Valorization of Pinecones as Biosorbents for Environmental Remediation of Zn-Contaminated Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemical Characterization of Pinecones
2.3. Physical Properties and Pinecone Surface Characteristics
2.4. Adsorption Studies
3. Results
3.1. Chemical Composition, Ash, and Cationic Content
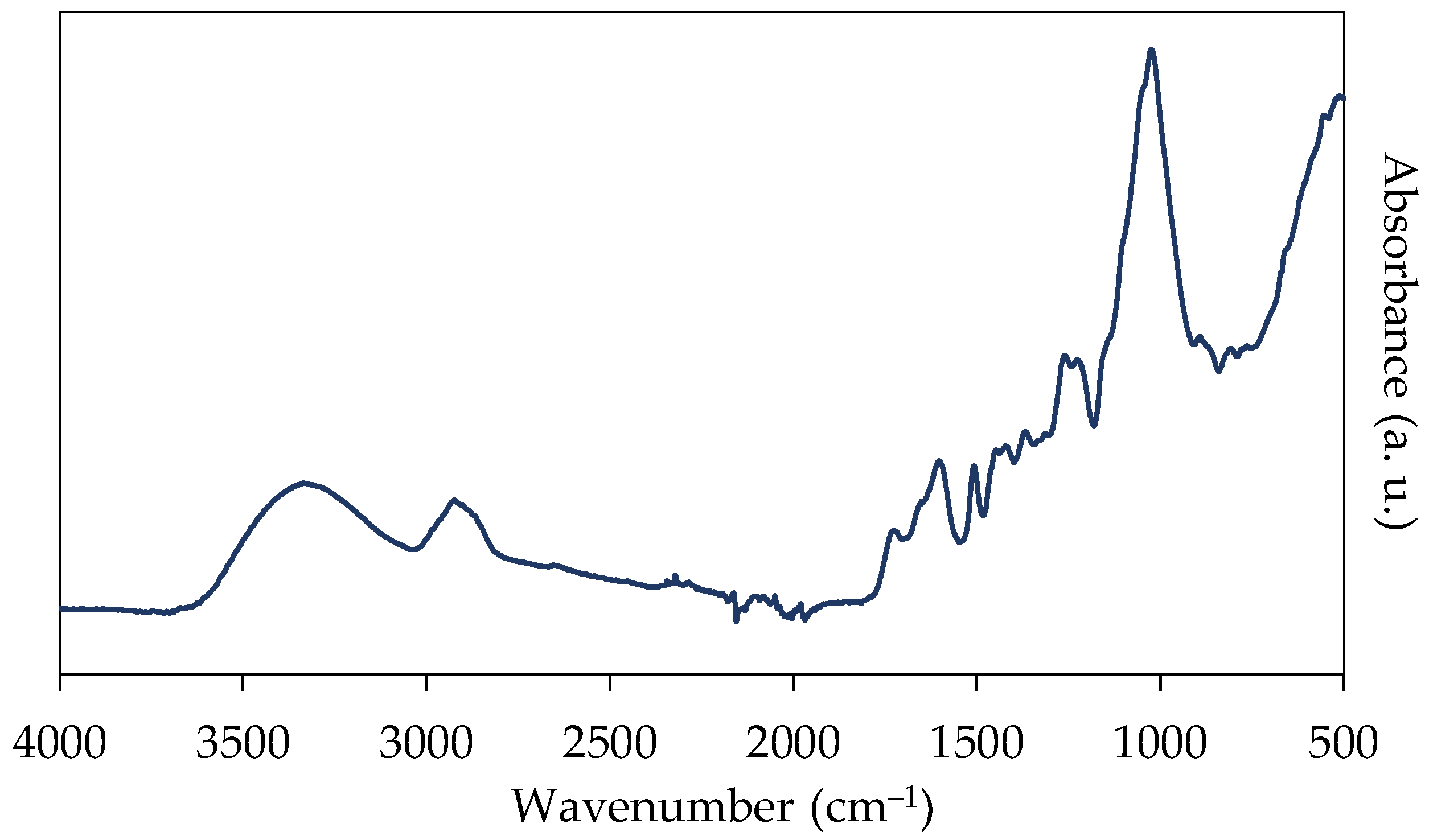
3.2. Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
3.3. Pinecone Surface Characteristics
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Powder X-Ray Diffraction (XRD)
3.3.3. Thermogravimetric Analysis (TGA)
3.4. Adsorption Tests
3.4.1. Effect of Particle Size
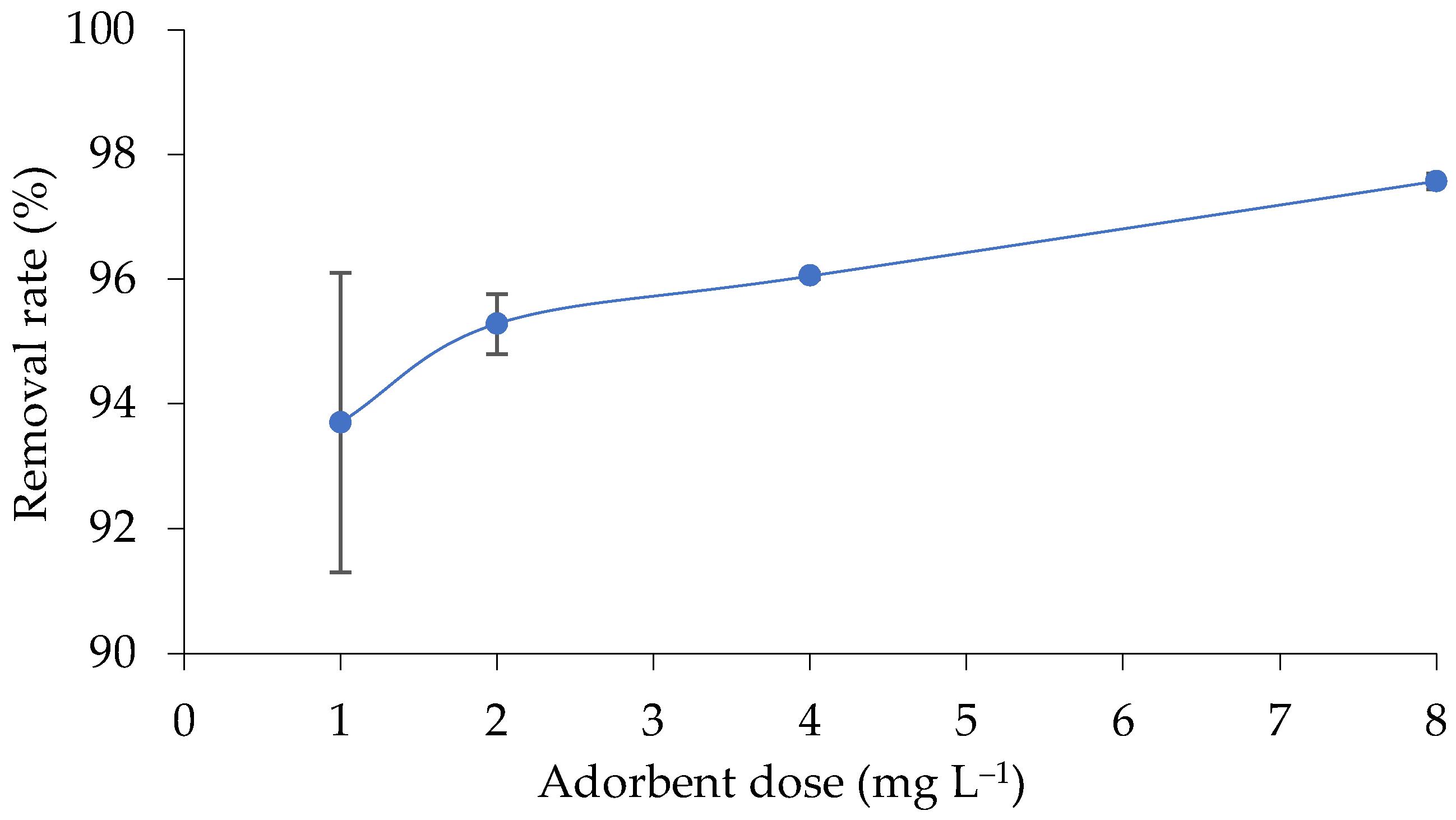
3.4.2. Effect of Adsorbent Dosage
3.4.3. Effect of pH
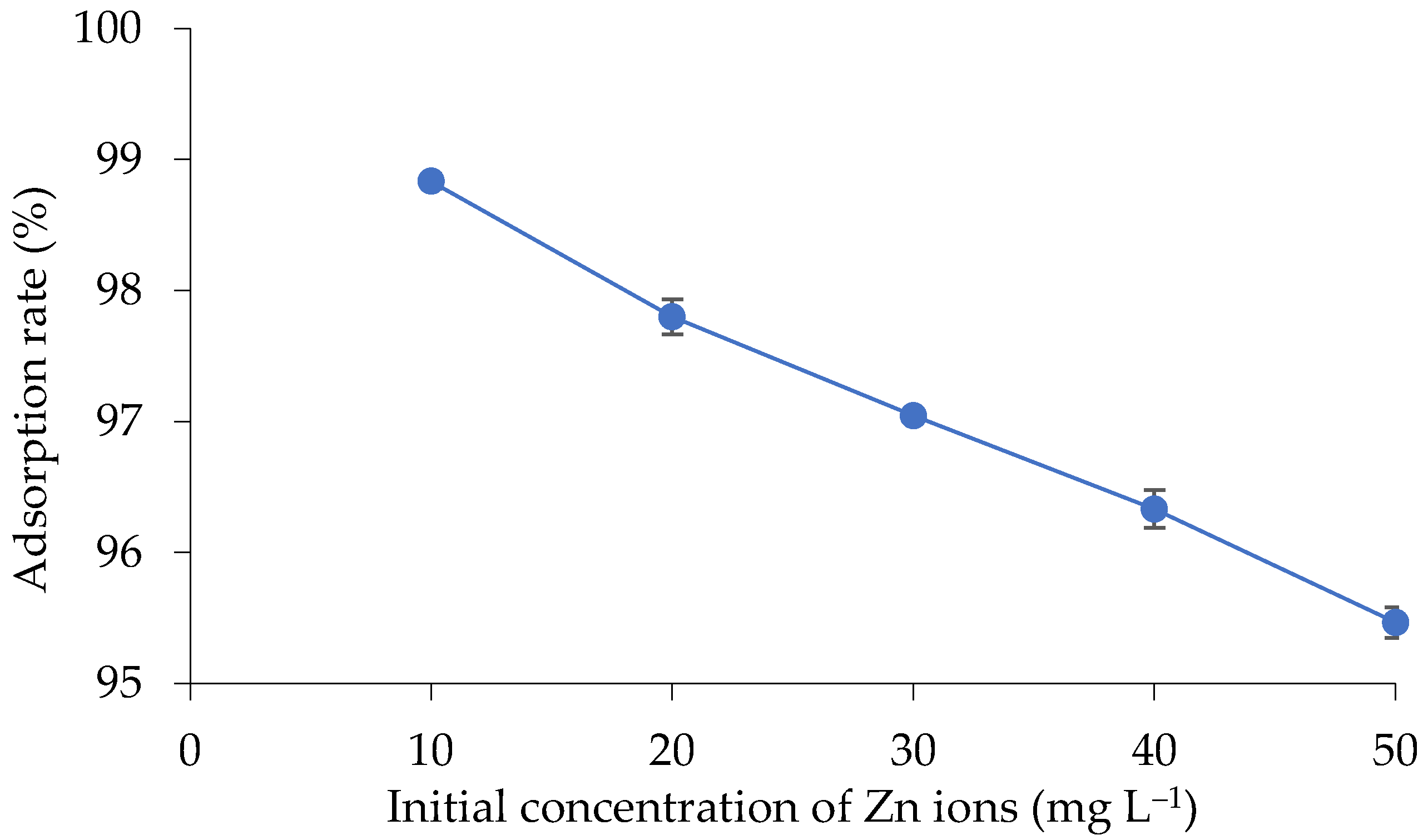
3.4.4. Adsorption Isotherms
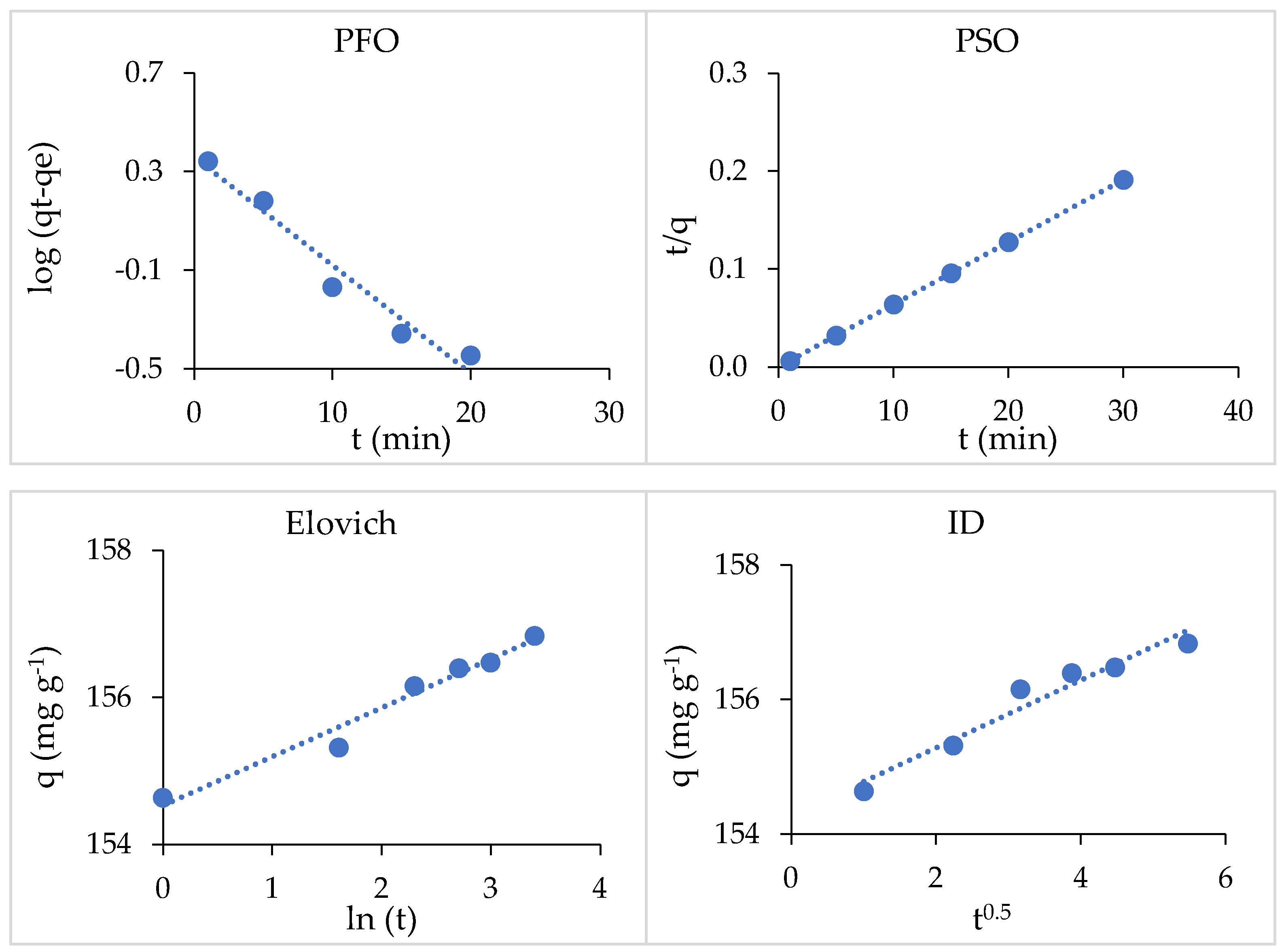
3.4.5. Adsorption Kinetics
4. Discussion
4.1. Chemical Characterization of Pinecones (SPCs)
4.2. Surface Morphology and Structural Analysis
4.3. Adsorption Process
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín-Lara, M.A.; Blázquez, G.; Calero, M.; Almendros, A.I.; Ronda, A. Binary Biosorption of Copper and Lead onto Pine Cone Shell in Batch Reactors and in Fixed Bed Columns. Int. J. Miner. Process. 2016, 148, 72–82. [Google Scholar] [CrossRef]
- Shaikhiev, I.G.; Kraysman, N.V.; Sverguzova, S.V. World Experience in Using Pine Cones to Remove Various Pollutants from Aquatic Environments. Mater. Int. 2025, 7, 29. [Google Scholar]
