Niobium-Based Catalysts in Advanced Oxidation Processes: A Systematic Review of Mechanisms, Material Engineering, and Environmental Applications
Abstract
1. Introduction
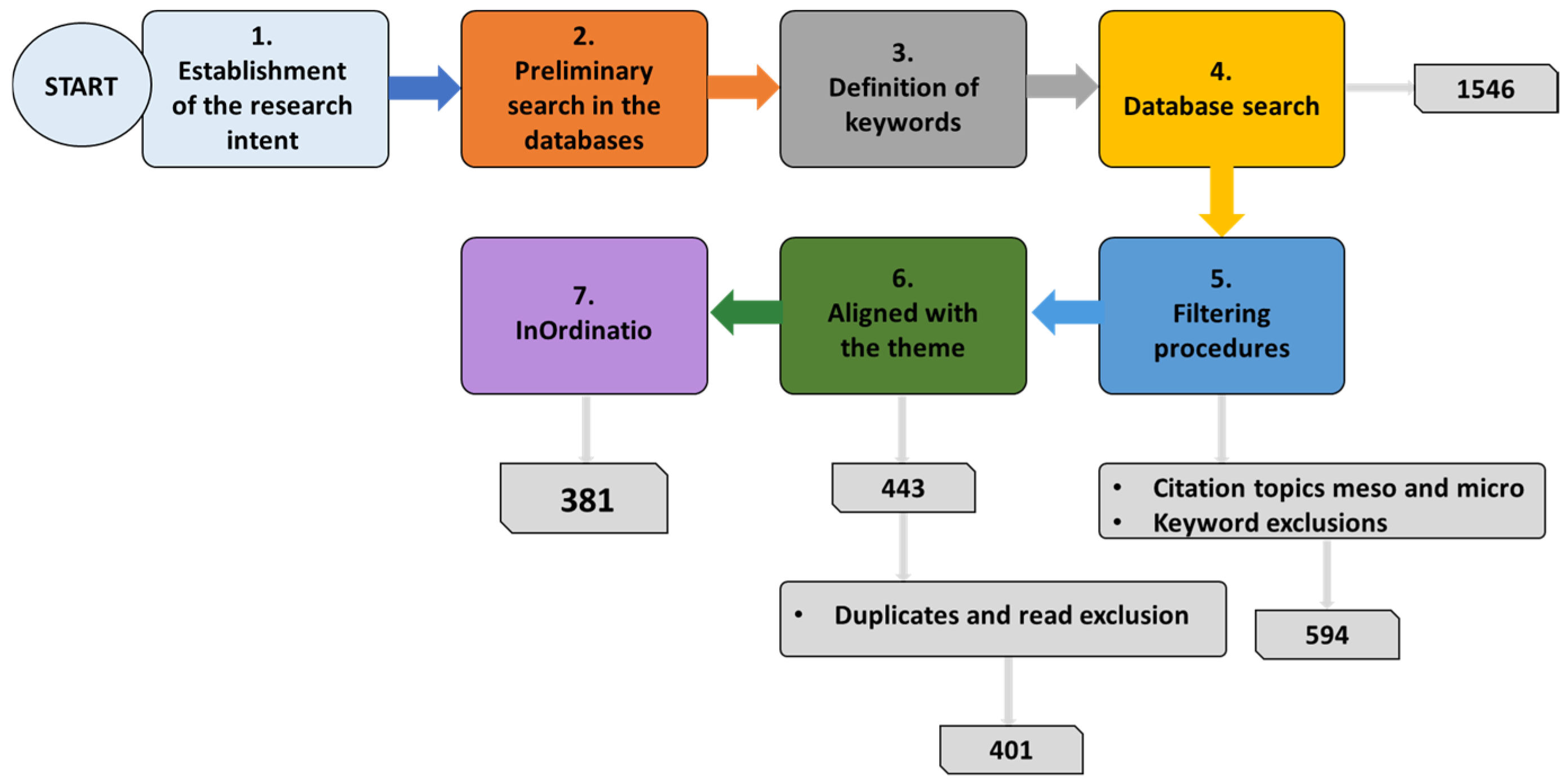
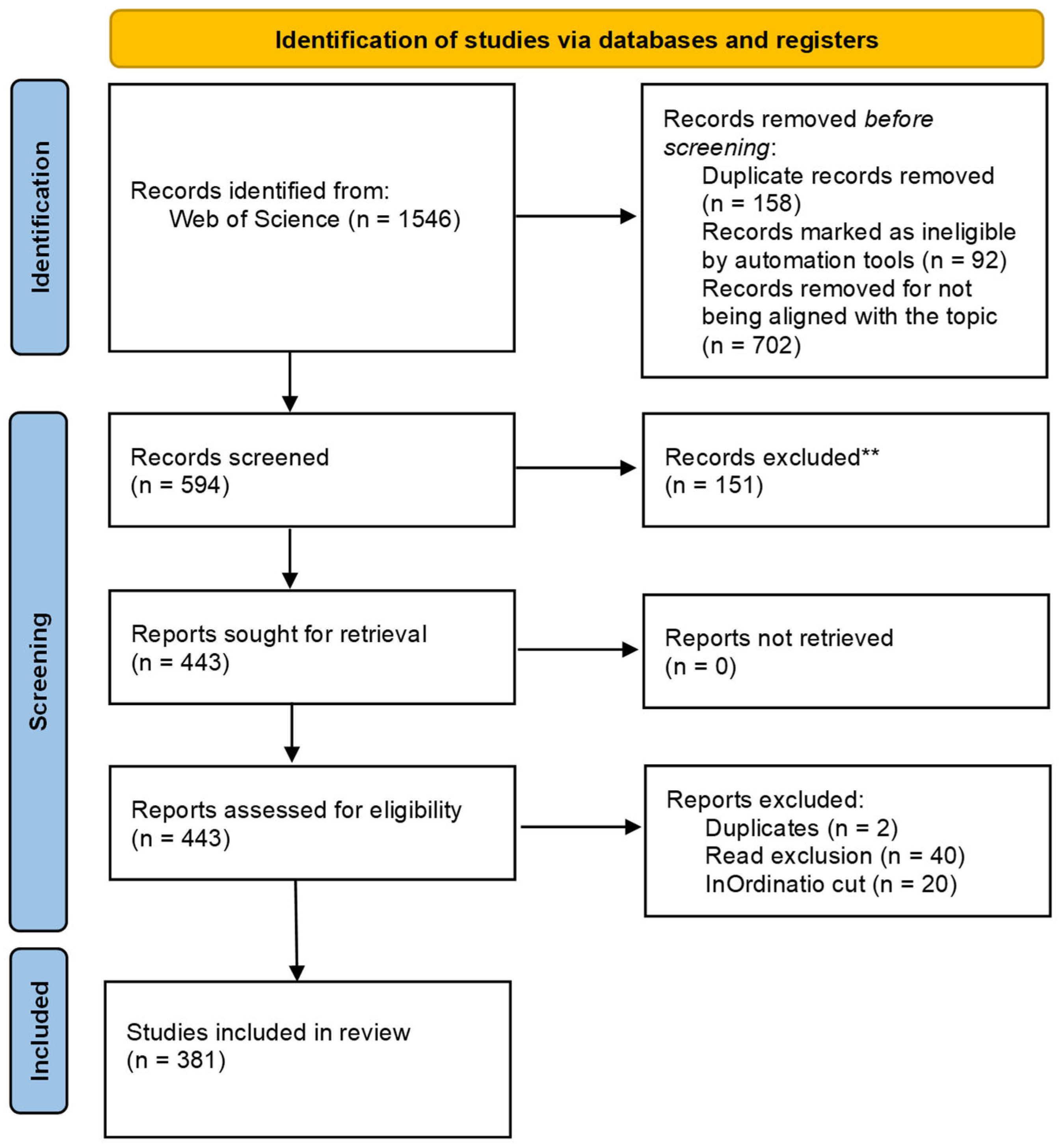
2. Scientometrics
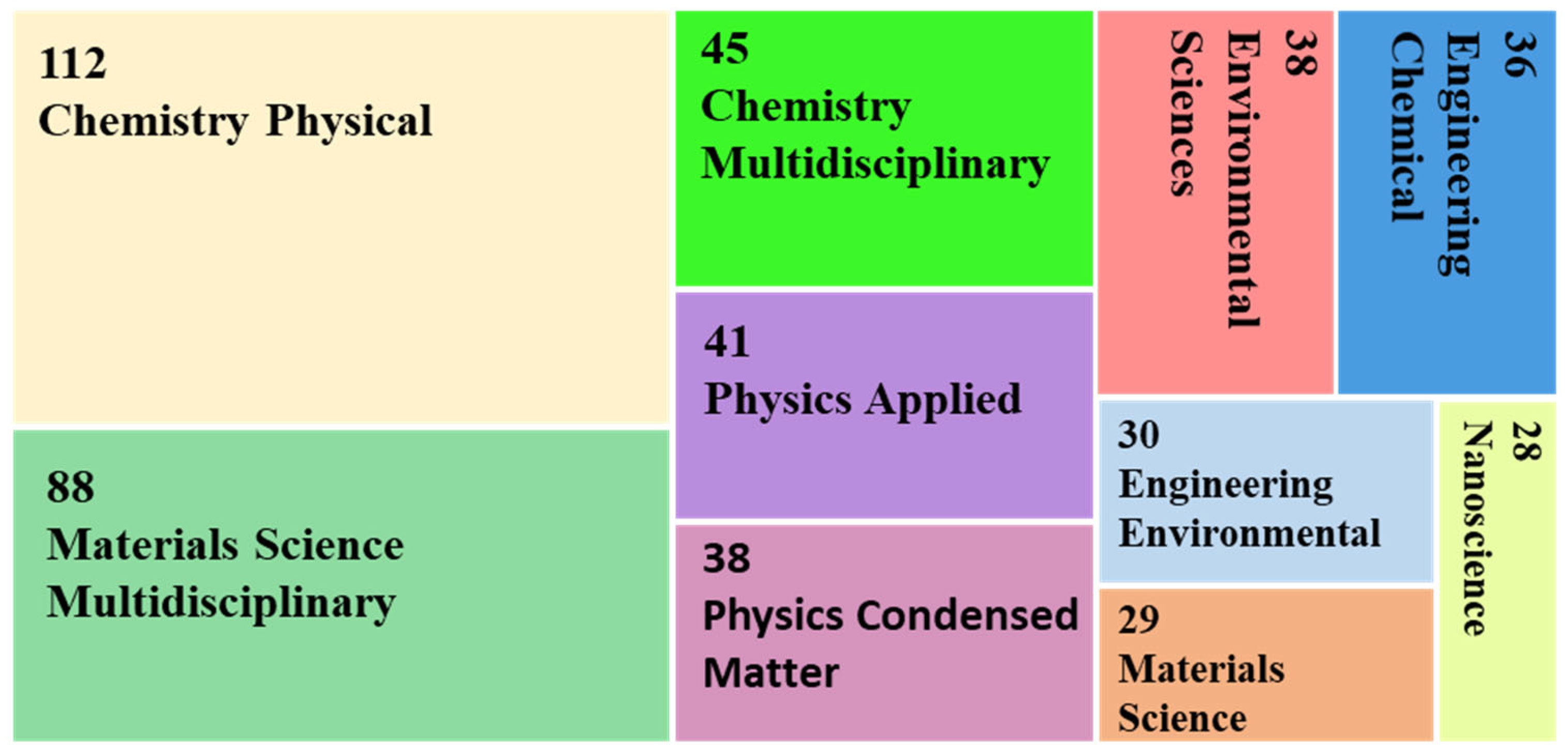
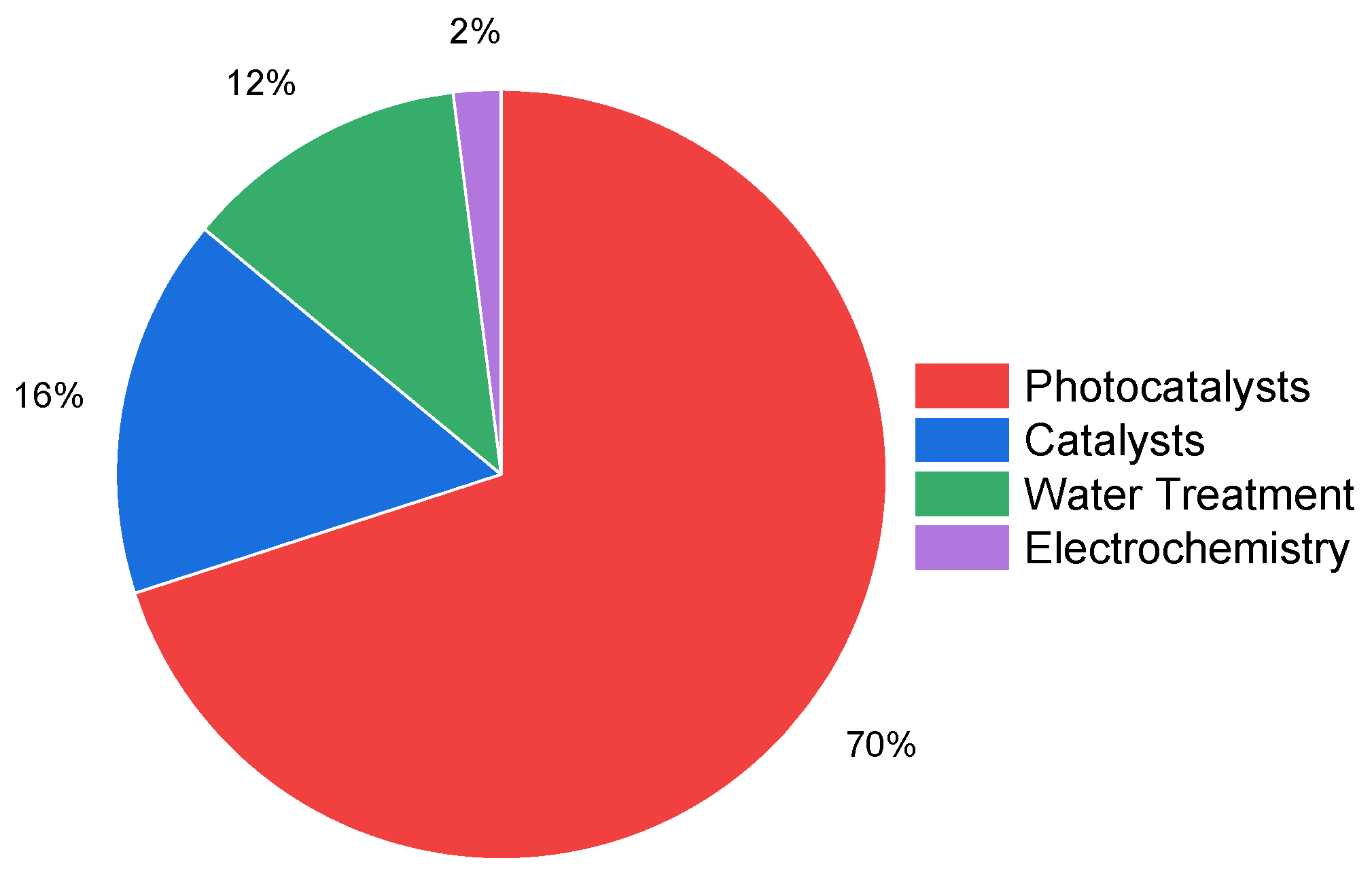
“((niobi* OR nb2o5) AND (removal OR degradation OR AOP) NOT (nitrobenzene))—Articles”
“((niobi* OR nb2o5) AND (removal OR degradation OR AOP) NOT (nitrobenzene))—Articles—Citation topics Meso 2.74 Photocatalysts 2.90 Water Treatment 2.62 Electrochemistry 2.41 Catalysts—Citation topics micro EXLCUDE Oxigen reduction reaction Supercapacitor Electrochromism Litium-ion Battery Litium-sulfur Batteries Adsorption”
- IF is the impact factor (or estimated CiteScore if IF is unavailable);
- Δ is the weight (0–10) assigned to the importance of IF;
- λ is the weight (0–10) assigned to the relevance of publication year;
- Ω is the weight (0–10) assigned to the annual average citation count;
- ResearchYear is the year of analysis;
- PubYear is the year of publication;
- HalfLife is the journal’s cited half-life;
- Cᵢ is the total number of citations retrieved from Google Scholar.
