Mulching and Fertilization Effects on Weed Dynamics under Conservation Agriculture-Based Maize Cropping in Zimbabwe †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Background to the Study

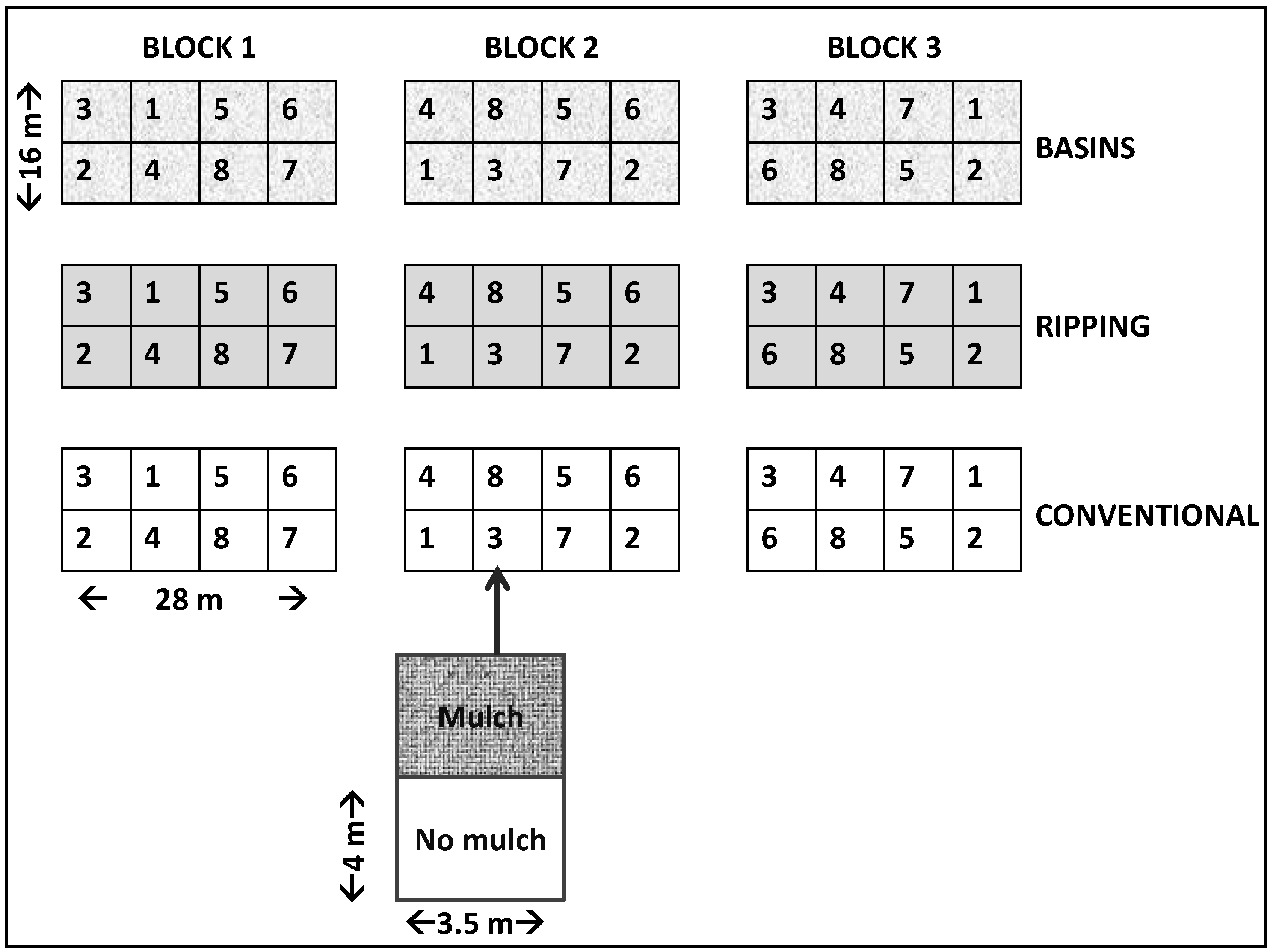
2.3. Experimental Layout, Treatments, and Agronomic Management
- (i)
- Conventional (tillage with conventional ox-drawn moldboard plow)
- (ii)
- Ripping (using ripper tine)
- (iii)
- Planting basins (basins—basin size = 15 cm × 15 cm × 15 cm)
| Plot No. | Season 1 (2011–2012) Treatments | Season 2 (2012–2013) Treatments | ||
|---|---|---|---|---|
| Test Crop | Fertilizer Rate and Type | Test Crop | Fertilizer Rate and Type | |
| 1 | Maize | High rate (120 kg·N·ha−1; 26 kg·P·ha−1) | Cowpea | high rate (17 kg·N·ha−1; 26 kg·P·ha−1) |
| 2 | Cowpea | High rate (17 kg·N·ha−1; 26 kg·P·ha−1) | Maize | high rate (120 kg·N·ha−1; 26 kg·P·ha−1) |
| 3 | Maize | Low rate (35 kg·N·ha−1; 14 kg·P·ha−1) | Cowpea | low rate (8 kg·N·ha−1; 14 kg·P·ha−1) |
| 4 | Cowpea | Low rate (8 kg·N·ha−1; 14 kg·P·ha−1) | Maize | low rate (35 kg N·ha−1; 14 kg·P·ha−1) |
| 5 | Maize | High rate (7 t manure ha−1 + 90 kg·N·ha−1; 26 kg·P·ha−1) | Cowpea | Residual fertility (high fertilizer rate) |
