Biogenic CO2, CH4, and N2O Emissions from Abalone Culture in Tidal Ponds
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Pond Management
2.3. Sampling and Environmental Factors Analysis
2.4. GHG Concentrations
2.5. GHG Fluxes
2.6. Statistic Analysis
3. Results
3.1. Environmental Parameters
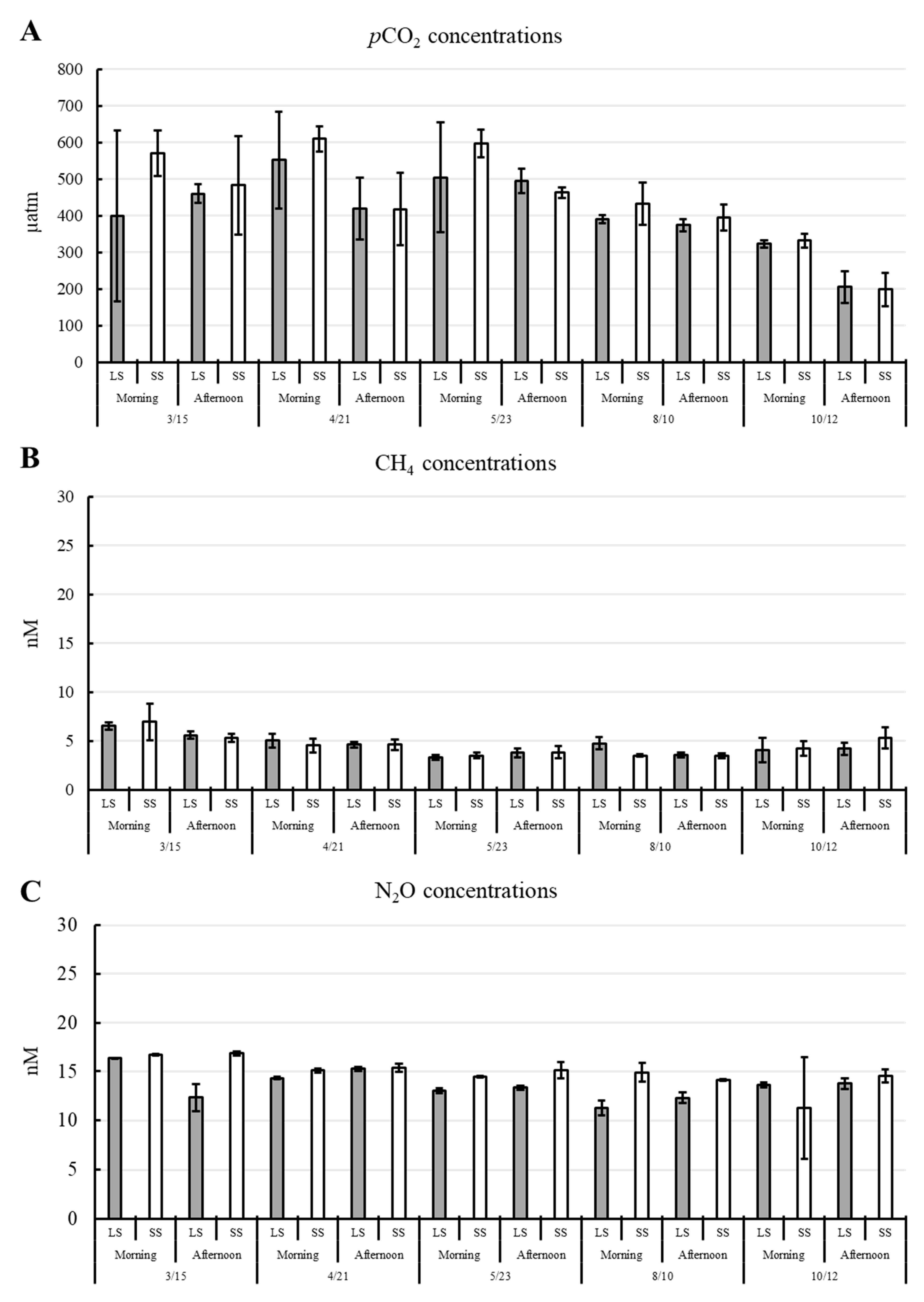
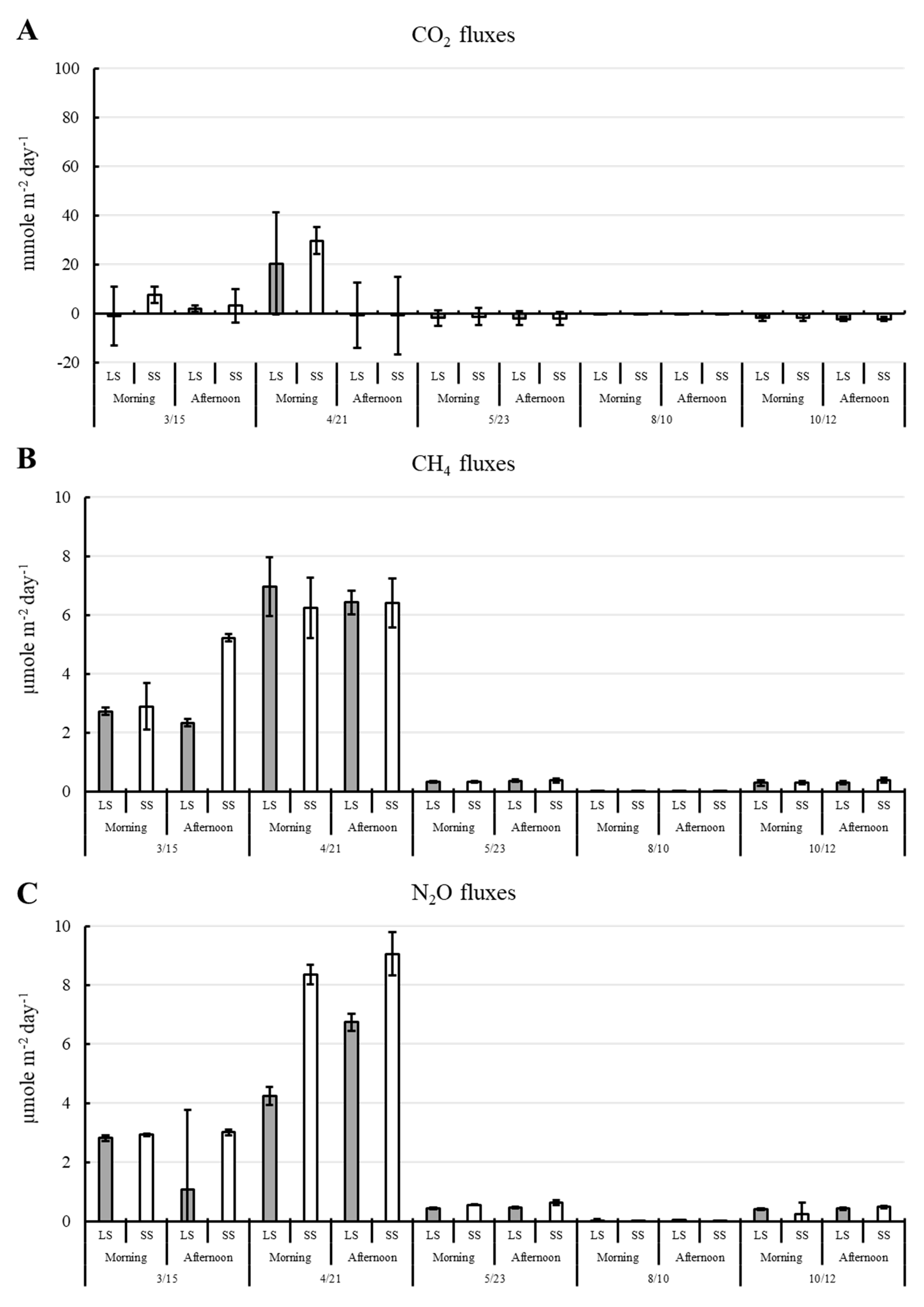
3.2. Variation in GHG Concentrations and Fluxes
3.3. Correlation Between GHG Fluxes and Environmental Factors
4. Discussion
4.1. Environmental Variables Contributing to the Dynamic of pCO2, CH4, and N2O Concentrations Throughout the Cultivation Period
4.2. Environmental Variables Contributing to the Dynamic of CO2, CH4, and N2O Fluxes Throughout the Cultivation Period
4.3. Management Practices Contributing to the Dynamic of CO2, CH4, and N2O Fluxes Throughout the Cultivation Period and Potential Strategies for Mitigating Emissions
4.4. CO2-eq Fluxes Throughout the Cultivation Period
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| CH4 | Methane |
| DIC | Dissolved inorganic carbon |
| DIN | Dissolved inorganic nitrogen |
| DIP | Dissolved inorganic phosphorus |
| ECD | Electron capture detector |
| FAO | Food and Agriculture Organization |
| FID | Flame ionization detector |
| GC | Gas chromatograph |
| GHG | Greenhouse gas |
| IMTA | Integrated Multi-Trophic Aquaculture |
| IPCC | Intergovernmental Panel on Climate Change |
| N2O | Nitrous oxide |
| TA | Total alkalinity |
| TOC | Total dissolved organic carbon |
References
- Cook, P. Worldwide abalone production: An update. N. Z. J. Mar. Freshw. Res. 2023, 5, 4–10. [Google Scholar] [CrossRef]
- Schubel, J.R.; Thompson, K. Farming the Sea: The Only Way to Meet Humanity’s Future Food Needs. Geohealth 2019, 3, 238–244. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- Dong, B.; Xi, Y.; Cui, Y.; Peng, S. Quantifying Methane Emissions from Aquaculture Ponds in China. Environ. Sci. Technol. 2023, 57, 1576–1583. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Huang, L.; Wang, C.; Gao, Y.; Peng, S.; Canadell, J.G.; Piao, S. Inventory of methane and nitrous oxide emissions from freshwater aquaculture in China. Commun. Earth Environ. 2024, 5, 531. [Google Scholar] [CrossRef]
- Weerathunga, V.; Liu, L.-L.; Yuan, F.-L.; Xu, S.X.; Kao, K.-J.; Huang, W.-J. Temporal variability of air-water gas exchange of carbon dioxide in clam and fish aquaculture ponds. Sci. Total Environ. 2024, 917, 170090. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Hasan, M.R.; Robb, D.H.F.; Mamun-Ur-Rashid, M. Quantifying greenhouse gas emissions from global aquaculture. Sci. Rep. 2020, 10, 11679. [Google Scholar] [CrossRef]
- Polymeni, S.; Skoutas, D.N.; Sarigiannidis, P.; Kormentzas, G.; Skianis, C. Smart Agriculture and Greenhouse Gas Emission Mitigation: A 6G-IoT Perspective. Electronics 2024, 13, 1480. [Google Scholar] [CrossRef]
