How Altitude Affects the Phenolic Potential of the Grapes of cv. ‘Fokiano’ (Vitis vinifera L.) on Ikaria Island
Abstract
1. Introduction
2. Materials and Methods
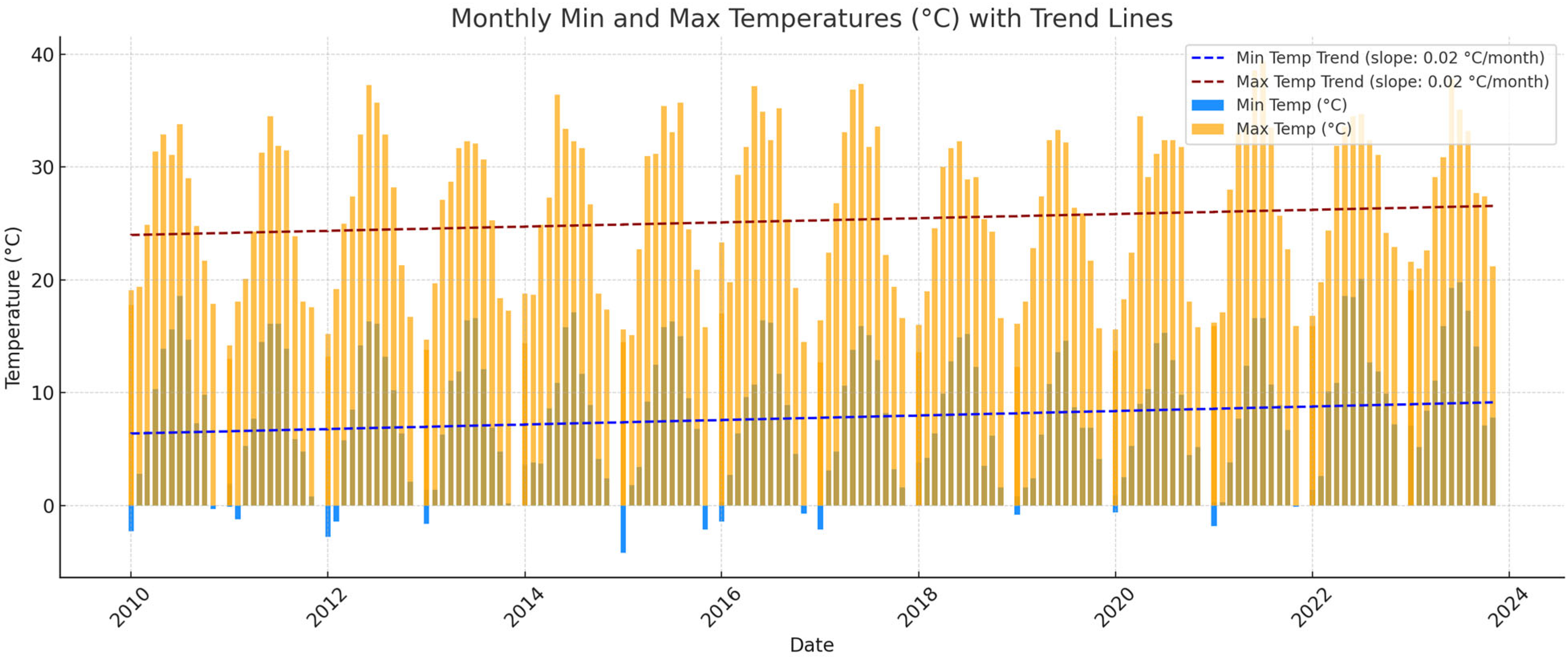
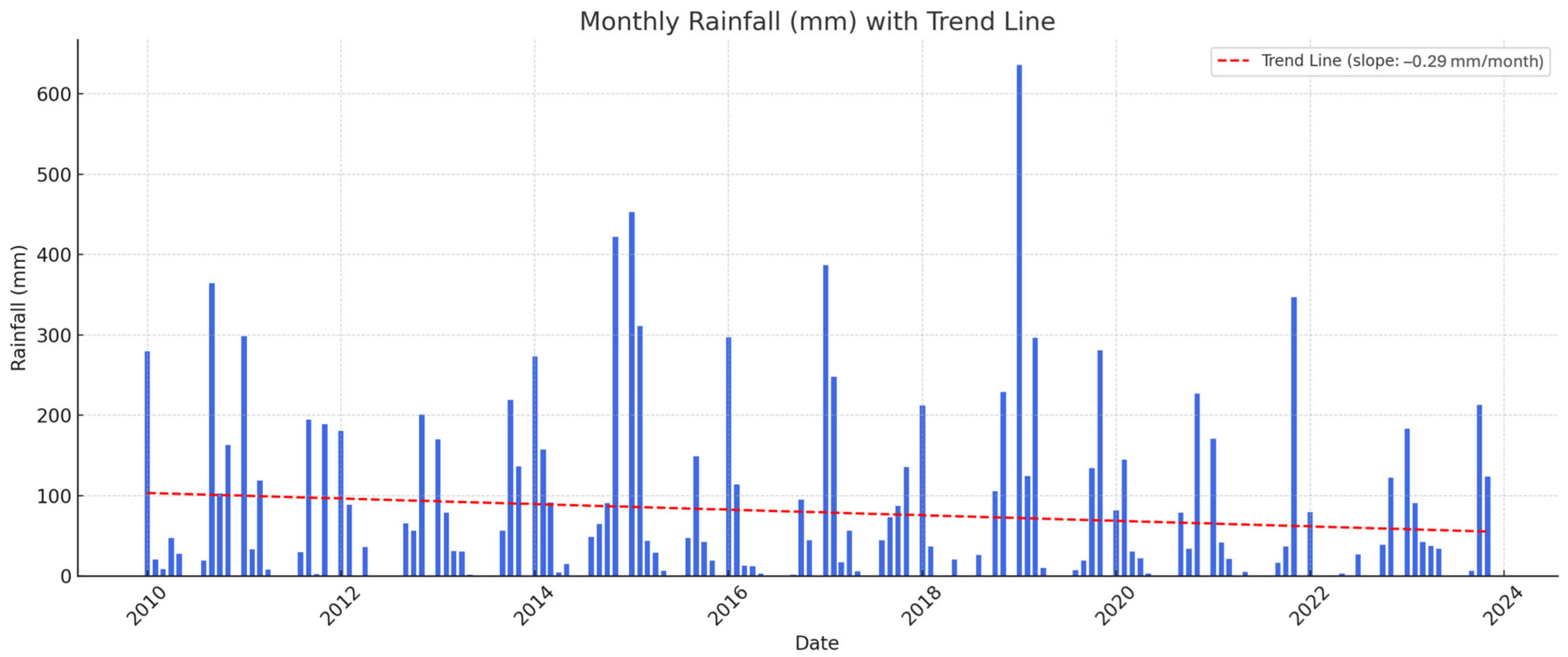
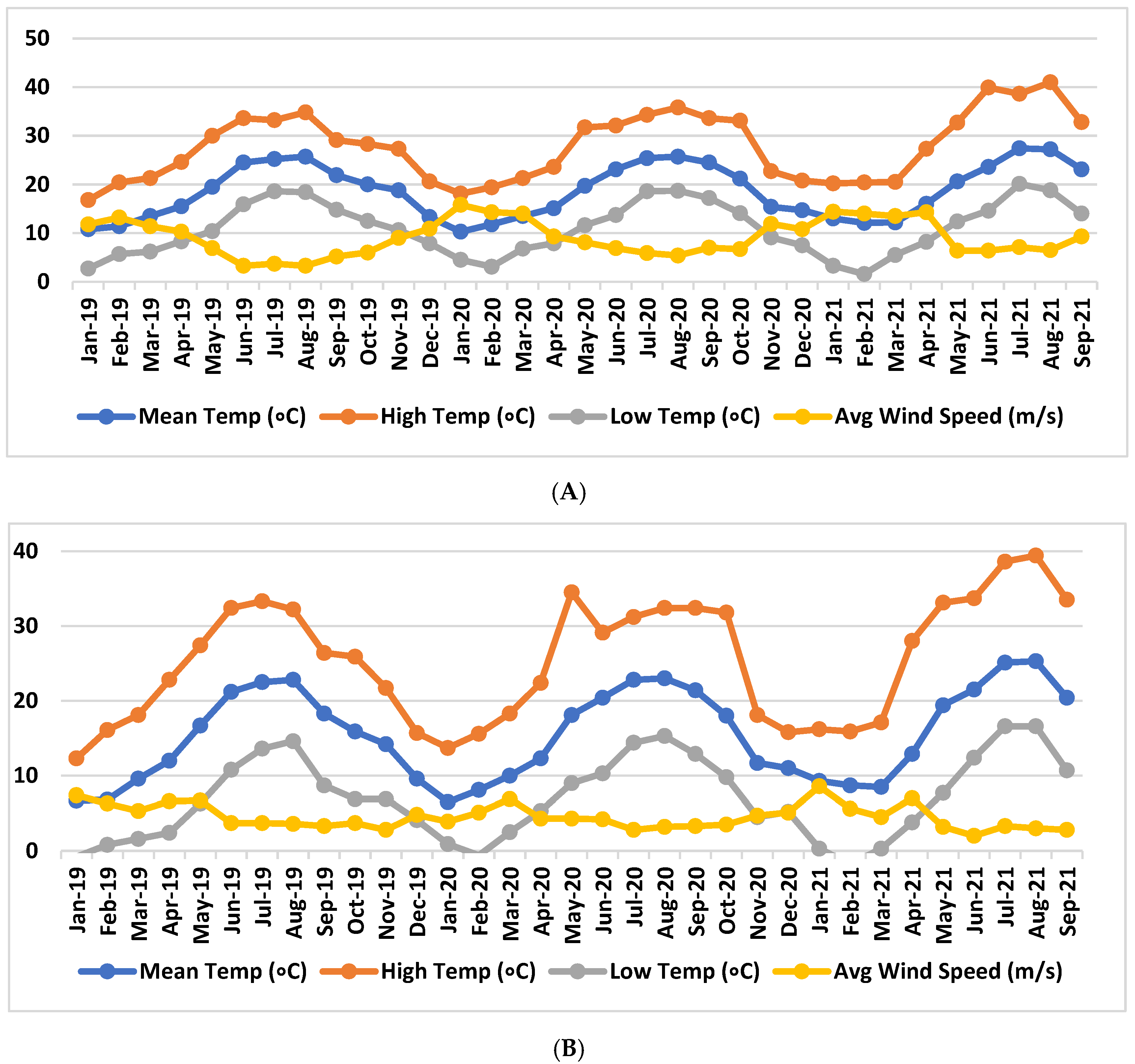
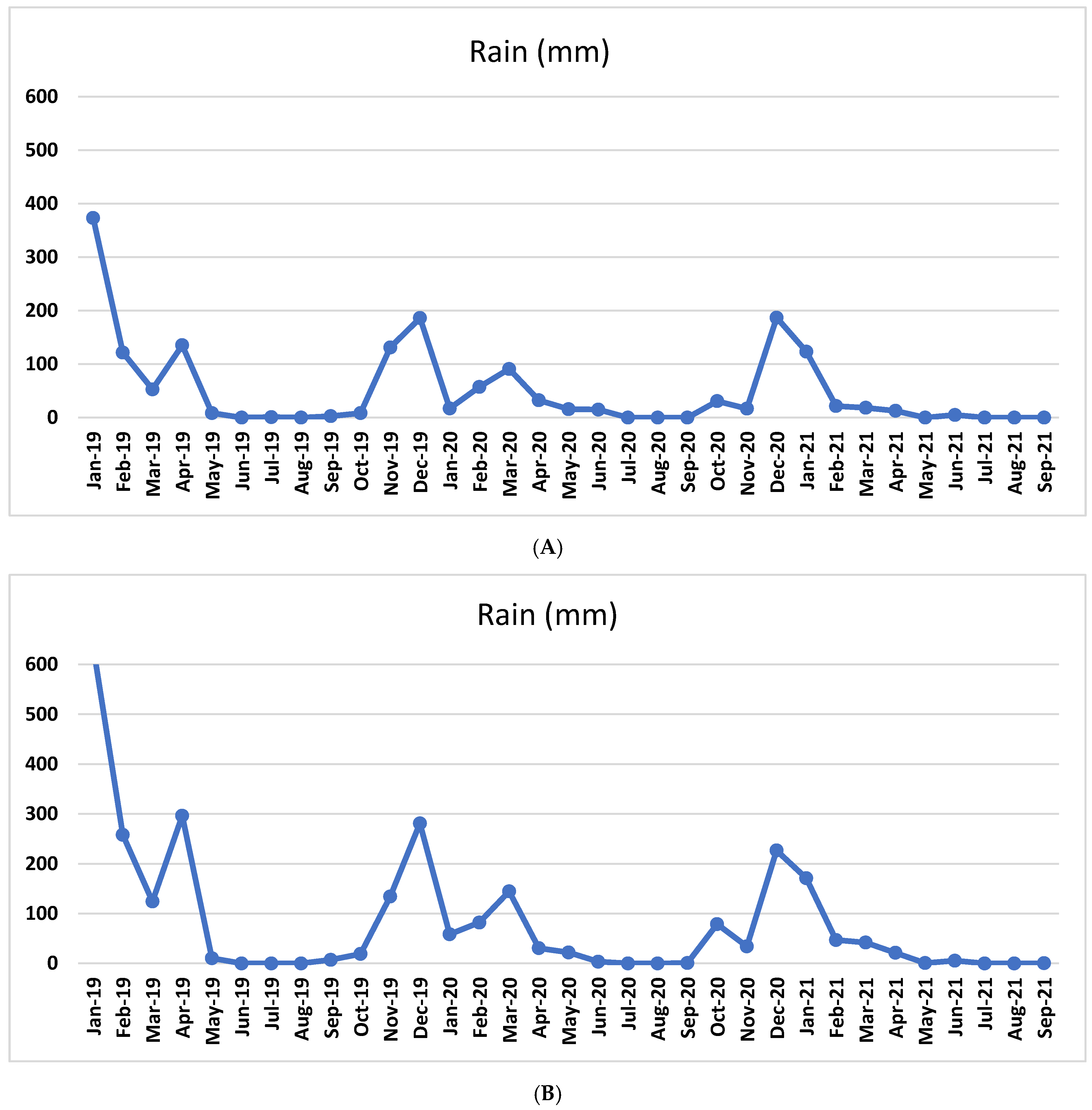
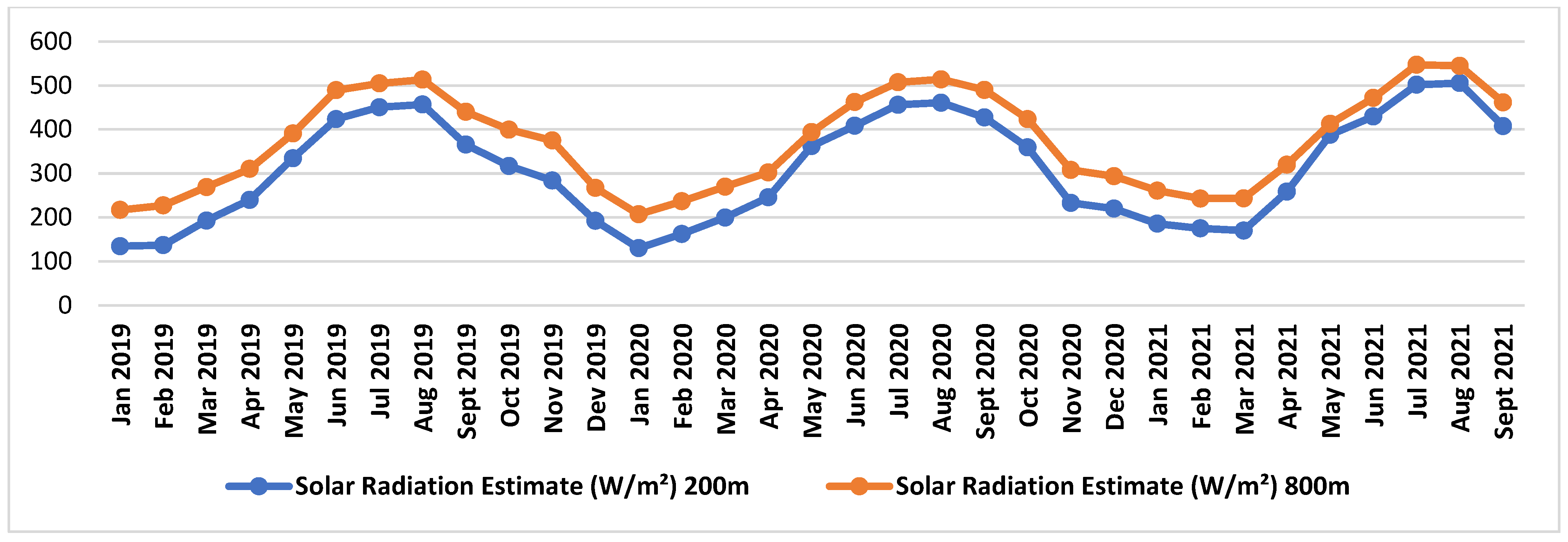
2.1. Plant Material, Vineyard Information, and Climatic Data
2.2. Grape Sampling and Sample Preparation
2.3. Grape and Berry Mechanical Properties—Characteristics of the Must
2.4. Determination of Phenolic Compounds
2.5. Determination of Antioxidant Activity
2.6. HPLC Analyses
2.7. Statistical Analysis
3. Results
3.1. Results on the Various Measurements
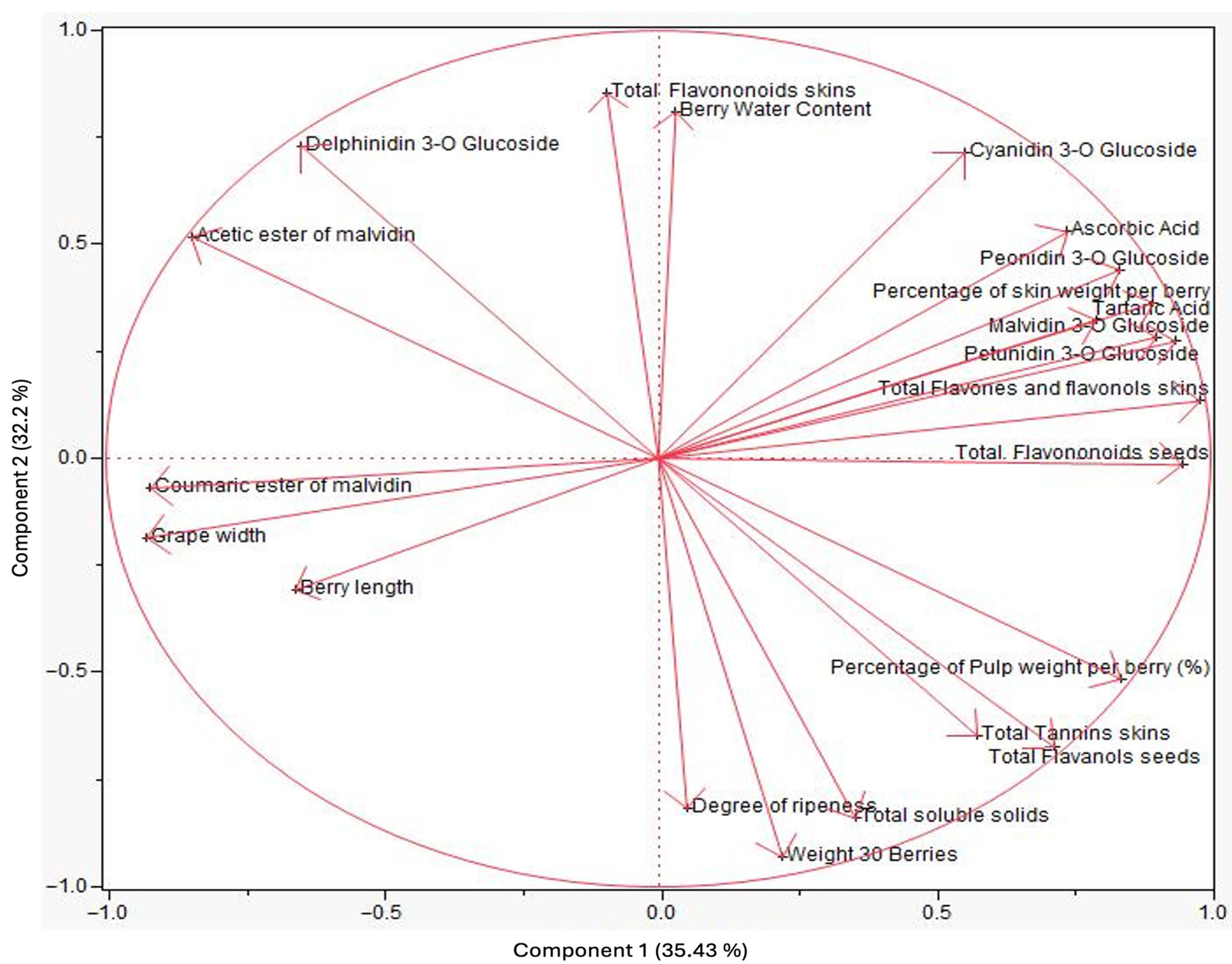
3.2. Principal Component Analysis (PCA)
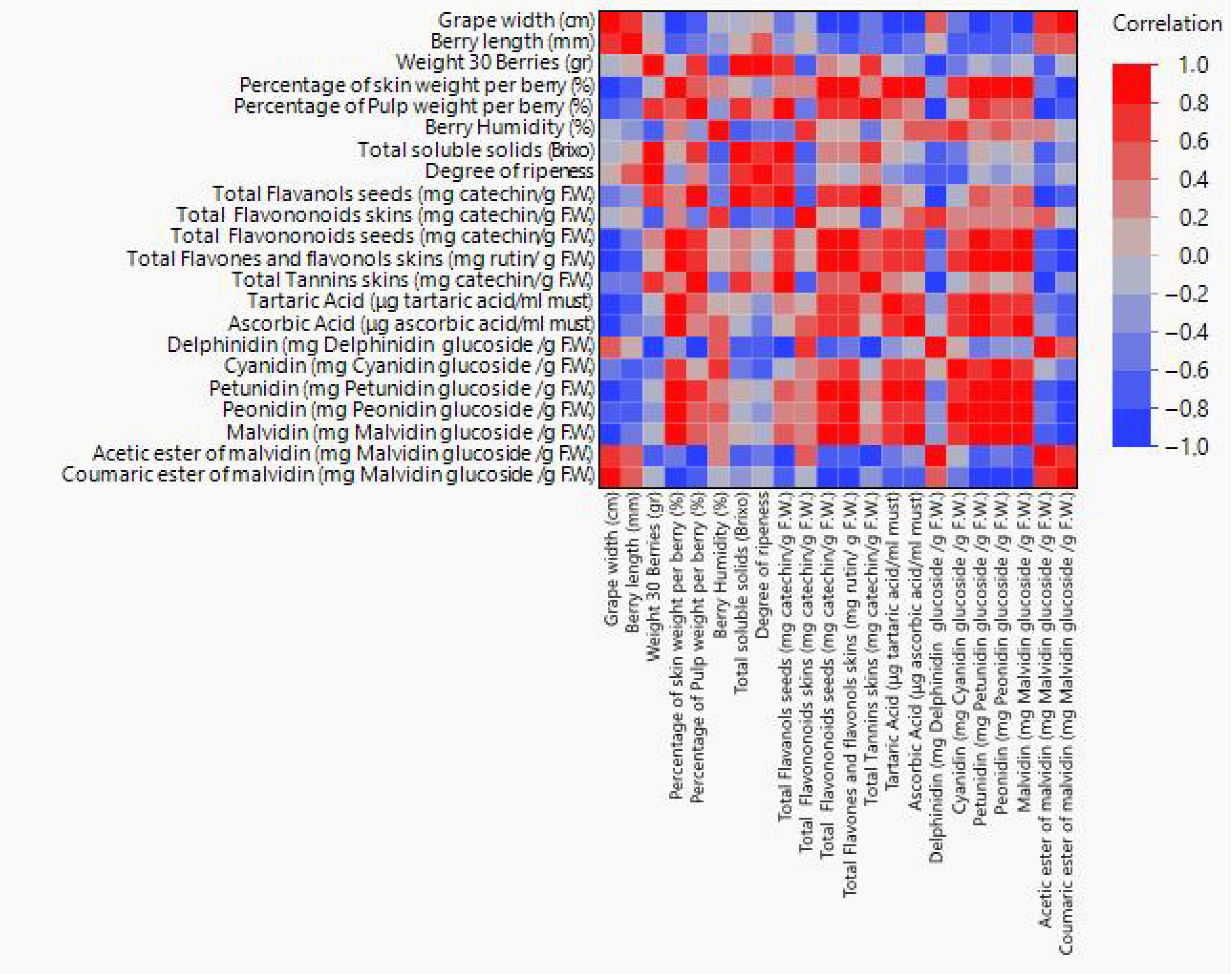
3.3. Correlation of Weighted Variables Pairwise
3.4. Partial Least Squares (PLS) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Naing, A.-H.; Kim, C.-K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Wang, Q.-J.; Spence, C. Wine complexity: An empirical investigation. Food Qual. Pref. 2018, 68, 238–244. [Google Scholar] [CrossRef]
- Gouot, J.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef]
- Keller, M. Managing grapevines to optimize fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-Viana, L.; Silva, M.F.; Bressan-Smith, R.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes, and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar] [CrossRef]