| 6 | Maize | Low rate (4 t manure ha−1 + 35 kg·N·ha−1; 14 kg·P·ha−1) | Cowpea | Residual fertility (low fertilizer rate) |
| 7 | Maize | Control–no fertilizer | Maize | Control–no fertilizer |
| 8 | Maize | high rate (120 kg·N·ha−1; 26 kg·P·ha−1) | Maize | high rate (120 kg·N·ha−1; 26 kg·P·ha−1) |
| Crop Type and Management | Crop Establishment Option | ||
|---|---|---|---|
| Conventional | Ripping | Basins | |
| MAIZE (SC 513 early-medium maturity)
Land preparation Mulching @30% cover Planting date Seeds per station Thinning Thinned to: | 28 November 2012 28 November 2012 29 November 2012 2 13 December 2012 1 | 3 November 2012 28 November 2012 29 November 2012 2 13 December 2012 1 | 3 November 2012 28 November 2012 29 November 2012 3 13 December 2012 2 |
| Target population
First fertilizer application Manure application Weeding Second fertilizer application Pesticide application Harvesting | 37,000 plants ha−1 Basal at planting At planting 6 weeks after crop emergence Top-dressing 6 weeks after crop emergence Kombat (2.5% Carbaryl) at 3–4 kg·ha−1 for maize stalk borer (Busseola fusca) at 6 weeks after emergence 28 April 2013 | ||
