Pesticide Degradation: Impacts on Soil Fertility and Nutrient Cycling
Abstract
1. Introduction
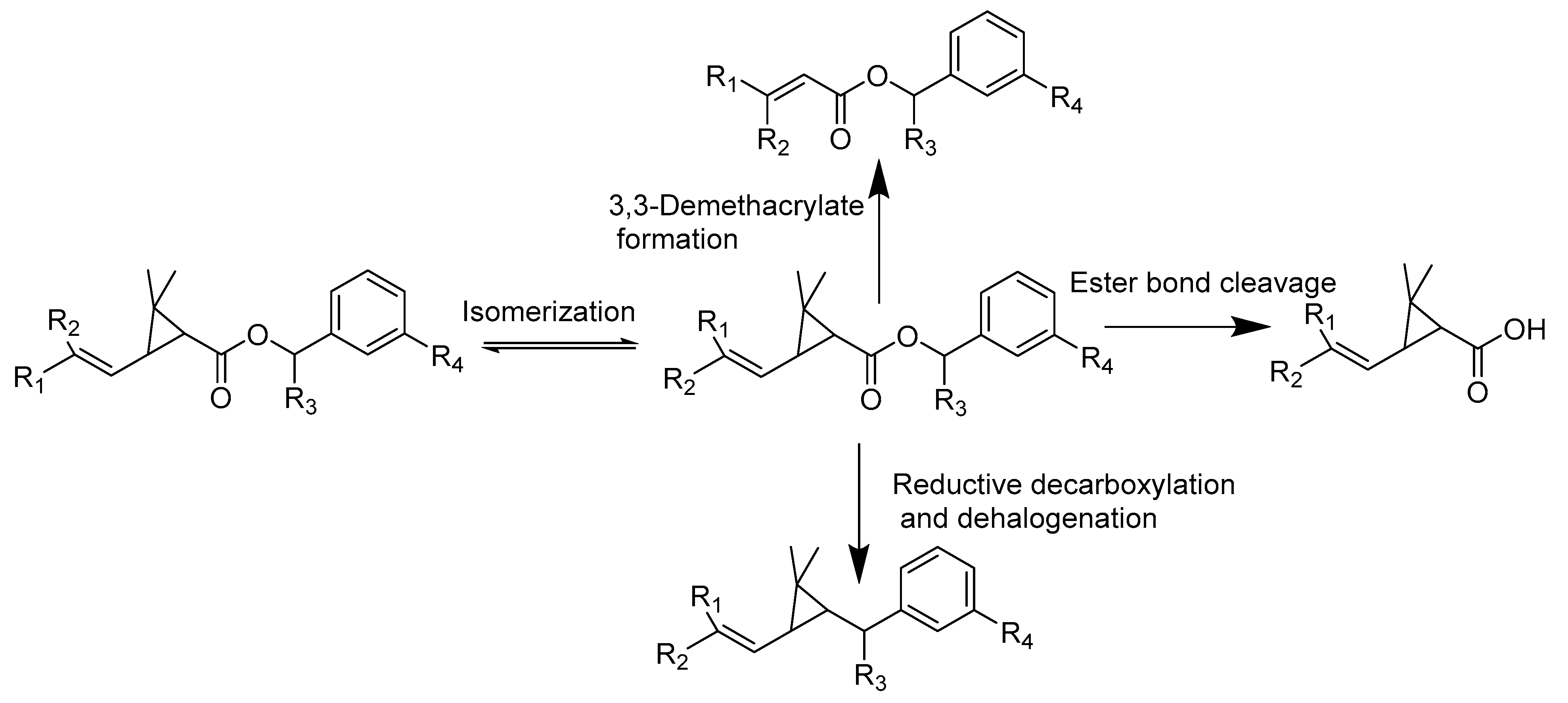
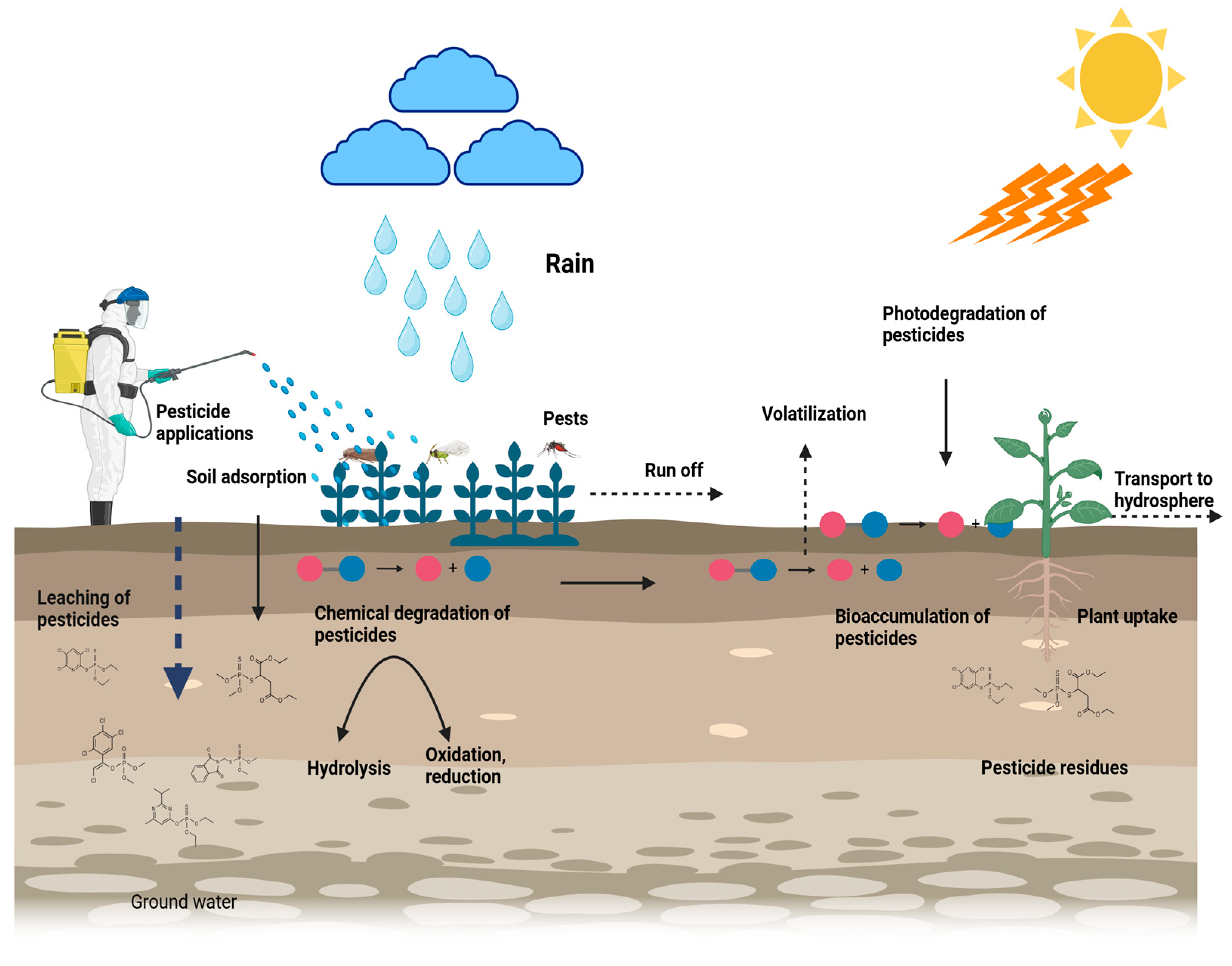
2. Pesticide Degradation Mechanisms in Soil
2.1. Abiotic Degradation
2.1.1. Photodegradation
2.1.2. Hydrolysis
2.1.3. Chemical Oxidation and Reduction
2.2. Biotic Degradation
2.2.1. Microbial Metabolism of Pesticides
2.2.2. Role of Soil Enzymes in Pesticide Breakdown
2.3. Factors Affecting Pesticide Degradation
2.3.1. Soil Type and Composition
2.3.2. Moisture Content and Temperature
2.3.3. Organic Matter Content
2.3.4. pH and Redox Potential
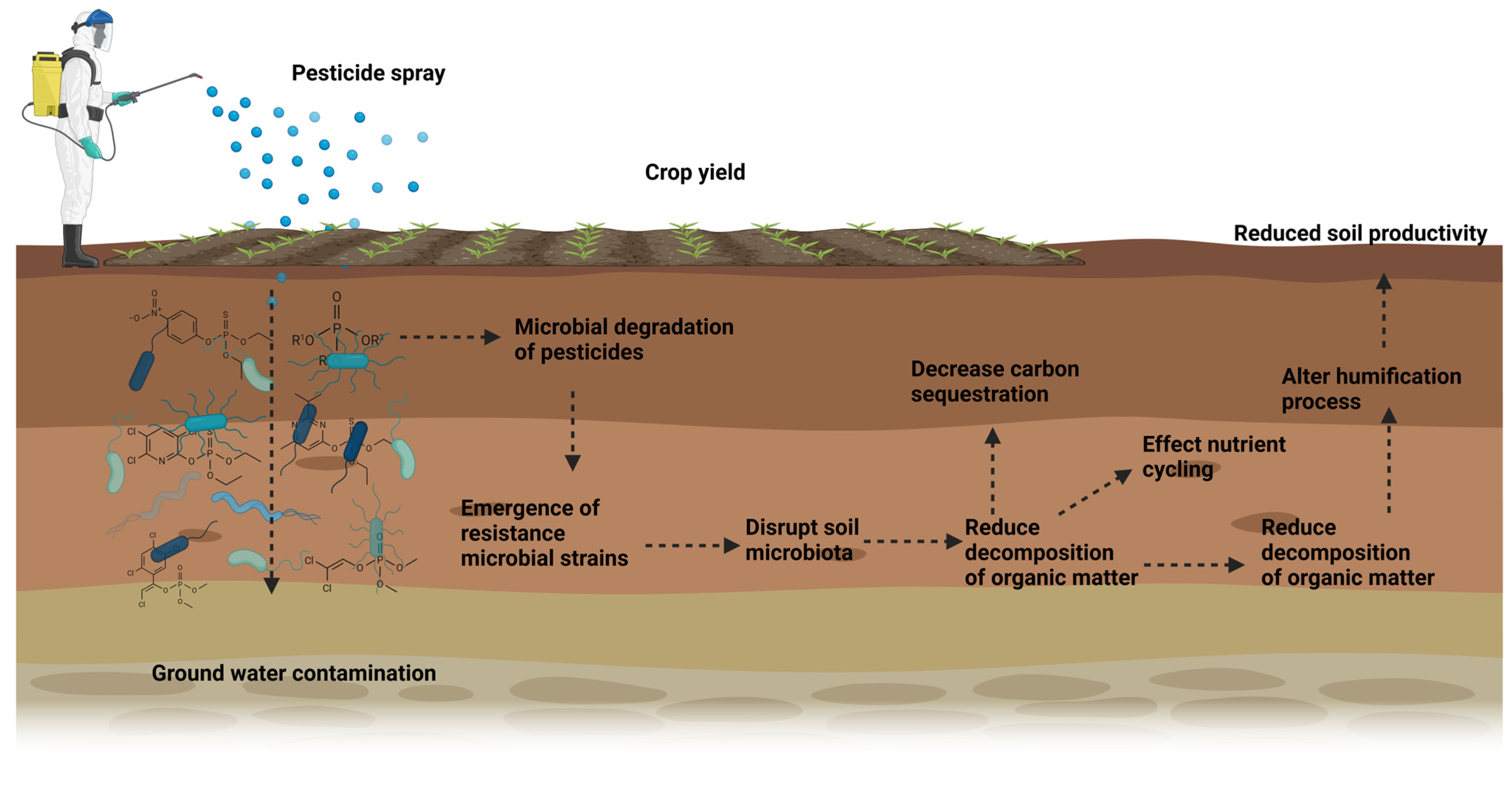
3. Influence of Pesticide Degradation on Soil Fertility
3.1. Effects of Various Pesticides on Soil Microbial Communities
Emergence of Pesticide-Resistant Microbial Strains
3.2. Impact on Soil Organic Matter Decomposition
3.2.1. Alterations in Carbon Cycling and Humus Formation
3.2.2. Potential Role of Pesticide Degradation Products in Soil Carbon Sequestration
3.3. Effects on Soil Physicochemical Properties
3.3.1. Changes in Soil Structure and Porosity
3.3.2. Influence on Water Retention and Infiltration Rates
3.3.3. Pesticide-Induced Alterations in Cation Exchange Capacity (CEC)
4. Influence of Pesticide Degradation on Nutrient Cycling
4.1. Nitrogen Cycle
4.1.1. Effects on Nitrogen Mineralization and Nitrification Processes
4.1.2. Impact of Pesticides on Nitrogen-Fixing Bacteria and Denitrifiers
4.2. Phosphorus Cycle
4.2.1. Alterations in Phosphatase Enzyme Activity and Phosphorus Availability
4.2.2. Role of Pesticide Residues in Phosphate Immobilization or Solubilization
4.3. Potassium and Micronutrient Cycling
5. Environmental and Agricultural Implications
5.1. Reduced Soil Productivity and Crop Yield
5.2. Long-Term Soil Degradation and Loss of Soil Biodiversity
5.3. Groundwater Contamination and Its Feedback on Soil Nutrients
5.4. Risks to Sustainable Agriculture and Food Security
5.5. Emerging Concerns: PFAS (Per- and Polyfluoroalkyl Substances)-Containing Pesticides and Their Soil Implications
6. Sustainable Management Strategies
6.1. Bioremediation and Microbial Inoculation for Pesticide Degradation
6.2. Use of Biochar and Organic Amendments to Enhance Soil Resilience
6.3. Development of Eco-Friendly and Biodegradable Pesticides
6.4. Integrated Pest Management (IPM) to Reduce Pesticide Dependency
6.5. Policy and Regulatory Frameworks for Sustainable Pesticide Use
7. Future Research Directions
7.1. Need for Long-Term Field Studies on Pesticide Degradation and Soil Health
7.2. Advances in Biotechnology for Enhancing Microbial Pesticide Breakdown
7.3. Development of Predictive Models for Pesticide–Soil Interactions Under Climate Change Scenarios
7.4. Assessment of Alternative Pest Control Strategies with Minimal Environmental Impact
7.5. Integration of Omics, Biosensors, and AI-Based Modeling in Pesticide Management
7.6. Soil Carbon Modeling for Future Studies