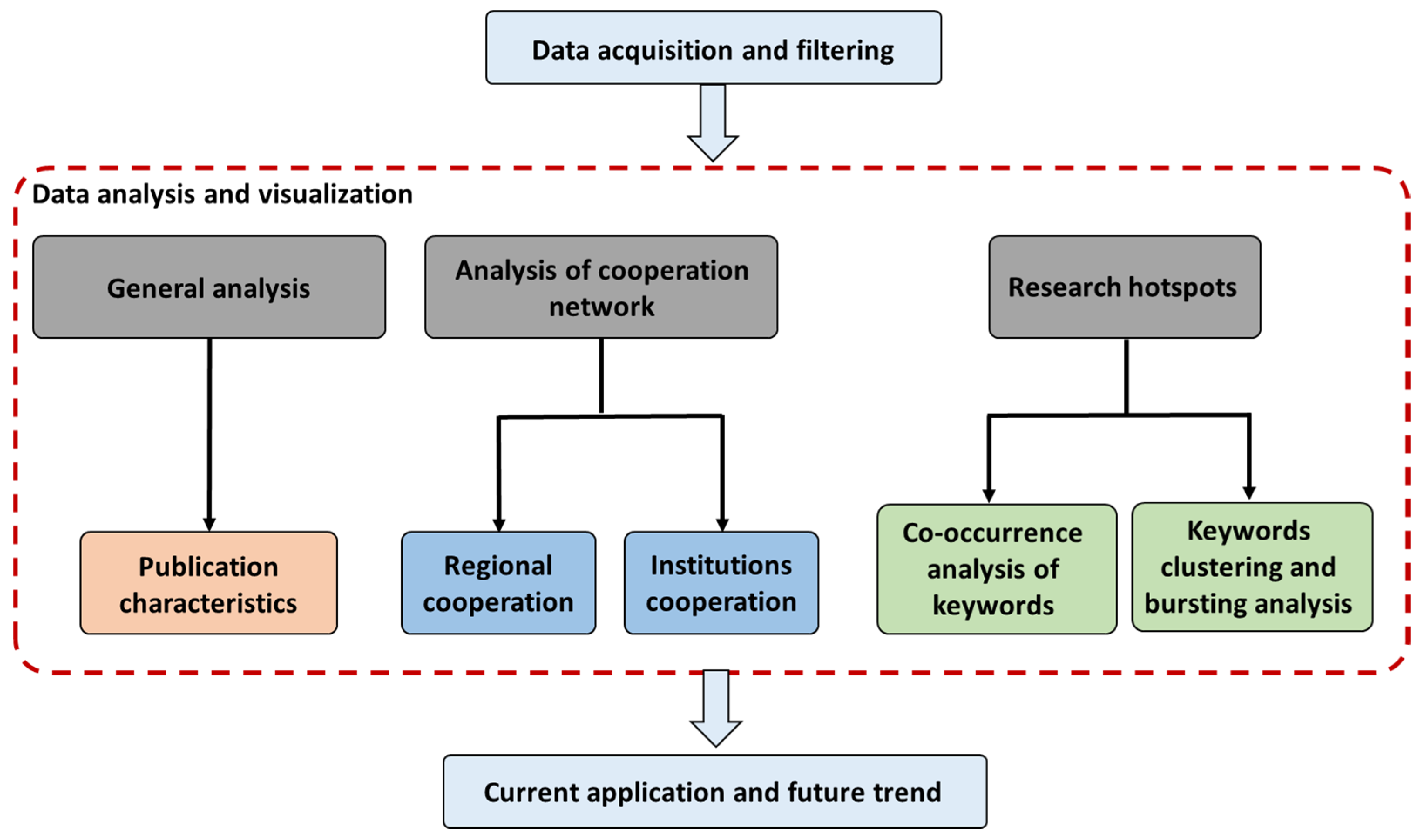
2.1. Statistical Analysis and Visualization
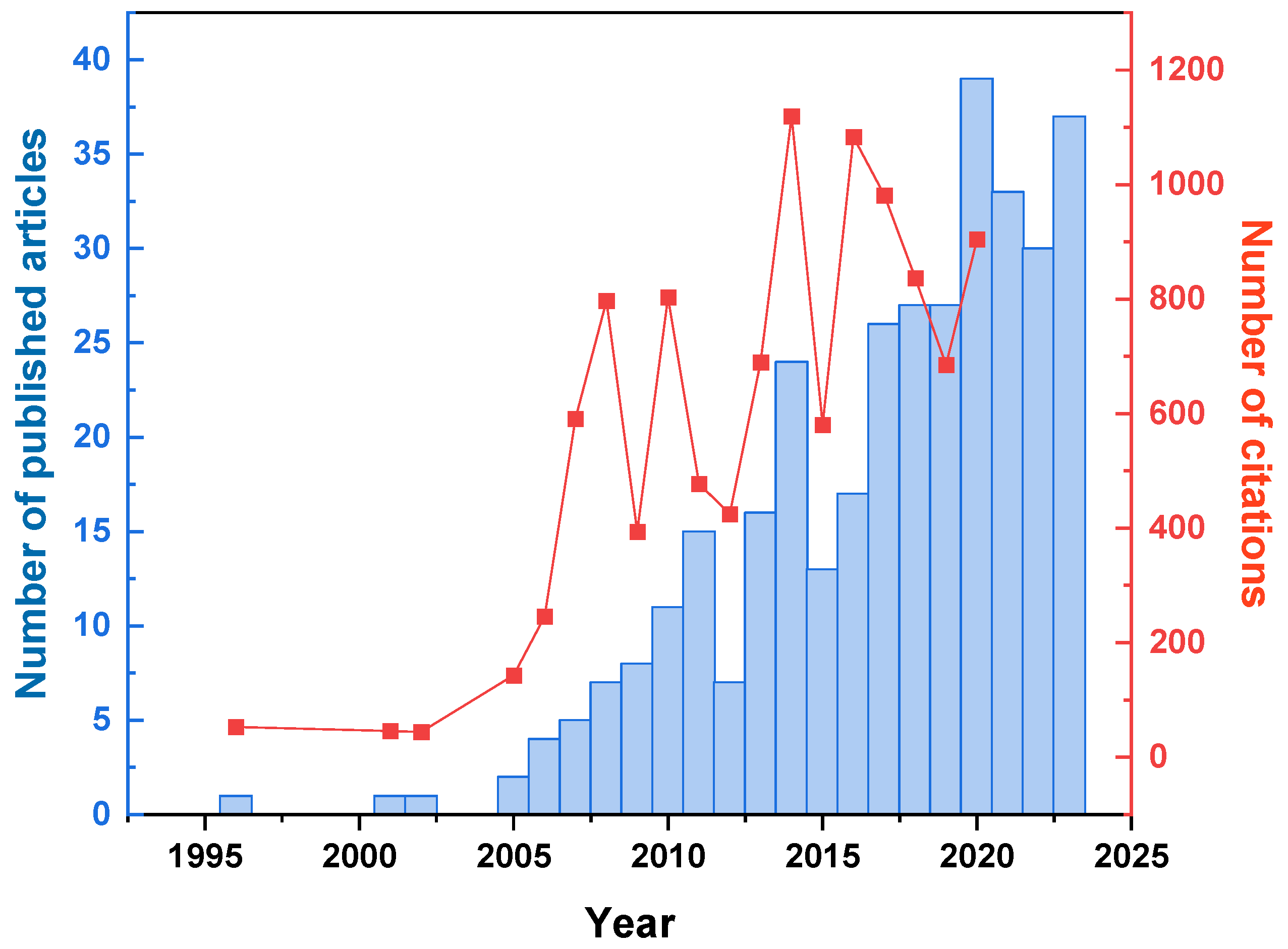
2.1.1. Publication Characteristics
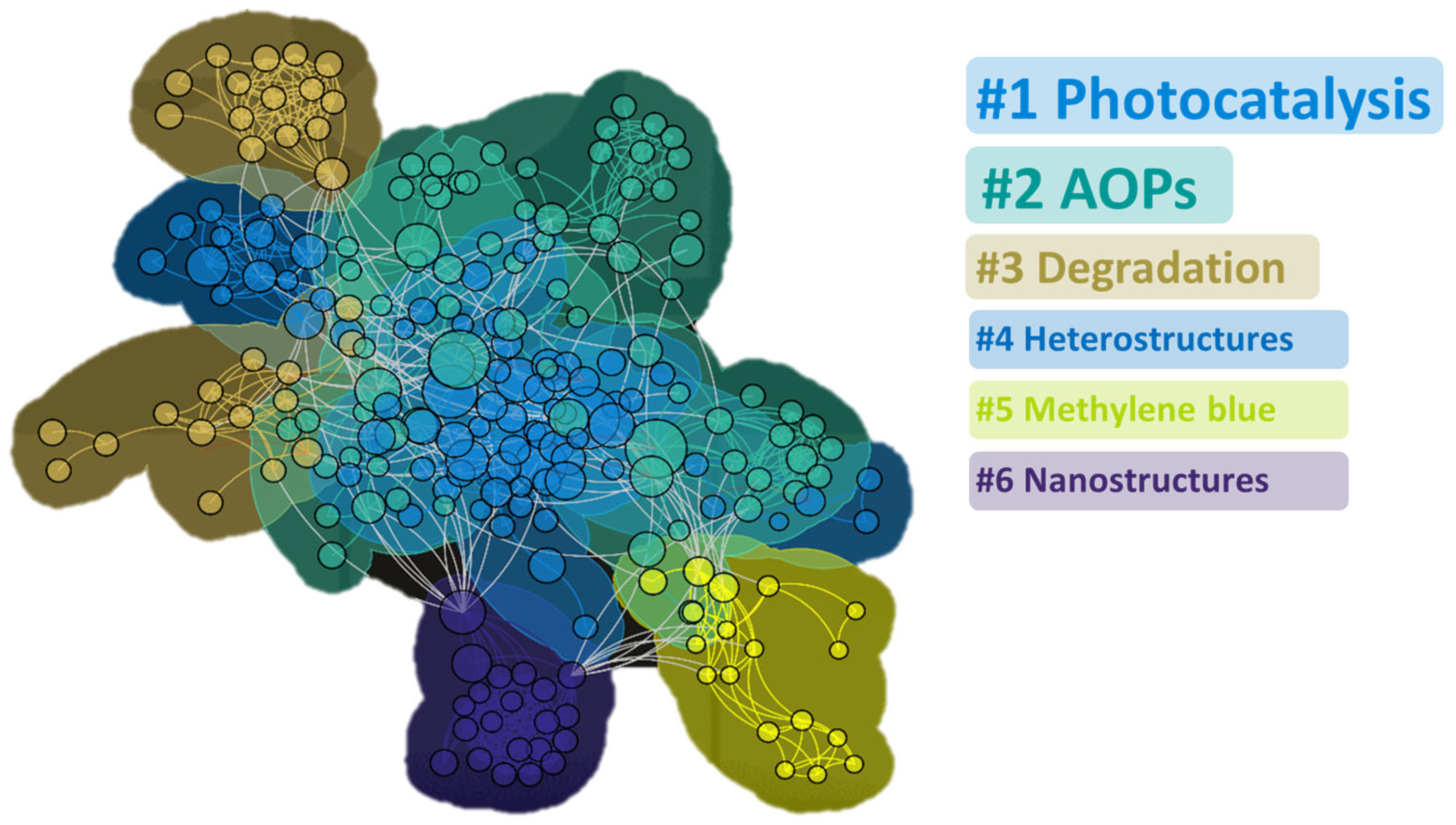
2.1.2. Analysis of Cooperation Network
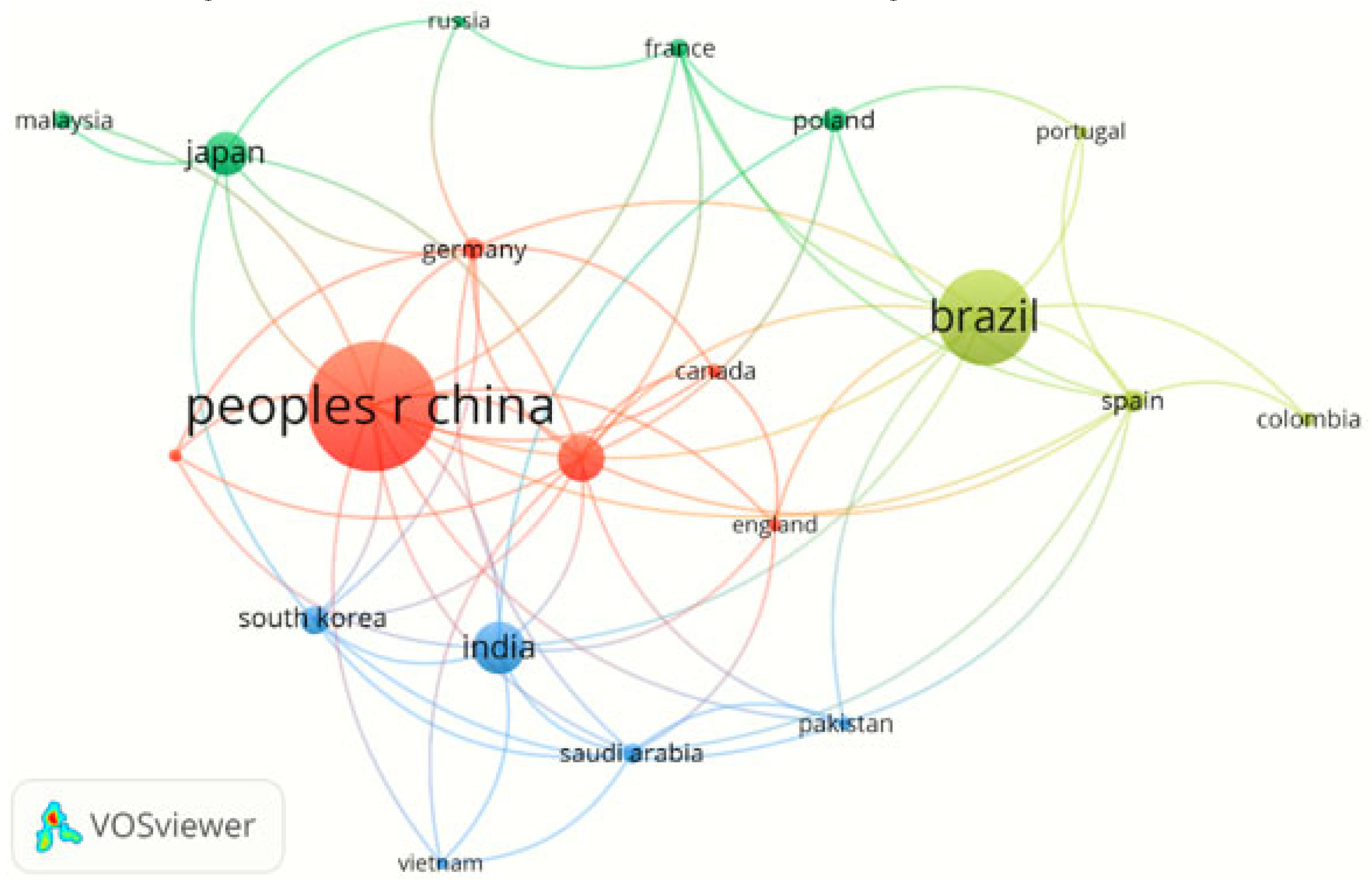
Research Contributions from Different Countries and Regions
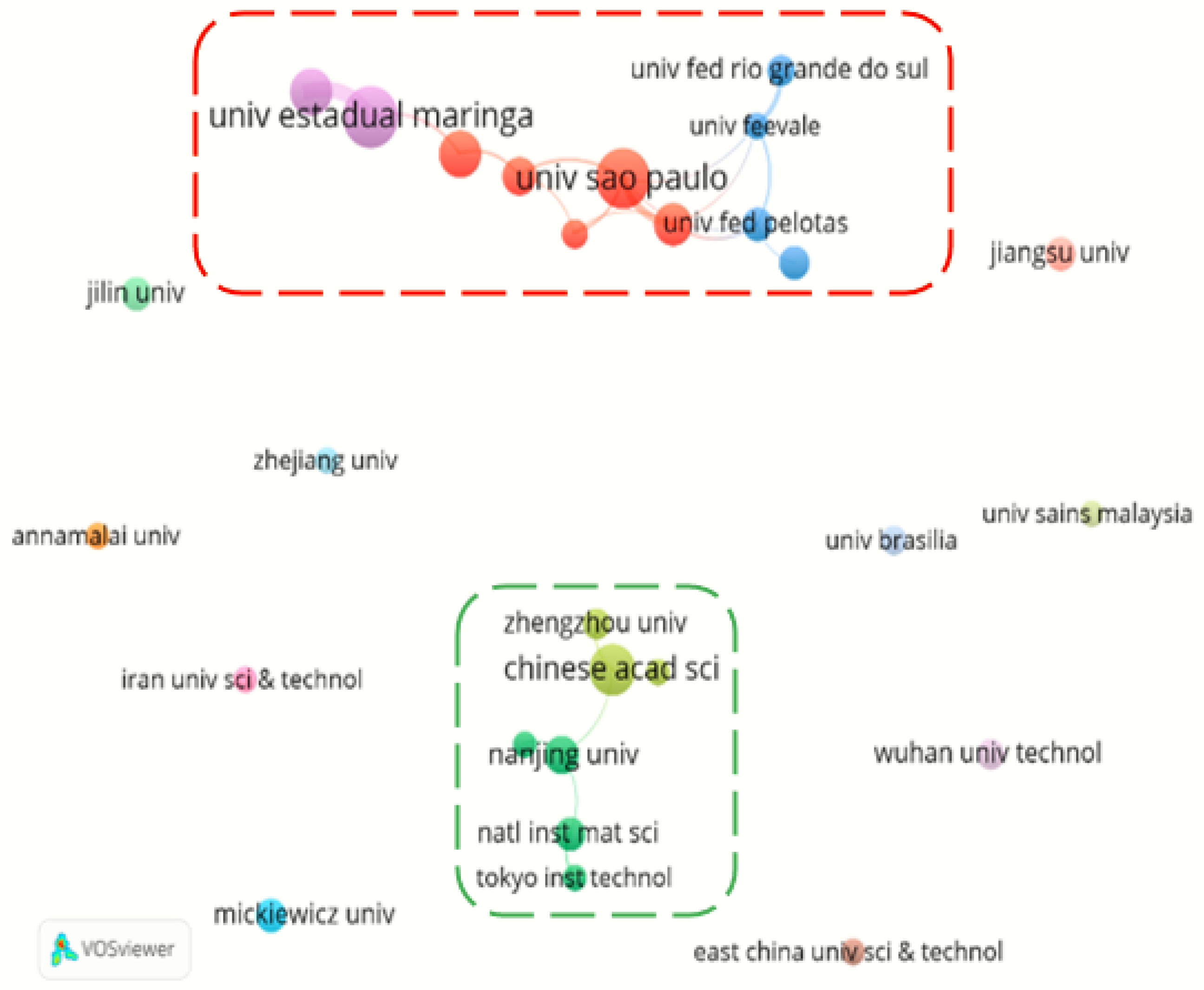
Institution Cooperation
Co-Occurrence Analysis of Keywords and Research Hotspots
3. Nb2O5: An Overview
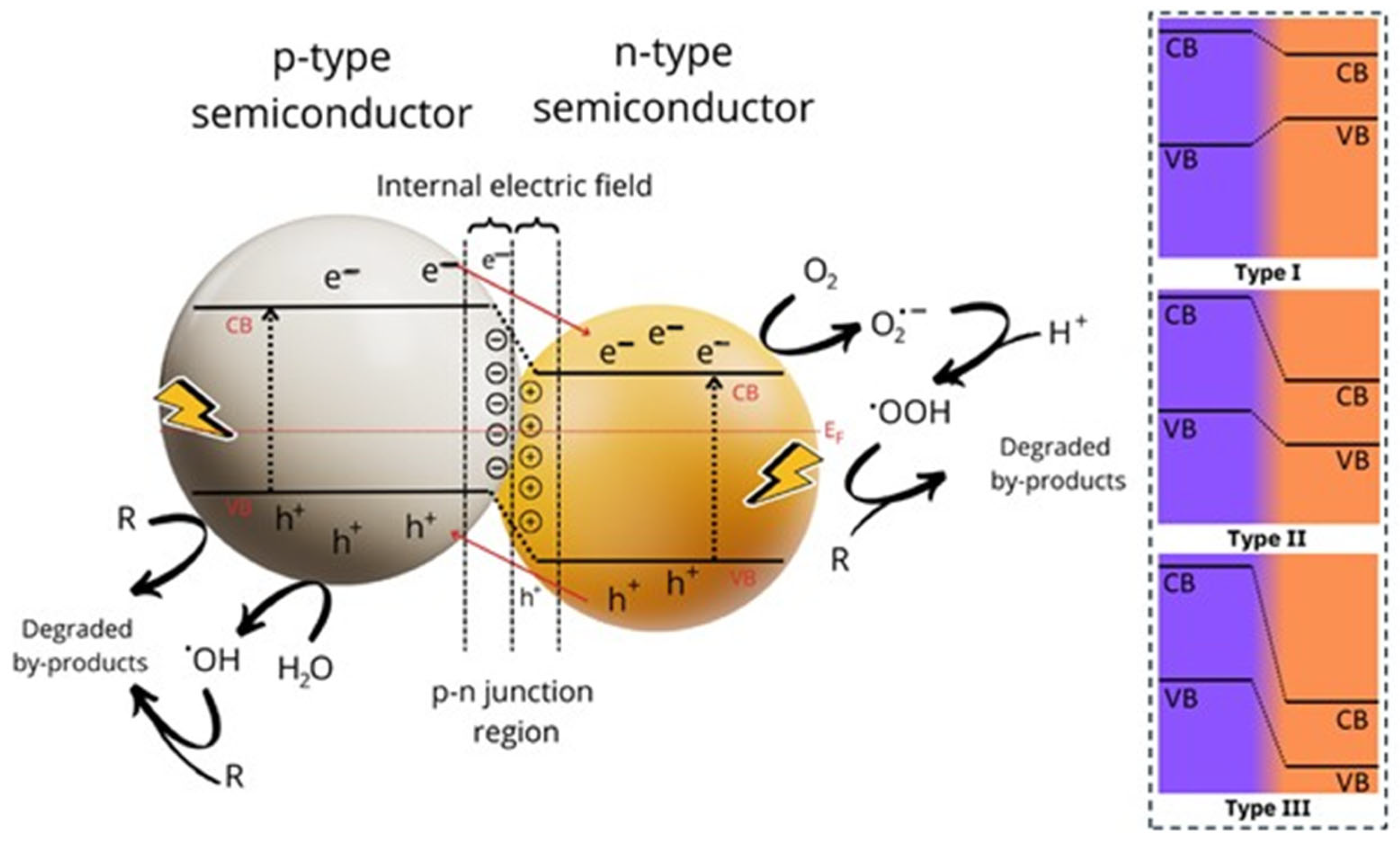
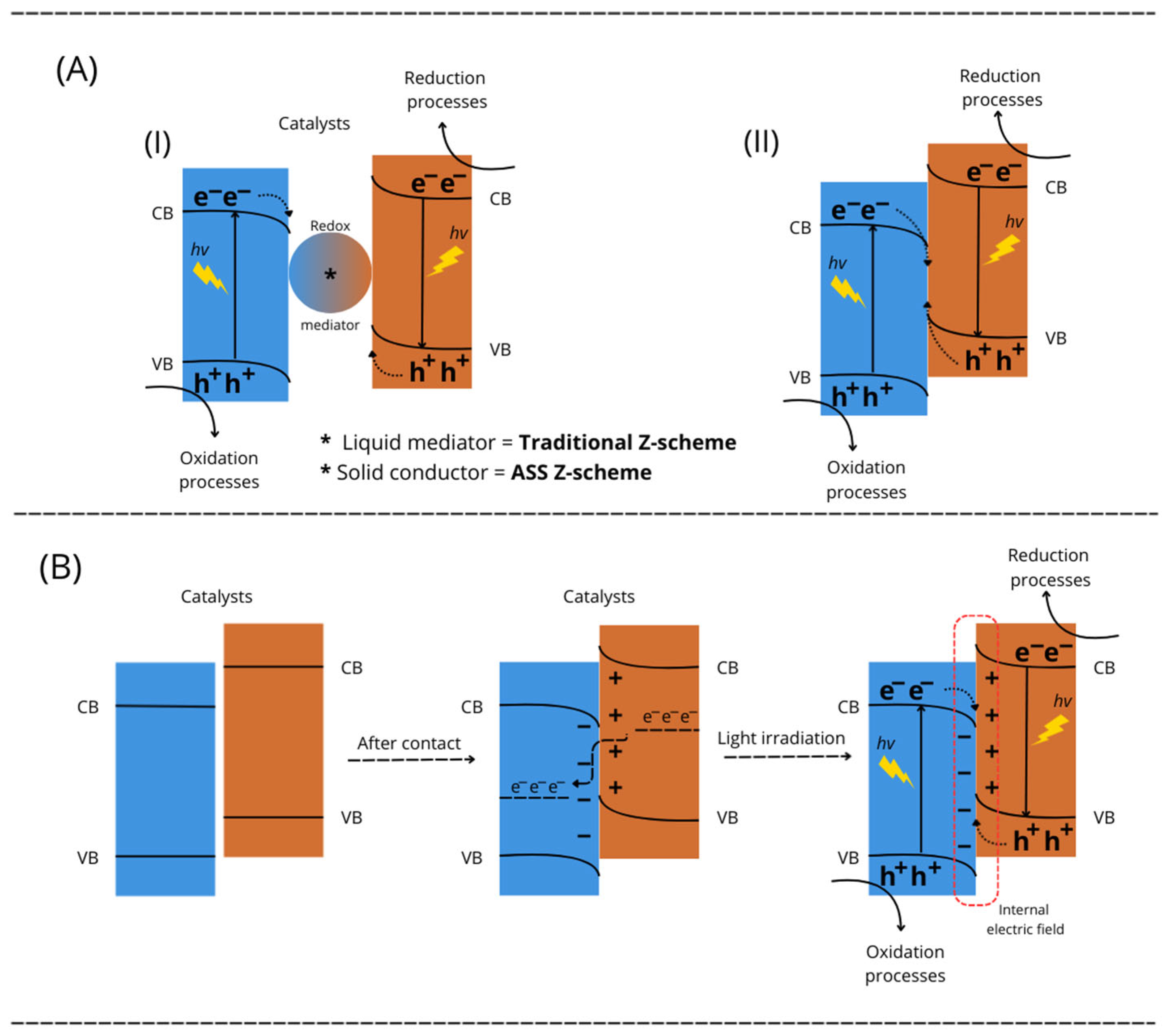
4. Mechanisms of Action of Nb2O5
5. Common Synthesis Methods of Nb2O5-Based Materials
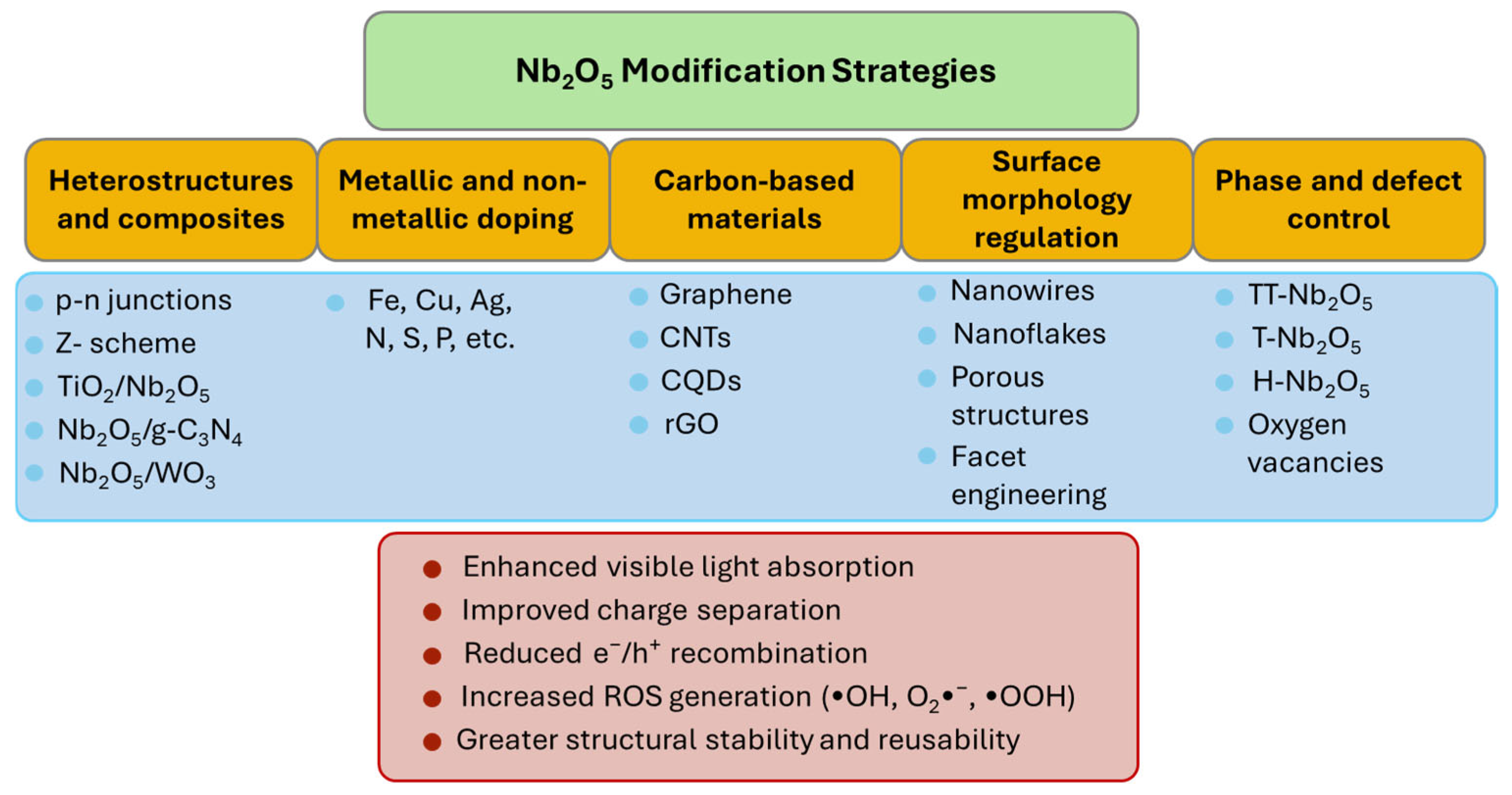
6. Modification Strategies and Research Progress Advancement on Nb for Pollutant Degradation
6.1. Heterostructures and Composites
Carbonaceous Modifications
6.2. Surface Morphology Regulation
7. Contaminant Removal
7.1. Dyes
7.2. Pharmaceuticals
7.3. Heavy Metals
7.4. Personal Care Products
7.5. Pesticides
7.6. Hormones
8. Environmental Implications
9. Current Limitations and Future Directions
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amebelu, A.; Ban, R.; Bhagwan, J.; Brown, J.; Chilengi, R.; Chandler, C.; Colford, J.M.; Cumming, O.; Curtis, V.; Evans, B.E.; et al. The Lancet Commission on Water, Sanitation and Hygiene, and Health. Lancet 2021, 398, 1469–1470. [Google Scholar] [CrossRef]
- Wolf, J.; Hubbard, S.; Brauer, M.; Ambelu, A.; Arnold, B.F.; Bain, R.; Bauza, V.; Brown, J.; Caruso, B.A.; Clasen, T.; et al. Effectiveness of Interventions to Improve Drinking Water, Sanitation, and Handwashing with Soap on Risk of Diarrhoeal Disease in Children in Low-Income and Middle-Income Settings: A Systematic Review and Meta-Analysis. Lancet 2022, 400, 48–59. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Gao, L.; Wang, T.; Liu, B.; Fang, D.; Gao, Y. Progress towards the Sustainable Development Goals Has Been Slowed by Indirect Effects of the COVID-19 Pandemic. Commun. Earth Environ. 2023, 4, 184. [Google Scholar] [CrossRef]
- Licci, S.; Marmonier, P.; Wharton, G.; Delolme, C.; Mermillod-Blondin, F.; Simon, L.; Vallier, F.; Bouma, T.J.; Puijalon, S. Scale-Dependent Effects of Vegetation on Flow Velocity and Biogeochemical Conditions in Aquatic Systems. Sci. Total Environ. 2022, 833, 155123. [Google Scholar] [CrossRef]
- Shevgan, M. Advanced Oxidation Technologies Market Analysis & Forecast: 2025–2032; Coherent Market Insights: Burlingame, CA, USA, 2025. [Google Scholar]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment–A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Ricardo, I.A.; Leitão, J.M.M.; Esteves da Silva, J.C.G. A Review on Advanced Oxidation Processes: From Classical to New Perspectives Coupled to Two- and Multi-Way Calibration Strategies to Monitor Degradation of Contaminants in Environmental Samples. Trends Environ. Anal. Chem. 2019, 24, e00072. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhou, C.Y.; Zhou, P.; Liu, Y.; Zhang, H.; Du, Y.; He, C.S.; Xiong, Z.K.; Lai, B. Exceptionally Accelerated Fe(III)/Fe(II) Redox Couple by Niobium Carbide MXene: A Green and Long-Lasting Enhanced Fenton Oxidation. Appl. Catal. B Environ. 2024, 342, 123385. [Google Scholar] [CrossRef]
- Roccamante, M.; Salmerón, I.; Ruiz, A.; Oller, I.; Malato, S. New Approaches to Solar Advanced Oxidation Processes for Elimination of Priority Substances Based on Electrooxidation and Ozonation at Pilot Plant Scale. Catal. Today 2020, 355, 844–850. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Nacheva, P.M. Effect of Electrolytes on the Simultaneous Electrochemical Oxidation of Sulfamethoxazole, Propranolol and Carbamazepine: Behaviors, by-Products and Acute Toxicity. Environ. Sci. Pollut. Res. 2019, 26, 6855–6867. [Google Scholar] [CrossRef]
- Abreu, E.; Fidelis, M.Z.; Fuziki, M.E.; Malikoski, R.M.; Mastsubara, M.C.; Imada, R.E.; Diaz de Tuesta, J.L.; Gomes, H.T.; Anziliero, M.D.; Baldykowski, B.; et al. Degradation of Emerging Contaminants: Effect of Thermal Treatment on Nb2o5 as Photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 419, 113484. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and Prospects of Advanced Oxidation Water Treatment Processes Using Catalytic Nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Mahdi, M.M.; Salim, E.T.; Obaid, A.S. A Comparison Study of Au@Nb2O5 Core–Shell Nanoparticle Using Two Different Laser Flounces. Plasmonics 2025, 20, 6313–6326. [Google Scholar] [CrossRef]
- Alias, N.; Hussain, Z.; Tan, W.K.; Kawamura, G.; Muto, H.; Matsuda, A.; Lockman, Z. Nanoporous Anodic Nb2O5 with Pore-in-Pore Structure Formation and Its Application for the Photoreduction of Cr(VI). Chemosphere 2021, 283, 131231. [Google Scholar] [CrossRef] [PubMed]
- Pagani, R.N.; Pedroso, B.; dos Santos, C.B.; Picinin, C.T.; Kovaleski, J.L. Methodi Ordinatio 2.0: Revisited under Statistical Estimation, and Presenting FInder and RankIn. Qual. Quant. 2023, 57, 4563–4602. [Google Scholar] [CrossRef]
- Sundarapandi Edward, I.E.; Ponpandi, R. Challenges, Strategies and Opportunities for Wind Farm Incorporated Power Systems: A Review with Bibliographic Coupling Analysis. Environ. Sci. Pollut. Res. 2022, 30, 11332–11356. [Google Scholar] [CrossRef] [PubMed]
- Zanata, A.C.; Bertan, F.A.B.; Bernardi, C.; dos Santos, C.S.; Ferreira, G.A.; De Oliveira, J.A.; Fernandes, M.D.C.C.; Anschau, A. Obtention of Microalgal Biomass Tolerant to Herbicides for Production of Bio-Fertilizers: A Review Based on Methodi Ordinatio Methodology. Orbital Electron. J. Chem. 2019, 11, 399–401. [Google Scholar] [CrossRef]
- da Silva, V.L.; Kovaleski, J.L.; Pagani, R.N.; Gomes, M.A.S. Industry 4.0 Implementations: A Systematic Review of Approaches and Main Applicabilities in the Broiler Meat Production Chain. Worlds Poult. Sci. J. 2023, 79, 563–579. [Google Scholar] [CrossRef]
- Batista, D.M.; Goulart, E.V.; de Oliveira, A.R.P.; Santos, P.F.; Valois, R.C.; Ferreira, M.G.S. ASSISTIVE AND EDUCATIONAL TECHNOLOGIES FOR CHILDREN WITH AUTISM SPECTRUM DISORDER: A BIBLIOMETRIC STUDY. Cogitare Enferm. 2024, 29, e95019. [Google Scholar] [CrossRef]
- Da Silva, V.L.; Kovaleski, J.L.; Pagani, R.N.; Gomes, M.A.S. Technology Transfer Model Oriented to Industry 4.0. Braz. J. Oper. Prod. Manag. 2024, 21, 1777. [Google Scholar] [CrossRef]
- Hernández-Contreras, M.; Cruz, J.C.; Gurrola, M.P.; Pamplona Solis, B.; Vega-Azamar, R.E. Application of Nanosilica in the Construction Industry: A Bibliometric Analysis Using Methodi Ordinatio. MethodsX 2024, 12, 102642. [Google Scholar] [CrossRef]
- Yoshino, R.T.; Pinto, M.M.A.; Pontes, J.; Treinta, F.T.; Justo, J.F.; Santos, M.M.D. Educational Test Bed 4.0: A Teaching Tool for Industry 4.0. Eur. J. Eng. Educ. 2020, 45, 1002–1023. [Google Scholar] [CrossRef]
- Zarhri, Z.; Rosado Martinez, W.; Dominguez Lepe, J.A.; Vega Azamar, R.E.; Chan Juarez, M.; Pamplona Solis, B.B. 30 Years of Rubberized Concrete Investigations (1990–2020). A Bibliometric Analysis. Rev. ALCONPAT 2022, 12, 1. [Google Scholar] [CrossRef]
- de Carvalho, G.D.G.; Sokulski, C.C.; da Silva, W.V.; de Carvalho, H.G.; de Moura, R.V.; de Francisco, A.C.; da Veiga, C.P. Bibliometrics and Systematic Reviews: A Comparison between the Proknow-C and the Methodi Ordinatio. J. Informetr. 2020, 14, 101043. [Google Scholar] [CrossRef]
- Barros, M.V.; Ferreira, M.B.; do Prado, G.F.; Piekarski, C.M.; Picinin, C.T. The Interaction between Knowledge Management and Technology Transfer: A Current Literature Review between 2013 and 2018. J. Technol. Transf. 2020, 45, 1585–1606. [Google Scholar] [CrossRef]
- Sierdovski, M.; Pilatti, L.A.; Rubbo, P. Organizational Competencies in the Development of Environmental, Social, and Governance (ESG) Criteria in the Industrial Sector. Sustainability 2022, 14, 13463. [Google Scholar] [CrossRef]
- Oliveira, J.R.P.; Ribas, L.S.; Napoli, J.S.; Abreu, E.; Diaz de Tuesta, J.L.; Gomes, H.T.; Tusset, A.M.; Lenzi, G.G. Green Magnetic Nanoparticles CoFe2O4@Nb5O2 Applied in Paracetamol Removal. Magnetochemistry 2023, 9, 200. [Google Scholar] [CrossRef]
- Regatieri, H.R.; Ando Junior, O.H.; Salgado, J.R.C. Systematic Review of Lithium-Ion Battery Recycling Literature Using ProKnow-C and Methodi Ordinatio. Energies 2022, 15, 1485. [Google Scholar] [CrossRef]
- Sarmento, A.L.C.; Sá, B.S.; Vasconcelos, A.G.; Arcanjo, D.D.R.; Durazzo, A.; Lucarini, M.; de Souza de Almeida Leite, J.R.; Sousa, H.A.; Kückelhaus, S.A.S. Perspectives on the Therapeutic Effects of Pelvic Floor Electrical Stimulation: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14035. [Google Scholar] [CrossRef]
- Lizot, M.; Júnior, P.P.A.; Trojan, F.; Magacho, C.S.; Thesari, S.S.; Goffi, A.S. Analysis of Evaluation Methods of Sustainable Supply Chain Management in Production Engineering Journals with High Impact. Sustainability 2019, 12, 270. [Google Scholar] [CrossRef]
- De Bail, R.F.; Kovaleski, J.L.; da Silva, V.L.; Pagani, R.N.; de Chiroli, D.M.G. Internet of Things in Disaster Management: Technologies and Uses. Environ. Hazards 2021, 20, 493–513. [Google Scholar] [CrossRef]
- Rosa, F.M.; Mota, T.F.M.; Busso, C.; de Arruda, P.V.; Brito, P.E.M.; Miranda, J.P.M.; Trentin, A.B.; Dekker, R.F.H.; Cunha, M.A.A. da Filamentous Fungi as Bioremediation Agents of Industrial Effluents: A Systematic Review. Fermentation 2024, 10, 143. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Xie, Y.; Han, X.; Zhang, S.; Zhu, Z.; Park, S.; Han, J. The Application of MnOx/TiO2 Catalyst in SCR-NH3 Reaction. Preprint 2023. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Khalid, M.U.; Rudokaite, A.; da Silva, A.M.H.; Kirsnyte-Snioke, M.; Stirke, A.; Melo, W.C.M.A. A Comprehensive Review of Niobium Nanoparticles: Synthesis, Characterization, Applications in Health Sciences, and Future Challenges. Nanomaterials 2025, 15, 106. [Google Scholar] [CrossRef]
- Lopes, O.F.; de Mendonça, V.R.; Silva, F.B.F.; Paris, E.C.; Ribeiro, C. NIOBIUM OXIDES: AN OVERVIEW OF THE SYNTHESIS OF Nb2O5AND ITS APPLICATION IN HETEROGENEOUS PHOTOCATALYSIS. Quim. Nova 2014, 38. [Google Scholar] [CrossRef]
- Ahmad, I.; Al-Qattan, A.; Iqbal, M.Z.; Anas, A.; Khasawneh, M.A.; Obaidullah, A.J.; Mahal, A.; Duan, M.; Al Zoubi, W.; Ghadi, Y.Y.; et al. A Systematic Review on Nb2O5-Based Photocatalysts: Crystallography, Synthetic Methods, Design Strategies, and Photocatalytic Mechanisms. Adv. Colloid Interface Sci. 2024, 324, 103093. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, S.; Jervis, R.; Shearing, P. Probing the Electrochemical Processes of Niobium Pentoxides (Nb2O5) for High-Rate Lithium-ion Batteries: A Review. ChemElectroChem 2024, 11, e202300581. [Google Scholar] [CrossRef]
- Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R. Synthesis and Structural Properties of Niobium Pentoxide Powders: A Comparative Study of the Growth Process. Mater. Res. 2016, 19, 1381–1387. [Google Scholar] [CrossRef]
- Su, K.; Liu, H.; Gao, Z.; Fornasiero, P.; Wang, F. Nb2O5-Based Photocatalysts. Adv. Sci. 2021, 8, 2003156. [Google Scholar] [CrossRef]
