ weight as a function of the total metal concentration in the HSP fraction of white sucker liver (males and females together) and gonads. Figure S2: Peak area proportions among MW pools for Cd, and peak areas normalized by organ weight as a function of the total metal concentration in the HSP fraction of white sucker liver (males and females together). Figure S3: Peak area proportions among the three major peaks in the VLMW pool for As, and peak areas normalized by organ weight as a function of the total metal concentration in the HSP fraction of white sucker liver (males and females together) and gonads. Figure S4: Peak area proportions among the peaks detected in the MMW and VLMW pools for Se.
Author Contributions
N.U., P.G.C.C. and P.C. conceived and designed the experiments; N.U. and S.J. performed the experiments; S.J. and N.U. analyzed the data; N.U. wrote the first draft of the paper; all authors contributed to the revision of subsequent drafts and the final manuscript.
Funding
The funding sponsor Stantec Consulting Ltd. had no role in sample analysis, in the interpretation of data; in the writing of the manuscript, and in the decision to publish the results. Stantec Consulting Ltd. provided assistance in field work for fish collection and contributed to the design of the larger project co-funded by NSERC and Stantec Consulting Ltd. through an NSERC Collaborative Research and Development grant and entitled “Development of subcellular distribution methods to identify indirect or direct effects of metals on aquatic organisms” (Grant number CRDPJ 485344-15).
Acknowledgments
The authors thank the industrial partner, Stantec Consulting Ltd., and the Natural Sciences and Engineering Research Council of Canada (NSERC) for co-funding this study. We would also like to thank M. Lerquet, O. Gosselin, L. Rozon-Ramilo and H. Sonnenberg who participated in the field work, and J. Perrault for her invaluable assistance in the INRS-ETE laboratory. PGCC was supported by the Canada Research Chairs program.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Total Metal Concentrations Determination in the Whole Liver and Gonads of Collected White Suckers
To determine total metal concentrations, samples of raw organs (≈0.5 g of wet weight) were freeze-dried for 72 h, weighed and digested overnight at room temperature in 1 mL of nitric acid (70%, v/v, Optima grade, Fisher Scientific). The samples were then heated at 70 °C for 2 h before addition of 500 µL of H2O2 (30%, v/v, Optima grade, Fisher Scientific) and heated again at 70 °C for 2 h. Digests were then completed with 8.5 mL ultrapure water and livers were diluted 100-fold. In parallel, reference material TORT-3 (Lobster Hepatopancreas, National Research Council of Canada) and digestion blanks were subjected to the same digestion procedure. Analyses were performed by ICP-MS (XSERIES 2, Thermo Fisher Scientific, Winsford, England, UK). Standard reference water (synthetic water [900-Q30-100, SCP Science]) was analyzed during each run (every twenty samples) and was quantitatively recovered. Analytical procedural blanks indicated no appreciable contamination. The recovery of TORT-3 reference samples (n = 5) was of 113 ± 6% for As, 104 ± 5% for Cd, 96 ± 5% for Cu, and 122 ± 8% for Se. No correction was applied.
References
- Wang, W.-X. Prediction of metal toxicity in aquatic organisms. Chin. Sci. Bull. 2013, 58, 194–202. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Hare, L. Metal detoxification in freshwater animals. Roles of metallothioneins. In Metallothioneins and Related Chelators; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Royal Society of Chemistry: Cambridge, UK, 2009; Volume 5, pp. 239–277. [Google Scholar]
- Mason, A.Z.; Jenkins, K.D. Metal detoxification in aquatic organisms. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D., Eds.; IUPAC Series on Analytical and Physical Chemistry of Environmental Systems; J. Wiley & Sons: Chichester, UK, 1995; pp. 479–608. [Google Scholar]
- Wallace, W.G.; Lee, B.-G.; Luoma, S.N. Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef]
- Wang, W.; Rainbow, P.S. Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ. Chem. 2006, 3, 395–399. [Google Scholar] [CrossRef]
- Amiard, J.-C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Vasak, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Roesijadi, G. Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat. Toxicol. 1992, 22, 81–113. [Google Scholar] [CrossRef]
- Giguère, A.; Campbell, P.G.C.; Hare, L.; Couture, P. Sub-cellular partitioning of cadmium, copper, nickel and zinc in indigenous yellow perch (Perca flavescens) sampled along a polymetallic gradient. Aquat. Toxicol. 2006, 77, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Urien, N.; Cooper, S.; Caron, A.; Sonnenberg, H.; Rozon-Ramilo, L.; Campbell, P.G.C.; Couture, P. Subcellular partitioning of metals and metalloids (As, Cd, Cu, Se and Zn) in liver and gonads of wild white suckers (Catostomus commersonii) collected downstream from a mining operation. Aquat. Toxicol. 2018, 202, 105–116. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 30 August 2018).
