Comparative Sorption of Methylene Blue onto Hydrophobic Clays
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical Reagents and Solutions
2.2. Cationic Exchange of Bentonite with Ammonium Salts
2.3. Adsorption Tests
2.4. Characterization of the Adsorbents
2.5. Data Analysis
3. Results and Discussion
3.1. Characterization of the Adsorbents
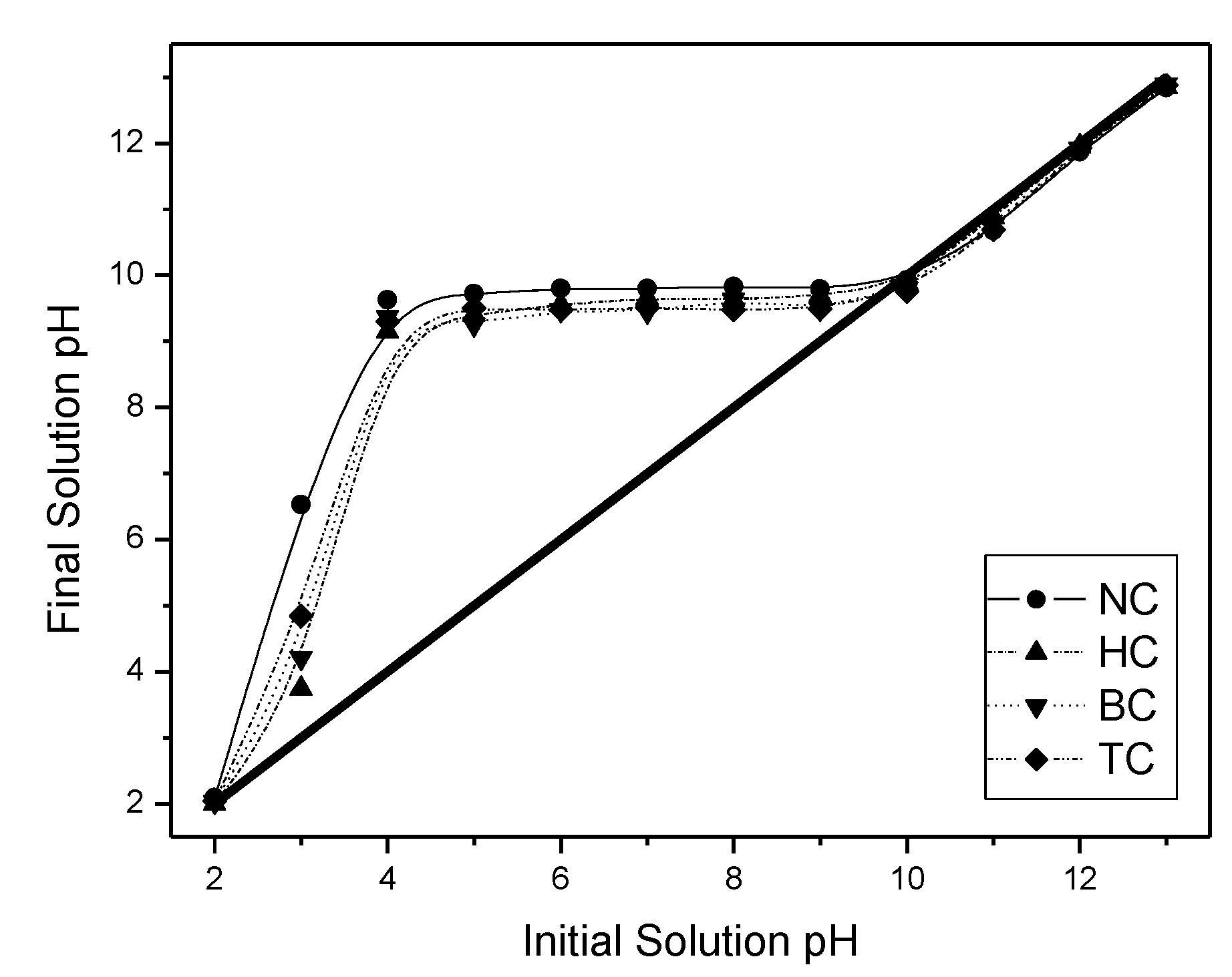
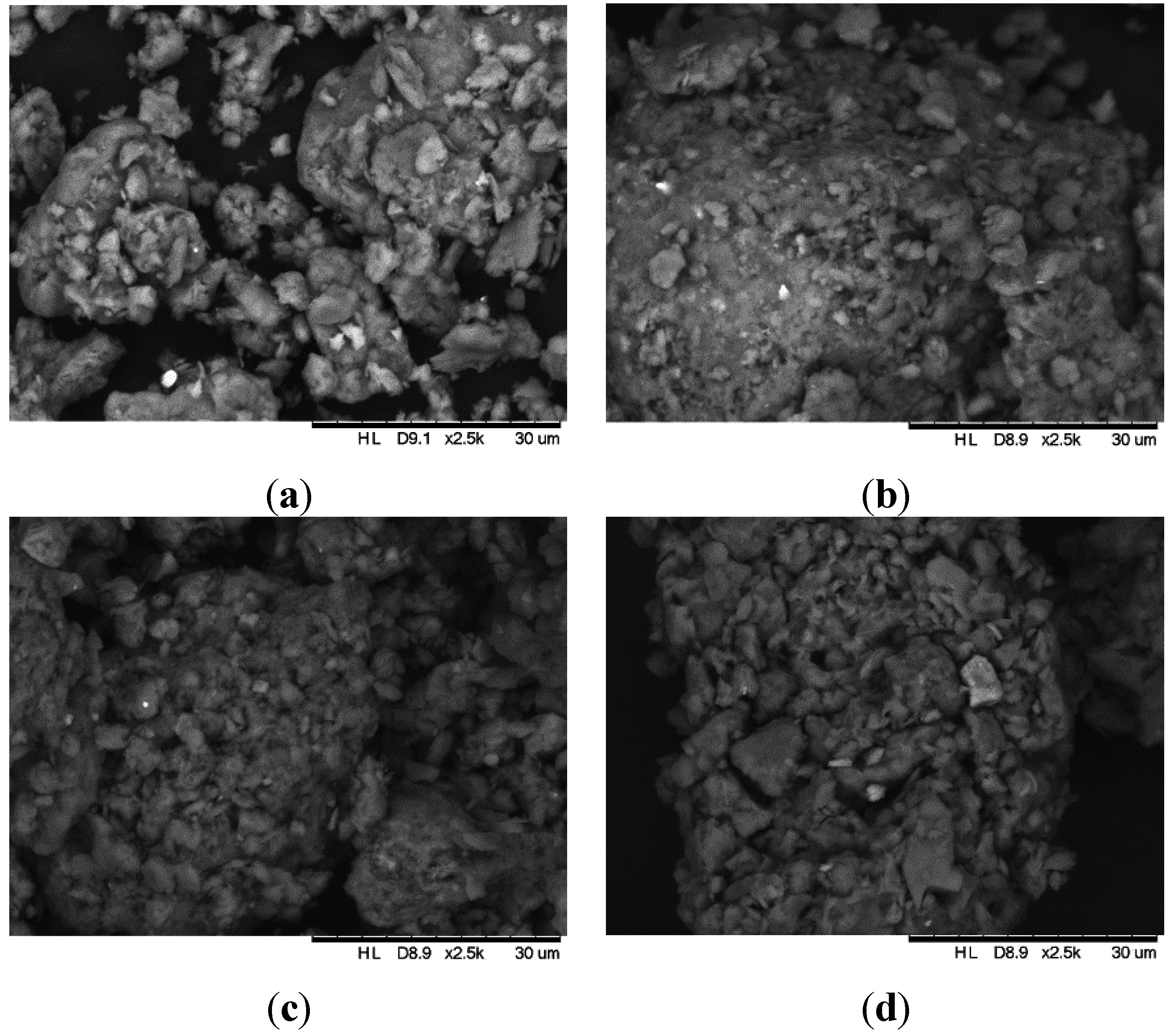
3.2. Adsorption Tests
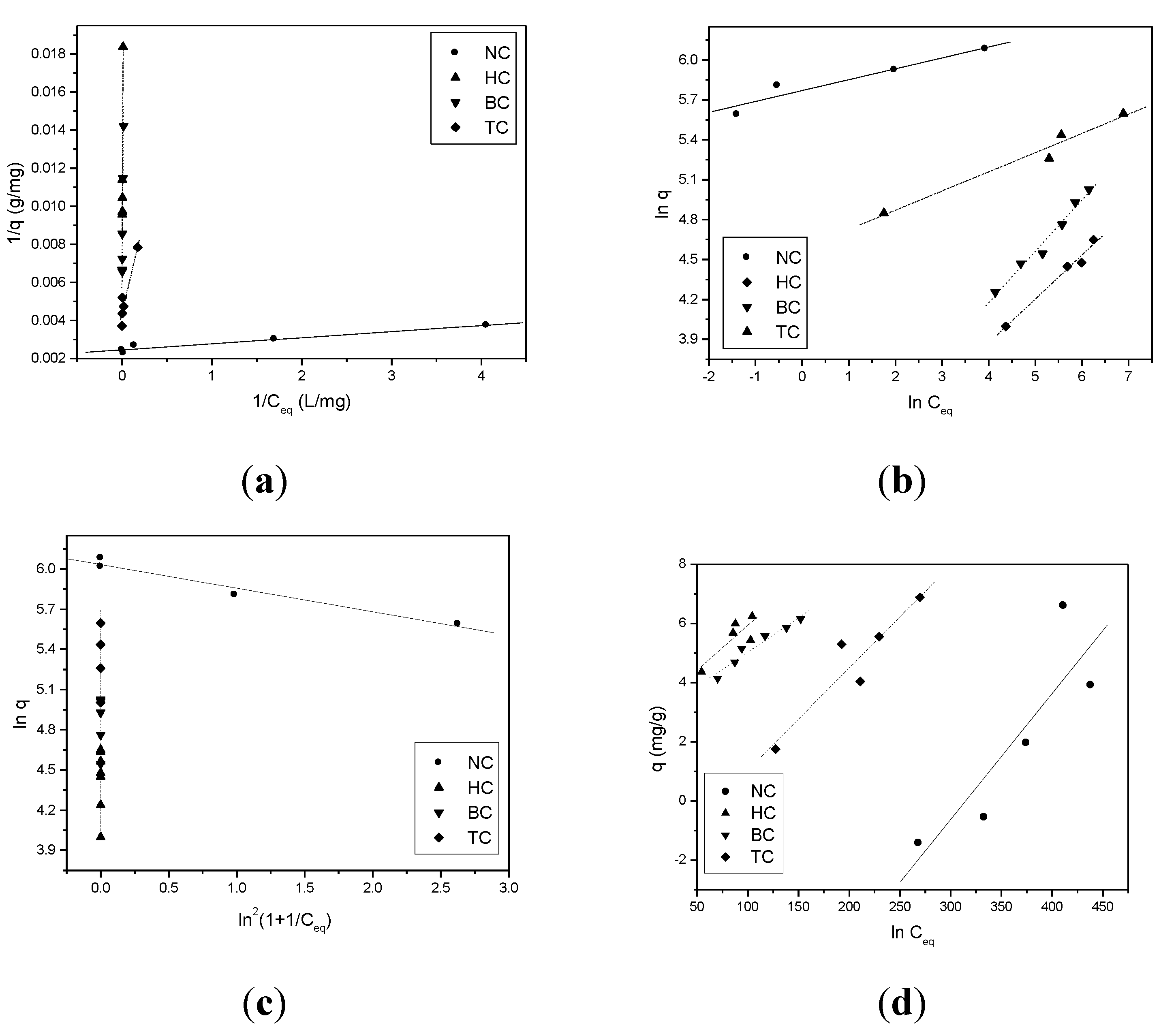
| Adsorption Isotherm | Parameter | NC | HC | BC | TC |
|---|---|---|---|---|---|
| Langmuir | qmax (mg/g) | 408 | 153 | 167 | 217 |
| b (L/mg) | 7.65 | 0.0074 | 0.011 | 0.248 | |
| p-value | 0.004 | 0.038 | <0.0001 | 0.026 | |
| R2 | 0.953 | 0.925 | 0.978 | 0.948 | |
| Freundlich | kF (mg/g) (L/mg)1/n | 320.3 | 12.88 | 13.92 | 90.55 |
| n | 12.24 | 3.04 | 2.59 | 6.54 | |
| p-value | 0.042 | 0.009 | <0.0001 | 0.011 | |
| R2 | 0.916 | 0.981 | 0.978 | 0.911 | |
| Dubinin-Radushkevich | qDR (mg/g) | 404.06 | 99.96 | 155.41 | 287.3 |
| B (mol2·J2) | 2.64 × 10−8 | 6.05 × 10−4 | 2.57 × 10−3 | 3.14 × 10−3 | |
| E (J/mol) | 4.35 | 0.03 | 0.014 | 0.012 | |
| p-value | 0.011 | 0.009 | 0.003 | 0.053 | |
| R2 | 0.91 | 0.848 | 0.959 | 0.898 | |
| Temkin | aT | 9.5 × 107 | 0.165 | 0.077 | 31.04 |
| bT (J/mol) | 139.28 | 106.6 | 60.79 | 97.94 | |