- Ye, W.; Sun, H.; Li, Y.; Zhang, J.; Zhang, M.; Gao, Z.; Yan, J.; Liu, J.; Wen, J.; Yang, H.; et al. Greenhouse gas emissions from fed mollusk mariculture: A case study of a Sinonovacula constricta farming system. Agric. Ecosyst. Environ. 2022, 336, 108029. [Google Scholar] [CrossRef]
- Yuan, J.; Xiang, J.; Liu, D.; Kang, H.; He, T.; Kim, S.; Lin, Y.; Freeman, C.; Ding, W. Rapid growth in greenhouse gas emissions from the adoption of industrial-scale aquaculture. Nat. Clim. Chang. 2019, 9, 318–322. [Google Scholar] [CrossRef]
- Tong, C.; Bastviken, D.; Tang, K.W.; Yang, P.; Yang, H.; Zhang, Y.; Guo, Q.; Lai, D.Y.F. Annual CO2 and CH4 fluxes in coastal earthen ponds with Litopenaeus vannamei in southeastern China. Aquaculture 2021, 545, 737229. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, X.; Dong, S.; Chen, Y.; Feng, J.; He, R.-P.; Zhang, K. Carbon dioxide fluxes from two typical mariculture polyculture systems in coastal China. Aquaculture 2020, 521, 735041. [Google Scholar] [CrossRef]
- Ray, N.E.; Maguire, T.J.; Al-Haj, A.N.; Henning, M.C.; Fulweiler, R.W. Low Greenhouse Gas Emissions from Oyster Aquaculture. Environ. Sci. Technol. 2019, 53, 9118–9127. [Google Scholar] [CrossRef]
- da Silva, M.G.; Packer, A.P.; Sampaio, F.G.; Marani, L.; Mariano, E.V.C.; Pazianotto, R.A.A.; Ferreira, W.J.; Alvalá, P.C. Impact of intensive fish farming on methane emission in a tropical hydropower reservoir. Clim. Chang. 2018, 150, 195–210. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, D. Accounting for Carbon Emissions from Fisheries in China and Analyzing the Decoupling Effect. Fishes 2025, 10, 79. [Google Scholar] [CrossRef]
- Mistri, M.; Munari, C. Clam farming generates CO2: A study case in the Marinetta lagoon (Italy). Mar. Pollut. Bull. 2012, 64, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Rossetti, E.; Mistri, M. Shell formation in cultivated bivalves cannot be part of carbon trading systems: A study case with Mytilus galloprovincialis. Mar. Environ. Res. 2013, 92, 264–267. [Google Scholar] [CrossRef]
- Xiao, Q.; Duan, H.; Qin, B.; Hu, Z.; Zhang, M.; Qi, T.; Lee, X. Eutrophication and temperature drive large variability in carbon dioxide from China’s Lake Taihu. Limnol. Oceanogr. 2022, 67, 379–391. [Google Scholar] [CrossRef]
- Conrad, R. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: A mini review. Pedosphere 2020, 30, 25–39. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global Change, Nitrification, and Denitrification: A Review. Glob. Biogeochem. Cycles 2005, 19, GB1007. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Jin, J.; Zhu, X.; Han, D.; Xie, S. Towards a low-carbon footprint: Current status and prospects for aquaculture. Water Biol. Secur. 2024, 3, 100290. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.-H.; Cheng, S.-Y.; Chen, J.-C. Effect of dissolved oxygen on the acid–base balance and ion concentration of Taiwan abalone Haliotis diversicolor supertexta. Aquaculture 2004, 231, 573–586. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Y.; Bastviken, D.; Lai, D.Y.F.; Yang, H.; Zhang, Y.F.; Guo, Q.Q.; Tan, L.; Tong, C. Large increase in diffusive greenhouse gas fluxes from subtropical shallow aquaculture ponds during the passage of typhoons. J. Hydrol. 2020, 583, 124643. [Google Scholar] [CrossRef]
