Comprehensive Instrumental Odor Analysis Using SIFT-MS: A Case Study
Abstract
1. Introduction
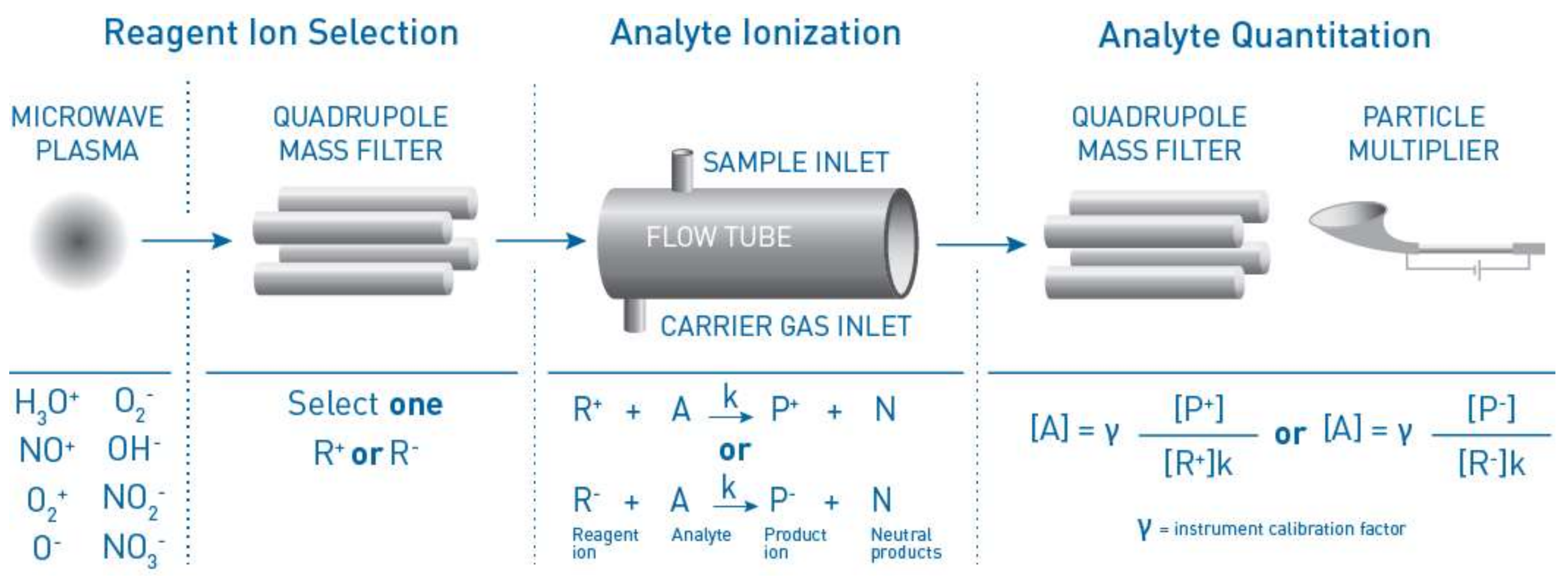