- Klaverkamp, J.F.; Dutton, M.D.; Majewski, H.S.; Hunt, R.V.; Wesson, L.J. Evaluating the effectiveness of metal pollution controls in a smelter by using metallothionein and other biochemical responses in fish. In Metal Ecotoxicology—Concepts and Applications; Newman, M.C., McIntosh, A.W., Eds.; Lewis Publishers Ltd.: Chelsea, MI, USA, 1991; pp. 33–64. [Google Scholar]
- Van Campenhout, K.; Infante, H.G.; Goemans, G.; Belpaire, C.; Adams, F.; Blust, R.; Bervoets, L. A field survey of metal binding to metallothionein and other cytosolic ligands in liver of eels using an on-line isotope dilution method in combination with size exclusion (SE) high pressure liquid chromatography (HPLC) coupled to Inductively Coupled Plasma time-of-flight Mass Spectrometry (ICP-TOFMS). Sci. Total Environ. 2008, 394, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Infante, H.G.; Campenhout, K.V.; Schaumlöffel, D.; Blust, R.; Adams, F.C. Multi-element speciation of metalloproteins in fish tissue using size-exclusion chromatography coupled “on-line” with ICP-isotope dilution-time-of-flight-mass spectrometry. Analyst 2003, 128, 651–657. [Google Scholar] [CrossRef]
- Krasnići, N.; Dragun, Z.; Erk, M.; Ramani, S.; Jordanova, M.; Rebok, K.; Kostov, V. Size-exclusion HPLC analysis of trace element distributions in hepatic and gill cytosol of Vardar chub (Squalius vardarensis Karaman) from mining impacted rivers in north-eastern Macedonia. Sci. Total Environ. 2018, 613–614, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Krasnići, N.; Dragun, Z.; Erk, M.; Raspor, B. Distribution of selected essential (Co, Cu, Fe, Mn, Mo, Se, and Zn) and nonessential (Cd, Pb) trace elements among protein fractions from hepatic cytosol of European chub (Squalius cephalus L.). Environ. Sci. Pollut. Res. 2013, 20, 2340–2351. [Google Scholar] [CrossRef] [PubMed]
- Infante, H.G.; Van Campenhout, K.; Blust, R.; Adams, F.C. Anion-exchange high performance liquid chromatography hyphenated to inductively coupled plasma-isotope dilution-time-of-flight mass spectrometry for speciation analysis of metal complexes with metallothionein isoforms in gibel carp (Carassius auratus gibelio) exposed to environmental metal pollution. J. Chromatogr. A 2006, 1121, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Rosabal, M.; Drevet, O.; Couture, P.; Campbell, P.G.C. Binding of trace elements (Ag, Cd, Co, Cu, Ni, and Tl) to cytosolic biomolecules in livers of juvenile yellow perch (Perca flavescens) collected from lakes representing metal contamination gradients. Environ. Toxicol. Chem. 2018, 37, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Krasnići, N.; Dragun, Z.; Erk, M.; Raspor, B. Distribution of Co, Cu, Fe, Mn, Se, Zn, and Cd among cytosolic proteins of different molecular masses in gills of European chub (Squalius cephalus L.). Environ. Sci. Pollut. Res. 2014, 13512–13521. [Google Scholar] [CrossRef]
- Albores, A.; Koropatnick, J.; Cherian, M.G.; Zelazowski, A.J. Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem. Biol. Interact. 1992, 85, 127–140. [Google Scholar] [CrossRef]
- Kreppel, H.; Bauman, J.W.; Liu, J.; McKim, J.M., Jr.; Klaassen, C.D. Induction of metallothionein by arsenicals in mice. Fundam. Appl. Toxicol. 1993, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; Santos, H.M.; Costa, P.M.; Peres, I.; Costa, M.H.; Capelo, J.L. Metallothionein responses in the Asiatic clam (Corbicula fluminea) after exposure to trivalent arsenic. Biomarkers 2007, 12, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, L.M.; Wang, W.X. Biotransformation and detoxification of inorganic arsenic in a marine juvenile fish Terapon jarbua after waterborne and dietborne exposure. J. Hazard. Mater. 2012, 221, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, X.-F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic Binding to Proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; MacDonald, T.C.; Saibu, Y.; George, G.N.; Niyogi, S.; (University of Saskatchewan, Saskatoon, SK, Canada). Personal communication, 2017.