- Cheng, Q.-F.; Liao, B.-K.; Tseng, H.-C. Shrimp mariculture may increase aquatic CO2, CH4, and N2O emissions in semi-indoor and indoor ponds. Mar. Pollut. Bull. 2025, 210, 117298. [Google Scholar] [CrossRef]
- Tzollas, N.; Zachariadis, G.; Anthemidis, A.; Stratis, J. A new approach to indophenol blue method for determination of ammonium in geothermal waters with high mineral content. Anal. Chem. 2010, 90, 115–126. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Z.; Jiang, Y.; Zhao, J.; Tang, S. Biological effect of cadmium in Daphnia magna: Influence of nitrogen and phosphorus. Fresenius Environ. Bull. 2012, 21, 2891–2895. [Google Scholar]
- Ormaza-González, F.I.; Statham, P.J. Determination of dissolved inorganic phosphorus in natural waters at nanomolar concentrations using a long capillary cell detector. Anal. Chim. Acta 1991, 244, 63–70. [Google Scholar] [CrossRef]
- Johan, F.; Mat Jafri, M.Z.; Lim, H.S.; Wan Omar, W.M. Laboratory measurement: Chlorophyll-a concentration measurement with acetone method using spectrophotometer. In Proceedings of the IEEE International Conference on Industrial Engineering and Engineering Management, Selangor, Malaysia, 9–12 December 2014; Volume 2015, pp. 744–748. [Google Scholar] [CrossRef]
- Pelletier, G.; Lewis, E.; Wallace, D. CO2Sys. xls: A Calculator for the CO2 System in Seawater for Microsoft Excel/VBA; Washington State Department of Ecology: Olympia, WA, USA; Brookhaven National Laboratory: Upton, NY, USA, 2007. [Google Scholar]
- Lueker, T.J.; Dickson, A.G.; Keeling, C.D. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 2000, 70, 105–119. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Kortelainen, P.; Sobek, S.; Müller, R.; Rantakari, M. Carbon Dioxide in Boreal Surface Waters: A Comparison of Lakes and Streams. Ecosystems 2012, 15, 1295–1307. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, X.; Duan, H.; Qi, T.; Qin, B.; Lee, X.; Hu, Z.; Wang, W.; Xiao, W.; Zhang, M. Eutrophic Lake Taihu as a significant CO2 source during 2000–2015. Water Res. 2020, 170, 115331. [Google Scholar] [CrossRef]
- Johnson, K.M.; Hughes, J.E.; Donaghay, P.L.; Sieburth, J.M. Bottle-calibration static head space method for the determination of methane dissolved in seawater. Anal. Chem. 1990, 62, 2408–2412. [Google Scholar] [CrossRef]