| COWPEA (CBC 2- erect variety)
Land preparation Mulching @30% cover Planting date Seeds per station | 28 November 2012 28 November 2012 29 November 2012 2 | 3 November 2012 28 November 2012 29 November 2012 2 | 3 November 2012 28 November 2012 29 November 2012 3 |
| Spacing
Fertilizer application Manure application Weeding Pesticide application Harvesting | 0.45 m between rows and 0.15 m within rows
Basal at planting At planting 6 weeks after crop emergence Dimethoate at 2 mL per liter of water at 30 days after emergence for aphids Dimethoate at 60 days after emergence 2 mL per liter of water at 30 days after emergence for aphids First harvesting beginning of March till all the pods were harvested end of April 2013 | ||
2.4. Weed Data Collection and Analysis
3. Results and Discussion
3.1. Weed Population Dynamics under Different Crop Establishment Options
| Species | Common Name | Relative Dominance Overall (%) |
|---|---|---|
| Herbaceous weeds | ||
| Galinsoga parviflora Cav. | Gallant soldier | 35–72 |
| Richardia scabra L. | Mexican clover | 20–50 |
| Acanthospermum hispidum DC | Bristly starbur | 20–30 |
| Bidens pilosa L. | Cobbler’s pegs | 10–15 |
| Commelina benghalensis L. | Tropical spiderwort | <10 |
| Crotalaria cylindrostachys Welw. Ex Baker | Crotalaria | <10 |
| Macrotyloma daltonii (Webb) Verdc. | Macrotyloma | <10 |
| Amaranthus thunbergii Moq. | Thunberg's amaranth | <5 |
| Leucas martinicensis (Jacq.) R.Br. | Whitewort | <5 |
| Hibiscus cannabinus L. | Java jute | <2 |
| Nicandra physalodes (L.) Gaertn. | Shoo-fly plant | <2 |
| Grasses | ||
| Cynodon nlemfuensis Vanderyst (Bogdan) | Stargrass | 50–100 |
| Cynodon dactylon (L.) Pers | Couch grass | 50–80 |
| Eleusine indica (L) Gaertn. | Wiregrass | <20 |
| Cyperus esculentus L. | Yellow nutsedge | <2 |
| Bulbostylis hispidula (Vahl) R.W. Haines | Hispidula | <2 |
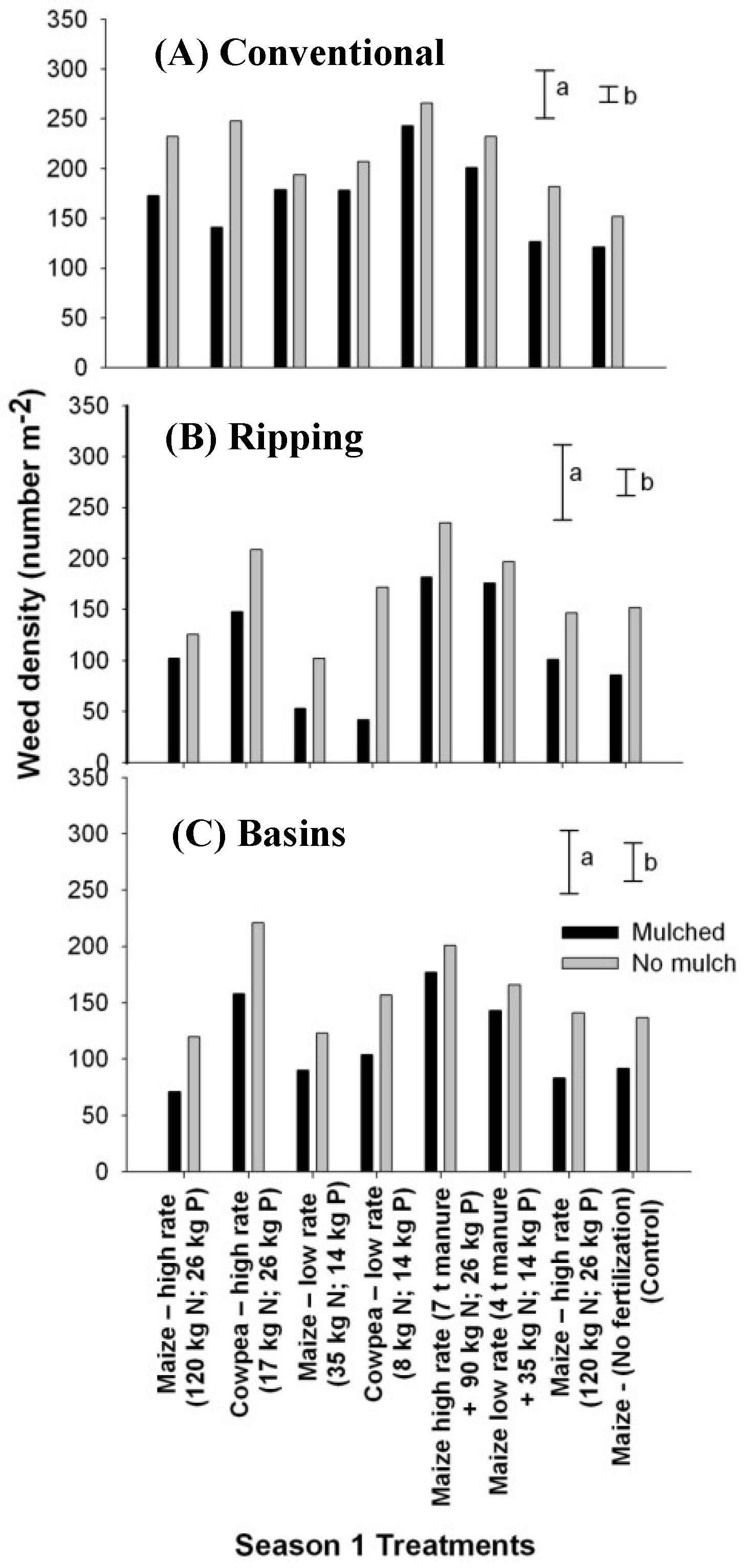
3.2. Fertility Effects on Weed Flora
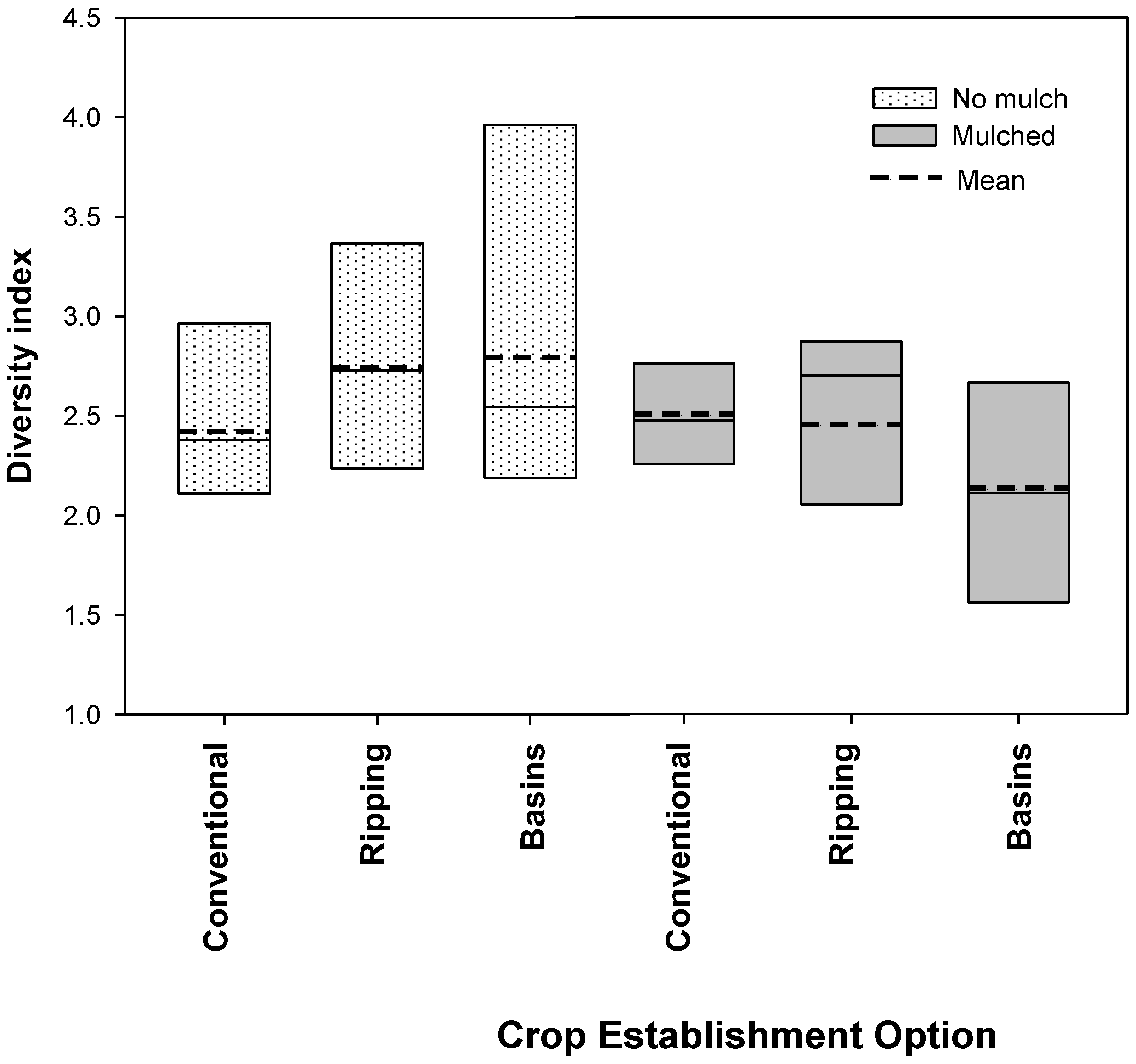
3.3. Mulching Suppressed Weed Density and Diversity
| Season 1 Treatments | Season 2 Treatments | Weed Biomass Season 2 (g·m−2) | |
|---|---|---|---|
| Mulch | No Mulch | ||
|
| 223 (44) a | 378 (63) b |
|
| 344 (28) b | 456 (41) e |
|
| 77 (23) c | 133 (38) c |
|
| 118 (51) c | 201 (59) a,d |