- Ballihaut, G.; Pécheyran, C.; Mounicou, S.; Preud’homme, H.; Grimaud, R.; Lobinski, R. Multimode detection (LA-ICP-MS, MALDI-MS and nanoHPLC-ESI-MS2) in 1D and 2D gel electrophoresis for selenium-containing proteins. Trends Anal. Chem. 2007, 26, 183–190. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. FEBS J. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Pappas, A.C.; Zoidis, E.; Surai, P.F.; Zervas, G. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Lemly, A.D. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ. Monit. Assess. 1993, 28, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.M.; DeForest, D.K.; Brooks, M.L.; Chapman, P.M.; Gilron, G.; Hoff, D.; Hopkins, W.A.; McIntyre, D.O.; Mebane, C.A.; Palace, V.P. Selenium toxicity to aquatic organisms. In Ecological Assessment of Selenium in the Aquatic Environment; Chapman, P.M., Adams, W.J., Brooks, M.L., Delos, C.G., Luoma, S.N., Maher, W.A., Ohlendorf, H.M., Presser, T.S., Shaw, D.P., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 141–231. ISBN 978-1-4398-2678-2. [Google Scholar]
- Muscatello, J.R.; Bennett, P.M.; Himbeault, K.T.; Belknap, A.M.; Janz, D.M. Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environ. Sci. Technol. 2006, 40, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, A.; Behne, D. Selenium-containing proteins in mammals and other forms of life. In Reviews of Physiology, Biochemistry and Pharmacology; Amara, S.G., Bamberg, E., Blaustein, M.P., Grunicke, H., Jahn, R., Lederer, W.J., Miyajima, A., Murer, H., Pfanner, N., Schultz, G., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–46. ISBN 3-540-43520-4. [Google Scholar]
- Mariotti, M.; Ridge, P.G.; Zhang, Y.; Lobanov, A.V.; Pringle, T.H.; Guigo, R.; Hatfield, D.L.; Gladyshev, V.N. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS ONE 2012, 7, e33066. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamashita, M. Identification of a Novel Selenium-containing Compound, Selenoneine, as the Predominant Chemical Form of Organic Selenium in the Blood of Bluefin Tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef] [PubMed]
- Anan, Y.; Ishiwata, K.; Suzuki, N.; Tanabe, S.; Ogra, Y. Speciation and identification of low molecular weight selenium compounds in the liver of sea turtles. J. Anal. At. Spectrom. 2011, 26, 80–85. [Google Scholar] [CrossRef]
- Adams, W.J.; Blust, R.; Borgmann, U.; Brix, K.V.; DeForest, D.K.; Green, A.S.; Meyer, J.S.; McGeer, J.C.; Paquin, P.R.; Rainbow, P.S.; et al. Utility of tissue residues for predicting effects of metals on aquatic organisms. Integr. Environ. Assess. Manag. 2011, 7, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Rosabal, M.; Mounicou, S.; Hare, L.; Campbell, P.G.C. Metal (Ag, Cd, Cu, Ni, Tl, and Zn) Binding to Cytosolic Biomolecules in Field-Collected Larvae of the Insect Chaoborus. Environ. Sci. Technol. 2016, 50, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).