- Weiss, R.F. Determinations of Carbon Dioxide and Methane by Dual Catalyst Flame Ionization Chromatography and Nitrous Oxide by Electron Capture Chromatography. J. Chromatogr. Sci. 1981, 19, 611–616. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, M.; Hu, Z.; Gao, Y.; Hu, C.; Liu, C.; Liu, S.; Zhang, Z.; Zhao, J.; Xiao, W.; et al. Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate: Methane flux in a shallow eutrophic lake. J. Geophys. Res. Biogeosci. 2017, 122, 1597–1614. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Lin, C.-C.; Pan, H.-J.; Han, Y.T.Y.; Gong, G.-C. Seasonal Distributions of Methane in a Populous Urban Coastal Sea Area. Front. Mar. Sci. 2022, 9, 843549. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Yuh Han, Y.T.; Lin, C.-C.; Gong, G.-C. Seasonal variations of nitrous oxide in a populous urban estuary and its adjacent sea. Front. Earth Sci. 2023, 11, 1112192. [Google Scholar] [CrossRef]
- Weiss, R.F.; Price, B.A. Nitrous oxide solubility in water and seawater. Mar. Chem. 1980, 8, 347–359. [Google Scholar] [CrossRef]
- Wiesenburg, D.A.; Guinasso, N.L., Jr. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J. Chem. Eng. Data 1979, 24, 356–360. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods 2014, 12, 351–362. [Google Scholar] [CrossRef]
- Banko-Kubis, H.; Wurl, O.; Mustaffa, N.I.H.; Ribas Ribas, M. Gas transfer velocities in Norwegian fjords and the adjacent North Atlantic waters. Oceanologia 2019, 61, 460–470. [Google Scholar] [CrossRef]
- Crusius, J.; Wanninkhof, R. Gas transfer velocities measured at low wind speed over a lake. Limnol. Oceanogr. 2003, 48, 1010–1017. [Google Scholar] [CrossRef]
- Gruca-Rokosz, R.; Koszelnik, P. Production pathways for CH4 and CO2 in sediments of two freshwater ecosystems in south-eastern Poland. PLoS ONE 2018, 13, e0199755. [Google Scholar] [CrossRef] [PubMed]
- Gruca-Rokosz, R.; Tomaszek, J.A.; Koszelnik, P.; Czerwieniec, E. Methane and Carbon Dioxide Fluxes at the Sediment-Water Interface in Reservoirs. Pol. J. Environ. Stud. 2011, 20, 81–86. [Google Scholar]
- Moriarty, D.J.W. The role of microorganisms in aquaculture ponds. Aquaculture 1997, 151, 333–349. [Google Scholar] [CrossRef]
- Crump, B.C.; Fine, L.M.; Fortunato, C.S.; Herfort, L.; Needoba, J.A.; Murdock, S.; Prahl, F.G. Quantity and quality of particulate organic matter controls bacterial production in the Columbia River estuary. Limnol. Oceanogr. 2017, 62, 2713–2731. [Google Scholar] [CrossRef]
- Geider, R.J. Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: Implications for physiology and growth of phytoplankton. New Phytol. 1987, 106, 1–34. [Google Scholar] [CrossRef]
- Clark, D.R.; Flynn, K.J. The relationship between the dissolved inorganic carbon concentration and growth rate in marine phytoplankton. Proc. Biol. Sci. 2000, 267, 953–959. [Google Scholar] [CrossRef]
- Weiss, R.F. The solubility of nitrogen, oxygen and argon in water and seawater. Deep Sea Res. Oceanogr. Abstr. 1970, 17, 721–735. [Google Scholar] [CrossRef]
- Staehr, P.A.; Bade, D.; Van de Bogert, M.C.; Koch, G.R.; Williamson, C.; Hanson, P.; Cole, J.J.; Kratz, T. Lake metabolism and the diel oxygen technique: State of the science. Limnol. Oceanogr. Methods 2010, 8, 628–644. [Google Scholar] [CrossRef]