|
| 402 (66) b,e | 511(101) e |
|
| 325 (47) b | 460 (53) e |
|
| 96 (20) c | 122 (42) c |
|
| 191 (31) a,d | 258 (69) a,d |
| SED | 43.6 | 55.1 | |
3.4. Implications of Mulching for Smallholder Farmers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mazvimavi, K.; Twomlow, S. Socioeconomic and institutional factors influencing adoption of conservation farming by vulnerable households in Zimbabwe. Agric. Syst. 2009, 101, 20–29. [Google Scholar] [CrossRef]
- Thierfelder, C.; Wall, P.C. Effects of conservation agriculture techniques on infiltration and soil water content in Zambia and Zimbabwe. Soil Tillage Res. 2009, 105, 217–227. [Google Scholar] [CrossRef]
- Mupangwa, W.; Twomlow, S.; Walker, S. Reduced tillage, mulching and rotational effects on maize (Zea. mays L.), cowpea (Vigna. unguiculata (Walp) L.) and sorghum (Sorghum bicolor L. (Moench)) yields under semi-arid conditions. Field Crop. Res. 2012, 132, 139–148. [Google Scholar] [CrossRef]
- FAO. Weed Control in Smallholder Conservation Agriculture, 2012. Available online: http://www.fao.org/ca/Training_materials/leaflet/weedcontrol.pdf (accessed on 20 July 2015).