| p-value | 0.055 | 0.075 | 0.001 | 0.019 | |
| R2 | 0.757 | 0.704 | 0.948 | 0.874 |
| Adsorbent | Specific Surface Area (m2/g) |
|---|---|
| NC | 1295 |
| HC | 486 |
| BC | 530 |
| TC | 689 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bello-Huitle, V.; Atenco-Fernandez, P.; Reyes-Mazzoco, R. Adsorption studies of methylene blue and phenol onto pecan and castile nutshells prepared by chemical activation. Rev. Mex. Ing. Quim. 2010, 9, 313–322. [Google Scholar]
- Aksu, Z.; Isoglu, I. Use of agricultural waste sugar beet pulp for the removal of Gemazol turquoise blue-G reactive dye from aqueous solution. J. Hazard. Mater. 2006, 137, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Chandran, B.; Nigam, P. Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res. 2002, 36, 2824–2830. [Google Scholar] [CrossRef]
- Gupta, V.; Suhas, K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Removal of methylene blue from aqueous solutions by yellow passion fruit waste. J. Hazard. Mater. 2010, 259, 249–257. [Google Scholar]
- Navarro, A.; Chang, E.; Chang, P.; Yoon, S.; Manrique, A. Separation of dyes from aqueous solutions by magnetic alginate beads. Trends Chromatogr. 2013, 8, 31–41. [Google Scholar]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 9, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Nigam, P.; Armour, G.; Banat, I.; Singh, D.; Marchant, R. Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresour. Technol. 2000, 72, 219–226. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Fundamentals and Applications of Biosorption Isotherms, Kinetics and Thermodynamics; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Volesky, B. Sorption and Biosorption; BV Sorbex: Montreal, Canada, 2003. [Google Scholar]
- Lazo, J.; Navarro, A.; Sun-Kou, R.; Llanos, B. Synthesis and Characterization of organophilic clays and their application as phenol adsorbents. Rev. Soc. Quim. Peru 2008, 74, 3–19. [Google Scholar]
- Navarro, A.; Cuizano, N.; Lazo, J.; Sun-Kou, R.; Llanos, B. Comparative study of the removal of phenolic compounds on biological and non-biological adsorbents. J. Hazard. Mater. 2008, 164, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Cuizano, N.; Lazo, J.; Sun-Kou, R.; Llanos, B. Insights into Removal of phenol from aqueous solutions by low cost adsorbents: Clays versus algae. Sep. Sci. Technol. 2009, 44, 2491–2509. [Google Scholar] [CrossRef]
- Deng, H.; Liu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Tan, W.; Lu, S.; Liu, F.; Feng, X.; He, Z.; Koopal, L. Determination of the point zero charge of manganese oxides with different methods including an improved salt. Soil Sci. 2008, 173, 277–286. [Google Scholar] [CrossRef]