- Nergiz, C.; Dönmez, İ. Chemical Composition and Nutritive Value of Pinus pinea L. Seeds. Food Chem. 2004, 86, 365–368. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Ramos, P.A.B.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal. Appl. Sci. 2018, 8, 2575. [Google Scholar] [CrossRef]
- Calama, R.; Gordo, J.; Mutke, S.; Conde, M.; Madrigal, G.; Garriga, E.; Arias, M.J.; Piqué, M.; Gandía, R.; Montero, G.; et al. Decline in Commercial Pine Nut and Kernel Yield in Mediterranean Stone Pine (Pinus pinea L.) in Spain. Iforest—Biogeosciences For. 2020, 13, 251. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Buyuksari, U.; Avci, E.; Koc, E. Utilization of Pine (Pinus pinea L.) Cone in Manufacture of Wood Based Composite. For. Ecol. Manag. 2009, 259, 65–70. [Google Scholar] [CrossRef]
- ICNF. Perfil Florestal—Portugal; Instituto da Conservação da Natureza e das Florestas: Lisbon, Portugal, 2021; p. 4. [Google Scholar]
- Almendros, A.I.; Martín-Lara, M.A.; Ronda, A.; Pérez, A.; Blázquez, G.; Calero, M. Physico-Chemical Characterization of Pine Cone Shell and Its Use as Biosorbent and Fuel. Bioresour. Technol. 2015, 196, 406–412. [Google Scholar] [CrossRef]
- Ben Amar, M.; Mallek, M.; Valverde, A.; Monclús, H.; Myers, T.G.; Salvadó, V.; Cabrera-Codony, A. Competitive Heavy Metal Adsorption on Pinecone Shells: Mathematical Modelling of Fixed-Bed Column and Surface Interaction Insights. Sci. Total Environ. 2024, 917, 170398. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, G.; Nemati, E.; Arab Chamjangali, M.; Ashrafi, M. Removal of Lead Ions from Aqueous Solutions Using Functionalized Pine Cone Powder. J. Iran. Chem. Soc. 2021, 18, 2369–2379. [Google Scholar] [CrossRef]