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Lamichhane, J.R. Pesticide Use and Risk Reduction in European Farming Systems with IPM: An Introduction to the Special Issue. Crop Prot. 2017, 97, 1–6. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The Benefits of Pesticides to Mankind and the Environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of Pesticides Degrading Microbial Communities and Their Environmental Impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Larson, W.E.; Pierce, F.J. The Dynamics of Soil Quality as a Measure of Sustainable Management. In Defining Soil Quality for a Sustainable Environment; Wiley: Hoboken, NJ, USA, 2015; pp. 37–51. ISBN 9780891189305. [Google Scholar]
- Javed, A.; Ali, E.; Binte Afzal, K.; Osman, A.; Riaz, S. Soil Fertility: Factors Affecting Soil Fertility, and Biodiversity Responsible for Soil Fertility. Int. J. Plant Anim. Environ. Sci. 2022, 12, 21–33. [Google Scholar] [CrossRef]
- Zaman, W.; Ayaz, A.; Puppe, D. Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions. Biology 2025, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; Semenov, A.M. In Search of Biological Indicators for Soil Health and Disease Suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
- Khader, E.H.; Muslim, S.A.; Saady, N.M.C.; Ali, N.S.; Salih, I.K.; Mohammed, T.J.; Albayati, T.M.; Zendehboudi, S. Recent Advances in Photocatalytic Advanced Oxidation Processes for Organic Compound Degradation: A Review. Desalination Water Treat. 2024, 318, 100384. [Google Scholar] [CrossRef]
- Scholtz, M.T.; Bidleman, T.F. Modelling of the Long-Term Fate of Pesticide Residues in Agricultural Soils and Their Surface Exchange with the Atmosphere: Part II. Projected Long-Term Fate of Pesticide Residues. Sci. Total Environ. 2007, 377, 61–80. [Google Scholar] [CrossRef]
- Marie, L.; Sylvain, P.; Benoit, G.; Maurice, M.; Gwenaël, I. Degradation and Transport of the Chiral Herbicide S-Metolachlor at the Catchment Scale: Combining Observation Scales and Analytical Approaches. Environ. Sci. Technol. 2017, 51, 13231–13240. [Google Scholar] [CrossRef]
- Robinson, D.E.; Mansingh, A.; Dasgupta, T.P. Fate and Transport of Ethoprophos in the Jamaican Environment. Sci. Total Environ. 1999, 237–238, 373–378. [Google Scholar] [CrossRef]
- Kruve, A.; Kiefer, K.; Hollender, J. Benchmarking of the Quantification Approaches for the Non-Targeted Screening of Micropollutants and Their Transformation Products in Groundwater. Anal. Bioanal. Chem. 2021, 413, 1549–1559. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran Toxicity and Its Microbial Degradation in Contaminated Environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef]
- Kafaei, R.; Arfaeinia, H.; Savari, A.; Mahmoodi, M.; Rezaei, M.; Rayani, M.; Sorial, G.A.; Fattahi, N.; Ramavandi, B. Organochlorine Pesticides Contamination in Agricultural Soils of Southern Iran. Chemosphere 2020, 240, 124983. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential Removal of Pesticides from Water by Molecular Imprinting on TiO2 Photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Barka, N.; Qourzal, S.; Assabbane, A.; Nounah, A.; Ait-Ichou, Y. Photocatalytic Degradation of an Azo Reactive Dye, Reactive Yellow 84, in Water Using an Industrial Titanium Dioxide Coated Media. Arab. J. Chem. 2010, 3, 279–283. [Google Scholar] [CrossRef]
- Nayak, S.; Muniz, J.; Sales, C.M.; Tikekar, R.V. Fructose as a Novel Photosensitizer: Characterization of Reactive Oxygen Species and an Application in Degradation of Diuron and Chlorpyrifos. Chemosphere 2016, 144, 1690–1697. [Google Scholar] [CrossRef]