- Kim, D. Measurement of point of zero charge of bentonite by solubilization technique and its dependence of surface potential on pH. Environ. Eng. Res. 2003, 8, 222–227. [Google Scholar]
- Rosas-Castor, J.; Garza-Gonzalez, M.; Garcia-Reyes, R.; Soto-Regalado, E.; Cerino-Cordova, F.; Garcia-Gonzalez, A.; Loredo-Medrano, J. Methylene blue biosorption by pericarp of corn, alfalfa, and agave bagasse wastes. Environ. Technol. 2014, 35, 1077–1090. [Google Scholar] [CrossRef]
- Olivella, M.; Fiol, N.; de la Torre, F.; Porchi, J.; Villaescusa, I. A mechanistic approach to methylene blue sorption on two vegetable wastes: Corn bark and grape stalks. Bioresources 2012, 7, 3340–3354. [Google Scholar]
- Kaewprasit, C.; Hequet, E.; Abidi, N.; Gourlot, J. Application of methylene blue adsorption to cotton fiber specific surface area measurement: Part I. Methodology. J. Cotton Sci. 1998, 2, 164–173. [Google Scholar]
- Pinzon-Bello, J. Specific surface of a bentonite by means of methylene blue adsorption. Rev. Colomb. Quim. 1997, 26, 1–14. [Google Scholar]
- Gregg, S.; Sing, K. Adsorption, Surface and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Graham, D. Characterization of physical adsorption systems. III. The separate effects of pore size and surface acidity upon the adsorbent capacities of activated carbons. J. Phys. Chem. 1955, 59, 896–900. [Google Scholar] [CrossRef]