- do Nascimento Silva, F.L.; Schmidt, E.M.; Messias, C.L.; Eberlin, M.N.; Sawaya, A.C.H.F. Quantitation of organic acids in wine and grapes by direct infusion electrospray ionization mass spectrometry. Anal. Methods 2015, 7, 53–62. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Pillet, J.; Egert, A.; Pieri, P.; Lecourieux, F.; Kappel, C.; Charon, J.; Gomès, E.; Keller, F.; Delrot, S.; Lecourieux, D. VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries. Plant Cell Physiol. 2012, 53, 1776–1792. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef]
- Pillet, J.; Berdeja, M.; Guan, L.; Delrot, S. Berry response to water, light and heat stresses. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; Géros, H., Chaves, M.M., Medrano Gil, H., Delrot, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 223–257. [Google Scholar]
- Murcia, G.; Pontin, M.; Reinoso, H.; Baraldi, R.; Bertazza, G.; Gómez-Talquenca, S.; Bottini, R.; Piccoli, P.N. ABA and GA3 increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters. Physiol. Plant. 2016, 156, 323–337. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Lecourieux, D. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing Cabernet Sauvignon grape berries. Front. Plant Sci. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Mansour, G.; Ghanem, C.; Mercenaro, L.; Nassif, N.; Hassoun, G.; Del Caro, A. Effects of altitude on the chemical composition of grapes and wine: A review. OENO One 2022, 56, 227–239. [Google Scholar] [CrossRef]