- Carignan, R.; Planas, D.; Vis, C. Planktonic production and respiration in oligotrophic Shield lakes. Limnol. Oceanogr. 2000, 45, 189–199. [Google Scholar] [CrossRef]
- Vinuganesh, A.; Kumar, A.; Prakash, S.; Korany, S.M.; Alsherif, E.A.; Selim, S.; AbdElgawad, H. Evaluation of growth, primary productivity, nutritional composition, redox state, and antimicrobial activity of red seaweeds Gracilaria debilis and Gracilaria foliifera under pCO2-induced seawater acidification. Mar. Pollut. Bull. 2022, 185, 114296. [Google Scholar] [CrossRef]
- Kohl, K.D.; Yahn, J. Effects of environmental temperature on the gut microbial communities of tadpoles. Environ. Microbiol. 2016, 18, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.F.; Oliveira, M.C.; Colepicolo, P. Diurnal Fluctuation of Nitrate Reductase Activity in the Marine Red Alga Gracilaria Tenuistipitata (RHODOPHYTA). J. Phycol. 1997, 33, 225–231. [Google Scholar] [CrossRef]
- Wang, X.; Tang, B.; Luo, X.; Ke, C.; Huang, M.; You, W.; Wang, Y. Effects of temperature, diet and genotype-induced variations on the gut microbiota of abalone. Aquaculture 2020, 524, 735269. [Google Scholar] [CrossRef]
- Weinberger, F.; Hoppe, H.-G.; Friedlander, M. Bacterial induction and inhibition of a fast mecrotic response in Gracilaria conferta (Rhodophyta). J. Appl. Phycol. 1997, 9, 277–285. [Google Scholar] [CrossRef]
- Jaffray, A.E.; Anderson, R.J.; Coyne, V.E. Investigation of Bacterial Epiphytes of the Agar-producing Red Seaweed Gracilaria gracilis (Stackhouse) Steentoft, Irvine et Farnham from Saldanha Bay, South Africa and Lüderitz, Namibia. Bot. Mar. 1997, 40, 569–576. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, Y.-J.; Song, W.Y.; Kim, T.-I.; Lee, W.C.; Kim, T.Y.; Kang, C.-K. Physiological responses of the abalone Haliotis discus hannai to daily and seasonal temperature variations. Sci. Rep. 2019, 9, 8019. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Park, G.-H.; Kim, D.; Kim, T.-W. Variations in Seawater pCO2 Associated With Vertical Mixing During Tropical Cyclone Season in the Northwestern Subtropical Pacific Ocean. Front. Mar. Sci. 2021, 8, 679314. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Y.; Lai, D.Y.F.; Tan, L.; Jin, B.; Tong, C. Fluxes of carbon dioxide and methane across the water–atmosphere interface of aquaculture shrimp ponds in two subtropical estuaries: The effect of temperature, substrate, salinity and nitrate. Sci. Total Environ. 2018, 635, 1025–1035. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Kosten, S.; Almeida, R.M.; Barbosa, I.; Mendonça, R.; Santos Muzitano, I.; Sobreira Oliveira-Junior, E.; Vroom, R.J.E.; Wang, H.-J.; Barros, N. Better assessments of greenhouse gas emissions from global fish ponds needed to adequately evaluate aquaculture footprint. Sci. Total Environ. 2020, 748, 141247. [Google Scholar] [CrossRef]