- Moin, M.; Moin, M.; Zhao, H.; Ahsan, Z.; Dong, L.; Penki, T.R.; Harika, V.K.; Thumu, U. Unveiling the Potential of Hetero-Atom Substitution in Niobium Oxide Hydride Materials: A Computational Insights to next-Generation Li-Ion Batteries. J. Energy Storage 2025, 131, 115883. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Beknalkar, S.A.; Chavan, G.T.; Ahir, N.A.; Dalavi, D.S.; Jeon, C.-W. Versatile Electrochromic Energy Storage Smart Window Utilizing Surfactant-Assisted Niobium Oxide Thin Films. Chem. Eng. J. 2024, 484, 149556. [Google Scholar] [CrossRef]
- Pehlivan, E.; Tepehan, F.Z.; Tepehan, G.G. Electrochromic Properties of Pure and Doped Nb2O5 Thin Films. Key Eng. Mater. 2004, 264–268, 375–378. [Google Scholar] [CrossRef]
- Sena, M.P.; de Lima, S.P.; Carvalho, L.S.; Ruiz, D.; Ballarini, A.; Martins, A.R. Synthesis, Characterization and Catalytic Evaluation of Cobalt and Niobium Oxide Solids Modified with Alkaline Earth Metals. Matéria 2020, 25. [Google Scholar] [CrossRef]
- Bledowski, M.; Wang, L.; Ramakrishnan, A.; Khavryuchenko, O.V.; Khavryuchenko, V.D.; Ricci, P.C.; Strunk, J.; Cremer, T.; Kolbeck, C.; Beranek, R. Visible-Light Photocurrent Response of TiO2–Polyheptazine Hybrids: Evidence for Interfacial Charge-Transfer Absorption. Phys. Chem. Chem. Phys. 2011, 13, 21511. [Google Scholar] [CrossRef]
- Genco, A.; García-López, E.I.; Megna, B.; Ania, C.; Marcì, G. Nb2O5 Based Photocatalysts for Efficient Generation of H2 by Photoreforming of Aqueous Solutions of Ethanol. Catal. Today 2025, 447, 115147. [Google Scholar] [CrossRef]
- Pang, R.; Wang, Z.; Li, J.; Chen, K. Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications. Materials 2023, 16, 6956. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present Applications of Titanium Dioxide for the Photocatalytic Removal of Pollutants from Water: A Review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Lopes, O.F.; De Mendonça, V.R.; Silva, F.B.F.; Paris, E.C.; Ribeiro, C. Óxidos de Nióbio: Uma Visão Sobre a Síntese Do Nb2O5e Sua Aplicação Em Fotocatálise Heterogênea. Quim. Nova 2015, 38, 106–117. [Google Scholar]
- Fidelis, M.Z.; Abreu, E.; Josué, T.G.; de Almeida, L.N.B.; Lenzi, G.G.; Dos Santos, O.A.A. Continuous Process Applied to Degradation of Triclosan and 2.8-Dichlorodibenzene-p-Dioxin. Environ. Sci. Pollut. Res. 2021, 28, 23675–23683. [Google Scholar] [CrossRef] [PubMed]
- Aimaiti, G.; Ma, Y.H.; Shi, Y.J.; Wang, X.; Wang, S.Y.; Wang, Z.H.; Li, Y.C.; Li, J.W.; Qi, X.H.; Chen, X. Nb2O5/Red Phosphorus S-Scheme Heterojunction Photocatalyst for Removal of Organic Contaminant and Cr(VI): Electrochemical Performance and Mechanism. Mater. Sci. Semicond. Process 2023, 160, 107421. [Google Scholar] [CrossRef]
- Crisóstomo, C.; Damaceno, F.; Barbosa, L.; Almeida, J.; Batista, W.V.; Malagutti, A.R.; de Mesquita, J.P.; Torres, J.; Iga, G.; de Oliveira, C.; et al. FACILE HYDROTHERMAL SYNTHESIS OF NIOBIUM PENTOXIDE SEMICONDUCTOR AND THEIR APPLICATION IN THE PHOTODEGRADATION OF DYES AND REDUCTION OF FREE FAT ACIDS IN WASTE OIL. Quim. Nova 2025, 48, e-20250015. [Google Scholar] [CrossRef]
- Maarisetty, D.; Komandur, J.; Sharma, S.; Baral, S.S.; Mohapatra, P. Unravelling the Rate Controlling Step in Degradation of Phenol on a Higher Potential Photocatalyst. J. Environ. Chem. Eng. 2020, 8, 103938. [Google Scholar] [CrossRef]
- Ruiyi, L.; Xiaoyue, L.; Zaijun, L. Nb2O5-Graphene Heterojunction Composite with Ultrahigh Photocatalytic Activity for Solar Light Driven Photodegradation of Ciprofloxacin. J. Photochem. Photobiol. A Chem. 2024, 446, 115188. [Google Scholar] [CrossRef]
- Chen, X.H.; Ren, J.Y.; Li, N.B.; Luo, H.Q. Constructing of CoP-Nb2O5 p-n Heterojunction with Built-in Electric Field to Accelerate the Charge Migration in Electrocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2023, 651, 760–768. [Google Scholar] [CrossRef]
- Osman, N.S.; Sulaiman, S.N.; Muhamad, E.N.; Mukhair, H.; Tan, S.T.; Abdullah, A.H. Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye. Catalysts 2021, 11, 458. [Google Scholar] [CrossRef]
- Silva, I.F.B.; Martins, A.R.; Krambrock, K.; Rosmaninho, M.G.; Binatti, I.; Moura, F.C.C. Understanding Photocatalytic Activity and Mechanism of Nickel-Modified Niobium Mesoporous Nanomaterials. J. Photochem. Photobiol. A Chem. 2020, 388, 112168. [Google Scholar] [CrossRef]
- Moradi, Z.; Jahromi, S.Z.; Ghaedi, M. Design of Active Photocatalysts and Visible Light Photocatalysis. Interface Sci. Technol. 2021, 32, 557–623. [Google Scholar]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Shao, X.; Wang, K.; Peng, L.; Li, K.; Wen, H.; Le, X.; Wu, X.; Wang, G. In-Situ Irradiated XPS Investigation on 2D/1D Cd0.5Zn0.5S/Nb2O5 S-Scheme Heterojunction Photocatalysts for Simultaneous Promotion of Antibiotics Removal and Hydrogen Evolution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129846. [Google Scholar] [CrossRef]
- Ramar, S.; Elango, P.; Velusamy, A.; Athinarayanan, B.; Jothi, V.K.; Hsu-Wei; Pattappan, D.; Gurusamy, A.; Lai, Y.-T. An Eco-Safety g-C3N4/Nb2O5/Ag Ternary Nanocomposite for Photocatalytic Degradation of Pharmaceutical Wastes and Dyes in Wastewater and Zebrafish Embryonic Assessment. J. Mol. Struct. 2024, 1317, 139127. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Peng, C.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. One-Pot Synthesis of Ru/Nb2O5@Nb2C Ternary Photocatalysts for Water Splitting by Harnessing Hydrothermal Redox Reactions. Appl. Catal. B 2022, 303, 120910. [Google Scholar] [CrossRef]
- Guo, X.; Duan, J.; Li, C.; Zhang, Z.; Wang, W. Fabrication of Highly Stabilized Zr Doped G-C3N4/Nb2O5 Heterojunction and Its Enhanced Photocatalytic Performance for Pollutants Degradation under Visible Light Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129474. [Google Scholar] [CrossRef]
- Qu, X.; Liu, M.; Gao, Z.; Zhai, H.; Ren, W.; Shi, L.; Du, F. A Novel Ternary Bi4NbO8Cl/BiOCl/Nb2O5 Architecture via in-Situ Solvothermal-Induced Electron-Trap with Enhanced Photocatalytic Activities. Appl. Surf. Sci. 2020, 506, 144688. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, X.; Gou, X.T.; Zhao, X.H.; Shi, L.; Qu, X.F. In Situ Formation of a Ternary Bi4NbO8Cl/BiOCl/Nb2O5 Photocatalyst and Its Enhanced Photocatalytic Performance. J. Mater. Sci. 2023, 58, 2539–2551. [Google Scholar] [CrossRef]
- Zanin, H.; Teófilo, R.F.; Peterlevitz, A.C.; Oliveira, U.; de Paiva, J.C.; Ceragioli, H.J.; Reis, E.L.; Baranauskas, V. Diamond Cylindrical Anodes for Electrochemical Treatment of Persistent Compounds in Aqueous Solution. J. Appl. Electrochem. 2013, 43, 323–330. [Google Scholar] [CrossRef]
- da Silva, S.W.; do Prado, J.M.; Heberle, A.N.A.; Schneider, D.E.; Rodrigues, M.A.S.; Bernardes, A.M. Electrochemical Advanced Oxidation of Atenolol at Nb/BDD Thin Film Anode. J. Electroanal. Chem. 2019, 844, 27–33. [Google Scholar] [CrossRef]
- Fidelis, M.Z.; Favaro, Y.B.; Santos, A.; Pereira, M.F.R.; Brackmann, R.; Lenzi, G.G.; Soares, O.; Andreo, O.A.B. Enhancing Ibuprofen and 4-Isobutylacetophenone Degradation: Exploiting the Potential of Nb2O5 Sol-Gel Catalysts in Photocatalysis, Catalytic Ozonation, and Photocatalytic Ozonation. J. Environ. Chem. Eng. 2023, 11, 110690. [Google Scholar] [CrossRef]
- Gonçalves, M.; Guerreiro, M.C.; Oliveira, L.C.A.; da Rocha, C.L. Materiais à base de óxido de ferro para oxidação de compostos presentes no efluente da despolpa do café. Quim. Nova 2008, 31, 1636–1640. [Google Scholar] [CrossRef][Green Version]
- Wolski, L.; Sobanska, K.; Munko, M.; Czerniak, A.; Pietrzyk, P. Unraveling the Origin of Enhanced Activity of the Nb2O5/H2O2 System in the Elimination of Ciprofloxacin: Insights into the Role of Reactive Oxygen Species in Interface Processes. ACS Appl. Mater. Interfaces 2022, 14, 31824–31837. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Decyk, P.; Sobańska, K.; Pietrzyk, P.; Sojka, Z. Search for Reactive Intermediates in Catalytic Oxidation with Hydrogen Peroxide over Amorphous Niobium(V) and Tantalum(V) Oxides. Appl. Catal. B 2015, 164, 288–296. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Han, P. Electrospinning Preparation of G-C3N4/Nb2O5 Nanofibers Heterojunction for Enhanced Photocatalytic Degradation of Organic Pollutants in Water. Sci. Rep. 2021, 11, 22950. [Google Scholar] [CrossRef]
- Jones, B.M.F.; Mamba, G.; Maruthamani, D.; Muthuraj, V. Honeycomb Nb2O5/RGO Wrapped on MoO3 Nanorods for Visible Light-Driven Degradation of Sulfasalazine and Ciprofloxacin in Water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129836. [Google Scholar] [CrossRef]
- Fidelis, M.Z.; Abreu, E.; Andreo, O.A.B.; Soares, O.S.G.P.; Janzen, F.C.; Lenzi, G.G. Application of Structured Sol-Gel Nb2O5 Catalyst in Photocatalytic Continuous Process Prototype for Ibuprofen Degradation. Top. Catal. 2024, 67, 828–842. [Google Scholar] [CrossRef]
- Fávaro, Y.B.; Fuziki, M.E.K.; Fidelis, M.Z.; Abreu, E.; Tusset, A.M.; Brackmann, R.; Lenzi, G.G. Sol-Gel and Pechini Niobium Modified: Synthesis, Characterization and Application in the 2,4-D Herbicide Degradation. J. Environ. Sci. Health Part B 2024, 59, 50–61. [Google Scholar] [CrossRef]
- Dias, D.T.; Rodrigues, A.O.; Pires, P.B.; Semianko, B.C.; Fuziki, M.E.K.; Lenzi, G.G.; Sabino, S.R.F. Photoacoustic Spectroscopy of Titanium Dioxide, Niobium Pentoxide, Titanium:Niobium, and Ruthenium-Modified Oxides Synthesized Using Sol-Gel Methodology. Appl. Spectrosc. 2024, 78, 1028–1042. [Google Scholar] [CrossRef]
- do Prado, N.T.; Oliveira, L.C.A. Nanostructured Niobium Oxide Synthetized by a New Route Using Hydrothermal Treatment: High Efficiency in Oxidation Reactions. Appl. Catal. B 2017, 205, 481–488. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Riemke, F.C.; Oliveira, M.E.; Carreno, N.L.V.; Morisso, F.D.P.; Teodoro, M.D.; Mastelaro, V.R.; Moreira, M.L.; Raubach, C.W.; et al. The Photocatalytic Performance of Fe Inserted in Nb2O5 Obtained by Microwave-Assisted Hydrothermal Synthesis: Factorial Design of Experiments. J. Photochem. Photobiol. A Chem. 2023, 435, 114294. [Google Scholar] [CrossRef]
- Wermuth, T.B.; Arcaro, S.; Venturini, J.; Hubert Ribeiro, T.M.; de Assis Lawisch Rodriguez, A.; Machado, E.L.; Franco de Oliveira, T.; Franco de Oliveira, S.E.; Baibich, M.N.; Bergmann, C.P. Microwave-Synthesized KNbO3 Perovskites: Photocatalytic Pathway on the Degradation of Rhodamine B. Ceram. Int. 2019, 45, 24137–24145. [Google Scholar] [CrossRef]
- Sultana, R.; Islam, K.; Chakraborty, S. Tuning Optical and Electrochemical Properties of Nb2O5 Thin Films via WO3 Doping. Trans. Electr. Electron. Mater. 2024, 26, 48–59. [Google Scholar] [CrossRef]
- Sathasivam, S.; Williamson, B.A.D.; Althabaiti, S.A.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. Chemical Vapor Deposition Synthesis and Optical Properties of Nb2O5Thin Films with Hybrid Functional Theoretical Insight into the Band Structure and Band Gaps. ACS Appl. Mater. Interfaces 2017, 9, 18031–18038. [Google Scholar] [CrossRef]