- Sun, R.-Z.; Cheng, G.; Li, Q.; He, Y.-N.; Wang, Y.; Lan, Y.-B.; Li, S.-Y.; Zhu, Y.-R.; Song, W.-F.; Zhang, X.; et al. Light-induced variation in phenolic compounds in Cabernet Sauvignon grapes (Vitis vinifera L.) involves extensive transcriptome reprogramming of biosynthetic enzymes, transcription factors, and phytohormonal regulators. Front. Plant Sci. 2017, 8, 547. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Q.; Jin, Z.; Mu, L.; Duan, C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011, 125, 77–83. [Google Scholar] [CrossRef]
- Liang, N.-N.; Zhu, B.-Q.; Han, S.; Wang, J.-H.; Pan, Q.-H.; Reeves, M.J.; Duanm, C.-Q.; He, F. Regional characteristics of anthocyanin and flavonol compounds from grapes of four Vitis vinifera varieties in five wine regions of China. Food Res. Int. 2014, 64, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Coklar, H. Antioxidant capacity and phenolic profile of berry, seed, and skin of Ekşikara (Vitis vinifera L.) grape: Influence of harvest year and altitude. Ιnt. J. Food Prop. 2017, 20, 2071–2087. [Google Scholar] [CrossRef]
- Gil, M.; Pontin, M.; Berli, F.; Bottini, R.; Piccoli, P. Metabolism of terpenes in the response of grape (Vitis vinifera L.) leaf tissues to UV-B radiation. Phytochemistry 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Jin, X.-D.; Wu, X.; Liu, X. Phenolic characteristics and antioxidant activity of Merlot and Cabernet Sauvignon wines increase with vineyard altitude in a high-altitude region. S. Afr. J. Enol. Vitic. 2017, 38, 132–143. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Morales, F.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Gomès, E.; Pascual, I. Climate change conditions (elevated CO2 and temperature) and UV-B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates. Plant Sci. 2015, 236, 168–176. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B alleviates the uncoupling effect of elevated CO2 and increased temperature on grape berry (Vitis vinifera cv. Tempranillo) anthocyanin and sugar accumulation. Aust. J. Grape Wine Res. 2016, 22, 87–95. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, T.-J.; Zheng, J.; Huang, X.-D.; Yu, Z.-C.; Peng, C.-L. Anthocyanins function as a light attenuator to compensate for insufficient photoprotection mediated by nonphotochemical quenching in young leaves of Acmena acuminatissima in winter. Photosynthetica 2018, 56, 445–454. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in Corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef]
- Stavrakakis, M.N. Ampelography; Embryo Publications: Athens, Greece, 2021. [Google Scholar]
- Iqbal, M. An Introduction to Solar Radiation, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Liou, K.N. An Introduction to Atmospheric Radiation, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes, 4th ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- European Commission Joint Research Centre (JRC). (n.d.). Photovoltaic Geographical Information System (PVGIS). Available online: https://re.jrc.ec.europa.eu/pvg_tools/en/ (accessed on 10 May 2023).