- Amar, M.B.; Walha, K.; Salvadó, V.; Salvestrini, S. Valorisation of Pine Cone as an Efficient Biosorbent for the Removal of Pb(II), Cd(II), Cu(II), and Cr(VI). Adsorpt. Sci. Technol. 2021, 2021, 6678530. [Google Scholar] [CrossRef]
- Şen, A.; Pereira, H.; Olivella, M.A.; Villaescusa, I. Heavy Metals Removal in Aqueous Environments Using Bark as a Biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.K.; Goyal, N.; Bobde, P.; Kwon, E.E.; Lin, K.-Y.A.; Chen, W.-H. A Critical Review on Biochar Production from Pine Wastes, Upgradation Techniques, Environmental Sustainability, and Challenges. Bioresour. Technol. 2023, 387, 129632. [Google Scholar] [CrossRef]
- Değirmen, G.; Kılıç, M.; Çepelioğullar, Ö.; Pütün, A.E. Removal of Copper(II) and Cadmium(II) Ions from Aqueous Solutions by Biosorption onto Pine Cone. Water Sci. Technol. 2012, 66, 564–572. [Google Scholar] [CrossRef]
- Gundogdu, A.; Ozdes, D.; Duran, C.; Bulut, V.N.; Soylak, M.; Senturk, H.B. Biosorption of Pb(II) Ions from Aqueous Solution by Pine Bark (Pinus brutia Ten.). Chem. Eng. J. 2009, 153, 62–69. [Google Scholar] [CrossRef]
- González-Feijoo, R.; Santás-Miguel, V.; Arenas-Lago, D.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Arias-Estévez, M.; Pérez-Rodríguez, P. Effectiveness of Cork and Pine Bark Powders as Biosorbents for Potentially Toxic Elements Present in Aqueous Solution. Environ. Res. 2024, 250, 118455. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) Ions, from Aqueous Solutions, by Adsorption onto Sawdust of Pinus sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Mandal, S.N.; Das, S.K. Adsorption of Zn(II) from Aqueous Solution by Using Different Adsorbents. Chem. Eng. J. 2006, 123, 43–51. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Paradelo, R.; Igrexas-Soto, A.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Garrote, G.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Valorization of Biosorbent Obtained from a Forestry Waste: Competitive Adsorption, Desorption and Transport of Cd, Cu, Ni, Pb and Zn. Ecotoxicol. Environ. Saf. 2016, 131, 118–126. [Google Scholar] [CrossRef]
- Morales-Barrera, L.; Cristiani-Urbina, E. Equilibrium Biosorption of Zn2+ and Ni2+ Ions from Monometallic and Bimetallic Solutions by Crab Shell Biomass. Processes 2022, 10, 886. [Google Scholar] [CrossRef]
- Morales-Barrera, L.; Flores-Ortiz, C.M.; Cristiani-Urbina, E. Single and Binary Equilibrium Studies for Ni2+ and Zn2+ Biosorption onto Lemna Gibba from Aqueous Solutions. Processes 2020, 8, 1089. [Google Scholar] [CrossRef]
- Santos, Y.T.d.C.; da Costa, G.P.; Menezes, J.M.C.; Nunes, J.V.S.; Hosseini-Bandegharaei, A.; Coutinho, H.D.M.; Júnior, D.S.; Filho, F.J.d.P.; Teixeira, R.N.P. Adsorption of Zn(II) IONS by Ziziphus joazeiro Barks in Aqueous Solutions. Results Chem. 2024, 7, 101339. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Multimetal Biosorption Modeling of Zn2+, Cu2+ and Ni2+ by Sargassum ilicifolium. Ecol. Eng. 2014, 71, 197–205. [Google Scholar] [CrossRef]
- T 204 cm-17; Solvent Extractives of Wood and Pulp, Test Method. Technical Association of the Pulp & Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2017.