- Williams, J.; Crutzen, P.J. Nitrous oxide from aquaculture. Nat. Geosci. 2010, 3, 143. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Cao, X.; Chen, J. Insights into simultaneous anammox and denitrification system with short-term pyridine exposure: Process capability, inhibition kinetics and metabolic pathways. Front. Environ. Sci. Eng. 2021, 15, 139. [Google Scholar] [CrossRef]
- Tan, L.; Ge, Z.; Zhou, X.; Li, S.; Li, X.; Tang, J. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Chang. Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Sharma, K.; Brotto, A.C.; Khanal, S.K. Nitrogen transformations in intensive aquaculture system and its implication to climate change through nitrous oxide emission. Bioresour. Technol. 2013, 130, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.K.; Oliveira, C.T.A.; Henry-Silva, G.G. Greenhouse gas emission flux (CO2, CH4, N2O) from marine shrimp (Litopenaeus vannamei) monoculture cultures in the Brazilian semi-arid region. Aquaculture 2024, 582, 740536. [Google Scholar] [CrossRef]
- Threlkeld, S.T. Reservoir limnology; Ecological perspectives (K. W. Thornton. B.L. Kimmel, and F. E. Payne [eds.]). Limnol. Oceanogr. 1990, 35, 1411–1412. [Google Scholar] [CrossRef]
- Woolf, D.K.; Land, P.E.; Shutler, J.D.; Goddijn-Murphy, L.M.; Donlon, C.J. On the calculation of air-sea fluxes of CO2 in the presence of temperature and salinity gradients. J. Geophys. Res. Ocean. 2016, 121, 1229–1248. [Google Scholar] [CrossRef]
- Hu, M.; Wilson, B.J.; Sun, Z.; Ren, P.; Tong, C. Effects of the addition of nitrogen and sulfate on CH4 and CO2 emissions, soil, and pore water chemistry in a high marsh of the Min River estuary in southeastern China. Sci. Total Environ. 2017, 579, 292–304. [Google Scholar] [CrossRef]
- Maeck, A.; DelSontro, T.; McGinnis, D.F.; Fischer, H.; Flury, S.; Schmidt, M.; Fietzek, P.; Lorke, A. Sediment Trapping by Dams Creates Methane Emission Hot Spots. Environ. Sci. Technol. 2013, 47, 8130–8137. [Google Scholar] [CrossRef]
- Boyd, C.E.; Wood, C.W.; Chaney, P.L.; Queiroz, J.F. Role of aquaculture pond sediments in sequestration of annual global carbon emissions. Environ. Pollut. 2010, 158, 2537–2540. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Bolton, N.W.; Gomes, L.E.d.O.; Nobrega, G.N. Shrimp ponds lead to massive loss of soil carbon and greenhouse gas emissions in northeastern Brazilian mangroves. Ecol. Evol. 2018, 8, 5530–5540. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Z.; Wu, S.; Li, S.; Li, Z.; Zou, J. Methane and Nitrous Oxide Emissions Reduced Following Conversion of Rice Paddies to Inland Crab–Fish Aquaculture in Southeast China. Environ. Sci. Technol. 2016, 50, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.T.; Drexl, M.; Tait, D.R.; Johnston, S.G.; Jeffrey, L.C. iAMES: An inexpensive, Automated Methane Ebullition Sensor. Environ. Sci. Technol. 2019, 53, 6420–6426. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M.; Audet, J.; Bastviken, D.; Cook, S.; Evans, C.D.; Grinham, A.; Holgerson, M.A.; Högbom, L.; Pickard, A.E.; Zieliński, P.; et al. Small artificial waterbodies are widespread and persistent emitters of methane and carbon dioxide. Glob. Chang. Biol. 2021, 27, 5109–5123. [Google Scholar] [CrossRef]