- Biniari, K.; Gerogiannis, O.; Daskalakis, I.; Bouza, D.; Stavrakaki, M. Study of some qualitative and quantitative characters of the grapes of indigenous Greek grapevine varieties (Vitis vinifera L.) using HPLC and spectrophotometric analyses. Not. Bot. Horti Agrobo. 2018, 46, 97–106. [Google Scholar] [CrossRef]
- Stavrakaki, M.; Biniari, K.; Daskalakis, I.; Bouza, D. Polyphenol content and antioxidant capacity of the skin extracts of berries from seven biotypes of the Greek grapevine cultivar Korinthiaki Staphis (Vitis vinifera L.). Aust. J. Crop Sci. 2018, 12, 1927–1936. [Google Scholar] [CrossRef]
- Bouza, D.; Tsavala, A.; Lappa, M.; Biniari, K.; Daskalakis, I.; Stavrakaki, M. The effect of rootstock on grape quality characters, phenolic and antioxidant potential of a biotype of cv ‘Korinthiaki Staphis’ (Vitis vinifera L.). Acta Hortic. 2024, 1418, 111–118. [Google Scholar] [CrossRef]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2020, 307, 125518. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.C.; Evans, M.E. Spectral evaluation of young red wines: Anthocyanin equilibria, total phenolic, free and molecular SO2, ‘chemical age’. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Vivas, N.; Nonier, M.F.; de Gaulejac, N.V.; Absalon, C.; Bertrand, A.; Mirabel, M. Determination of proanthocyanidins in wine and oak wood using p-dimethylaminocinnamaldehyde. J. Sci. Food Agric. 1994, 64, 153–160. [Google Scholar]
- Bonvehi, J.S.; Coll, F.V.; Jorda, R.E. The composition, active components, and bacteriostatic activity of propolis in dietetics. J. Am. Oil Chem. Soc. 1994, 71, 529–532. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimized tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Roussos, P.A.; Pontikis, C.A. Phenolic compounds in olive explants and their contribution to browning during the establishment stage in vitro. Eur. J. Hortic. Sci. 2001, 66, 298–303. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kontoudakis, N.; González, E.; Gil, M.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of wine pH on changes in color and polyphenol composition induced by micro-oxygenation. J. Agric. Food Chem. 2011, 59, 1974–1984. [Google Scholar] [CrossRef]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of non-irrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of elevated temperature in grapevine. II. Juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Acevedo, D.C.A.; Hilbert, G.; Rivière, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.-C.; Delrot, S.; Mérillon, J.-M.; et al. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC–MS and LC–NMR. Anal. Chim. Acta 2012, 732, 145–152. [Google Scholar] [CrossRef]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.-R.; He, F.; Xiao, H.-L.; Duan, C.-Q.; Pan, Q.-H. Accumulation pattern of flavonoids in Cabernet Sauvignon grapes grown in a low-latitude and high-altitude region. S. Afr. J. Enol. Vitic. 2016, 36, 32–43. [Google Scholar] [CrossRef]
- Muñoz, F.A.; Urvieta, R.A.; Buscema, F.; Rasse, M.; Fontana, A.R.; Berli, F.J. Phenolic characterization of Cabernet Sauvignon wines from different geographical indications of Mendoza, Argentina: Effects of plant material and environment. Trends Plant Sci. 2021, 5, 1523. [Google Scholar] [CrossRef]
- Urvieta, R.; Jones, G.; Buscema, F.; Bottini, R.; Fontana, A. Terroir and vintage discrimination of Malbec wines based on phenolic composition across multiple sites in Mendoza, Argentina. Sci. Rep. 2021, 11, 2863. [Google Scholar] [CrossRef]
- Piazena, H.; Häder, D.P. Solar UV-B and UV-A irradiance in arid high-mountain regions: Measurements on the island of Tenerife as compared to previous tropical Andes data. J. Geophys. Res.-Biogeosci. 2009, 114, 37–52. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming—Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef] [PubMed]
- de Rosas, I.; Ponce, M.T.; Malovini, E.; Deis, L.; Cavagnaro, B.; Cavagnaro, P. Loss of anthocyanins and modification of the anthocyanin profiles in grape berries of Malbec and Bonarda grown under high temperature conditions. Plant Sci. 2017, 258, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A. Genetic and environmental impacts on the biosynthesis of anthocyanins in grapes. Hort. J. 2018, 87, 1–17. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.Á.; Diago, M.P.; Tomás-Las-Heras, R.; Monforte, L.; Soriano, G.; Martínez-Abaigar, J.; Núñez-Olivera, E. Effects of ambient solar UV radiation on grapevine leaf physiology and berry phenolic composition along one entire season under Mediterranean field conditions. Plant Physiol. Biochem. 2016, 109, 374–386. [Google Scholar] [CrossRef]
- Mateus, N.; Marques, S.; Gonçalves, A.C.; Machado, J.M.; De Freitas, V. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro Valley during ripening: Influence of cultivation altitude. Am. J. Enol. Vitic. 2001, 52, 115–121. [Google Scholar] [CrossRef]