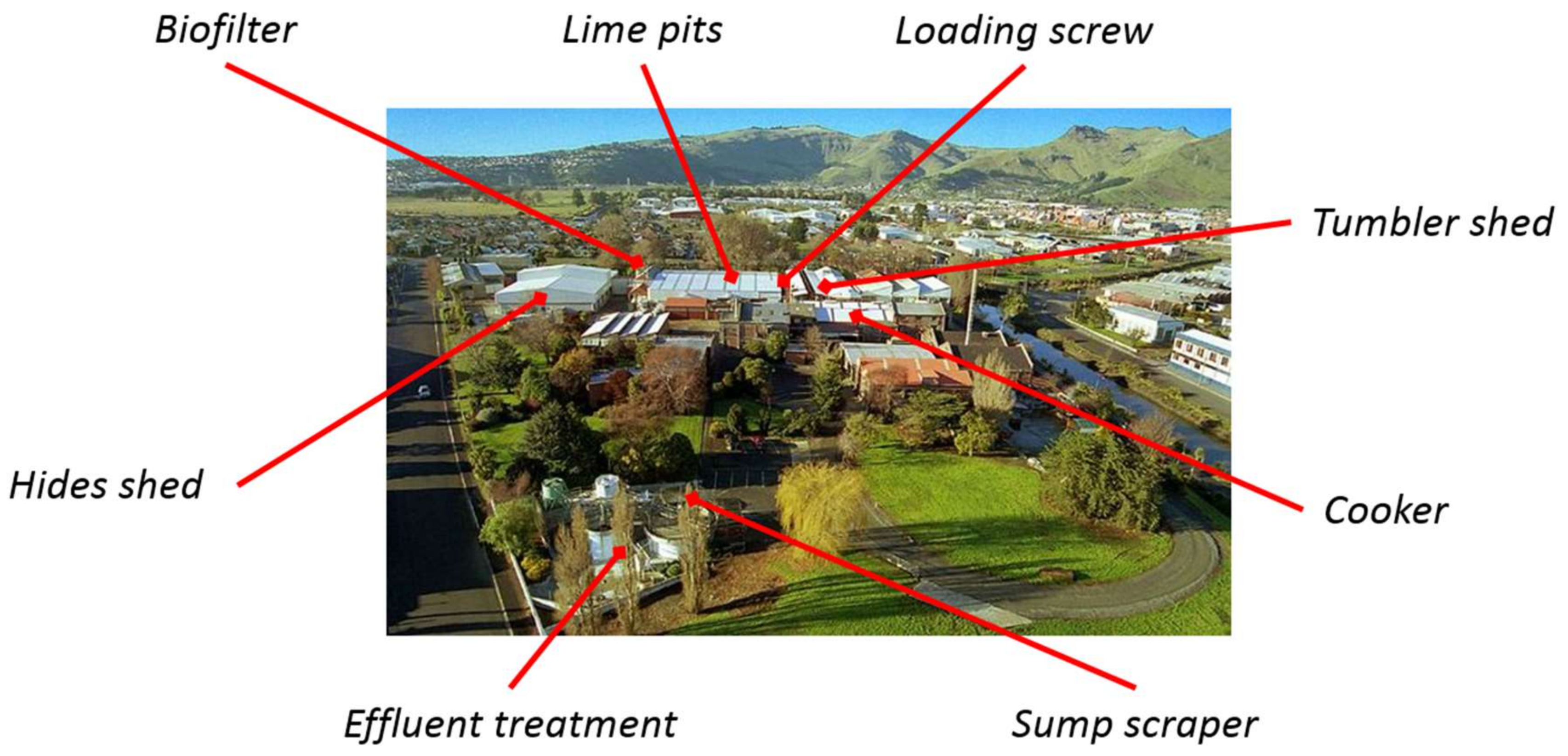
2. Materials and Methods
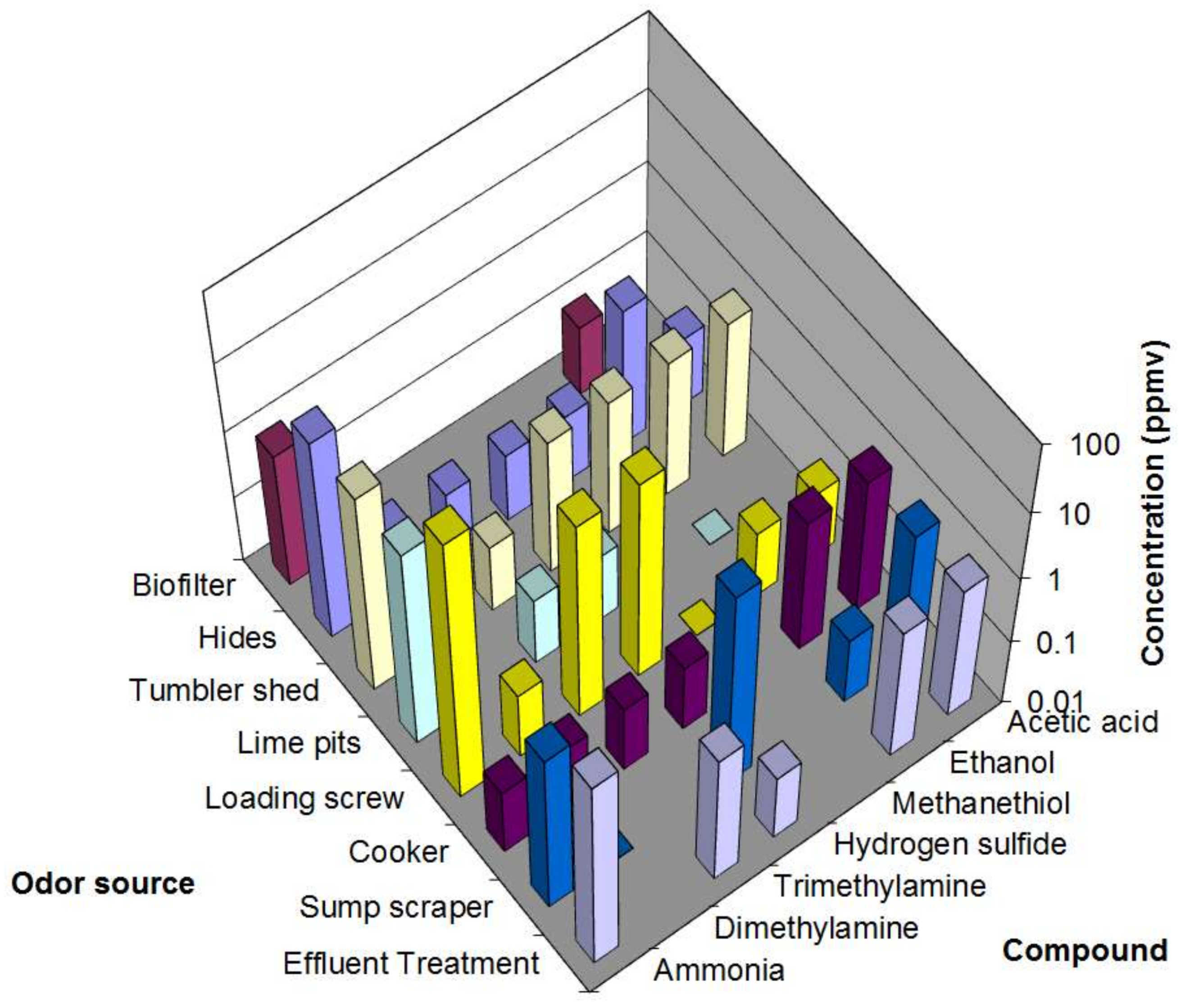
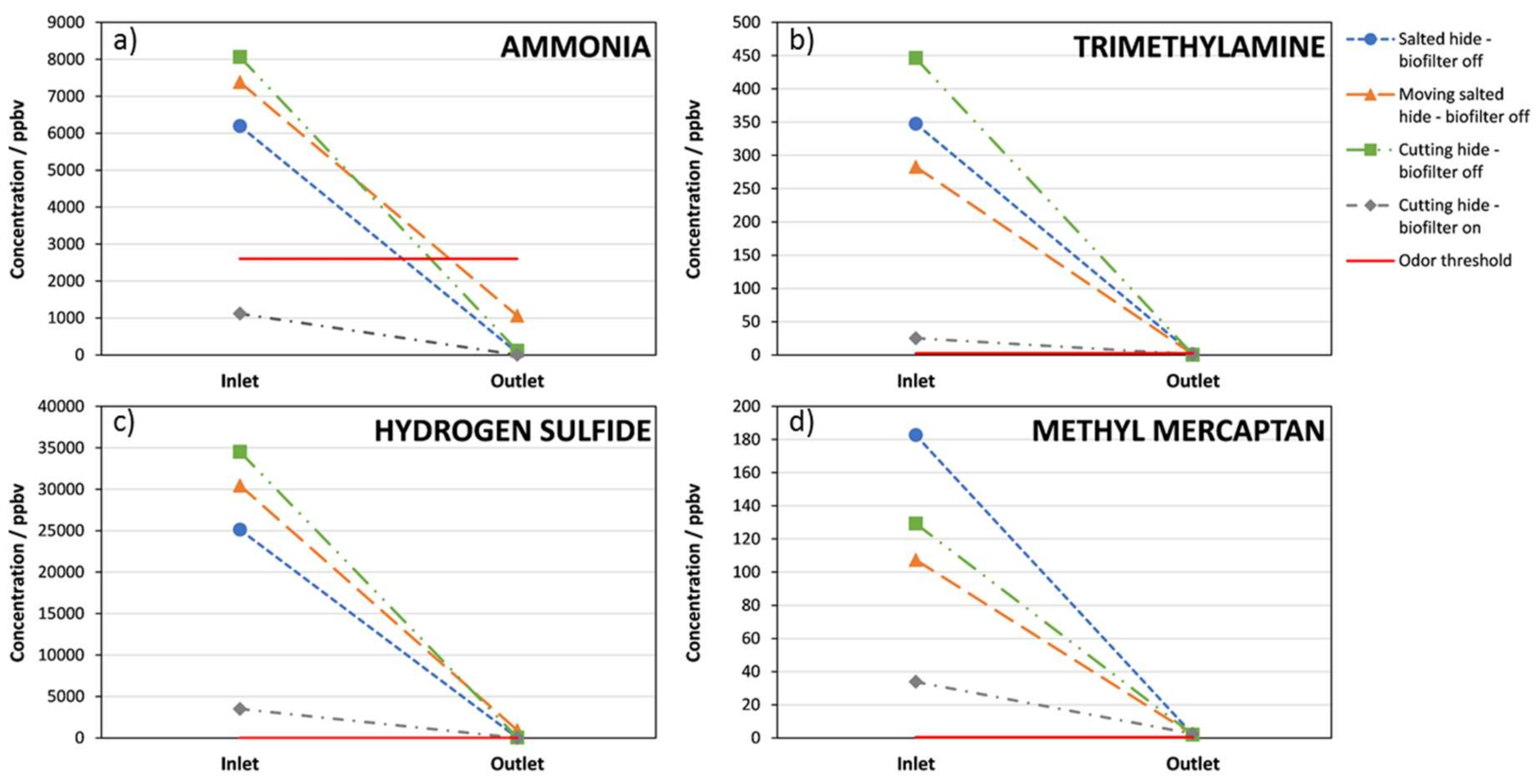