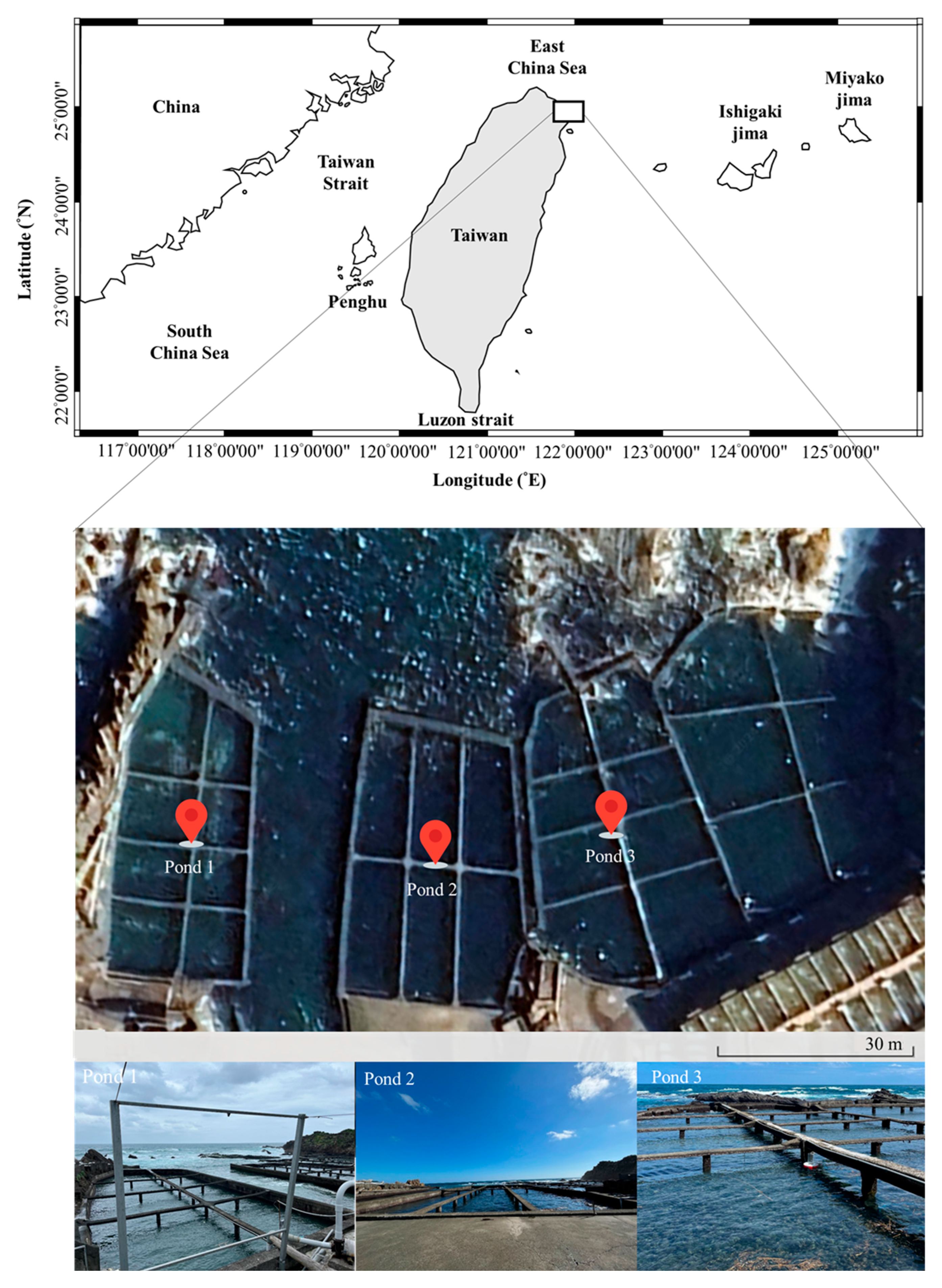

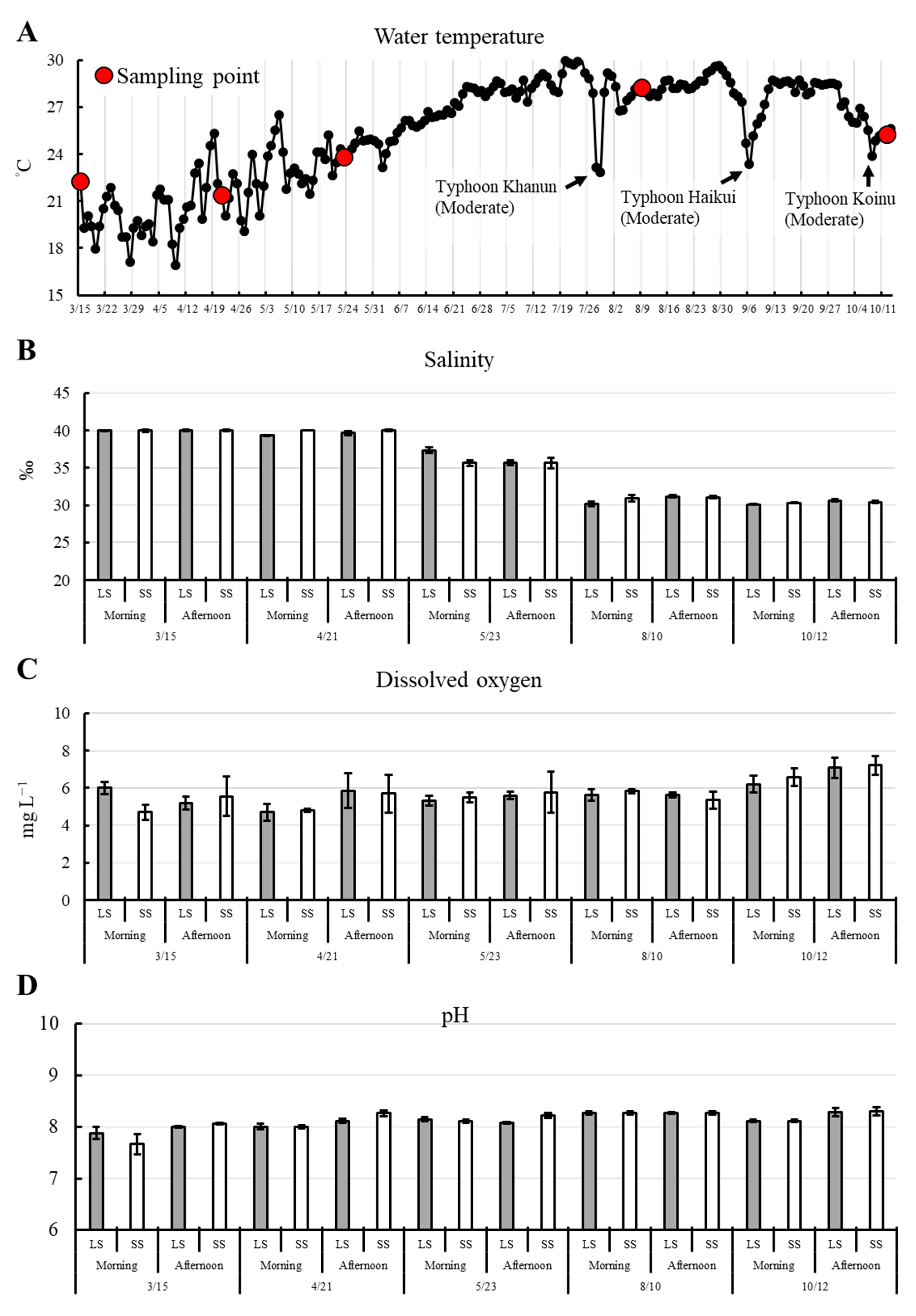
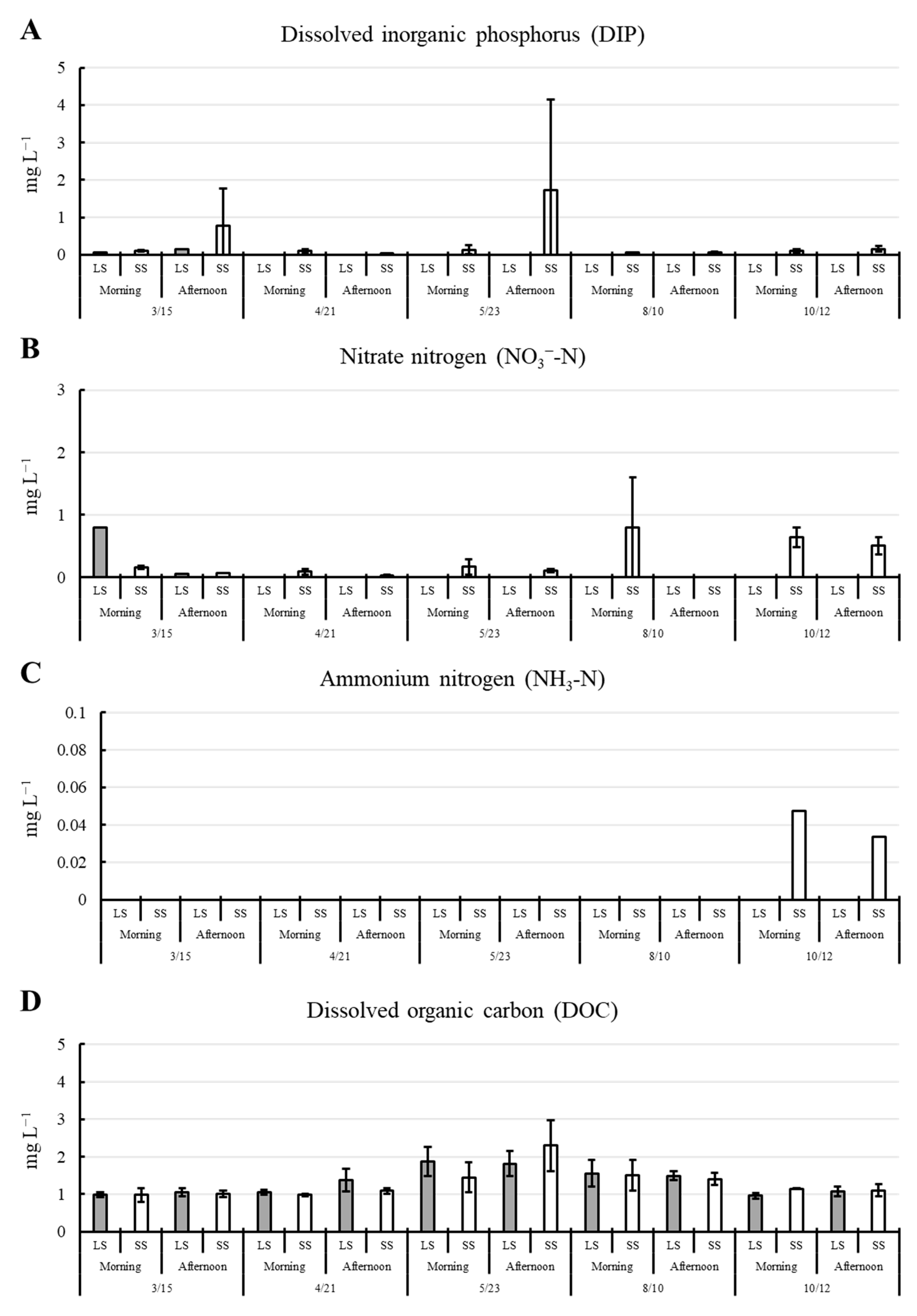
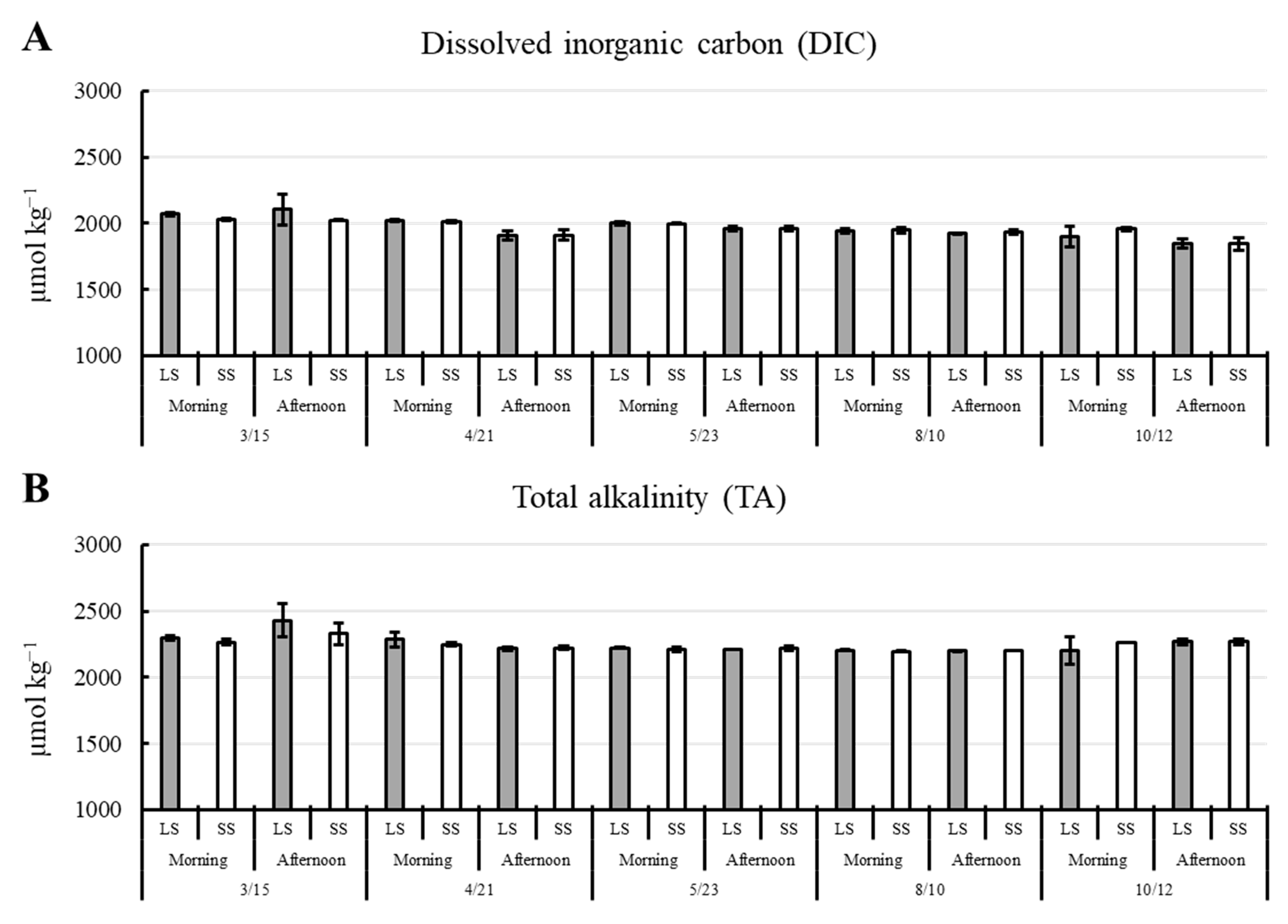
| Parameters | Pond 1 | Pond 2 | Pond 3 |
|---|---|---|---|
| Area (m2) | 1800 | 1660 | 1700 |
| Depth (m) | 2–4 | 2–4 | 2–4 |
| Volume (tons) | 2700 | 2490 | 2550 |
| Culture month | 8 | 8 | 8 |
| Survival rate (%) | 95.0 | 95.0 | 95.0 |
| Initial weight (g/unit Abalone) | 2–3 | 2–3 | 2–3 |
| Initial stocking weight (kg) | 400–600 | 400–600 | 400–600 |
| Final weight (g/unit Abalone) | 24–28 | 24–28 | 24–28 |
| Final harvest weight (tons) | 4.56–5.32 | 4.56–5.32 | 4.56–5.32 |
| Variables | Factor | n | df | p-Value |
|---|---|---|---|---|
| Water temperature | Location | 120 | 1 | 0.954 |
| Period | 1 | 0.633 | ||
| Seasons | 3 | p < 0.001 | ||
| Salinity | Location | 120 | 1 | 0.943 |
| Period | 1 | 0.695 | ||
| Seasons | 3 | p < 0.001 | ||
| pH | Location | 120 | 1 | 0.557 |
| Period | 1 | 0.130 | ||
| Seasons | 3 | p < 0.001 | ||
| Dissolved oxygen | Location | 120 | 1 | 0.903 |
| Period | 1 | 0.539 | ||
| Seasons | 3 | p < 0.001 | ||
| Dissolved organic carbon | Location | 120 | 1 | 0.227 |
| Period | 1 | 0.980 | ||
| Seasons | 3 | p < 0.001 | ||
| Dissolved inorganic carbon | Location | 120 | 1 | 0.844 |
| Period | 1 | 0.424 | ||
| Seasons | 3 | p < 0.001 | ||
| Total alkalinity | Location | 120 | 1 | 0.655 |
| Period | 1 | 0.500 | ||
| Seasons | 3 | p < 0.001 | ||
| Ammonium nitrogen | Location | 120 | 1 | 0.475 |
| Period | 1 | 0.677 | ||
| Seasons | 3 | p < 0.001 | ||
| Nitrate nitrogen | Location | 120 | 1 | 0.560 |
| Period | 1 | <0.05 (0.018) | ||
| Seasons | 3 | p < 0.001 | ||
| Dissolved inorganic phosphorus | Location | 120 | 1 | 0.802 |
| Period | 1 | 0.235 | ||
| Seasons | 3 | 0.149 | ||
| pCO2 concentration | Location | 120 | 1 | 0.209 |
| Period | 1 | <0.05 (0.007) | ||
| Seasons | 3 | p < 0.001 | ||
| CH4 concentration | Location | 120 | 1 | 0.980 |
| Period | 1 | 0.822 | ||
| Seasons | 3 | <0.05 (0.001) | ||
| N2O concentration | Location | 120 | 1 | <0.05 (0.001) |
| Period | 1 | 0.280 | ||
| Seasons | 3 | <0.05 (0.005) | ||
| CO2 flux | Location | 120 | 1 | 0.328 |
| Period | 1 | 0.445 | ||
| Seasons | 3 | <0.05 (0.003) | ||
| CH4 flux | Location | 120 | 1 | 0.643 |
| Period | 1 | 0.691 | ||
| Seasons | 3 | p < 0.001 | ||
| N2O flux | Location | 120 | 1 | <0.05 (0.001) |
| Period | 1 | 0.341 | ||
| Seasons | 3 | p < 0.001 |
| Environmental Variables | CO2 Flux | CH4 Flux | N2O Flux | |||
|---|---|---|---|---|---|---|
| Pearson Correlation | p-Value | Pearson Correlation | p-Value | Pearson Correlation | p-Value | |
| Greenhouse gas flux | ||||||
| CO2 flux | - | 0.528 * | <0.001 | 0.442 * | 0.003 | |
| CH4 flux | 0.528 * | <0.001 | - | 0.982 * | <0.001 | |
| N2O flux | 0.442 * | 0.003 | 0.982 * | <0.001 | - | |