- Mateus, N.; Machado, J.M.; De Freitas, V. Development changes of anthocyanins in Vitis vinifera grapes grown in the Douro Valley and concentration in respective wines. J. Sci. Food Agric. 2002, 82, 1689–1695. [Google Scholar] [CrossRef]
- Mateus, N.; Proença, S.; Ribeiro, P.; Machado, J.M.; De Freitas, V. Grape and wine polyphenolic composition of red Vitis vinifera varieties concerning vineyard altitude. CYTA J. Food 2001, 3, 102–110. [Google Scholar] [CrossRef]
- Pajovic, R.; Raicevic, D.; Popovic, T.; Sivilotti, P.; Lisjak, K.; Vanzo, A. Polyphenolic characterization of Vranac, Kratosija and Cabernet Sauvignon (Vitis vinifera L. cv.) grapes and wines from different vineyard locations in Montenegro. S. Afr. J. Enol. Vitic. 2014, 35, 139–148. [Google Scholar] [CrossRef]

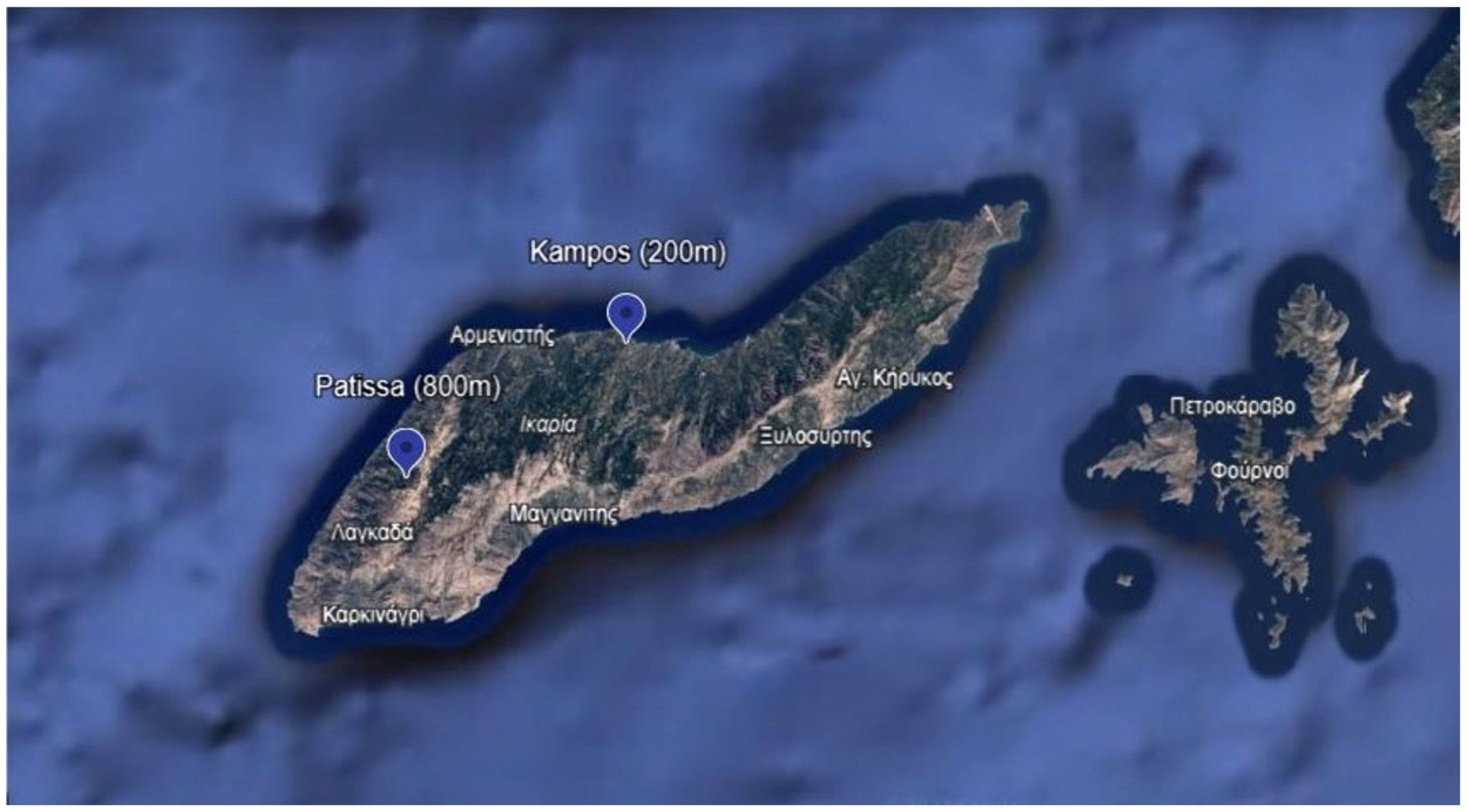
| Code | Vineyard Name | Vineyard Characteristics |
|---|---|---|
| V800P | Patissa | Altitude 800 m, organic vineyard, 20-year-old vines, grafted on R110 rootstock, head-trained |
| V200K | Kampos | Altitude 200 m, organic vineyard, 20-year-old vines, grafted on R110 rootstock, head-trained |
| Years | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Measurements | V800P | V200K | V800P | V200K | V800P | V200K |
| Grape bunch weight (g) | 131 ± 20 b | 229 ± 20 a | 107 ± 4 b | 241 ± 4 a | 148 ± 5 b | 274 ± 7 a |
| Grape bunch length (cm) | 14 ± 1 a | 13.2 ± 0.5 a | 7.1 ± 0.7 a | 7.1 ± 0.7 a | 9 ± 1 a | 9.6 ± 0.3 a |
| Grape bunch width (cm) | 7.11 ± 1 b | 8.2 ± 0.4 a | 11.5 ± 0.8 b | 16.9 ± 0.9 a | 16 ± 1 b | 21.3 ± 0.3 a |
| Berry length (mm) | 14 ± 3 b | 18 ± 2 a | 16.4 ± 0.5 a | 17.9 ± 0.4 a | 18 ± 1 a | 18.9 ± 0.4 a |
| Berry width (mm) | 15 ± 2 b | 17 ± 1 a | 14.9 ± 0.2 b | 16.5 ± 0.3 a | 16.95 ± 0 b | 18.5 ± 0.5 a |
| Weight of 30 berries (g) | 119 ± 6 b | 142 ± 1 a | 143 ± 13 b | 163 ± 2 a | 112 ± 2 b | 121 ± 5 a |
| Percentage of skin weight per berry (%) | 14.1 ± 0.5 a | 12.3 ± 0.3 b | 10.3 ± 0.6 a | 8.7 ± 0.6 b | 9.7 ± 0.3 a | 7.1 ± 0.2 b |
| Percentage of seed weight per berry (%) | 1.49 ± 0.14 b | 1.94 ± 0.04 a | 1.64 ± 0.07 b | 1.96 ± 0.17 a | 1.55 ± 0.05 b | 2.41 ± 0.13 a |
| Percentage of pulp weight per berry (%) | 84.4 ± 0.6 a | 85.8 ± 0.2 a | 88.1 ± 0.5 a | 89.3 ± 0.8 a | 88.7 ± 0.3 a | 90.5 ± 0.3 a |
| Berry water content (%) | 73.2 ± 0.5 a | 68 ± 1 b | 64 ± 2 a | 63 ± 4 a | 72 ± 1 a | 70.2 ± 0.3 a |
| Years | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Measurements | V800P | V200K | V800P | V200K | V800P | V200K |
| Titratable acidity (g tartaric L−1 must) | 7.6 ± 0.3 a | 5.75 ± 0.01 b | 7.1 ± 0.2 a | 5.6 ± 0.2 b | 6.9 ± 0.2 a | 5.8 ± 0.2 b |
| Total soluble solids (°Brix) | 20.90 ± 0.06 b | 24.67 ± 0.07 a | 22.86 ± 0.06 b | 26.20 ± 0.01 a | 20.01 ± 0.03 a | 20.00 ± 0.05 a |
| pH | 3.77 ± 0.01 b | 3.95 ± 0.03 a | 3.63 ± 0.01 b | 3.94 ± 0.02 a | 3.31 ± 0.01 b | 3.64 ± 0.00 a |
| Fructose (g L−1 must) | 121.7 ± 0.9 b | 131 ± 3 a | 129 ± 20 b | 149.2 ± 17 a | 123 ± 44 a | 121 ± 75 a |
| Glucose (g L−1 must) | 127.6 ± 0.9 a | 134 ± 3 a | 139 ± 5 a | 142 ± 6 a | 127 ± 1 a | 125.2 ± 0.5 a |
| Degree of ripeness | 2.7 ± 0.1 b | 4.29 ± 0.02 a | 3.2 ± 0.1 b | 4.7 ± 0.2 a | 2.9 ± 0.2 a | 3.4 ± 1.8 a |
| Years | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Measurements | V800P | V200K | V800P | V200K | V800P | V200K |
| Total phenolics, seeds (mg gallic acid g−1 F.W.) | 20.9 ± 0.4 b | 24.4 ± 1.5 a | 22.6 ± 0.3 a | 20.3 ± 0.1 b | 24.22 ± 0.05 | 22.9 ± 0.1 b |
| Total phenolics, skins (mg gallic acid g−1 F.W.) | 4.5 ± 0.1 a | 2.2 ± 0.1 b | 3.9 ± 0.1 a | 3.6 ± 0.2 b | 3.9 ± 1.9 a | 2.1 ± 1.3 b |
| Total flavanols, skins (mg catechin g−1 F.W.) | 4.5 ± 0.1 b | 4.9 ± 0.3 a | 10.8 ± 0.1 a | 10.3 ± 0.2 a | 5.4 ± 0.2 a | 4.9 ± 0.2 b |
| Total flavanols, seeds (mg catechin g−1 F.W.) | 20.14 ± 0.04 b | 24.7 ± 0.4 a | 20.9 ± 0.1 b | 30.7 ± 1.7 a | 22.3 ± 0.1 a | 16.50 ± 0.01 b |
| Total flavonoids, skins (mg catechin g−1 F.W.) | 25.09 ± 0.16 a | 26.7 ± 0.6 a | 17.9 ± 0.2 a | 12.9 ± 0.3 b | 29.1 ± 1.6 a | 25.4 ± 0.2 b |
| Total flavonoids, seeds mg catechin g−1 F.W.) | 84 ± 2 a | 62.8 ± 0.4 b | 80.0 ± 0.2 a | 69.2 ± 0.9 b | 82.2 ± 0.1 a | 61.65 ± 0.01 b |
| Total flavones and flavonols, skins (mg rutin g−1 F.W.) | 1.56 ± 0.04 a | 1.20 ± 0.01 b | 0.92 ± 0.01 a | 0.80 ± 0.02 b | 0.52 ± 0.01 a | 0.45 ± 0.01 b |
| Total flavones and flavonols, seeds (mg rutin g−1 F.W.) | 0.39 ± 0.02 b | 0.59 ± 0.02 a | 0.42 ± 0.01 a | 0.25 ± 0.02 b | 0.54 ± 0.01 b | 0.75 ± 0.01 a |
| Τotal orthodiphenols, skins (mg caffeic acid g−1 F.W.) | 0.63 ± 0.01 a | 0.57 ± 0.02 b | 0.50 ± 0.01 a | 0.42 ± 0.03 b | 0.55 ± 0.03 a | 0.56 ± 0.02 a |
| Τotal orthodiphenols, seeds (mg caffeic acid g−1 F.W.) | 1.87 ± 0.03 b | 2.19 ± 0.07 a | 1.19 ± 0.02 a | 1.13 ± 0.01 a | 1.36 ± 0.57 a | 1.35 ± 0.29 a |
| Τotal tannins, skins (mg catechin g−1 F.W.) | 11.13 ± 0.03 a | 9.8 ± 0.6 a | 13.83 ± 0.03 a | 12.9 ± 0.1 a | 6.0 ± 0.1 b | 7.2 ± 0.2 a |