- Chuma, E.; Mombeshora, B.G.; Murwira, H.K.; Chikuvire, J. The dynamics of soil fertility management in Zimbabwe. In Nutrients on the Move. Soil Fertility Dynamics in African Farming Systems; Hilhorst, T., Muchena, F., Eds.; International Institute for Environment and Development: London, UK, 2000; pp. 45–64. [Google Scholar]
- Mashingaidze, A.B. Improving Weed Management and Crop Productivity in Maize Systems in Zimbabwe. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2004; p. 196. [Google Scholar]
- Nyamangara, J.; Mashingaidze, N.; Masvaya, E.N.; Nyengerai, K.; Kunzekweguta, M.; Tirivavi, R.; Mazvimavi, K. Weed growth and labor demand under hand-hoe based reduced tillage in smallholder farmers’ fields in Zimbabwe. Agric. Ecosyst. Environ. 2014, 187, 146–154. [Google Scholar] [CrossRef]
- Mapfumo, P.; Mtambanengwe, F.; Nezomba, H.; Manzeke, M.G. Options for adaptation of conservation Agriculture practices on nutrient-depleted soils by smallholder farmers in southern Africa. In 1st ACCA Africa Congress on Conservation Agriculture. Book of Condensed Papers; ACT/NORAD: Nairobi, Kenya, 2014; pp. 118–120. [Google Scholar]
- Ndah, H.T.; Schuler, J.; Uthes, S.; Zander, P.; Traore, K.; Gama, M.S.; Nyagumbo, I.; Triomphe, B.; Sieber, S.; Corbeels, M. Adoption Potential of Conservation Agriculture Practices in Sub-Saharan Africa: Results from Five Case Studies. Environ. Manag. 2014, 53, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Kombiok, J.M.; Alhassan, A.Y. Tillage effects on subsequent weed types, population and biomass in maize cropped in Northern Savanna zone of Ghana. J. Sustain. Agric. 2007, 30, 47–57. [Google Scholar] [CrossRef]
- Mashingaidze, N. Weed Dynamics in Low-Input Dryland Smallholder Conservation Agriculture Systems in Semi-Arid Zimbabwe. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2013; p. 188. [Google Scholar]
- Mashingaidze, N.; Madakadze, I.C.; Twomlow, S.J. Response of weeds and maize to planting basin tillage in semi-arid Zimbabwe. In Proceedings of the African Crop Science Conference, Cape Town, South Africa, 28 September–2 October 2009; pp. 259–261.
- FAO Document Repository. Hot Pepper Seed and Crop Production in the Bahamas. Mulching. Available online: http://www.fao.org/docrep/007/y5259e/y5259e09.htm# (accessed on 13 January 2014).
- Vincent, V.; Thomas, R.G. An Agroecological Survey of Southern Rhodesia: Part 1—Agro-Ecological Survey; Government Printer: Salisbury, Rhodesia, 1961.
- World Reference Base for Soils, FAO/ISRIC/ISSS. In World Soil Resources Report No. 84; Food and Agriculture Organization: Rome, Italy, 1998.
- Tittonell, P.; Scopel, E.; Andrieu, N.; Posthumus, H.; Mapfumo, P.; Corbeels, M.; van Halsemaf, G.E.; Lahmar, R.; Lugandu, S.; Rakotoriosa, J.; et al. Agroecology-based aggradation-conservation agriculture (ABACO): Targeting innovations to combat soil degradation and food insecurity in semi-arid Africa. Field Crop. Res. 2012, 132, 168–174. [Google Scholar] [CrossRef]
- Weaver, J.E. The Quadrat Method in Teaching Ecology. Plant World 1918, 21, 267–283. [Google Scholar]
- Wheater, C.P.; Bell, J.R.; Cook, P.A. Practical Field Ecology: Project Guide; John Wiley and Sons: Chichester, UK, 2011. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; p. 680. [Google Scholar]
- VSN. GenStat for Windows, 14th ed.; VSN International: Hempstead, UK, 2011. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. The Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chivinge, O.A. Weed science technological needs for communal areas of Zimbabwe. Zambezia 1990, 17, 133–143. [Google Scholar]
- Munguri, W.M.; Mariga, I.K.; Chivinge, O.A. Optimising cattle manure utilisation in Chinyika Resettlement Area, Zimbabwe. In Proceedings of the International Crop Science Conference for East and Southern Africa, Blantyre, Malawi, 19–24 February 1995.