- Singh, K.; Abhimanyu; Sonu, S.; Chaudhary, V.; Raizada, P.; Rustagi, S.; Singh, P.; Thakur, P.; Kumar, V.; Kaushik, A. Defect and Heterostructure Engineering Assisted S-Scheme Nb2O5 Nanosystems-Based Solutions for Environmental Pollution and Energy Conversion. Adv. Colloid. Interface Sci. 2024, 332, 103273. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Wang, L.; Guo, H. Efficient Nb2O5@g-C3N4 Heterostructures for Enhanced Photocatalytic CO2 Reduction with Highly Selective Conversion to CH 4. Inorg. Chem. Front. 2024, 11, 123–132. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, S.; Lin, S.; Shan, B.; Zhou, X.; Zhao, J.; Qi, C.; Yang, P. Promoting the Performance of Nb2O5 by Doping Transition Metal Oxide for Catalytic Degradation of Monochlorobenzene and Toluene. J. Mater. Res. Technol. 2023, 25, 3642–3653. [Google Scholar] [CrossRef]
- Ücker, C.L.; Rodrigues, F.S.M.; Riemke, F.C.; Morisso, F.D.P.; Teodoro, M.D.; Mastelaro, V.R.; Ferrer, M.M.; Raubach, C.W.; da Cava, S.S. Surface Modification of T-Nb2O5 with Low-Crystallinity Nb2O5 to Enhance Photocatalytic Degradation of Rhodamine B. Ceram. Int. 2023, 49, 34333–34338. [Google Scholar] [CrossRef]
- Zu, D.Y.; Song, H.R.; Wang, Y.W.; Chao, Z.; Li, Z.; Wang, G.; Shen, Y.M.; Li, C.P.; Ma, J. One-Pot in-Situ Hydrothermal Synthesis of CdS/Nb2O5/Nb2C Heterojunction for Enhanced Visible-Light-Driven Photodegradation. Appl. Catal. B Environ. 2020, 277, 119140. [Google Scholar] [CrossRef]
- Wolski, L.; Ziolek, M. Insight into Pathways of Methylene Blue Degradation with H2O2 over Mono and Bimetallic Nb, Zn Oxides. Appl. Catal. B 2018, 224, 634–647. [Google Scholar] [CrossRef]
- Zulkiflee, A.; Mansoob Khan, M.; Yusuf Khan, M.; Khan, A.; Hilni Harunsani, M. Nb2O5/BiOCl Composite as a Visible-Light-Active Photocatalyst for the Removal of RhB Dye and Photoelectrochemical Studies. J. Photochem. Photobiol. A Chem. 2024, 446, 115177. [Google Scholar] [CrossRef]
- Sacco, O.; Murcia, J.J.; Lara, A.E.; Hernández-Laverde, M.; Rojas, H.; Navío, J.A.; Hidalgo, M.C.; Vaiano, V. Pt-TiO2-Nb2O5 Heterojunction as Effective Photocatalyst for the Degradation of Diclofenac and Ketoprofen. Mater. Sci. Semicond. Process 2020, 107, 104839. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, J.; Zhang, S.; Xiong, Q.; Huang, B.; Wang, J.; Gong, W. Preparation of Nanosized Bi3NbO7 and Its Visible-Light Photocatalytic Property. J. Hazard. Mater. 2009, 172, 986–992. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Rabeah, J.; Brückner, A.; Cao, H. Visible-Light Photocatalytic Ozonation Using Graphitic C3N4Catalysts: A Hydroxyl Radical Manufacturer for Wastewater Treatment. Acc. Chem. Res. 2020, 53, 1024–1033. [Google Scholar] [CrossRef]
- Nogueira, M.V.; Lustosa, G.M.M.M.; Kobayakawa, Y.; Kogler, W.; Ruiz, M.; Monteiro Filho, E.S.; Zaghete, M.A.; Perazolli, L.A. Nb-Doped TiO2 Photocatalysts Used to Reduction of CO2 to Methanol. Adv. Mater. Sci. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Tee, S.Y.; Kong, J.; Koh, J.J.; Teng, C.P.; Wang, X.; Wang, X.; Teo, S.L.; Thitsartarn, W.; Han, M.-Y.; Seh, Z.W. Structurally and Surficially Activated TiO2 Nanomaterials for Photochemical Reactions. Nanoscale 2024, 16, 18165–18212. [Google Scholar] [CrossRef]
- Wang, S.; Yun, J.-H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Xiao, M.; Wang, L. Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. J. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; de O, M.; Ramakrishnappa, T.; Teixeira, S.R.; Dupont, J. Ionic Liquid–Assisted Hydrothermal Synthesis of Nb/TiO2 Nanocomposites for Efficient Photocatalytic Hydrogen Production and Photodecolorization of Rhodamine B under UV-Visible and Visible Light Illuminations. Mater. Today Chem. 2019, 12, 373–385. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Lopes, O.F.; Neto, A.B.S.; Ribeiro, C. Enhanced Cr(VI) Photoreduction in Aqueous Solution Using Nb2O5/CuO Heterostructures under UV and Visible Irradiation. Chem. Eng. J. 2017, 312, 220–227. [Google Scholar] [CrossRef]
- Serenario, M.E.D.; Santos, B.A.F.; Petrucelli, A.C.F.; Souza, R.C.; Moreira, G.P.C.; Miranda, L.R.M.; Bueno, A.H.S. Anti-Corrosion Coatings Based on Nb2O5—A Comparison Between Two Coatings Technology: Thermal Spray Coating and Epoxy Paint. Mater. Res. 2022, 25, e20210515. [Google Scholar] [CrossRef]
- Goswami, T.; Kumar, S.; Bheemaraju, A.; Reddy, K.M.; Sharma, A.K.; Kataria, A.; Shrivastav, A. TiO2 Nanoparticles and Nb2O5 Nanorods Immobilized RGO for Efficient Visible-Light Photocatalysis and Catalytic Reduction. Catal. Lett. 2023, 153, 605–621. [Google Scholar] [CrossRef]
- Ücker, C.L.; Riemke, F.; Goetzke, V.; Moreira, M.L.; Raubach, C.W.; Longo, E.; Cava, S. Facile Preparation of Nb2O5/TiO2 Heterostructures for Photocatalytic Application. Chem. Phys. Impact 2022, 4, 100079. [Google Scholar] [CrossRef]
- Jin, F.; Ma, X.; Guo, S.-Q. F-Doped Hierarchical Nb2O5: Structural, Optical and Photocatalytic Performance. Mater. Lett. 2023, 347, 134664. [Google Scholar] [CrossRef]
- Lim, J.; Murugan, P.; Lakshminarasimhan, N.; Kim, J.Y.; Lee, J.S.; Lee, S.H.; Choi, W. Synergic Photocatalytic Effects of Nitrogen and Niobium Co-Doping in TiO2 for the Redox Conversion of Aquatic Pollutants under Visible Light. J. Catal. 2014, 310, 91–99. [Google Scholar] [CrossRef]
- Rajan, S.T.; Senthilnathan, J.; Arockiarajan, A. Sputter-Coated N-Enriched Mixed Metal Oxides (Ta2O5-Nb2O5-N) Composite: A Resilient Solar Driven Photocatalyst for Water Purification. J. Hazard. Mater. 2023, 452, 131283. [Google Scholar] [CrossRef]
- Fogaça, L.Z.; Vicentini, J.C.M.; de Freitas, C.F.; Braga, T.L.; da Silva, F.A.; de Souza, M.; Baesso, M.L.; Caetano, W.; Batistela, V.R.; Scaliante, M.H.N.O. Nanocomposites of Nb2O5 and ZnO with Reduced Graphene Oxide for Heterogeneous Photocatalysis of Dyes. Catal Commun 2023, 185, 106799. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, J.; Zhang, Q.; Chen, G.; Zhou, L.; Li, L. 3D Graphene Aerogel Composite of 1D-2D Nb2O5-g-C3N4 Heterojunction with Excellent Adsorption and Visible-Light Photocatalytic Performance. J. Colloid. Interface Sci. 2020, 563, 131–138. [Google Scholar] [CrossRef]
- Sethuraman, S.; Marimuthu, A.; Kattamuthu, R.; Karuppasamy, G. Highly Surface Active Niobium Doped G-C3N4/g-C3N4 Heterojunction Interface towards Superior Photocatalytic and Selective Ammonia Response. Appl. Surf. Sci. 2021, 561, 150077. [Google Scholar] [CrossRef]
- Wang, Y.; Su, N.; Liu, J.; Lin, Y.; Wang, J.; Guo, X.; Zhang, Y.; Qin, Z.; Liu, J.; Zhang, C.; et al. Enhanced Visible-Light Photocatalytic Properties of SnO2 Quantum Dots by Niobium Modification. Results Phys. 2022, 37, 105515. [Google Scholar] [CrossRef]
- Cui, C.; Guo, R.; Ren, E.; Xiao, H.; Lai, X.; Qin, Q.; Jiang, S.; Shen, H.; Zhou, M.; Qin, W. Facile Hydrothermal Synthesis of Rod-like Nb2O5/Nb2CTx Composites for Visible-Light Driven Photocatalytic Degradation of Organic Pollutants. Environ. Res. 2021, 193, 110587. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Duan, J.; Zhang, X.; Zhang, H.; Liu, X.; Feng, Y.; Zheng, M. Heterojunction Architecture of Nb2O5/g-C3N4 for Enhancing Photocatalytic Activity to Degrade Organic Pollutants and Deactivate Bacteria in Water. Chin. Chem. Lett. 2022, 33, 3792–3796. [Google Scholar] [CrossRef]
- Du, Y.C.; Zhang, S.H.; Wang, J.S.; Wu, J.S.; Dai, H.X. Nb2O5 Nanowires in-Situ Grown on Carbon Fiber: A High-Efficiency Material for the Photocatalytic Reduction of Cr(VI). J. Environ. Sci. 2018, 66, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Berenguer, A.; Velasco, L.F.; Velo-Gala, I.; Ania, C.O. Photochemistry of Nanoporous Carbons: Perspectives in Energy Conversion and Environmental Remediation. J. Colloid. Interface Sci. 2017, 490, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Huang, G.; Zhang, Z.; Han, L.; Song, W.; Li, M.; Zhang, Y. Facile Synthesis of Urchin-like Hierarchical Nb2O5 Nanospheres with Enhanced Visible Light Photocatalytic Activity. J. Alloys Compd. 2017, 728, 19–28. [Google Scholar] [CrossRef]
- Qi, S.; Zuo, R.; Liu, Y.; Wang, Y. Synthesis and Photocatalytic Activity of Electrospun Niobium Oxide Nanofibers. Mater. Res. Bull. 2013, 48, 1213–1217. [Google Scholar] [CrossRef]
- Khan, S.U.; Perini, J.A.L.; Hussain, S.; Khan, H.; Khan, S.; Boldrin Zanoni, M.V. Electrochemical Preparation of Nb2O5 Nanochannel Photoelectrodes for Enhanced Photoelectrocatalytic Performance in Removal of RR120 Dye. Chemosphere 2020, 257, 127164. [Google Scholar] [CrossRef]
- Breault, T.M.; Bartlett, B.M. Composition Dependence of TiO2:(Nb,N)-x Compounds on the Rate of Photocatalytic Methylene Blue Dye Degradation. J. Phys. Chem. C 2013, 117, 8611–8618. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Gaffarinejad, A.; Rahimi, R. Porous P-NiO/n-Nb2O5 Nanocomposites Prepared by an EISA Route with Enhanced Photocatalytic Activity in Simultaneous Cr(VI) Reduction and Methyl Orange Decolorization under Visible Light Irradiation. J. Hazard. Mater. 2015, 286, 64–74. [Google Scholar] [CrossRef]
- Valerio, T.L.; Maia, G.A.R.; Gonçalves, L.F.; Viomar, A.; Banczek, E.d.P.; Rodrigues, P.R.P. Study of the Nb2O5 Insertion in ZnO to Dye-Sensitized Solar Cells. Materials Research 2019, 22, e20180864. [Google Scholar] [CrossRef]
- Peng, C.; Xie, X.; Xu, W.; Zhou, T.; Wei, P.; Jia, J.; Zhang, K.; Cao, Y.; Wang, H.; Peng, F.; et al. Engineering Highly Active Ag/Nb2O5@Nb2CT (MXene) Photocatalysts via Steering Charge Kinetics Strategy. Chem. Eng. J. 2021, 421, 128766. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.; Yang, J.; Wang, J.; Xu, L. Bird’s Nest-like Nb2O5: Preparation, Characterization and Multifunctional Photocatalytic Application. Mater. Technol. 2024, 39, 2364552. [Google Scholar] [CrossRef]
- Ücker, C.L.; Gularte, L.T.; Fernandes, C.D.; Goetzke, V.; Moreira, E.C.; Raubach, C.W.; Moreira, M.L.; Cava, S.S. Investigation of the Properties of Niobium Pentoxide for Use in Dye-sensitized Solar Cells. J. Am. Ceram. Soc. 2019, 102, 1884–1892. [Google Scholar] [CrossRef]
- Fidelis, M.Z.; de Paula, E.; Abreu, E.; Fuziki, M.E.K.; Santos, O.A.A.D.; Brackmann, R.; Lenzi, G.G. Nb2O5: Percentage Effect of T/H Phase and Evaluation of Catalytic Activity, a Preliminary Study. Catal. Res. 2023, 3, 023. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium Oxides and Niobates Physical Properties: Review and Prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; Olusegun, S.J.; Gabriel, J.B.; Costa, R.C.V.; Mohallem, N.D.S. The Role of Crystalline Nb2O5 Nanoparticles for Enhanced Dye Adsorption and Photodegradation. Ceram. Int. 2023, 49, 6164–6176. [Google Scholar] [CrossRef]
- Ücker, C.L.; Riemke, F.C.; de Andrade Neto, N.F.; de Santiago, A.A.G.; Siebeneichler, T.J.; Carreño, N.L.V.; Moreira, M.L.; Raubach, C.W.; Cava, S. Influence of Nb2O5 Crystal Structure on Photocatalytic Efficiency. Chem. Phys. Lett. 2021, 764, 138271. [Google Scholar] [CrossRef]
- Suzuki, N.; Athar, T.; Huang, Y.-T.; Shimasaki, K.; Miyamoto, N.; Yamauchi, Y. Synthesis of Mesoporous Nb2O5 with Crystalline Walls and Investigation of Their Photocatalytic Activity. J. Ceram. Soc. Jpn. 2011, 119, 405–411. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 Catalysts. Nano Rev. 2012, 3, 17631. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive Review of Emerging Contaminants: Detection Technologies, Environmental Impact, and Management Strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Ejiohuo, O.; Onyeaka, H.; Akinsemolu, A.; Nwabor, O.F.; Siyanbola, K.F.; Tamasiga, P.; Al-Sharify, Z.T. Ensuring Water Purity: Mitigating Environmental Risks and Safeguarding Human Health. Water Biol. Secur. 2025, 4, 100341. [Google Scholar] [CrossRef]