- Kafilzadeh, F.; Ebrahimnezhad, M.; Tahery, Y. Isolation and Identification of Endosulfan-Degrading Bacteria and Evaluation of Their Bioremediation in Kor River, Iran. Osong Public Health Res. Perspect. 2015, 6, 39–46. [Google Scholar] [CrossRef]
- Linn, D.M. Sorption and Degradation of Pesticides and Organic Chemicals in Soil. In Proceedings of the a Symposium Sponsored by Divisions S-3, S-1, S-2, and A-5 of the Soil Science Society of America and American Society of Agronomy, Denver, CO, USA, 30 October 1991; Soil Science Society of America/American Society of Agronomy: Madison, WI, USA, 1993; ISBN 0891188037. [Google Scholar]
- Fernández-López, M.G.; Popoca-Ursino, C.; Sánchez-Salinas, E.; Tinoco-Valencia, R.; Folch-Mallol, J.L.; Dantán-González, E.; Laura Ortiz-Hernández, M. Enhancing Methyl Parathion Degradation by the Immobilization of Burkholderia sp. Isolated from Agricultural Soils. Microbiologyopen 2017, 6, e00507. [Google Scholar] [CrossRef]
- Murphy, E.M.; Zachara, J.M.; Smith, S.C.; Phillips, J.L. The Sorption of Humic Acids to Mineral Surfaces and Their Role in Contaminant Binding. Sci. Total Environ. 1992, 117–118, 413–423. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial Degradation of Pesticide Residues and an Emphasis on the Degradation of Cypermethrin and 3-Phenoxy Benzoic Acid: A Review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Laura, M.; Snchez-Salinas, E.; Dantn Gonzlez, E.; Luisa, M. Pesticide Biodegradation: Mechanisms, Genetics and Strategies to Enhance the Process. In Biodegradation–Life of Science; InTech: London, UK, 2013; pp. 252–276. [Google Scholar]
- Mamta; Rao, R.J.; Wani, K.A. Bioremediation of Pesticides under the Influence of Bacteria and Fungi. In Handbook of Research on Uncovering New Methods for Ecosystem Management Through Bioremediation; IGI Global: Hershey, PA, USA, 2015; pp. 51–72. ISBN 9781466686830. [Google Scholar]
- Sariwati, A.; Purnomo, A.S.; Kamei, I. Abilities of Co-Cultures of Brown-Rot Fungus Fomitopsis pinicola and Bacillus subtilis on Biodegradation of DDT. Curr. Microbiol. 2017, 74, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Doolotkeldieva, T.; Konurbaeva, M.; Bobusheva, S. Microbial Communities in Pesticide-Contaminated Soils in Kyrgyzstan and Bioremediation Possibilities. Environ. Sci. Pollut. Res. 2018, 25, 31848–31862. [Google Scholar] [CrossRef] [PubMed]
- Luiz Meleiro Porto, A.; Zelayarán Melgar, G.; Consiglio Kasemodel, M.; Nitschke, M. Biodegradation of Pesticides. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: London, UK, 2011; Volume 1, pp. 407–438. [Google Scholar]
- Jenks, B.M.; Roeth, F.W.; Martin, A.R.; Mccallister, D.L. Influence of Surface and Subsurface Soil Properties on Atrazine Sorption and Degradation. Weed Sci. 1998, 46, 132–138. [Google Scholar] [CrossRef]
- Spark, K.M.; Swift, R.S. Investigation of the Interaction between Pesticides and Humic Sunstances Using Fluorescence Spectroscopy. Sci. Total Environ. 1994, 152, 9–17. [Google Scholar] [CrossRef]
- Kulovaara, M. Distribution of DDT and Benzo[a]Pyrene between Water and Dissolved Organic Matter in Natural Humic Water. Chemosphere 1993, 27, 2333–2340. [Google Scholar] [CrossRef]
- Devitt, E.C.; Wiesner, M.R. Dialysis Investigations of Atrazine-Organic Matter Interactions and the Role of a Divalent Metal. Environ. Sci. Technol. 1998, 32, 232–237. [Google Scholar] [CrossRef]