| Water properties | ||||||
| Water temperature | −0.290 * | 0.002 | −0.663 * | <0.001 | −0.474 * | 0.001 |
| Salinity | 0.348 * | <0.001 | 0.768 * | <0.001 | 0.615 * | <0.001 |
| pH | −0.532 * | <0.001 | −0.364 * | 0.021 | NS | 0.345 |
| Dissolved oxygen | −0.592 * | <0.001 | −0.384 * | 0.005 | NS | 0.373 |
| Dissolved organic carbon | −0.250 * | 0.013 | −0.434 * | <0.001 | −0.362 * | 0.030 |
| Dissolved inorganic carbon | 0.366 * | 0.001 | NS | 0.053 | NS | 0.336 |
| Total alkalinity | NS | 0.792 | NS | 0.068 | NS | 0.946 |
| NH3-N concentration | NS | 0.469 | NS | 0.122 | NS | 0.470 |
| NO2-N concentration | ND | ND | ND | |||
| NO3-N concentration | NS | 0.459 | NS | 0.109 | NS | 0.258 |
| DIP concentration | NS | 0.964 | NS | 0.474 | NS | 0.531 |
| Chlorophyll a concentration | ND | ND | ND | |||
| Wind speed | 0.471 * | <0.001 | 0.976 * | <0.001 | 0.914 * | <0.001 |
| Culture Period | CO2 Flux (mmol m−2 day−1) | CH4 Flux (mmol m−2 day−1) | N2O Flux (mmol m−2 day−1) | Wind Speed (m s−1) |
|---|---|---|---|---|
| Spring | 2.95 ± 7.14 a | 2.72 ± 0.46 × 10−3 a | 2.24 ± 1.73 × 10−3 a | 1.30 ± 0.00 a |
| Summer | 5.19 ± 15.47 b | 3.43 ± 3.17 × 10−3 a | 2.91 ± 3.82 × 10−3 a | 1.45 ± 0.86 a |
| Autumn | −0.06 ± 0.06 a | 0.01 ± 0.00 × 10−3 c | 0.02 ± 0.00 × 10−3 b | 0.10 ± 0.00 c |
| Winter | −2.06 ± 1.04 c | 0.32 ± 0.08 × 10−3 b | 0.39 ± 0.22 × 10−3 b | 0.50 ± 0.00 b |
| Average | 2.19 ± 10.83 | 2.11 ± 2.81 × 10−3 | 1.65 ± 2.73 × 10−3 | 0.84 ± 0.64 |
| CO2 Flux | CH4 Flux | N2O Flux | |||
|---|---|---|---|---|---|
| CO2-eq (mg CO2-eq m−2 day−1) | GWP20 | Range | −0.32–0.96 | 0.05–42.90 × 10−3 | −12.54–59.36 × 10−3 |
| Average | 0.05 ± 0.25 | 10.50 ± 13.94 × 10−3 | 10.23 ± 16.96 × 10−3 | ||
| Total Average | 0.02 ± 0.09 | ||||
| GWP100 | Range | −0.32–0.96 | 0.02–14.53 × 10−3 | −12.54–59.36 × 10−3 | |
| Average | 0.05 ± 0.25 | 3.56 ± 4.72 × 10−3 | 10.23 ± 16.96 × 10−3 | ||
| Total Average | 0.02 ± 0.09 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Chou, W.-C.; Tseng, H.-C.; Shiu, R.-F.; Lee, M.-C.; Nan, F.-H.; Yeh, H.-Y. Biogenic CO2, CH4, and N2O Emissions from Abalone Culture in Tidal Ponds. Environments 2025, 12, 313. https://doi.org/10.3390/environments12090313
Chen Y-J, Chou W-C, Tseng H-C, Shiu R-F, Lee M-C, Nan F-H, Yeh H-Y. Biogenic CO2, CH4, and N2O Emissions from Abalone Culture in Tidal Ponds. Environments. 2025; 12(9):313. https://doi.org/10.3390/environments12090313
Chicago/Turabian StyleChen, Yi-Jung, Wen-Chen Chou, Hsiao-Chun Tseng, Ruei-Feng Shiu, Meng-Chou Lee, Fan-Hua Nan, and Han-Yang Yeh. 2025. "Biogenic CO2, CH4, and N2O Emissions from Abalone Culture in Tidal Ponds" Environments 12, no. 9: 313. https://doi.org/10.3390/environments12090313
APA StyleChen, Y.-J., Chou, W.-C., Tseng, H.-C., Shiu, R.-F., Lee, M.-C., Nan, F.-H., & Yeh, H.-Y. (2025). Biogenic CO2, CH4, and N2O Emissions from Abalone Culture in Tidal Ponds. Environments, 12(9), 313. https://doi.org/10.3390/environments12090313