- Das, S.; Parida, V.K.; Tiwary, C.S.; Gupta, A.K.; Chowdhury, S. Emerging Contaminants in the Aquatic Environment: Fate, Occurrence, Impacts, and Toxicity. In Bioremediation of Emerging Contaminants in Water; ACS: Washington, DC, USA, 2024; pp. 1–32. [Google Scholar]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of Emerging Contaminants and Associated Human Health Effects. Biomed. Res. Int. 2015, 2015, 404796. [Google Scholar] [CrossRef] [PubMed]
- Mitoraj, D.; Lamdab, U.; Kangwansupamonkon, W.; Pacia, M.; Macyk, W.; Wetchakun, N.; Beranek, R. Revisiting the Problem of Using Methylene Blue as a Model Pollutant in Photocatalysis: The Case of InVO4/BiVO4 Composites. J. Photochem. Photobiol. A Chem. 2018, 366, 103–110. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules 2014, 20, 88–110. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid. Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Alegbe, E.O.; Uthman, T.O. A Review of History, Properties, Classification, Applications and Challenges of Natural and Synthetic Dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Alzain, H.; Kalimugogo, V.; Hussein, K. A Review of Environmental Impact of Azo Dyes. Int. J. Res. Rev. 2023, 10, 64–689. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for Degradation of Dyes in Industrial Effluents: Opportunities and Challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- de Carvalho, G.S.G.; de Siqueira, M.M.; do Nascimento, M.P.; de Oliveira, M.A.L.; Amarante, G.W. Nb2O5 Supported in Mixed Oxides Catalyzed Mineralization Process of Methylene Blue. Heliyon 2020, 6, e04128. [Google Scholar] [CrossRef]
- Le Luu, T.; Ngan, P.T.K. Fabrication of High Performance Ti/SnO2-Nb2O5 Electrodes for Electrochemical Textile Wastewater Treatment. Sci. Total Environ. 2023, 860, 160366. [Google Scholar] [CrossRef]
- Rangel, E.M.; Riemke, F.C.; Ücker, C.L.; Raubach, C.W.; Adebayo, M.A.; Machado Machado, F. Photodegradation of Acid Yellow 23 BY Nb2O5 Supported on Eco-Friendly Glass Foams. J. Clean. Prod. 2022, 371, 133231. [Google Scholar] [CrossRef]
- Hass Caetano Lacerda, E.; Monteiro, F.C.; Kloss, J.R.; Fujiwara, S.T. Bentonite Clay Modified with Nb2O5: An Efficient and Reused Photocatalyst for the Degradation of Reactive Textile Dye. J. Photochem. Photobiol. A Chem. 2020, 388, 112084. [Google Scholar] [CrossRef]
- Pereira da Costa, G.; Rafael, R.A.; Soares, J.C.S.; Gaspar, A.B. Synthesis and Characterization of ZnO-Nb2O5 Catalysts for Photodegradation of Bromophenol Blue. Catal. Today 2020, 344, 240–246. [Google Scholar] [CrossRef]
- Hamzad, S.; Kumar, K.Y.; Prashanth, M.K.; Radhika, D.; Parashuram, L.; Alharti, F.A.; Jeon, B.H.; Raghu, M.S. Boron Doped RGO from Discharged Dry Cells Decorated Niobium Pentoxide for Enhanced Visible Light-Induced Hydrogen Evolution and Water Decontamination. Surf. Interfaces 2023, 36, 102544. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Facile Preparation of New Nanohybrids for Enhancing Photocatalytic Activity toward Removal of Organic Dyes under Visible Light Irradiation. J. Phys. Chem. Solids 2020, 140, 109271. [Google Scholar] [CrossRef]
- Iborra-Torres, A.; Kulak, A.N.; Palgrave, R.G.; Hyett, G. Demonstration of Visible Light-Activated Photocatalytic Self-Cleaning by Thin Films of Perovskite Tantalum and Niobium Oxynitrides. ACS Appl. Mater. Interfaces 2020, 12, 33603–33612. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.S.; Bolzon, L.B.; Pedroso, C.P.; Moura, A.O.; Costa, L.L. Nb2O5 as Efficient and Recyclable Photocatalyst for Indigo Carmine Degradation. Appl. Catal. B 2008, 82, 219–224. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Ramalho, T.C.; Souza, E.F.; Gonçalves, M.; Oliveira, D.Q.L.; Pereira, M.C.; Fabris, J.D. Catalytic Properties of Goethite Prepared in the Presence of Nb on Oxidation Reactions in Water: Computational and Experimental Studies. Appl. Catal. B 2008, 83, 169–176. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Oliveira, H.S.; Mayrink, G.; Mansur, H.S.; Mansur, A.A.P.; Moreira, R.L. One-Pot Synthesis of CdS@Nb2O5 Core-Shell Nanostructures with Enhanced Photocatalytic Activity. Appl. Catal. B 2014, 152–153, 403–412. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, W.; Li, Q.; Gao, S.; Shang, J.K. Passivated N-p Co-Doping of Niobium and Nitrogen into Self-Organized TiO2 Nanotube Arrays for Enhanced Visible Light Photocatalytic Performance. Appl. Catal. B 2014, 144, 343–352. [Google Scholar] [CrossRef]
- Wolski, L.; Sobańska, K.; Walkowiak, A.; Akhmetova, K.; Gryboś, J.; Frankowski, M.; Ziolek, M.; Pietrzyk, P. Enhanced Adsorption and Degradation of Methylene Blue over Mixed Niobium-Cerium Oxide—Unraveling the Synergy between Nb and Ce in Advanced Oxidation Processes. J. Hazard. Mater. 2021, 415, 125665. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Silva, F.N.; da Silva, M.L.C.P.; Campos, T.M.B.; Thim, G.P.; Rodrigues, L.A. Methylene Blue Photodegradation Employing Hexagonal Prism-Shaped Niobium Oxide as Heterogeneous Catalyst: Effect of Catalyst Dosage, Dye Concentration, and Radiation Source. Mater. Chem. Phys. 2018, 214, 95–106. [Google Scholar] [CrossRef]
- Kumari, N.; Gaurav, K.; Samdarshi, S.K.; Bhattacharyya, A.S.; Paul, S.; Rajbongshi, B.M.; Mohanty, K. Dependence of Photoactivity of Niobium Pentoxide (Nb2O5) on Crystalline Phase and Electrokinetic Potential of the Hydrocolloid. Sol. Energy Mater. Sol. Cells 2020, 208, 110408. [Google Scholar] [CrossRef]
- Ferraz, N.P.; Nogueira, A.E.; Marcos, F.C.F.; Machado, V.A.; Rocca, R.R.; Assaf, E.M.; Asencios, Y.J.O. CeO2–Nb2O5 Photocatalysts for Degradation of Organic Pollutants in Water. Rare Met. 2020, 39, 230–240. [Google Scholar] [CrossRef]
- Awais, M.; Khursheed, S.; Tehreem, R.; Sirajuddin; Mok, Y.S.; Siddiqui, G.U. PH Regulated Rapid Photocatalytic Degradation of Methylene Blue Dye via Niobium-Nitrogen Co-Doped Titanium Dioxide Nanostructures under Sunlight. Appl. Catal. A Gen. 2022, 643, 118764. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, O.P. NbC/C Heterojunction for Efficient Photodegradation of Methylene Blue under Visible Irradiation. Sol. Energy 2019, 183, 398–409. [Google Scholar] [CrossRef]
- Breault, T.M.; Bartlett, B.M. Lowering the Band Gap of Anatase-Structured TiO2 by Coalloying with Nb and N: Electronic Structure and Photocatalytic Degradation of Methylene Blue Dye. J. Phys. Chem. C 2012, 116, 5986–5994. [Google Scholar] [CrossRef]
- Carvalho, K.T.G.; Nogueira, A.E.; Lopes, O.F.; Byzynski, G.; Ribeiro, C. Synthesis of G-C3N4/Nb2O5 Heterostructures and Their Application in the Removal of Organic Pollutants under Visible and Ultraviolet Irradiation. Ceram. Int. 2017, 43, 3521–3530. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Aziz, A.R.; Wan Daud, W.M.A.; Embong, Z. Niobium Substituted Magnetite as a Strong Heterogeneous Fenton Catalyst for Wastewater Treatment. Appl. Surf. Sci. 2015, 351, 175–187. [Google Scholar] [CrossRef]
- da Silva, A.L.; Dondi, M.; Hotza, D. Self-Cleaning Ceramic Tiles Coated with Nb2O5-Doped-TiO2 Nanoparticles. Ceram. Int. 2017, 43, 11986–11991. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Muche, D.N.F.; Dey, S.; Hotza, D.; Castro, R.H.R. Photocatalytic Nb2O5-Doped TiO2 Nanoparticles for Glazed Ceramic Tiles. Ceram. Int. 2016, 42, 5113–5122. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, O.P. Visible Irradiation Induced Photodegradation by NbC/C Nanocomposite Derived from Smoked Cigarette Litter (Filters). Sol. Energy 2018, 163, 167–176. [Google Scholar] [CrossRef]
- Matos, J.; Lanfredi, S.; Montaña, R.; Nobre, M.A.L.; de Córdoba, M.C.F.; Ania, C.O. Photochemical Reactivity of Apical Oxygen in KSr2Nb5O15 Materials for Environmental Remediation under UV Irradiation. J. Colloid. Interface Sci. 2017, 496, 211–221. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Bacani, R.; da Silva, M.L.C.P.; Campos, T.M.B.; Thim, G.P.; Rodrigues, L.A. Effect of Nb/C Ratio in the Morphological, Structural, Optical and Photocatalytic Properties of Novel and Inexpensive Nb2O5/Carbon Xerogel Composites. Ceram. Int. 2018, 44, 6645–6652. [Google Scholar] [CrossRef]
- Oliveira, H.S.; Almeida, L.D.; De Freitas, V.A.A.; Moura, F.C.C.; Souza, P.P.; Oliveira, L.C.A. Nb-Doped Hematite: Highly Active Catalyst for the Oxidation of Organic Dyes in Water. Catal. Today 2015, 240, 176–181. [Google Scholar] [CrossRef]
- Silva, A.C.; Oliveira, D.Q.L.; Oliveira, L.C.A.; Anastácio, A.S.; Ramalho, T.C.; Lopes, J.H.; Carvalho, H.W.P.; Torres, C.E.R. Nb-Containing Hematites Fe2-XNbxO3: The Role of Nb5+ on the Reactivity in Presence of the H2O2 or Ultraviolet Light. Appl. Catal. A Gen. 2009, 357, 79–84. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface Aging Behaviour of Fe-Based Amorphous Alloys as Catalysts during Heterogeneous Photo Fenton-like Process for Water Treatment. Appl. Catal. B 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Photocatalytic Activity of TiO2/Nb2O5/PANI and TiO2/Nb2O5/RGO as New Nanocomposites for Degradation of Organic Pollutants. J. Hazard. Mater. 2018, 351, 147–159. [Google Scholar] [CrossRef]
- Batista, L.M.B.; dos Santos, A.J.; da Silva, D.R.; de Alves, A.P.M.; Garcia-Segura, S.; Martínez-Huitle, C.A. Solar Photocatalytic Application of NbO2OH as Alternative Photocatalyst for Water Treatment. Sci. Total Environ. 2017, 596–597, 79–86. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Batista, L.M.B.; Martínez-Huitle, C.A.; de Alves, A.P.M.; Garcia-Segura, S. Niobium Oxide Catalysts as Emerging Material for Textile Wastewater Reuse: Photocatalytic Decolorization of Azo Dyes. Catalysts 2019, 9, 1070. [Google Scholar] [CrossRef]
- Patil, B.N.; Naik, D.B.; Shrivastava, V.S. Photocatalytic Degradation of Hazardous Ponceau-S Dye from Industrial Wastewater Using Nanosized Niobium Pentoxide with Carbon. Desalination 2011, 269, 276–283. [Google Scholar] [CrossRef]
- Souza, R.P.; Freitas, T.K.F.S.; Domingues, F.S.; Pezoti, O.; Ambrosio, E.; Ferrari-Lima, A.M.; Garcia, J.C. Photocatalytic Activity of TiO2, ZnO and Nb2O5 Applied to Degradation of Textile Wastewater. J. Photochem. Photobiol. A Chem. 2016, 329, 9–17. [Google Scholar] [CrossRef]
- Domingues, F.S.; Geraldino, H.C.L.; de Freitas, T.K.F.S.; de Almeida, C.A.; de Figueiredo, F.F.; Garcia, J.C. Photocatalytic Degradation of Real Textile Wastewater Using Carbon Black-Nb 2 O 5 Composite Catalyst under UV/Vis Irradiation. Environ. Technol. 2021, 42, 2335–2349. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Cheng, L.; Chen, J.; Hou, W.; Ding, W. A Comparison of H+-Restacked Nanosheets and Nanoscrolls Derived from K4Nb6O17 for Visible-Light Degradation of Dyes. J. Energy Chem. 2014, 23, 136–144. [Google Scholar] [CrossRef]