- Fitch, A.; Du, J. Solute Transport in Clay Media: Effect of Humic Acid. Environ. Sci. Technol. 1996, 30, 12–15. [Google Scholar] [CrossRef]
- Miller, J.J.; Foroud, N.; Hill, B.D.; Lindwall, C.W. Herbicides in surface runoff and groundwater under surface irrigation in southern Alberta. Can. J. Soil Sci. 1995, 75, 145–148. [Google Scholar] [CrossRef]
- Moreau-Kervévan, C.; Mouvet, C. Adsorption and Desorption of Atrazine, Deethylatrazine, and Hydroxyatrazine by Soil Components. J. Environ. Qual. 1998, 27, 46–53. [Google Scholar] [CrossRef]
- Andréa, M.M.; Peres, T.B.; Luchini, L.C.; Pettinelli, A. Impact of Long-Term Pesticide Applications on Some Soil Biological Parameters. J. Environ. Sci. Health B 2000, 35, 297–307. [Google Scholar] [CrossRef]
- Omar, S.A.; Abdel-Sater, M.A. microbial populations and enzyme activities in soil treated with pesticides. Water Air Soil Pollut. 2001, 127, 49–63. [Google Scholar] [CrossRef]
- Luo, Y.; Atashgahi, S.; Rijnaarts, H.H.M.; Comans, R.N.J.; Sutton, N.B. Influence of Different Redox Conditions and Dissolved Organic Matter on Pesticide Biodegradation in Simulated Groundwater Systems. Sci. Total Environ. 2019, 677, 692–699. [Google Scholar] [CrossRef]
- Robinson, J.M.; Liddicoat, C.; Muñoz-Rojas, M.; Breed, M.F. Restoring Soil Biodiversity. Curr. Biol. 2024, 34, R393–R398. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Malacrinò, A.; Wisniewski, M.; Cacciola, S.O.; Schena, L. Metabarcoding: A Powerful Tool to Investigate Microbial Communities and Shape Future Plant Protection Strategies. Biol. Control. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Opande, T.; Kong, M.; Feng, D.; Wen, Y.H.; Okoth, N.; Yatoo, A.M.; Khalil, F.M.A.; Elrys, A.S.; Meng, L.; Zhang, J. Edaphic Factors Mediate the Response of Nitrogen Cycling and Related Enzymatic Activities and Functional Genes to Heavy Metals: A Review. Ecotoxicol. Environ. Saf. 2025, 290, 117766. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Doolette, C.L.; Vasileiadis, S.; Drigo, B.; Wyrsch, E.R.; Djordjevic, S.P.; Donner, E.; Karpouzas, D.G.; Lombi, E. Pesticide Effects on Nitrogen Cycle Related Microbial Functions and Community Composition. Sci. Total Environ. 2022, 807, 150734. [Google Scholar] [CrossRef]
- Belotti, E. Assessment of a Soil Quality Criterion by Means of a Field Survey. Appl. Soil Ecol. 1998, 10, 51–63. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bhattacharyya, P.; Pal, R. Effect of Arsenic Contamination on Microbial Biomass and Its Activities in Arsenic Contaminated Soils of Gangetic West Bengal, India. Environ. Int. 2004, 30, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Megharaj, M.; Kantachote, D.; Singleton, I.; Naidu, R. Effects of Long-Term Contamination of DDT on Soil Microflora with Special Reference to Soil Algae and Algal Transformation of DDT. Environ. Pollut. 2000, 109, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gallori, E.; Casalone, E.; Colella, C.M.; Daly, S.; Polsinelli, M. 1,8-Naphthalic Anhydride Antidote Enhances the Toxic Effects of Captan and Thiram Fungicides on Azospirillum brasilense Cells. Res. Microbiol. 1991, 142, 1005–1012. [Google Scholar] [CrossRef]