- T 203; Alpha-, beta- and gamma-cellulose in pulp. Technical Association of the Pulp & Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2008.
- T 222 om-21; Acid Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp & Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2002.
- Bangaraiah, P.; Sarath Babu, B.; Abraham Peele, K.; Rajeswara Reddy, E.; Venkateswarulu, T.C. Removal of Multiple Metals Using Tamarindus Indica as Biosorbent through Optimization of Process Variables: A Statistical Approach. Int. J. Environ. Sci. Technol. 2020, 17, 1835–1846. [Google Scholar] [CrossRef]
- Gonultas, O.; Ucar, M.B. Characteristics of Pinus. Lignocellulose 2013, 2, 262–269. [Google Scholar]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of Non-Timber by-Products from Maritime Pine (Pinus pinaster, Ait) for Particleboard Production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Ucar, M.B.; Ucar, G. Lipophilic Extractives and Main Components of Black Pine Cones. Chem. Nat. Compd. 2008, 44, 380–383. [Google Scholar] [CrossRef]
- Momčilović, M.; Purenović, M.; Bojić, A.; Zarubica, A.; Ranđelović, M. Removal of Lead(II) Ions from Aqueous Solutions by Adsorption onto Pine Cone Activated Carbon. Desalination 2011, 276, 53–59. [Google Scholar] [CrossRef]
- Ouafi, R.; Omor, A.; Gaga, Y.; Akhazzane, M.; Taleb, M.; Rais, Z. Pine Cones Powder for the Adsorptive Removal of Copper Ions from Water. Chem. Ind. Chem. Eng. Q. 2021, 27, 341–354. [Google Scholar] [CrossRef]