- Qaraah, F.A.; Mahyoub, S.A.; Hezam, A.; Qaraah, A.; Drmosh, Q.A.; Xiu, G. Construction of 3D Flowers-like O-Doped g-C3N4-[N-Doped Nb2O5/C] Heterostructure with Direct S-Scheme Charge Transport and Highly Improved Visible-Light-Driven Photocatalytic Efficiency. Chin. J. Catal. 2022, 43, 2637–2651. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Almeida, S.R.; Moreira, E.C.; Ferrer, M.M.; Jardim, P.L.G.; Moreira, M.L.; Raubach, C.W.; Cava, S. Photocatalytic Degradation of Rhodamine B Using Nb2O5 Synthesized with Different Niobium Precursors: Factorial Design of Experiments. Ceram. Int. 2021, 47, 20570–20578. [Google Scholar] [CrossRef]
- Prabhakarrao, N.; Rao, T.S.; Lakshmi, K.V.D.; Divya, G.; Jaishree, G.; Raju, I.M.; Alim, S.A. Enhanced Photocatalytic Performance of Nb Doped TiO2/Reduced Graphene Oxide Nanocomposites over Rhodamine B Dye under Visible Light Illumination. Sustain. Environ. Res. 2021, 31, 37. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, Y.; Xu, L.; Yang, X.; Hu, C.; Zhang, L.; Fan, Y.; Hou, W. Thermostable Nitrogen-Doped HTiNbO5 Nanosheets with a High Visible-Light Photocatalytic Activity. Nano Res. 2011, 4, 635–647. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Reis, M.O.; Pires, M.S.; Ruotolo, L.A.M.; Ramalho, T.C.; Oliveira, C.R.; Lacerda, L.C.T.; Nogueira, F.G.E. Zn-Doped Nb2O5 Photocatalysts Driven by Visible-Light: An Experimental and Theoretical Study. Mater. Chem. Phys. 2019, 228, 160–167. [Google Scholar] [CrossRef]
- Dai, Q.; Yuan, B.; Guo, M.; Zhang, K.; Chen, X.; Song, Z.; Nguyen, T.T.; Wang, X.; Lin, S.; Fan, J.; et al. A Novel Nano-Fibriform C- Modified Niobium Pentoxide by Using Cellulose Templates with Highly Visible-Light Photocatalytic Performance. Ceram. Int. 2020, 46, 13210–13218. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Quijo, M.V.; Marcos, F.C.F.; Nogueira, A.E.; Rocca, R.R.; Assaf, E.M. Photocatalytic Activity of Nb Heterostructure (NaNbO3/Na2Nb4O11) and Nb/Clay Materials in the Degradation of Organic Compounds. Sol. Energy 2019, 194, 37–46. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Qiu, Y.; Leung, C.F.; He, J.; Liu, G.; Lau, T.C. Synthesis of Nitrogen-Doped KNbO3 Nanocubes with High Photocatalytic Activity for Water Splitting and Degradation of Organic Pollutants under Visible Light. Chem. Eng. J. 2013, 226, 123–130. [Google Scholar] [CrossRef]
- Gupta, A.; Mittal, M.; Singh, M.K.; Suib, S.L.; Pandey, O.P. Low Temperature Synthesis of NbC/C Nano-Composites as Visible Light Photoactive Catalyst. Sci. Rep. 2018, 8, 13597. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.; Liu, M.S.; Du, P.H.; Dang, C.Y.; Liang, J.L.; Li, Y.Y. Fabrication of Niobium Doped Titanate Nanoflakes with Enhanced Visible-Light-Driven Photocatalytic Activity for Efficient Ibuprofen Degradation. Chin. Chem. Lett. 2019, 30, 2177–2180. [Google Scholar] [CrossRef]
- Lenzi, G.G.; Abreu, E.; Fuziki, M.E.K.; Fidelis, M.Z.; Brackmann, R.; de Tuesta, J.L.D.; Gomes, H.T.; dos Santos, O.A.A. 17 α-Ethinylestradiol Degradation in Continuous Process by Photocatalysis Using Ag/Nb2O5 Immobilized in Biopolymer as Catalyst. Top. Catal. 2022, 65, 1225–1234. [Google Scholar] [CrossRef]
- Fernandes, C.H.M.; Goulart, L.A.; Gonçalves, R.; Santos, G.O.S.; Zanoni, M.V.B.; Mascaro, L.H.; Lanza, M.R.V. Effective Photoelectrocatalysis of Levofloxacin Antibiotic with Ti/IrO2-Nb2O5 in Environmental Samples. Electrochim. Acta 2024, 475, 143586. [Google Scholar] [CrossRef]
- Liao, W.N.; Yang, Z.Q.; Wang, Y.; Li, S.; Wang, C.Y.; Zhou, Z.L. Novel Z-Scheme Nb2O5/C3N5 Photocatalyst for Boosted Degradation of Tetracycline Antibiotics by Visible Light-Assisted Activation of Persulfate System. Chem. Eng. J. 2023, 478, 147346. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, H.; Ren, Y.H.; Wang, C.; Weng, Z.W.; Yue, B.; He, H.Y. Construction of G-C3N4-MNb2O5 Composites with Enhanced Visible Light Photocatalytic Activity. Nanomaterials 2018, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.H.; Wang, B.; Yang, J.N.; Zhao, J.W.; Yu, X.; Tian, Y.J. Construction of ZnS1-x Layers Coated Nb2O5−x Mesocrystals for Boosted Removal of Organic Contaminant. Ceram. Int. 2023, 49, 37861–37871. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Li, C.S.; Zhang, G.Y.; Meng, Y.D.; Yin, B.X.; Zhao, Y.; Shi, W.D. Efficient and Stable Nb2O5 Modified g-C3N4 Photocatalyst for Removal of Antibiotic Pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Wu, J.Z.; Li, W.; Guan, S.Y.; Chen, X.H.; Gao, H.Y.; Liu, X.L. Study on the Performance of Vanadium Doped NaNbO3 Photocatalyst Degradation Antibiotics. Inorg. Chem. Commun. 2021, 131, 108669. [Google Scholar] [CrossRef]
- Heberle, A.N.A.; García-Gabaldón, M.; Ortega, E.M.; Bernardes, A.M.; Pérez-Herranz, V. Study of the Atenolol Degradation Using a Nb/BDD Electrode in a Filter-Press Reactor. Chemosphere 2019, 236, 124318. [Google Scholar] [CrossRef]
- Tong, Q.Q.; Qiu, C.T.; Zheng, G.J.; Zhu, Q.Q.; Zhou, S.M.; Wang, Y.K.; Shi, L.; Wang, H.F.; He, D.B.; Sadakane, M.; et al. Higher Acidity Promoted Photodegradation of Fluoroquinolone Antibiotics under Visible Light by Strong Interaction with a Niobium Oxide Based Zeolitic Octahedral Metal Oxide. Appl. Catal. A Gen. 2023, 662, 119284. [Google Scholar] [CrossRef]
- Valim, R.B.; Carneiro, J.F.; Lourenço, J.C.; Hammer, P.; dos Santos, M.C.; Rodrigues, L.A.; Bertazzoli, R.; Lanza, M.; Rocha, R.D. Synthesis of Nb2O5/C for H2O2 Electrogeneration and Its Application for the Degradation of Levofloxacin. J. Appl. Electrochem. 2024, 54, 581–595. [Google Scholar] [CrossRef]
- Rameel, M.I.; Wali, M.; Al-Humaidi, J.Y.; Liaqat, F.; Khan, M.A. Enhanced Photocatalytic Degradation of Levofloxacin over Heterostructured C3N4/Nb2O5 System under Visible Light. Heliyon 2023, 9, e20479. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, E.T.; Moreira, A.J.; Sa, M.C.; Freschi, G.P.G.; Joya, M.R.; Li, M.S.; Paris, E.C. Potential of Nb2O5 Nanofibers in Photocatalytic Degradation of Organic Pollutants. Environ. Sci. Pollut. Res. 2021, 28, 69401–69415. [Google Scholar] [CrossRef] [PubMed]
- Fuziki, M.E.K.; Abreu, E.; Napoli, J.S.; Nunes, S.C.; Brackmann, R.; Machado, T.C.S.; Semianko, B.C.; Lenzi, G.G. Cu/Nb2O5, Fe/Nb2O5 and Cu-Fe/Nb2O5 Applied in Salicylic Acid Degradation: Parameters Studies and Photocatalytic Activity. J. Environ. Sci. HEALTH PART A-TOXIC/Hazard. Subst. Environ. Eng. 2022, 57, 797–812. [Google Scholar] [CrossRef]
- Orsetti, F.R.; Bukman, L.; Santos, J.S.; Nagay, B.E.; Rangel, E.C.; Cruz, N.C. Methylene Blue and Metformin Photocatalytic Activity of CeO2-Nb2O5 Coatings Is Dependent on the Treatment Time of Plasma Electrolytic Oxidation on Titanium. Appl. Surf. Sci. Adv. 2021, 6, 100143. [Google Scholar] [CrossRef]
- Welter, J.B.; da Silva, S.W.; Schneider, D.E.; Rodrigues, M.A.S.; Ferreira, J.Z. Performance of Nb/BDD Material for the Electrochemical Advanced Oxidation of Prednisone in Different Water Matrix. Chemosphere 2020, 248, 126062. [Google Scholar] [CrossRef]
- Wen, Z.J.; Ren, S.Y.; Zhang, Y.Y.; Li, J.Y.; Zhang, Z.G.; Wang, A.M. Performance of Anode Materials in Electro-Fenton Oxidation of Cefoperazone in Chloride Medium: New Insight into Simultaneous Mineralization and Toxic Byproducts Formation. J. Clean. Prod. 2022, 377, 134225. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Gao, J.L.; Chen, S.G.; Li, L.; Xu, J.H.; Li, D.; Liu, Y.F.; Quan, X.; Fu, X.; Xie, Y.Z.; et al. Advanced Electrooxidation of Florfenicol Using 3D Printed Nb2O5/Ti Electrodes: Degradation Efficiency, Dehalogenation Performance, and Toxicity Reduction. Chem. Eng. J. 2023, 474, 145561. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Selvi, M.; Balasubramaniyan, S.; Jagatheesan, R. Sphere-like Nb2O5 Nanoparticles by Waste Brassica Oleracea Leaf Extract for Lead Removal and Photocatalytic Degradation of Methylene Blue Dye. J. Indian. Chem. Soc. 2024, 101, 101377. [Google Scholar] [CrossRef]
- de Sousa, C.M.; Cardoso, V.L.; Batista, F.R.X. A Coupled Photocatalytic System Using Niobium Oxide and Microalga: Cr (VI)-Contaminated Wastewater Treatment. J. Photochem. Photobiol. A Chem. 2023, 439, 114602. [Google Scholar] [CrossRef]
- Li, Z.; Sun, D.; Yang, F.; Zhao, J.; Wu, X.; Zhao, S. Efficient Removal of Hg0 by Regulating the Bonding Degree between Cuo and Carrier Nb2O5. SSRN Electron. J. 2022, 332, 125796. [Google Scholar] [CrossRef]
- Honghu, L.; Jiangjun, H.; He, W. Catalytic Oxidation Removal of Gaseous Elemental Mercury in Flue Gas over Niobium-loaded Catalyst. Can. J. Chem. Eng. 2016, 94, 1486–1494. [Google Scholar] [CrossRef]
- Agrafioti, K.A.; Panagiotopoulos, N.T.; Moularas, C.; Deligiannakis, Y.; Prouskas, C.; Soukouli, P.P.; Evangelakis, G.A. Development of Ti-Based Nanocomposite Oxide Thin Films with CuO and Nb2O5 Additions Suitable for Catalytic Applications. Thin Solid. Film. 2023, 775, 139864. [Google Scholar] [CrossRef]
- Wang, T.N.; Wang, J.S.; Wu, J.S.; Du, Y.C.; Li, Y.L.; Li, H.Y.; Yang, Y.L.; Jia, X.J. Visible-Light Responsive Cr(VI) Reduction by Carbonyl Modification Nb3O7(OH) Nanoaggregates. J. Mater. Sci. 2018, 53, 12065–12078. [Google Scholar] [CrossRef]
- de Moraes, N.P.; de Siervo, A.; Campos, T.M.; Thim, G.P.; Rodrigues, L.A. Structure-Directing Ability of the Kraft-Lignin/Cellulose Carbon Xerogel for the Development of C-Nb2O5 Sunlight-Active Photocatalysts. J. Photochem. Photobiol. A Chem. 2023, 441, 114697. [Google Scholar] [CrossRef]
- Yang, J.; Hao, J.Y.; Xu, S.Y.; Dai, J.; Wang, Y.; Pang, X.C. Visible-Light-Driven Photocatalytic Degradation of 4-CP and the Synergistic Reduction of Cr(VI) on One-Pot Synthesized Amorphous Nb2O5 Nanorods/Graphene Heterostructured Composites. Chem. Eng. J. 2018, 353, 100–114. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Prashanth, M.K.; Shanavaz, H.; Parashuram, L.; Alharti, F.A.; Jeon, B.H.; Raghu, M.S. Green and Facile Synthesis of Strontium Doped Nb2O5/RGO Photocatalyst: Efficacy towards H2 Evolution, Benzophenone-3 Degradation and Cr (VI) Reduction. Catal. Commun. 2023, 173, 106560. [Google Scholar] [CrossRef]
- Alias, N.; Hussain, Z.; Tan, W.K.; Kawamura, G.; Muto, H.; Matsuda, A.; Lockman, Z. Photoreduction of Cr(VI) in Wastewater by Anodic Nanoporous Nb2O5 Formed at High Anodizing Voltage and Electrolyte Temperature. Environ. Sci. Pollut. Res. 2022, 29, 60600–60615. [Google Scholar] [CrossRef]
- Josué, T.G.; Almeida, L.N.B.; Lopes, M.F.; Santos, O.A.A.; Lenzi, G.G. Cr (VI) Reduction by Photocatalyic Process: Nb2O5 an Alternative Catalyst. J. Environ. Manag. 2020, 268, 110711. [Google Scholar] [CrossRef]
- Yang, X.P.; Zou, R.Y.; Huo, F.; Cai, D.C.; Xiao, D. Preparation and Characterization of Ti/SnO2-Sb2O3-Nb2O5/PbO2 Thin Film as Electrode Material for the Degradation of Phenol. J. Hazard. Mater. 2009, 164, 367–373. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Unuabonah, E.I.; Alfred, M.O.; Ogunlaja, A.; Ogunlaja, O.O.; Omorogie, M.O.; Olukanni, O.D. Toxicity and Removal of Parabens from Water: A Critical Review. Sci. Total Environ. 2021, 792, 148092. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, A.; Chowdhury, A.; Biswas, A.; Roy, S.; Majumdar, S.; Paul, S. Harmful Effect of Personal Care Products on Ecosystem and the Possible Alternative Approach. Biocatal. Agric. Biotechnol. 2024, 57, 103065. [Google Scholar] [CrossRef]