- Pleasant, J.M.; Schlather, K.J. Incidence of weed seed in cow manure and its importance as weed source for cropland. Weed Technol. 2000, 8, 304–310. [Google Scholar]
- Rupende, E.; Chivinge, O.A.; Mariga, I.K. The effect of curing cattle manure on survival of weed seeds and nutrient release. In Proceedings of the biennial weed science conference of East Africa, Morogoro, Tanzania, 18–22 September 1995.
- Van Oudtshoorn, F. Guide to Grasses of Southern Africa; Briza Publications: Cape Town, South Africa, 2002; pp. 228–228. [Google Scholar]
- Halvorson, W.L.; Guertin, P. USGS Weed in the West Project: Status of Introduced Plants in Southern Arizona Parks. Factsheet for: Cynodon. dactylon (L.) Pers.; US Geological Survey: Tucson, AZ, USA, 2003; p. 12.
- Mashingaidze, N.; Madakadze, I.C.; Twomlow, S. Response of weed flora to conservation agriculture systems and weeding intensity in semi-arid Zimbabwe. Afr. J. Agric. Res. 2012, 7, 5069–5082. [Google Scholar] [CrossRef]
- Marais, J.N. Weed Competition in Maize with Reference to Peasant Farming. Fort Hare Papers, University of Fort Hare, Alice, South Africa, 1983; pp. 72 and 208. [Google Scholar]
- Nzuma, J.K. Manure management options for increasing crop production in the smallholder sectors of Zimbabwe. Ph.D. Thesis, University of Zimbabwe, Harare, Zimbabwe, 2002. [Google Scholar]
- Mtambanengwe, F.; Mapfumo, P. Combating food insecurity on sandy soils in Zimbabwe: The legume challenge. Symbiosis 2009, 48, 25–36. [Google Scholar] [CrossRef]
- Nezomba, H.; Mtambanengwe, F.; Tittonell, P.; Mapfumo, P. Point of no return? Rehabilitating degraded soils for increased crop productivity on smallholder farms in eastern Zimbabwe. Geoderma 2015, 239, 143–155. [Google Scholar] [CrossRef]
- FAO. The Economics of Conservation Agriculture. Available online: ftp://ftp.fao.org/agl/agll/docs/ecconsagr.pdf (accessed on 21 July 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mtambanengwe, F.; Nezomba, H.; Tauro, T.; Chagumaira, C.; Manzeke, M.G.; Mapfumo, P. Mulching and Fertilization Effects on Weed Dynamics under Conservation Agriculture-Based Maize Cropping in Zimbabwe. Environments 2015, 2, 399-414. https://doi.org/10.3390/environments2030399
Mtambanengwe F, Nezomba H, Tauro T, Chagumaira C, Manzeke MG, Mapfumo P. Mulching and Fertilization Effects on Weed Dynamics under Conservation Agriculture-Based Maize Cropping in Zimbabwe. Environments. 2015; 2(3):399-414. https://doi.org/10.3390/environments2030399
Chicago/Turabian StyleMtambanengwe, Florence, Hatirarami Nezomba, Tonny Tauro, Christopher Chagumaira, Muneta G. Manzeke, and Paul Mapfumo. 2015. "Mulching and Fertilization Effects on Weed Dynamics under Conservation Agriculture-Based Maize Cropping in Zimbabwe" Environments 2, no. 3: 399-414. https://doi.org/10.3390/environments2030399
APA StyleMtambanengwe, F., Nezomba, H., Tauro, T., Chagumaira, C., Manzeke, M. G., & Mapfumo, P. (2015). Mulching and Fertilization Effects on Weed Dynamics under Conservation Agriculture-Based Maize Cropping in Zimbabwe. Environments, 2(3), 399-414. https://doi.org/10.3390/environments2030399