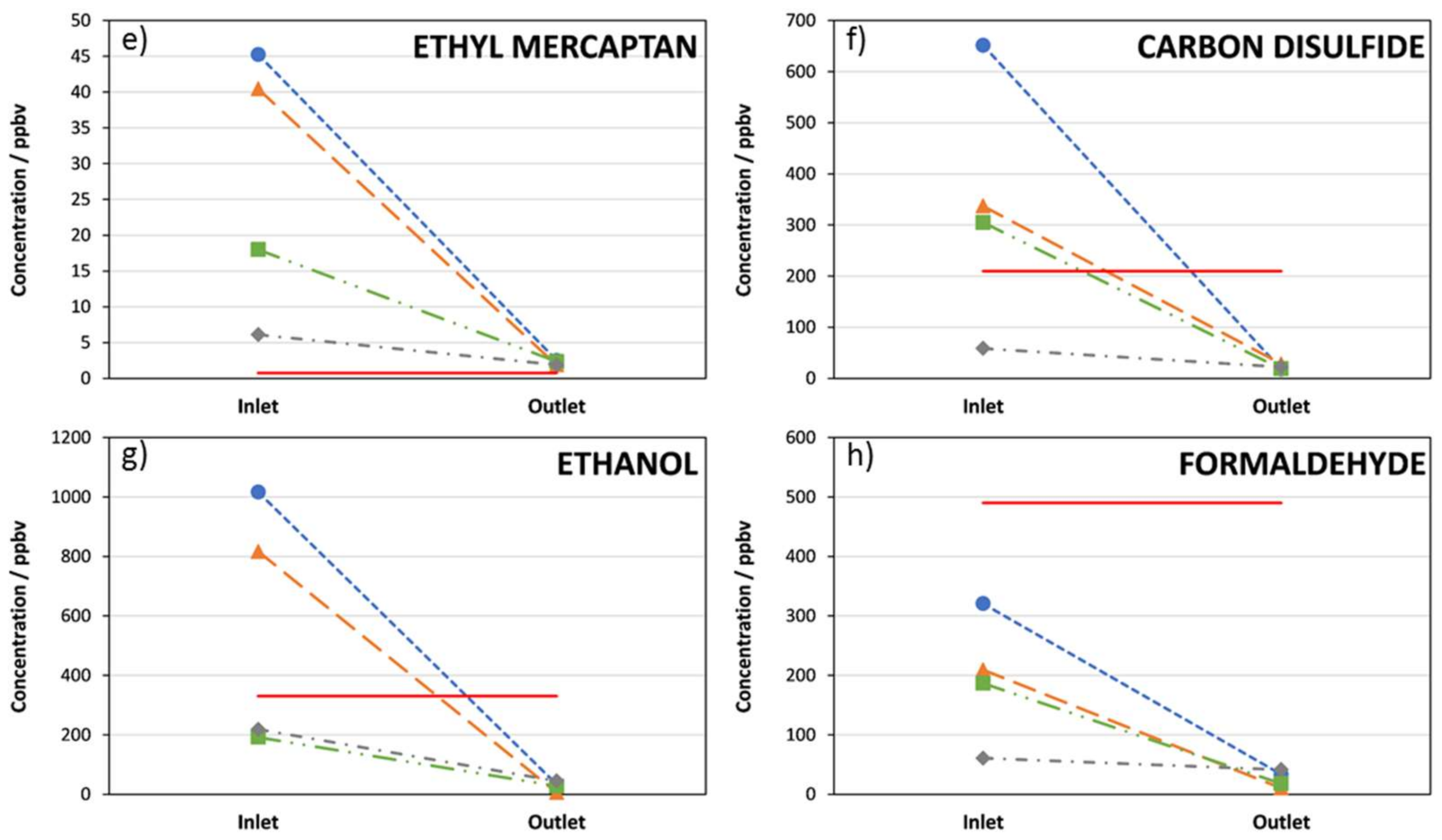
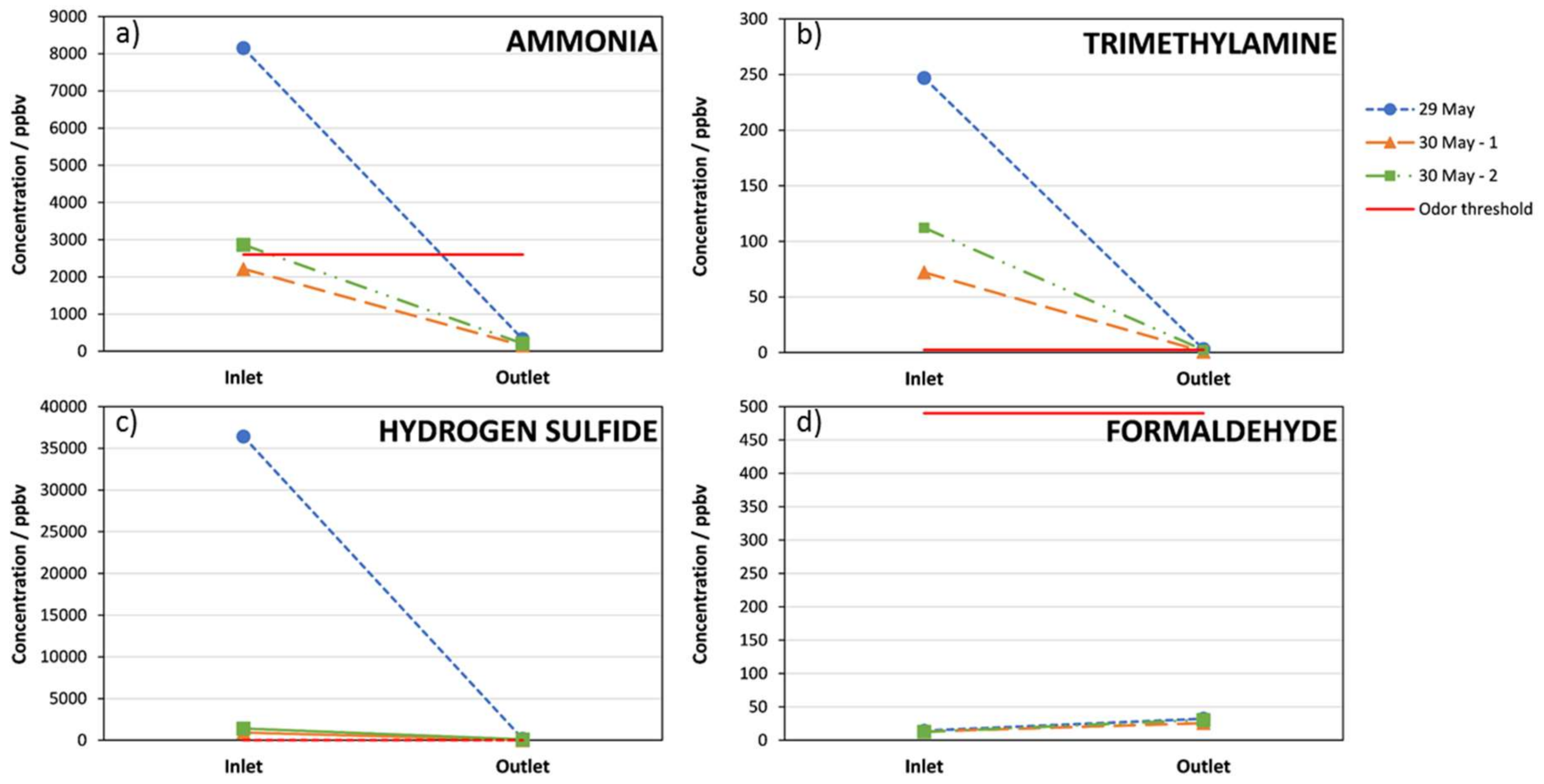
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Araneda, R.C.; Kini, A.D. The molecular receptive range of an odorant receptor. Nat. Neurosci. 2000, 3, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Powers, W. The Science of Smell Part 1: Odor Perception and Physiological Response. Iowa State University, 2004. Available online: http://www.aerisa.com/wp-content/uploads/2014/10/3-Science-of-Smell-Parts-1-4-Iowa-State-PM1963.pdf (accessed on 20 March 2018).