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and Personal Care Products as Emerging Contaminants: Need for Combined Treatment Strategy. J. Hazard. Mater. Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Ren, Y.Z.; Franke, M.; Anschuetz, F.; Ondruschka, B.; Ignaszak, A.; Braeutigam, P. Sonoelectrochemical Degradation of Triclosan in Water. Ultrason. Sonochem 2014, 21, 2020–2025. [Google Scholar] [CrossRef]
- Silva, L.M.; Silva, L.R.; Motheo, A.J. Using Niobium/BDD Anode-Based Multi-Cell Flow Reactor for the Electrochemical Oxidation of Methyl Paraben in the Presence of Surfactants. J. Water Process Eng. 2021, 44, 102439. [Google Scholar] [CrossRef]
- Fuziki, M.E.K.; Ribas, L.S.; Abreu, E.; Fernandes, L.; dos Santos, O.A.A.; Brackmann, R.; de Tuesta, J.L.D.; Tusset, A.M.; Lenzi, G.G. N-Doped TiO2-Nb2O5 Sol–Gel Catalysts: Synthesis, Characterization, Adsorption Capacity, Photocatalytic and Antioxidant Activity. Catalysts 2023, 13, 1233. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.D.; Qiu, J.P.; Wen, Z.R.; Luo, X.H.; Bian, C.Q.; Chen, J.; Luo, M.F. Insights into the Photocatalytic Degradation of Triclosan over Amorphous Nb2O5 Catalysts. Mater. Res. Express 2020, 7, 112502. [Google Scholar] [CrossRef]
- Castro, D.C.; Cavalcante, R.P.; Jorge, J.; Martines, M.A.U.; Oliveira, L.C.S.; Casagrande, G.A.; Machulek, A. Synthesis and Characterization of Mesoporous Nb2O5 and Its Application for Photocatalytic Degradation of the Herbicide Methylviologen. J. Braz. Chem. Soc. 2016, 27, 303–313. [Google Scholar] [CrossRef]
- Paris, E.C.; Malafatti, J.O.D.; Sciena, C.R.; Neves Junior, L.F.; Zenatti, A.; Escote, M.T.; Moreira, A.J.; Freschi, G.P.G. Nb2O5 Nanoparticles Decorated with Magnetic Ferrites for Wastewater Photocatalytic Remediation. Environ. Sci. Pollut. Res. 2021, 28, 23731–23741. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.G.; Asencios, Y.J.O. Development of a Solid Catalyst Based on Pt Supported on Heterostructure (NaNbO3/NaNb3O8/NiO) Applied to the Photodegradation of Phenol in Seawater. Catalysts 2022, 12, 1565. [Google Scholar] [CrossRef]
- Zakaria, W.F.W.; Jalil, A.A.; Hassan, N.S.; Ibrahim, M.; Azami, M.S. Visible-Light Driven Photodegradation of Phenol over Niobium Oxide-Loaded Fibrous Silica Titania Composite Catalyst. J. Chem. Technol. Biotechnol. 2020, 95, 2638–2647. [Google Scholar] [CrossRef]
- Ma, J.; Qin, G.T.; Wei, W.; Xiao, T.L.; Liu, S.M.; Jiang, L. Anti-Corrosion Porous RuO2/NbC Anodes for the Electrochemical Oxidation of Phenol. RSC Adv. 2019, 9, 17373–17381. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R.; Gomathisankar, P. Phenol-Photodegradation on ZrO2. Enhancement by Semiconductors. Spectrochim. ACTA PART A Mol. Biomol. Spectrosc. 2012, 92, 201–206. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Satoshi, I.; Abdullah, A.Z.; Mohamed, A.R. Enhanced Sunlight Photocatalytic Performance over Nb2O5/ZnO Nanorod Composites and the Mechanism Study. Appl. Catal. A Gen. 2014, 471, 126–135. [Google Scholar] [CrossRef]
- Bai, J.; Huang, Y.L.; Wei, D.; Fan, Z.; Seo, H.J. Synthesis and Characterization of Semiconductor Heterojunctions Based on Zr6Nb2O17 Nanoparticles. Mater. Sci. Semicond. Process 2020, 112, 105010. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Mohamed, A.R. Fabrication of ZnO Nanorods via a Green Hydrothermal Method and Their Light Driven Catalytic Activity towards the Erasure of Phenol Compounds. Mater. Lett. 2016, 167, 141–144. [Google Scholar] [CrossRef]
- Ma, J.; Wei, W.; Qin, G.T.; Jiang, L.; Wong, N.H.; Sunarso, J.; Liu, S.M. Electrochemical Oxidation of Phenol in a PtRu/NbC Membrane-Based Catalytic Nanoreactor. J. Environ. Chem. Eng. 2023, 11, 111128. [Google Scholar] [CrossRef]
- Gargouri, O.D.; Samet, Y.; Abdelhedi, R. Electrocatalytic Performance of PbO2 Films in the Degradation of Dimethoate Insecticide. Water SA 2013, 39, 31–37. [Google Scholar] [CrossRef]
- Reddy, G.R.; Balasubramanian, S.; Chennakesavulu, K. Zeolite Encapsulated Active Metal Composites and Their Photocatalytic Studies for Rhodamine-B, Reactive Red-198 and Chloro-Phenols. RSC Adv. 2015, 5, 81013–81023. [Google Scholar] [CrossRef]
- Tsai, S.J.; Cheng, S.F. Physico-Chemical Properties of Nb/Ti Binary Oxides and Their Photo-Catalytic Activities. J. Chin. Chem. Soc. 2001, 48, 1009–1016. [Google Scholar] [CrossRef]
- Zhang, D.F.; Meng, X.; Meng, Y.; Pu, X.P.; Ge, B.; Li, W.Z.; Dou, J.M. One-Pot Molten Salt Synthesis of CdNb2O6/Cd2Nb2O7 Heterojunction Photocatalysts with Enhanced Photocatalytic Properties. Sep. Purif. Technol. 2017, 186, 282–289. [Google Scholar] [CrossRef]
- Grzegorzek, M.; Wartalska, K.; Kowalik, R. Occurrence and Sources of Hormones in Water Resources—Environmental and Health Impact. Environ. Sci. Pollut. Res. 2024, 31, 37907–37922. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical Degradation of Estrone Using a Boron-Doped Diamond Anode in a Filter-Press Reactor. Electrochim. Acta 2016, 197, 186–193. [Google Scholar] [CrossRef]
- Nippes, R.P.; Gomes, A.D.; Macruz, P.D.; de Souza, M. Photocatalytic Removal of 17β-Estradiol from Water Using a Novel Bimetallic NiCu/Nb2O5 Catalyst. Environ. Sci. Pollut. Res. 2023, 30, 103731–103742. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.F.; Barrett, D.H.; Carrer, H.; Figueroa, S.J.A.; Teixeira-Neto, E.; Curvelo, A.A.S.; Rodella, C.B. Morphological, Structural, and Chemical Properties of Thermally Stable Ni-Nb2O5 for Catalytic Applications. J. Phys. Chem. C 2019, 123, 3130–3143. [Google Scholar] [CrossRef]
- Ko, J.S.; Le, N.Q.; Schlesinger, D.R.; Johnson, J.K.; Xia, Z. Novel Niobium-Doped Titanium Oxide Towards Electrochemical Destruction of Forever Chemicals. Sci. Rep. 2021, 11, 18020. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, F.D.; Tonetto, G.M. Nb2O5 Monolith as an Efficient and Reusable Catalyst for Textile Wastewater Treatment. Sustain. Environ. Res. 2021, 31, 35. [Google Scholar] [CrossRef]
| Countries/Regions | Number of Publications | % |
|---|---|---|
| China | 201 | 33.8 |
| Brazil | 132 | 22.2 |
| India | 56 | 9.4 |
| USA | 50 | 8.4 |
| Japan | 46 | 7.7 |
| South Korea | 25 | 4.2 |
| Poland | 19 | 3.1 |
| Spain | 18 | 3.0 |
| Germany | 17 | 2.8 |
| Saudi Arabia | 15 | 2.5 |
| Affiliations | Publication Count |
|---|---|
| Universidade Estadual De Maringa (Brazil) | 25 |
| Chinese Academy Of Sciences (China) | 23 |
| Universidade De Sao Paulo (Brazil) | 23 |
| Universidade Tecnologica Federal Do Parana (Brazil) | 19 |
| Universidade Federal De Sao Carlos (Brazil) | 16 |
| Universidade Federal De Minas Gerais (Brazil) | 15 |
| Centre National De La Recherche Scientifique (France) | 13 |
| Adam Mickiewicz University (Poland) | 11 |
| Nanjing University (China) | 11 |
| Universidade Estadual Paulista (Brazil) | 11 |
| Universidade Federal De Lavras (Brazil) | 11 |
| National Institute For Materials Science (Japan) | 10 |
| Empresa Brasileira De Pesquisa Agropecuaria (Brazil) | 9 |
| Jiangsu University (China) | 9 |
| Jilin University (China) | 9 |
| United States Department Of Energy (DOE) (USA) | 9 |
| Universidade Federal De Pelotas (Brazil) | 9 |
| Universidade Federal Do Rio Grande Do Norte (Brazil) | 9 |
| Consejo Superior De Investigaciones Cientificas (Spain) | 8 |
| Universidade Federal Do Rio Grande Do Sul (Brazil) | 8 |
| Wuhan University Of Technology (China) | 8 |
| Zhengzhou University (China) | 8 |
| East China University Of Science Technology (China) | 7 |
| Universidade De Brasilia (Brazil) | 7 |
| Universidade Estadual De Campinas (Brazil) | 7 |
| Material | AOP | Pollutant | Dye Removal | Reference |
|---|---|---|---|---|
| Ti/SnO2-Nb2O5 electrodes | Electrochemical | 232 Pt-Co | 83% (30 min) | [142] |
| Bi3NbO7 | Photocatalysis (Visible light (vis)) | Acid red G | 88% (120 min) | [91] |
| Nb2O5/glass foams | Photocatalysis (UV-C) | Acid yellow 23 BY | 91.1% (300 min) | [143] |
| Bentonite clay/Nb2O5 | Photocatalysis (UV) | Blue 19 | 98% (120 min) | [144] |
| ZnO-Nb2O5 | Photocatalysis (UV) | Bromophenol blue | 81% (120 min) | [145] |
| NbO-BRGO | Photocatalysis (UV–Vis) | Crystal Violet (CV) | 97% (90 min) | [146] |
| TiO2/Nb2O5/SnO2/RGO | Photocatalysis (Visible light) | CV and MO | 100% and 95% (120 min) | [147] |
| SrNbO2N | Photocatalysis (Visible light) | DCIP | 46% (180 min) | [148] |
| Nb2O5 | Photocatalysis (UV) | Indigo Carmine | 85% (90 min) | [149] |
| Nb2O5 and ZnNb2O6 | H2O2 | MB | 100% (60 min)/91% (240 min) | [88] |
| Nb2O5-OX catalyst | Photocatalysis (UV)/Fenton | 86% (300 min) | [78] | |
| Nb-substituted goethite | Fenton | 85% (120 min) | [150] | |
| CdS@Nb2O5 | Photocatalysis (UV–Vis) | 70% (120 min) | [151] | |
| Ti–Nb alloy | Photocatalysis (Visible light) | 82% (120 min) | [152] | |
| NbCeOx | H2O2 | 92% (120 min) | [153] | |
| Hexagonal prism-shaped niobium oxide | Photocatalysis (UV) | 100% (120 min) | [154] | |
| Nb2O5 (1000 °C) | Photocatalysis (UV–Vis) | 58% (250 min) | [155] | |
| 2.0 CeO2–Nb2O5 | Photocatalysis (UV–Vis)/Photocatalysis (Vis + H2O2) | 82% (150 min)/100% (150 min) | [156] | |
| Nb-N-TiO2 | Photocatalysis (Solar light) | 97% (45 min) | [157] | |
| Phosphate-doped Nb2O5 | H2O2 | 78% (120 min) | [71] | |
| NbC/C (800 °C) | Photocatalysis (Visible light) | 100% (8 h) | [158] | |
| TiO2:Nb | Photocatalysis (UV–Vis) | 93% (120 min) | [159] | |
| g-C3N4/Nb2O5 | Photocatalysis (UV–Vis) | 70% (210 min)/44% (210 min) | [160] | |
| Fe3−xNbxO4 | Fenton | 90% (120 min) | [161] | |
| Nb2O5-doped TiO2 (600 °C) | Photocatalysis (UV) | 85% (90 min) | [162] | |
| Nb2O5-doped TiO2 | Photocatalysis (UV–Vis) | 100% (2.5 h)/87% (7 h) | [163] | |
| NbC/C—cigarette litter | Photocatalysis (Visible light) | 54.38% (8 h) | [164] | |
| KSrNb-6 | Photocatalysis (UV) | 40% (300 min) | [165] | |
| XC-wNb | Photocatalysis (Visible light) | 60% (300 min) | [166] | |
| Nb-doped hematite | Fenton | 75% (120 min) | [167] | |
| Fe2−xNbxO3 | Photocatalysis (UV–Vis) | 70% (60 min) | [168] | |
| Fe73.5Si13.5B9Cu1Nb3—alloy ribbon | Fenton | MB and Methyl Orange (MO) | 85% (20 min) | [169] |
| TiO2/Nb2O5/PANI and TiO2/Nb2O5/RGO | Photocatalysis (Visible light) | 97% and 94% (240 min)/95% and 92% (240 min) | [170] | |
| p-NiO/n-Nb2O5 nanocomposites | Photocatalysis (Visible light) | MO | 94% (180 min) | [116] |
| NbO2OH | Photocatalysis (Solar light) | 100% (10 min) | [171] | |
| Nb2O5 | Photocatalysis (Solar light) + H2O2 | 98% (40 min) | [172] | |
| Nb2O5 with CAC | Photocatalysis (UV–Vis) | Ponceau-S | 100% (120 min) | [173] |
| Ta2O5-Nb2O5-N | Photocatalysis (Solar light) | P-Rosaniline Hydrochloride | 50 min (100%) | [103] |
| Nb2O5 (500 °C) | Photocatalysis (UV) | Real textile effluent | 80% (300 min) | [174] |
| Nb2O5:CB | Photocatalysis (UV–Vis) | 93% (5 h) | [175] | |
| Nb2O5 nanoparticles—oxidant-peroxo method (OPM) | Photocatalysis (UV–Vis) | RhB | 70% (250 min)/37% (250 min) | [36] |
| H+/nanoscrolls, H+/nanosheets, K4Nb6O17, HxK4−xNb6O17, Nb2O5 | Photocatalysis (Visible light) | 99% (20 min), 99% (20 min), 8% (40 min), 16% (40 min), 24% (40 min) | [176] | |
| Nb—S-scheme OCN-[N-NBO/C] nanocomposites | Photocatalysis (Visible light) | 99% (30 min) | [177] | |