| Τotal tannins, seeds (mg catechin g−1 F.W.) | 35.8 ± 0.4 b | 37 ± 4 a | 39 ± 1 a | 38.8 ± 0.1 a | 35 ± 1 b | 36.8 ± 0.1 a |
| FRAP, skins (mg Trolox g−1 F.W.) | 57.82 ± 0 6 a | 42.6 ± 0.7 b | 63 ± 4 a | 43.8 ± 0.3 b | 60 ± 1 b | 63 ± 2 a |
| FRAP, seeds (mg Trolox g−1 F.W.) | 91.3 ± 0.4 a | 80 ± 2 b | 90.01 ± 0.17 a | 84.5 ± 0.4 b | 93.9 ± 0.2 a | 85 ± 5 b |
| DPPH, skins (mg Trolox g−1 F.W.) | 16.9 ± 0.2 a | 15 ± 1 b | 14.4 ± 0.3 a | 11.6 ± 0.9 b | 17 ± 2 a | 11 ± 3 b |
| DPPH, seeds (mg Trolox g−1 F.W.) | 67.6 ± 0.8 a | 71 ± 1 a | 86 ± 1 a | 78.48 ± 0.17 b | 90 ± 1 a | 84.7 ± 0.9 b |
| Years | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Measurements | V800P | V200K | V800P | V200K | V800P | V200K |
| Total anthocyanins (mg malvidin g−1 F.W.) | 5.6 ± 0.4 a | 2.3 ± 0.1 b | 4.89 ± 0.03 a | 2.13 ± 0.01 b | 4.51 ± 0.02 a | 4.43 ± 0.03 a |
| Delphinidin (mg delphinidin glucoside g−1 F.W.) | 0.25 ± 0.01 a | 0.17 ± 0.01 b | 0.17 ± 0.03 a | 0.13 ± 0.05 b | 0.49 ± 0.01 a | 0.38 ± 0.01 b |
| Cyanidin (mg cyanidin glucoside g−1 F.W.) | 1.55 ± 0.11 a | 0.53 ± 0.05 b | 0.48 ± 0.03 a | 0.35 ± 0.01 b | 0.75 ± 0.02 a | 0.46 ± 0.03 b |
| Petunidin (mg petunidin glucoside g−1 F.W.) | 0.09 ± 0.002 a | 0.08 ± 0.001 b | 0.05 ± 0.005 a | 0.03 ± 0.001 b | 0.03 ± 0.001 a | 0.02 ± 0.001 b |
| Peonidin (mg peonidin glucoside g−1 F.W.) | 0.77 ± 0.06 a | 0.42 ± 0.04 b | 0.20 ± 0.01 a | 0.23 ± 0.01 a | 0.21 ± 0.01 a | 0.16 ± 0.01 b |
| Malvidin (mg malvidin glucoside g−1 F.W.) | 0.58 ± 0.03 a | 0.49 ± 0.01 b | 0.092 ± 0.004 a | 0.089 ± 0.002 a | 0.015 ± 0.001 b | 0.024 ± 0.003 a |
| Acetic ester of malvidin (mg malvidin glucoside g−1 F.W.) | 0.021 ± 0.001 a | 0.020 ± 0.001 a | 0.025 ± 0.002 a | 0.018 ± 0.002 b | 0.059 ± 0.007 a | 0.055 ± 0.024 a |
| Coumaric ester of malvidin (mg malvidin glucoside g−1 F.W.) | 0.211 ± 0.064 a | 0.151 ± 0.064 b | 0.75 ± 0.026 a | 0.61 ± 0.057 b | 0.83 ± 0.013 b | 0.92 ± 0.012 a |
| Tartaric acid (μg mL−1 must) | 9379 ± 37 a | 8758 ± 29 b | 8426 ± 19 a | 7698 ± 15 b | 8088 ± 16 a | 7245 ± 15 b |
| Malic acid (μg mL−1 must) | 5476 ± 28 a | 4804 ± 30 b | 5801 ± 16 a | 4702 ± 12 b | 5425 ± 13 a | 4231.5 ± 14 b |
| Ascorbic acid (μg mL−1 must) | 164 ± 6 a | 150 ± 3 b | 137 ± 1 a | 116.2 ± 0.1 b | 131.5 ± 0.02 a | 123.7 ± 0.01 b |
| Succinic acid (μg mL−1 must) | 15.5 ± 0.4 b | 19.5 ± 0.6 a | 22.08 ± 0.04 a | 21.37 ± 0.08 a | 14.39 ± 0.23 b | 18.07 ± 0.24 a |
| Fumaric acid (μg mL−1 must) | 3.42 ± 0.14 b | 3.56 ± 0.22 a | 2.35 ± 0.22 a | 2.2 ± 0.1 a | 3.20 ± 0.01 a | 2.82 ± 0.03 b |
| Variables/Measurements | Eigenvectors/Principal Components | ||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| Acetic ester of malvidin (mg malvidin glucoside g−1 F.W.) | −0.119 | −0.237 | 0.006 | 0.033 | −0.047 |
| Ascorbic acid (μg ascorbic acid mL−1 must) | 0.223 | −0.025 | 0.037 | 0.072 | 0.158 |
| Berry water content (%) | 0.101 | −0.174 | 0.091 | −0.178 | −0.068 |
| Berry length (mm) | −0.189 | −0.012 | 0.172 | 0.189 | −0.012 |
| Berry width (mm) | −0.139 | −0.077 | 0.199 | −0.113 | −0.096 |
| Coumaric ester of malvidin (mg malvidin glucoside g−1 F.W.) | −0.202 | −0.111 | −0.148 | −0.016 | 0.077 |
| Cyanidin (mg cyanidin glucoside g−1 F.W.) | 0.211 | −0.089 | −0.050 | −0.219 | −0.070 |
| Degree of ripeness | −0.096 | 0.205 | 0.159 | 0.039 | −0.157 |
| Delphinidin (mg delphinidin glucoside g−1 F.W.) | −0.046 | −0.257 | −0.005 | 0.008 | −0.173 |
| DPPH, seeds (mg Trolox g−1 F.W.) | −0.171 | −0.105 | −0.143 | 0.199 | 0.002 |
| DPPH, skins (mg Trolox g−1 F.W.) | 0.191 | −0.085 | −0.093 | 0.239 | −0.229 |
| FRAP, seeds (mg Trolox g−1 F.W.) | 0.051 | −0.123 | −0.246 | −0.104 | −0.131 |
| FRAP, skins (mg Trolox g−1 F.W.) | −0.021 | −0.185 | −0.191 | −0.091 | 0.342 |
| Fructose (g L−1 must) | −0.074 | 0.212 | −0.010 | −0.016 | −0.309 |
| Fumaric Acid (μg fumaric acid mL−1 must) | 0.159 | −0.117 | 0.200 | 0.101 | −0.095 |
| Glucose (g L−1 must) | −0.035 | 0.159 | −0.066 | 0.280 | 0.086 |
| Grape length (cm) | 0.196 | −0.048 | 0.206 | −0.065 | 0.015 |
| Grape weight (g) | −0.140 | 0.039 | 0.276 | −0.170 | −0.026 |
| Grape width (cm) | −0.227 | −0.078 | 0.004 | −0.148 | −0.053 |
| Malic acid (μg malic acid mL−1 must) | 0.131 | −0.014 | −0.259 | 0.233 | 0.005 |
| Malvidin (mg malvidin glucoside g−1 F.W.) | 0.223 | 0.058 | 0.140 | −0.045 | 0.041 |
| Peonidin (mg peonidin glucoside g−1 F.W.) | 0.233 | 0.015 | 0.047 | −0.186 | −0.039 |
| Percentage of pulp weight per berry (%) | 0.120 | 0.232 | −0.047 | 0.021 | 0.059 |
| Percentage of seed weight per berry (%) | −0.165 | 0.015 | 0.220 | −0.168 | 0.248 |
| Percentage of skin weight per berry (%) | 0.241 | 0.036 | 0.007 | 0.063 | −0.035 |
| Petunidin (mg petunidin glucoside g−1 F.W.) | 0.235 | 0.059 | 0.060 | 0.067 | 0.128 |
| pH | 0.040 | 0.193 | 0.143 | −0.214 | 0.016 |
| Succinic acid (μg succinic acid mL−1 must) | −0.089 | 0.209 | −0.030 | 0.111 | 0.345 |
| Tartaric acid (μg tartaric acid mL−1 must) | 0.216 | 0.028 | −0.032 | 0.077 | −0.038 |