- Omar, S.A.; Abd-Alla, M.H. Effect of Pesticides on Growth, Respiration and Nitrogenase Activity of Azotobacter and Azospirillum. World J. Microbiol. Biotechnol. 1992, 8, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mallek, A.Y.; Moharram, A.M.; Abdel-Kader, M.I.A.; Omar, S.A. Effect of Soil Treatment with the Organophosphorus Insecticide Profenfos on the Fungal Flora and Some Microbial Activities. Microbiol. Res. 1994, 149, 167–171. [Google Scholar] [CrossRef]
- Singh, G.; Wright, D. Effects of Herbicides on Nodulation, Symbiotic Nitrogen Fixation, Growth and Yield of Pea (Pisum sativum). J. Agric. Sci. 1999, 133, 21–30. [Google Scholar] [CrossRef]
- Khan, M.; Zaidi, A.; Rizvi, P. Biotoxic Effects of Herbicides on Growth, Nodulation, Nitrogenase Activity, and Seed Production in Chickpeas. Commun. Soil Sci. Plant Anal. 2006, 37, 1783–1793. [Google Scholar] [CrossRef]
- Nweke, C.O.; Ntinugwa, C.; Obah, I.F.; Ike, S.C.; Eme, G.E.; Opara, E.C.; Okolo, J.C.; Nwanyanwu, C.E. In Vitro Effects of Metals and Pesticides on Dehydrogenase Activity in Microbial Community of Cowpea (Vigna unguiculata) Rhizoplane. Afr. J. Biotechnol. 2007, 6, 290–295. [Google Scholar]
- Bending, G.D.; Rodríguez-Cruz, M.S.; Lincoln, S.D. Fungicide Impacts on Microbial Communities in Soils with Contrasting Management Histories. Chemosphere 2007, 69, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bearson, B.L.; Douglass, C.H.; Duke, S.O.; Moorman, T.B.; Tranel, P.J. Effects of Glyphosate on Antibiotic Resistance in Soil Bacteria and Its Potential Significance: A Review. J. Environ. Qual. 2024, 54, 160–180. [Google Scholar] [CrossRef]
- Qian, H.; Hu, B.; Wang, Z.; Xu, X.; Hong, T. Effects of Validamycin on Some Enzymatic Activities in Soil. Environ. Monit. Assess. 2007, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, T.P.; He, W.; Megharaj, M.; Naidu, R. Effect of Insecticide Fenamiphos on Soil Microbial Activities in Australian and Ecuadorean Soils. J. Environ. Sci. Health B 2009, 44, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Surya Kalyani, S.; Sharma, J.; Dureja, P.; Singh, S. Lata Influence of Endosulfan on Microbial Biomass and Soil Enzymatic Activities of a Tropical Alfisol. Bull. Environ. Contam. Toxicol. 2010, 84, 351–356. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Wang, F.; Liang, W. Toxicological Effects of Dimethomorph on Soil Enzymatic Activity and Soil Earthworm (Eisenia fetida). Chemosphere 2017, 169, 316–323. [Google Scholar] [CrossRef]
- Menon, P.; Gopal, M.; Prasad, R. Influence of Two Insecticides, Chlorpyrifos and Quinalphos, on Arginine Ammonification and Mineralizable Nitrogen in Two Tropical Soil Types. J. Agric. Food Chem. 2004, 52, 7370–7376. [Google Scholar] [CrossRef]
- Ramudu, A.C.; Mohiddin, G.J.; Srinivasulu, M.; Madakka, M.; Rangaswamy, V. Impact of Fungicides Chlorothalonil and Propiconazole on Microbial Activities in Groundnut (Arachis hypogaea L.) Soils. ISRN Microbiol. 2011, 2011, 623404. [Google Scholar] [CrossRef]
- Li, K.; Xing, B.; Torello, W.A. Effect of Organic Fertilizers Derived Dissolved Organic Matter on Pesticide Sorption and Leaching. Environ. Pollut. 2005, 134, 187–194. [Google Scholar] [CrossRef]
- Dolaptsoglou, C.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U.; Eleftherohorinos, I.; Voudrias, E.A. Influence of Different Organic Amendments on the Degradation, Metabolism, and Adsorption of Terbuthylazine. J. Environ. Qual. 2007, 36, 1793–1802. [Google Scholar] [CrossRef]