- Sahin, H.T.; Yalcin, O.U. Conifer Cones: An Alternative Raw Material for Industry. J. Pharm. Res. Int. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Almendros, A.I.; Calero, M.; Ronda, A.; Martín-Lara, M.A.; Blázquez, G. Influence of Nickel during the Thermal Degradation of Pine Cone Shell. Study of the Environmental Implications. J. Clean. Prod. 2018, 183, 403–414. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zhao, M.; Ma, H.; Chen, D.; Zhang, Y.; Zhou, J. Comparative Study on the Pyrolysis Behaviors of Pine Cone and Pretreated Pine Cone by Using TGA–FTIR and Pyrolysis-GC/MS. ACS Omega 2021, 6, 3490–3498. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, Y.-X.; Lu, H.-H.; Yang, R.-Q.; Yang, S.-M. Competitive Adsorption of Pb(II), Cu(II), and Zn(II) Ions onto Hydroxyapatite-Biochar Nanocomposite in Aqueous Solutions. J. Solid State Chem. 2018, 261, 53–61. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Total extractives (%) | 9.4 ± 0.39 |
| Dichloromethane extractives (%) | 3.6 ± 0.25 |
| Ethanol extractives (%) | 4.8 ± 0.86 |
| Water extractives (%) | 1.0 ± 0.67 |
| Total lignin (%) | 37.0 ± 0.44 |
| Klason lignin (%) | 36.9 ± 0.42 |
| Soluble lignin (%) | 0.1 ± 0.03 |
| Holocellulose (%) | 68.6 ± 0.38 |
| α-cellulose (%) | 49.0 ± 0.81 |
| Hemicelluloses (%) | 19.6 ± 1.07 |
| Ashes (%) | 1.4 ± 0.2 |
| pHpzc | 5.2 |
| Element | |||||
|---|---|---|---|---|---|
| Ca | Na | Mg | Zn | K | |
| Content (mg g−1) | 0.15 | 0.20 | 0.46 | 0.001 | 1.72 |
| Granulometry (Mesh) | Particle Size (mm) | Removal Rate (%) |
|---|---|---|
| 40–60 | 0.420–0.250 | 95.3 |
| 60–80 | 0.250–0.177 | 96.1 |
| <80 | <0.177 | 96.4 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| R2 | qmax (mg g−1) | KL (L mg−1) | R2 | n | KF (mg g−1) |