| Total flavonoids, seeds (mg catechin g−1 F.W.) | 0.198 | 0.131 | 0.124 | 0.065 | 0.072 |
| Total flavonoids, skins (mg catechin g−1 F.W.) | 0.083 | −0.216 | 0.150 | 0.165 | −0.033 |
| Total acidity (g tartaric L−1 must) | 0.154 | −0.100 | −0.231 | −0.014 | 0.065 |
| Total anthocyanins (mg malvidin g−1 F.W.) | 0.091 | −0.172 | −0.190 | −0.144 | 0.260 |
| Total flavanols, seeds (mg catechin g−1 F.W.) | 0.073 | 0.256 | −0.008 | 0.004 | −0.037 |
| Total flavanols, skins (mg catechin g−1 F.W.) | −0.096 | 0.178 | −0.225 | 0.077 | 0.064 |
| Total flavones and flavonols, seeds (mg rutin g−1 F.W.) | −0.057 | −0.179 | 0.204 | 0.100 | 0.272 |
| Total flavones and flavonols, skins (mg rutin g−1 F.W.) | 0.228 | 0.101 | 0.023 | −0.081 | 0.059 |
| Total phenolics, seeds (mg gallic acid g−1 F.W.) | −0.012 | −0.112 | 0.111 | 0.538 | 0.080 |
| Total phenolics, skins (mg gallic acid g−1 F.W.) | 0.111 | 0.005 | −0.294 | −0.087 | −0.236 |
| Total soluble solids (°Brix) | −0.027 | 0.243 | 0.045 | 0.090 | −0.167 |
| Weight of 30 berries (g) | −0.067 | 0.246 | −0.002 | 0.055 | 0.025 |
| Τotal orthodiphenols, skins (mg caffeic acid g−1 F.W.) | 0.168 | −0.161 | 0.108 | −0.028 | 0.183 |
| Τotal orthodiphenols, seeds (mg caffeic acid g−1 F.W.) | 0.180 | −0.006 | 0.241 | 0.118 | 0.034 |
| Τotal tannins, seeds (mg catechin g−1 F.W.) | −0.058 | 0.145 | −0.059 | −0.093 | 0.248 |
| Τotal tannins, skins (mg catechin g−1 F.W.) | 0.050 | 0.226 | −0.151 | −0.073 | 0.195 |
| Eigenvalue | 15.94 | 13.62 | 7.42 | 2.12 | 1.69 |
| Individual variation explained | 35.43 | 30.27 | 16.49 | 4.72 | 3.77 |
| Cumulative variation explained | 35.43 | 65.71 | 82.2 | 86.93 | 90.71 |
| Independent Variables | ||
| 1 | 2 | |
| Altitude | −0.015 | 0.298 |
| Cultivation period (Year) | 0.254 | 0.040 |
| Dependent Variables | ||
| 1 | 2 | |
| Acetic ester of malvidin | −0.175 | 0.137 |
| Ascorbic acid | 0.184 | 0.062 |
| Berry water content | 0.030 | 0.109 |
| Berry length | −0.169 | −0.120 |
| Berry width | −0.147 | −0.077 |
| Coumaric ester of malvidin | −0.204 | 0.093 |
| Cyanidin | 0.153 | 0.142 |
| Degree of ripeness | −0.024 | −0.247 |
| Delphinidin | −0.119 | 0.178 |
| DPPH, seeds | −0.175 | 0.100 |
| DPPH, skins | 0.141 | 0.165 |
| FRAP, seeds | 0.011 | 0.217 |
| FRAP, skins | −0.070 | 0.213 |
| Fructose | 0.079 | −0.029 |
| Fumaric acid | 0.097 | 0.038 |
| Glucose | 0.043 | −0.059 |
| Grape length | 0.149 | −0.009 |
| Grape weight | −0.116 | −0.199 |
| Grape width | −0.219 | −0.011 |
| Malic acid | 0.115 | 0.171 |
| Malvidin | 0.206 | −0.048 |
| Peonidin | 0.202 | 0.028 |
| Percentage of pulp weight per berry | 0.176 | −0.113 |
| Percentage of seed weight per berry | −0.143 | −0.165 |
| Percentage of skin weight per berry | 0.218 | 0.039 |
| Petunidin | 0.219 | −0.006 |
| pH | 0.091 | −0.201 |
| Succinic acid | −0.010 | −0.163 |
| Tartaric acid | 0.195 | 0.056 |
| Total flavonoids, seeds | 0.209 | −0.097 |
| Total flavonoids, skins | 0.003 | 0.112 |
| Total acidity | 0.107 | 0.220 |
| Total anthocyanins | 0.030 | 0.232 |
| Total flavanols, seeds | 0.141 | −0.161 |
| Total flavanols, skins | −0.022 | −0.049 |
| Total flavones and flavonols, seeds | −0.106 | 0.017 |
| Total flavones and flavonols, skins | 0.226 | −0.023 |
| Total phenolics, seeds | −0.044 | 0.036 |
| Total phenolics, skins | 0.102 | 0.165 |
| Total soluble solids | 0.051 | −0.201 |
| Total orthodiphenols, skins | 0.093 | 0.109 |
| Total orthodiphenols, seeds | 0.148 | −0.057 |
| Τotal tannins, seeds | −0.003 | −0.096 |
| Total tannins, skins | 0.116 | −0.082 |
| Weight of 30 berries | 0.018 | −0.194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalakis, I.; Stavrakaki, M.; Vardaka, K.; Nikolaou, S.; Koukoufiki, S.; Giannakou, T.; Bouza, D.; Biniari, K. How Altitude Affects the Phenolic Potential of the Grapes of cv. ‘Fokiano’ (Vitis vinifera L.) on Ikaria Island. Environments 2025, 12, 320. https://doi.org/10.3390/environments12090320
Daskalakis I, Stavrakaki M, Vardaka K, Nikolaou S, Koukoufiki S, Giannakou T, Bouza D, Biniari K. How Altitude Affects the Phenolic Potential of the Grapes of cv. ‘Fokiano’ (Vitis vinifera L.) on Ikaria Island. Environments. 2025; 12(9):320. https://doi.org/10.3390/environments12090320
Chicago/Turabian StyleDaskalakis, Ioannis, Maritina Stavrakaki, Katerina Vardaka, Stavroula Nikolaou, Stefania Koukoufiki, Theodora Giannakou, Despoina Bouza, and Katerina Biniari. 2025. "How Altitude Affects the Phenolic Potential of the Grapes of cv. ‘Fokiano’ (Vitis vinifera L.) on Ikaria Island" Environments 12, no. 9: 320. https://doi.org/10.3390/environments12090320
APA StyleDaskalakis, I., Stavrakaki, M., Vardaka, K., Nikolaou, S., Koukoufiki, S., Giannakou, T., Bouza, D., & Biniari, K. (2025). How Altitude Affects the Phenolic Potential of the Grapes of cv. ‘Fokiano’ (Vitis vinifera L.) on Ikaria Island. Environments, 12(9), 320. https://doi.org/10.3390/environments12090320