- Kumar, N.; Mukherjee, I.; Varghese, E. Adsorption–Desorption of Tricyclazole: Effect of Soil Types and Organic Matter. Environ. Monit. Assess. 2015, 187, 61. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.F.; Letey, J.; Farmer, W.J. Estimating the Potential for Facilitated Transport of Napropamide by Dissolved Organic Matter. Soil Sci. Soc. Am. J. 2006, 70, 24–30. [Google Scholar] [CrossRef]
- Huang, X.; Lee, L.S. Effects of Dissolved Organic Matter from Animal Waste Effluent on Chlorpyrifos Sorption by Soils. J. Environ. Qual. 2001, 30, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, M.; Letey, J.; Farmer, W.J.; Williams, C.F.; Nelson, S.D. Soluble and Solid Organic Matter Effects on Atrazine Adsorption in Cultivated Soils. Soil Sci. Soc. Am. J. 2003, 67, 1140–1146. [Google Scholar] [CrossRef]
- Gao, J.P.; Maguhn, J.; Spitzauer, P.; Kettrup, A. Distribution of Pesticides in the Sediment of the Small Teufelsweiher Pond (Southern Germany). Water Res. 1997, 31, 2811–2819. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and Water-Extractable Organic Matter in Soils: A Review on the Influence of Land Use and Management Practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Cox, L.; Cecchi, A.; Celis, R.; Hermosín, M.C.; Koskinen, W.C.; Cornejo, J. Effect of Exogenous Carbon on Movement of Simazine and 2,4-D in Soils. Soil Sci. Soc. Am. J. 2001, 65, 1688–1695. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Cox, L.; Hermosín, M.C.; Cornejo, J. Organic Amendments Affecting Sorption, Leaching and Dissipation of Fungicides in Soils. Pest. Manag. Sci. 2006, 62, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Dolaptsoglou, C.; Karpouzas, D.G.; Menkissoglu-Spiroudi, U.; Eleftherohorinos, I.; Voudrias, E.A. Influence of Different Organic Amendments on the Leaching and Dissipation of Terbuthylazine in a Column and a Field Study. J. Environ. Qual. 2009, 38, 782–791. [Google Scholar] [CrossRef]
- Charbonneau, A.; Lucotte, M.; Moingt, M.; Blakney, A.J.C.; Morvan, S.; Bipfubusa, M.; Pitre, F.E. Fertilisation of Agricultural Soils with Municipal Biosolids: Glyphosate and Aminomethylphosphonic Acid Inputs to Québec Field Crop Soils. Sci. Total Environ. 2024, 922, 171290. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Loffredo, E.; D’Orazio, V.; Brunetti, G.; Miano, T.M.; La Cava, P. Adsorption of Pesticides by Humic Acids from Organic Amendments and Soils. In Humic Substances and Chemical Contaminants; Wiley: Hoboken, NJ, USA, 2015; pp. 129–153. ISBN 9780891188759. [Google Scholar]
- Sánchez, M.E.; Estrada, I.B.; Martínez, O.; Martín-Villacorta, J.; Aller, A.; Morán, A. Influence of the Application of Sewage Sludge on the Degradation of Pesticides in the Soil. Chemosphere 2004, 57, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Zadeh, F.; Samsuri, A.W.; Radziah, O.; Dzolkhifli, O.; Seh-Bardan, B.J. Degradation and Leaching of Napropamide in BRIS Soil Amended with Chicken Dung and Palm Oil Mill Effluent. Clean. 2012, 40, 599–606. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Brown, C.D. Factors Influencing Degradation of Pesticides in Soil. J. Agric. Food Chem. 2007, 55, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, R. Factors Limiting Bioremediation Technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Zhong, W.H.; Cai, Z.C. Long-Term Effects of Inorganic Fertilizers on Microbial Biomass and Community Functional Diversity in a Paddy Soil Derived from Quaternary Red Clay. Appl. Soil Ecol. 2007, 36, 84–91. [Google Scholar] [CrossRef]