- Kim, T.; Yang, D.; Kim, J.; Musaev, H.; Navarro, A. Comparative adsorption of highly porous and raw adsorbents for the elimination of copper (II) ions from wastewaters. Trends Chromatogr. 2013, 8, 97–108. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sponza, A.D.; Fernandez, N.J.; Yang, D.; Ortiz, K.A.; Navarro, A.E. Comparative Sorption of Methylene Blue onto Hydrophobic Clays. Environments 2015, 2, 388-398. https://doi.org/10.3390/environments2030388
Sponza AD, Fernandez NJ, Yang D, Ortiz KA, Navarro AE. Comparative Sorption of Methylene Blue onto Hydrophobic Clays. Environments. 2015; 2(3):388-398. https://doi.org/10.3390/environments2030388
Chicago/Turabian StyleSponza, Alvaro D., Natalia J. Fernandez, David Yang, Karla A. Ortiz, and Abel E. Navarro. 2015. "Comparative Sorption of Methylene Blue onto Hydrophobic Clays" Environments 2, no. 3: 388-398. https://doi.org/10.3390/environments2030388
APA StyleSponza, A. D., Fernandez, N. J., Yang, D., Ortiz, K. A., & Navarro, A. E. (2015). Comparative Sorption of Methylene Blue onto Hydrophobic Clays. Environments, 2(3), 388-398. https://doi.org/10.3390/environments2030388