| Graphene aerogel composite Nb2O5-g-C3N4/rGA | Photocatalysis (Visible light) | 95% (100 min) | [105] | |
| Nb2O5/Nb2CTx composites | Photocatalysis (Visible light) | 98.5 (120 min) | [108] | |
| Nb2O5/BiOCl composite | Photocatalysis (Visible light) | 96.7% (120 min) | [89] | |
| Microwave-assisted hydrothermal Nb2O5 | Photocatalysis (UV) | 98.9% (60 min) | [178] | |
| KNbO3 perovskite nanostructures | Photocatalysis (UV) | 40% (180 min) | [80] | |
| 1% Fe-Nb2O5 | Photocatalysis (UV) | 100% (60 min) | [79] | |
| Pseudohexagonal Nb2O5/Orthorhombic Nb2O5 | Photocatalysis (UV) | 97% and 57% (150 min) | [124] | |
| Bi4NbO8Cl/BiOCl/Nb2O5 | Photocatalysis (UV–Vis) | 98% (90 min) | [65] | |
| g-C3N4/Nb2O5 nanofibers | Photocatalysis (Visible light) | 100% (120 min) | [73] | |
| Nb2O5 nanospheres | Photocatalysis (Visible light) | 99.9% (15 min) | [112] | |
| Nb doped TiO2/RGO | Photocatalysis (Visible light) | 98% (90 min) | [179] | |
| N-doped HTiNbO5 | Photocatalysis (Visible light) | 95% (100 min) | [180] | |
| Zn-doped Nb2O5 | Photocatalysis (UV–Vis) | 85% (180 min)/78% (180 min) | [181] | |
| Nb/TiO2 NPs | Photocatalysis (Solar light/UV) | 65% (120 min)/70% (120 min) | [96] | |
| F–C/Nb2O5 | Photocatalysis (Visible light) | 73% (60 min) | [182] | |
| NaNbO3/Na2Nb4O11 | Photocatalysis (UV) | 95% (250 min) | [183] | |
| Nitrogen-doped KNbO3 nanocubes | Photocatalysis (Visible light) | RhB and Orange G | 90% (18 h)/84% (24 h) | [184] |
| NbC/C nano-composites | Photocatalysis (Visible light) | RhB, MB, and MO | 78.6%, 67.8%, and 57.1% (120 min) | [185] |
| Nb2O5/g-C3N4 | Photocatalysis (Visible light) | 94%, 87%, and 15% (60 min) | [109] | |
| Nb2O5NC | Photocatalysis (UV) | RR120 | 98% (120 min) | [114] |
| Material | AOP | Pollutant | Pollutant Removal | Reference |
|---|---|---|---|---|
| Nb/BDD electrode | Electrochemical oxidation | Ibuprofen | 80% (300 min) | [67] |
| Structured Sol–Gel Nb2O5 Structured Sol–Gel Nb2O5 Nb-TNFs | Photocatalysis (UV) | 92% (300 min) | [69] | |
| Catalytic ozonation | 100% (30min) | |||
| Photocatalytic ozonation (UV) | 100% (12 min) | |||
| Non-calcined Nb2O5 Ag/Nb2O5 Immobilized in Biopolymer | Photocatalysis (UV) | 100% (300 min) | [75] | |
| Photocatalysis Continuous (UV) | 36% (40 min) | |||
| Nb2O5/RGO wrapped on MoO3 nanorods | Photocatalysis (vis) | 95% (240 min) | [186] | |
| Ti/IrO2–Nb2O5 electrode Nb2O5/C3N4 (NOCN) p–n g-C3N4-mNb2O5 ZnS1−x layers coated Nb2O5−x | Photocatalysis (UV) | Ibuprofen | 44.5% (120 min) | [11] |
| Acetylsalicylic acid | 46% (120 min) | |||
| Paracetamol | 55% (120 min) | |||
| 17α-etinylestradiol | 88.7% (120 min) | |||
| Nb2O5/g-C3N4 Nb2O5/Nb2CTX V/NaNbO3 | Photocatalysis (UV) | 17α-etinylestradiol | 77.7% (120 min) | [187] |
| Photocatalysis (UV) | 69.2% (120 min) | |||
| Photocatalysis Continuous (UV) | 37.3% (120 min) | |||
| Nb/BDD electrode Nb/BDD electrode | Photocatalysis (VIS) | Sulfasalazine | 88.2% (60 min) | [74] |
| Ciprofloxacin | 85.4% (60 min) | |||
| Cd0.5Zn0.5S/Nb2O5 ZOMO–NbOₓ Nb2O5/H2O2 | Photoelectrochemical (UV–Vis) | Levofloxacin | 100% (90 min) | [188] |
| Photolysis | 100% (90 min) | |||
| Electrochemical | <10% (90 min) | |||
| Nb2O5/C | Photocatalytic-assisted activation of persulfate (UV) | Tetracycline | 95% (160 min) | [189] |
| C3N4/Nb2O5 | Photocatalysis (vis) | 97.5% (180 min) | [190] | |
| Fe2O3/Nb2O5 | Photocatalysis (vis) | 70% (80 min) | [191] | |
| Nb/BDD electrode | Photocatalysis (UV–Vis) | 90% (60 min) | [192] | |
| Nb/BDD electrode | Photocatalysis (vis) | 91.2% (180 min) | [108] | |
| Nb2O5 nanofibers Fe/Nb2O5 Cu/Nb2O5 + B51AA50:D52 | Photocatalysis (vis) | Tetracycline | 60% (90 min) | [193] |
| Ciprofloxacin | 71% (150 min) | |||
| Enrofloxacin | 64.6% (150 min) | |||
| Fe/Nb2O5 | Electrochemical oxidation | Atenelol | 100% (240 min) | [194] |
| Cu–Fe/Nb2O5 | Electrochemical oxidation | Atenelol | 100% (240 min) | [68] |
| Zr-doped g-C3N4/Nb2O5 CoFe2O4@Nb2O5 | Photocatalysis (vis) | Cephalexin | 78% (60 min) | [61] |
| Ciprofloxacin | 83% (60 min) | |||
| CeO2–Nb2O5 | Photocatalysis (vis) | Ciprofloxacin | 98% (60 min) | [195] |
| Pt–TiO2– Nb2O5 | H2O2 | 95% (30 min) | [71] | |
| Nb/BDD electrode | Electrochemical generation of H2O2 | Levofloxacin | 96% (270 min) | [196] |
| Nb/BDD electrode | Photocatalysis (vis) | 91% (120 min) | [197] | |
| Nb2O5/Ti electrodes Nb/BDD electrode Structured Sol–Gel Nb2O5 | hv + H2O2 + Fe/Nb | Caffeine | 100% (50 min) | [70] |
| Heterogeneous Fenton | <10% (120 min) | |||
| hv + H2O2 + Fe/Nb | Catechol | 94% (240 min) | ||
| Heterogeneous Fenton | 95% (240 min) | |||
| Structured Sol–Gel Nb2O5 Nb-TNFs Non-calcined Nb2O5 | Electrochemical oxidation | Sulfamethoxazole | 88.8% (150 min) | [10] |
| Propranolol | 96% (150 min) | |||
| Carbamazepine | 82.5% (150 min) | |||
| Ag/Nb2O5 immobilized in biopolymer Nb2O5/RGO wrapped on MoO3 nanorods Ti/IrO2–Nb2O5 electrode Nb2O5/C3N5 (NOCN) p–n | Electro-Fenton process | Pentachlorophenol | 100% (75 min) | [9] |
| Terbutryn | 84.1% (75 min) | |||
| Chlorofenvinphos | 46.2% (75 min) | |||
| Diclofenac | 100% (75 min) | |||
| g-C3N4-mNb2O5 | Photocatalysis (UV) | Fluoxetine | 78% (90 min) | [198] |
| ZnS1−x layers coated Nb2O5−x | Photocatalysis (vis) | Triclosan | 90% (200 min) | [50] |
| Nb2O5/g-C3N4 | Photocatalysis (UV) | Salicylic acid | 23% (120 min) | [199] |
| Nb2O5/Nb2CTX | <10% (120 min) | |||
| V/NaNbO3 | 22% (120 min) | |||
| Nb/BDD electrode | Photocatalysis (vis) | Levofloxacin | 80.15% (240 min) | [64] |
| Nb/BDD electrode | Photocatalysis (UV) | Paracetamol | 97.5% (60 min) | [27] |
| Cd0.5ZnS0.5/Nb2O5 | Photocatalysis (UV) | Metformin | 67% (210 min) | [200] |
| ZOMO–NbOₓ Nb2O5/H2O2 | Photocatalysis (UV) | Diclofenac | 100% (30 min) | [90] |
| Ketoprofen | 100% (60 min) | |||
| Nb2O5/C | Electrochemical oxidation | Prednisone | 78% (240 min) | [201] |
| C3N4/Nb2O5 | Electro-Fenton process | Cefoperazone | 96.5% (60 min) | [202] |
| Fe2O3/Nb2O5 | Electrochemical oxidation | Defluorination | 82.5% (120 min) | [203] |
| Nb/BDD electrode | Photocatalysis (vis) | Carbamazepine | 92 (120%) | [87] |
| Material | AOP | Pollutant | Heavy Metal Removal | Reference |
|---|---|---|---|---|
| Nb2O5 nanospheres | Photocatalysis (Visible light) | Pb | 100% (80 min) | [206] |
| Nb2O5/microalgae C. reinhardtii | Photocatalysis (UV) | Cr(VI) | 71% (120 min) | [207] |
| Cu0.5/Nb2O5 | Photocatalysis (UV) | Hg | 95% (200 min; 150 °C) | [208] |
| Nb-Co-Ce/Al2O3 (electrode) | Oxidation | 75% (60 min) | [209] | |
| Nb-doped TiO2 | Oxidação | Id | 70% (90 min) | [102] |
| Photoreduction (UV) | Cd | 100% (120 min) | ||
| Ti57Nb26Cu17 | Photoreduction (Solar light) | Cr | 97% (50 min) | [210] |
| Nb3O7(OH) | Photoreduction (UV) | 90% (50 min) | [211] | |
| K3NbO2F4 | Photoreduction (Solar light) | 30% (3 h) | [212] | |
| Nb2O5 nanorods/graphene | Photoreduction (UV) | 95% (4 h) | [213] | |
| Nb2O5/RGO | Photoreduction (UV) | 87.7% (240 min) | [214] | |
| Nb2O5/CuO | Photoreduction (Solar light) | 84% (240 min) | [97] | |
| Nb2O5 (anodizing) | Photoreduction (UV) | 92% (120 min) | [215] | |
| p-NiO/n- Nb2O5 | Photoreduction (UV) | 97% (180 min) | [116] | |
| Nb2O5/red phosphorus | Photoreduction (UV) | 97% (30 min) | [51] | |
| Nb2O5 nanowires/carbon fibers | Photoreduction (UV) | 99.9% (60 min) | [110] | |
| Nb2O5 (anodic nanoporous) | Photoreduction (UV) | 100% (45 min) | [14] | |
| Nb2O5 | Photoreduction (UV) | 90% (120 min) | [216] |
| Material | AOP | Pollutant | PCP Removal | Reference |
|---|---|---|---|---|
| Nb-C/Fe(III) | Fenton | Dimethyl phthalate (DMP) | 100% (60 min, pH 3.2) | [8] |
| Diamond-coated Nb (electrode) | Sonoelectrochemical | Triclosan | 92% (15 min) | [221] |
| Nb2O5/Ti 3D printed electrode | Electrooxidation | Florfenicol | 97.0% (120 min) | [203] |
| Nb2O5/RGO Sr doped | Photocatalysis (UV) | Benzophenone-3 | 94.6% (120 min) | [214] |
| Fe/Nb2O5-immobilized | Photocatalysis (UV) | Triclosan 2.8-dichlorodibenzene-p-dioxin | 95% (15 min) 99% (15 min) | [50] |
| Cu/Nb2O5 Fe/Nb2O5 Cu-Fe/Nb2O5 | Photocatalysis (UV) | Salicylic Acid | 29% (400 °C, 120 min, pH 3.3) 27% (400 °C, 120 min, pH 7.0) 22% (400 °C, 120 min, pH 7.0) | [199] |
| Niobium BDD/anode | Electrooxidation | Methy paraben | 99% (120 min) | [222] |
| TiO2-Nb2O5 | Photocatalysis (UV) | Salicylic Acid | 72.9% (pH 5.0, 30 min) | [223] |
| Amorphous Nb2O5 | Photocatalysis (UV) | Triclosan | 99.9% (10 min) | [224] |
| Material | AOP | Pollutant | Pollutant Removal | Reference |
|---|---|---|---|---|
| Zr6Nb2O17/Nb2O5 heterojunction | Photocatalysis (UV-B and Visible light) | Phenol | 90% (150 min)/91% (360 min) | [232] |
| Nb2O5/ZnO nanocomposites | Photocatalysis (UV-A) | 100% (60 min) | [233] | |
| PtRu/NbC membrane anode | Electrochemical oxidation | 99.7% (6 min) | [234] | |
| Nb/PbO2 anode | Electrochemical oxidation | Dimethoate | 90% COD removal (8 h) | [235] |
| NbPd-zeolite nanocomposites | Photocatalysis (Visible light) | 2-chloro phenol and 4-chloro phenol | 97% (270 min)/87% (180 min) | [236] |
| Nb/Ti binary oxides | Photocatalysis (UV-B) | Phenol, tetrahydrofuran and | 94.6%/56.8%/51.4% | [237] |
| Cyclohexanol | (100 min) | |||
| CdNb2O6/Cd2Nb2O7 heterojunction | Photocatalysis (UV-A and Visible light) | Phenol | 100% (70 min)/97% (120 min) | [238] |
| Material | AOP | Pollutant | Hormones Removal | Reference |
|---|---|---|---|---|
| Nb/BDD anode | Electrooxidation | Estrone | 98% (30 min, filter-press reactor) | [240] |
| NiCu/Nb2O5 | Photocatalysis (UV-A) | 17β-estradiol | 82% (180 min) | [241] |
| Nb2O5 | Photocatalysis (UV) | 17α-ethinylestradiol | 88.7% (120 min) | [11] |
| Ag/Nb2O5 | Photocatalysis (UV) | 17α-ethinylestradiol | 77.7% (120 min) | [187] |
| KNbO3 | Photocatalysis (UV) | Bisphenol A (BPA) | 78.7% (1200 min) | [184] |
| Bi4NbO8Cl/BiOCl/Nb2O5 | Photocatalysis (UV–Vis) | Bisphenol A | 67% (240 min) | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidelis, M.Z.; Faria, J.; Santacruz, W.; Lima, T.S.; Lenzi, G.G.; Motheo, A.J. Niobium-Based Catalysts in Advanced Oxidation Processes: A Systematic Review of Mechanisms, Material Engineering, and Environmental Applications. Environments 2025, 12, 311. https://doi.org/10.3390/environments12090311
Fidelis MZ, Faria J, Santacruz W, Lima TS, Lenzi GG, Motheo AJ. Niobium-Based Catalysts in Advanced Oxidation Processes: A Systematic Review of Mechanisms, Material Engineering, and Environmental Applications. Environments. 2025; 12(9):311. https://doi.org/10.3390/environments12090311
Chicago/Turabian StyleFidelis, Michel Z., Julia Faria, William Santacruz, Thays S. Lima, Giane G. Lenzi, and Artur J. Motheo. 2025. "Niobium-Based Catalysts in Advanced Oxidation Processes: A Systematic Review of Mechanisms, Material Engineering, and Environmental Applications" Environments 12, no. 9: 311. https://doi.org/10.3390/environments12090311
APA StyleFidelis, M. Z., Faria, J., Santacruz, W., Lima, T. S., Lenzi, G. G., & Motheo, A. J. (2025). Niobium-Based Catalysts in Advanced Oxidation Processes: A Systematic Review of Mechanisms, Material Engineering, and Environmental Applications. Environments, 12(9), 311. https://doi.org/10.3390/environments12090311