- Buettner, A. Springer Handbook of Odor; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Capelli, L.; Sironi, S.; Del Rosso, R.; Gullot, J.M. Measuring odours in the environment vs. dispersion modelling: A review. Atmos. Environ. 2013, 79, 731–743. [Google Scholar] [CrossRef]
- Pickenhagen, W. History of Odor and Odorants. In Springer Handbook of Odor; Buettner, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Chapter 1; pp. 1–9. [Google Scholar]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical identification of key odor components in livestock waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Van Harrevald, P.; Heeres, P.; Harssema, H. A review of 20 years of standardization of odor concentration measurements by dynamic olfactometry in Europe. J. Waste Manag. Assoc. 1999, 49, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Mannebeck, D. Olfactometers according to EN 13725. In Springer Handbook of Odor; Buettner, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Chapter 24; pp. 545–551. [Google Scholar]
- Govardhan, K.; Grace, A.N. Metal/metal oxide doped semiconductor-based metal oxide gas sensors—A review. Sens. Lett. 2016, 14, 741–750. [Google Scholar] [CrossRef]
- James, D.; Scott, S.M.; Ali, Z.; O’Hare, W.T. Chemical sensors for electronic nose systems. Microchim. Acta 2005, 149, 1–17. [Google Scholar] [CrossRef]
- Escuderos, M.E.; Sanchez, S.; Jiminez, A. Quartz crystal microbalance (QCM) sensor array selection for olive oil sensory evaluation. Food Chem. 2011, 124, 857–862. [Google Scholar] [CrossRef]
- Guthrie, B. Machine Olfaction. In Springer Handbook of Odor; Buettner, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Chapter 21; pp. 459–482. [Google Scholar]
- Beauchamp, J.; Zardin, E. Odorant Detection by On-line Chemical Ionization Mass Spectrometry. In Springer Handbook of Odor; Buettner, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Chapter 18; pp. 355–393. [Google Scholar]
- Cagliero, C.; Sgorbini, B.; Cordero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C. Enantioselective Gas Chromatography with Cyclodextrin in Odorant analysis. In Springer Handbook of Odor; Buettner, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 409–433. [Google Scholar]
- Burdack-Freitag, A.; Heinlein, A.; Mayer, F. Material Odor Emissions and Indoor Air Quality. In Springer Handbook of Odor; Buettner, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 563–577. [Google Scholar]
- Guffanti, P.; Pifferi, V.; Falciola, L.; Ferrante, V. Analysis of odours from concentrated animal feeding operations: A review. Atmos. Environ. 2018, 175, 100–108. [Google Scholar] [CrossRef]
- Smith, D.; Spanel, P. Selected Ion Flow Tube Mass Spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef] [PubMed]
- Spanel, P.; Smith, D. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2011, 30, 236–267. [Google Scholar] [CrossRef] [PubMed]
- McEwan, M.J. Direct Analysis Mass Spectrometry in Ion/Molecule Attachment Reaction Mass Spectrometry; Fujii, T., Ed.; Springer Science & Business Media: New York, NY, USA, 2015; Chapter 8; pp. 263–318. [Google Scholar]
- Hera, D.; Langford, V.S.; McEwan, M.J.; McKellar, T.J.; Milligan, D.B. Negative reagent ions for real time detection using SIFT-MS. Environments 2017, 4, 16. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Giminez, B.; Lpez-Cabellero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources; a review. Food Hydrocolloids 2001, 25, 1813–1827. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Ann. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shu, Z. The extraction of collagen protein from pig skin. J. Chem. Pharm. Res. 2015, 6, 683–687. [Google Scholar]
- Augustin, O.G.; Little, S. A new odour control option for sewer and pump stations. In Proceedings of the 6th International Conference on Sewer Processes and Networks, Surfers Paradise and Networks, Surfers Paradise, Gold Coast, Australia, 7–10 November 2010. [Google Scholar]