- Lo, C.C. Effect of Pesticides on Soil Microbial Community. J. Environ. Sci. Health B 2010, 45, 348–359. [Google Scholar] [CrossRef]
- Lancaster, S.H.; Hollister, E.B.; Senseman, S.A.; Gentry, T.J. Effects of Repeated Glyphosate Applications on Soil Microbial Community Composition and the Mineralization of Glyphosate. Pest. Manag. Sci. 2010, 66, 59–64. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural Soils, Pesticides and Microbial Diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Schäffer, A.; Kästner, M.; Trapp, S. A Unified Approach for Including Non-Extractable Residues (NER) of Chemicals and Pesticides in the Assessment of Persistence. Environ. Sci. Eur. 2018, 30, 51. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Smreczak, B.; Weber, J.; Mielnik, L.; Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Jamroz, E.; Debicka, M.; Kocowicz, A.; et al. The Interaction of Pesticides with Humin Fractions and Their Potential Impact on Non-Extractable Residue Formation. Molecules 2023, 28, 7146. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J. Soil Organic Matter as a Nanoporous Sorbent of Organic Pollutants. Adv. Colloid Interface Sci. 1998, 76, 445–467. [Google Scholar] [CrossRef]
- Barriuso, E.; Benoit, P.; Dubus, I.G. Formation of Pesticide Nonextractable (Bound) Residues in Soil: Magnitude, Controlling Factors and Reversibility. Environ. Sci. Technol. 2008, 42, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Marioara Nicoleta, F.; Popescu, R.; Nicoleta Filimon, M.; Octavian Voia, S.; Dumitrescu, G.; Ciochina, L.P.; Mituletu, M.; Vlad, D.C. The Effect of Some Insecticides on Soil Microorganisms Based on Enzymatic and Bacteriological Analyses. Rom. Biotechnol. Lett. 2015, 20, 10439–10447. [Google Scholar]
- Chung, N.; Alexander, M. Differences in Sequestration and Bioavailability of Organic Compounds Aged in Dissimilar Soils. Environ. Sci. Technol. 1998, 32, 855–860. [Google Scholar] [CrossRef]
- Lal, R. Sequestering Carbon in Soils of Agro-Ecosystems. Food Policy 2011, 36, 33–39. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Effect of Pesticides on Plant Growth Promoting Traits of Greengram-Symbiont, Bradyrhizobium sp. Strain MRM6. Bull. Environ. Contam. Toxicol. 2011, 86, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Coleman, D.C.; Hendrix, P.F.; Beare, M.H. Biotic Manipulation Effects on Soil Carbohydrates and Microbial Biomass in a Cultivated Soil. Soil Biol. Biochem. 1995, 27, 1127–1135. [Google Scholar] [CrossRef]
- Chen, S.K.; Edwards, C.A. A Microcosm Approach to Assess the Effects of Fungicides on Soil Ecological Processes and Plant Growth: Comparisons of Two Soil Types. Soil Biol. Biochem. 2001, 33, 1981–1991. [Google Scholar] [CrossRef]
- Fravel, D.R.; Deahl, K.L.; Stommel, J.R. Compatibility of the Biocontrol Fungus Fusarium Oxysporum Strain CS-20 with Selected Fungicides. Biol. Control 2005, 34, 165–169. [Google Scholar] [CrossRef]