| 0.96 | 7.92 | 1.15 | 0.99 | 0.54 | 0.08 |
| PFO | PSO | Elovich | ID | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 (L min−1) | qe (mg g−1) | qe exp (mg g−1) | R2 | K2 (g mg−1.min) | h (mg g−1.min) | qe (mg g−1) | qe exp (mg g−1) | R2 | a | b | R2 | C | kdif | R2 |
| 0.04 | 1.43 | 156.84 | 0.96 | 0.006 | 1.00 | 156.25 | 156.84 | 1 | 0.66 | 154.53 | 0.97 | 154.28 | 0.50 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macena, M.; Cruz-Lopes, L.; Grosche, L.; Esteves, B.; Santos-Vieira, I.; Pereira, H. Valorization of Pinecones as Biosorbents for Environmental Remediation of Zn-Contaminated Wastewaters. Environments 2025, 12, 284. https://doi.org/10.3390/environments12080284
Macena M, Cruz-Lopes L, Grosche L, Esteves B, Santos-Vieira I, Pereira H. Valorization of Pinecones as Biosorbents for Environmental Remediation of Zn-Contaminated Wastewaters. Environments. 2025; 12(8):284. https://doi.org/10.3390/environments12080284
Chicago/Turabian StyleMacena, Morgana, Luísa Cruz-Lopes, Lucas Grosche, Bruno Esteves, Isabel Santos-Vieira, and Helena Pereira. 2025. "Valorization of Pinecones as Biosorbents for Environmental Remediation of Zn-Contaminated Wastewaters" Environments 12, no. 8: 284. https://doi.org/10.3390/environments12080284
APA StyleMacena, M., Cruz-Lopes, L., Grosche, L., Esteves, B., Santos-Vieira, I., & Pereira, H. (2025). Valorization of Pinecones as Biosorbents for Environmental Remediation of Zn-Contaminated Wastewaters. Environments, 12(8), 284. https://doi.org/10.3390/environments12080284