- Althoff, H.; Mizzi, R.; Augustin, O. New odour control technology deals with difficult odours. A case study from one of Europe’s largest Waste Water Treatment Plants. In Proceedings of the Australia’s National Water Conference and Exhibition, Sydney, Australia, 8–10 May 2012. [Google Scholar]
- Spanel, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of aldehydes and ketones. Int. J. Mass Spectrom. Ion Process. 1997, 165–166, 25–37. [Google Scholar] [CrossRef]
- Spanel, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+ and O2+ with some organosulfur molecules. Int. J Mass Spectrom. 1998, 176, 167–176. [Google Scholar] [CrossRef]
- Williams, T.L.; Adams, N.G.; Babcock, L.M. Selected ion flow tube studies of H3O+ (H2O)0.1 reactions with sulfides and thiols. Int. J. Mass Spectrom. 1998, 172, 149–159. [Google Scholar] [CrossRef]
- Spanel, P.; Smith, D. Selected ion flow tube studies H3O+, NO+ and O2+ with several amines and some other nitrogen containing molecules. Int. J. Mass Spectrom. 1998, 176, 203–211. [Google Scholar] [CrossRef]
- Midey, A.J.; Arnold, S.T.; Viggiano, A.A. Reactions of H3O+ (H2O)n with formaldehyde and acetaldehyde. J. Phys. Chem. A 2000, 104, 2706–2709. [Google Scholar] [CrossRef]
- Spanel, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of carboxylic acids and esters. Int. J. Mass Spectrom. Ion Process. 1998, 172, 137–147. [Google Scholar] [CrossRef]
- Langford, V.S.; Graves, I.; McEwan, M.J. Rapid monitoring of volatile compounds: A comparison between gas chromatography/mass spectrometry and selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Prince, B.J.; Milligan, D.B.; McEwan, M.J. Application of selected ion flow tube mass spectrometry to real time atmospheric monitoring. Rapid Commun. Mass Spectrom. 2010, 24, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Boeker, P.; Leppert, J.; Lammers, P.S. Comparison of odorant losses at the ppb-level from sampling bags of NalophanTM and TedlarTM and from adsorption tubes. Chem. Eng. Trans. 2014, 40, 157–162. [Google Scholar]
- Traube, S.L.; Anhalt, J.C.; Zahn, J.A. Bias of Tedlar bags in the measurement of agricultural odorants. J. Environ. Qual. 2006, 35, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J. Odour Thresholds: Compilation of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter and Partners: Utrecht, The Netherlands, 2011. [Google Scholar]
- US Occupational Safety and Health Administration Lists 8 Hour Exposure Safety Level for Formaldehyde as 750 ppbv. Available online: www.osha.gov (accessed on 20 March 2018).
- Dung, T.T.; Oh, Y.; Choi, S.-J.; Kim, I.-D.; Oh, M.-K. Applications and advances in bioelectronic noses for odor sensing. Sensors 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Lovascio, S.; Penza, M. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langford, V.S.; McEwan, M.J.; Askey, M.; Barnes, H.A.; Olerenshaw, J.G. Comprehensive Instrumental Odor Analysis Using SIFT-MS: A Case Study. Environments 2018, 5, 43. https://doi.org/10.3390/environments5040043
Langford VS, McEwan MJ, Askey M, Barnes HA, Olerenshaw JG. Comprehensive Instrumental Odor Analysis Using SIFT-MS: A Case Study. Environments. 2018; 5(4):43. https://doi.org/10.3390/environments5040043
Chicago/Turabian StyleLangford, Vaughan S., Murray J. McEwan, Mary Askey, Helena A. Barnes, and James G. Olerenshaw. 2018. "Comprehensive Instrumental Odor Analysis Using SIFT-MS: A Case Study" Environments 5, no. 4: 43. https://doi.org/10.3390/environments5040043
APA StyleLangford, V. S., McEwan, M. J., Askey, M., Barnes, H. A., & Olerenshaw, J. G. (2018). Comprehensive Instrumental Odor Analysis Using SIFT-MS: A Case Study. Environments, 5(4), 43. https://doi.org/10.3390/environments5040043