- Monkiedje, A.; Ilori, M.O.; Spiteller, M. Soil Quality Changes Resulting from the Application of the Fungicides Mefenoxam and Metalaxyl to a Sandy Loam Soil. Soil Biol. Biochem. 2002, 34, 1939–1948. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Effect of Selected Pesticides on Soil Microflora Involved in Organic Matter and Nitrogen Transformations: Pot Experiment. Pol. J. Ecol. 2007, 55, 207–220. [Google Scholar]
- Min, H.; Ye, Y.F.; Chen, Z.Y.; Wu, W.X.; Yufeng, D. Effects of Butachlor on Microbial Populations and Enzyme Activities in Paddy Soil. J. Environ. Sci. Health B 2001, 36, 581–595. [Google Scholar] [CrossRef]
- El-Ghamry, A.M.; Xu, J.M.; Huang, C.Y.; Gan, J. Microbial Response to Bensulfuron-Methyl Treatment in Soil. J. Agric. Food Chem. 2002, 50, 136–139. [Google Scholar] [CrossRef]
- Niewiadomska, A. Effect of Carbendazim, Imazetapir and Thiram on Nitrogenase Activity, the Number of Microorganisms in Soil and Yield of Red Clover (Trifolium pratense L.). Pol. J. Environ. Stud. 2004, 13, 403–410. [Google Scholar]
- Fox, J.E.; Gulledge, J.; Engelhaupt, E.; Burow, M.E.; Mclachlan, J.A. Pesticides Reduce Symbiotic Efficiency of Nitrogen-Fixing Rhizobia and Host Plants. Proc. Natl. Acad. Sci. USA 2007, 104, 10282–10287. [Google Scholar] [CrossRef] [PubMed]
- Bishnu, A.; Chakraborty, A.; Chakrabarti, K.; Saha, T. Ethion Degradation and Its Correlation with Microbial and Biochemical Parameters of Tea Soils. Biol. Fertil. Soils 2012, 48, 19–29. [Google Scholar] [CrossRef]
- Tejada, M. Evolution of Soil Biological Properties after Addition of Glyphosate, Diflufenican and Glyphosate+diflufenican Herbicides. Chemosphere 2009, 76, 365–373. [Google Scholar] [CrossRef]
- Strandberg, M.; Scott-Fordsmand, J.J. Effects of Pendimethalin at Lower Trophic Levels—A Review. Ecotoxicol. Environ. Saf. 2004, 57, 190–201. [Google Scholar] [CrossRef]
- Jie, C.; Chen, J.-Z.; Tan, M.-Z.; Gong, Z.-T. Soil Degradation: A Global Problem Endangering Sustainable Development. J. Geogr. Sci. 2002, 12, 243–252. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine Pesticides, Their Toxic Effects on Living Organisms and Their Fate in the Environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Durska, G. Fungicide Effect on Nitrogenase Activity in Methylotrophic Bacteria. Pol. J. Microbiol. 2004, 53, 155–158. [Google Scholar]
- Sukul, P. Enzymatic Activities and Microbial Biomass in Soil as Influenced by Metalaxyl Residues. Soil Biol. Biochem. 2006, 38, 320–326. [Google Scholar] [CrossRef]
- Perfect, T.J.; Cook, A.G.; Critchley, B.R.; Russell-Smith, A. The Effect of Crop Protection with DDT on the Microarthropod Population of a Cultivated Forest Soil in the Sub-Humid Tropics. Pedobiologia 1981, 21, 7–18. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Santos, J.B.; Jakelaitis, A.; Silva, A.A.; Costa, M.D.; Manabe, A.; Silva, M.C.S. Action of Two Herbicides on the Microbial Activity of Soil Cultivated with Common Bean (Phaseolus vulgaris) in Conventional-till and No-till Systems. Weed Res. 2006, 46, 284–289. [Google Scholar] [CrossRef]
- Elliott, J.A.; Cessna, A.J.; Nicholaichuk, W.; Tollefson, L.C. Leaching Rates and Preferential Flow of Selected Herbicides through Tilled and Untilled Soil. J. Environ. Qual. 2000, 29, 1650–1656. [Google Scholar] [CrossRef]
- Komorowicz, I.; Gramowska, H.; Barałkiewicz, D. Estimation of the Lake Water Pollution by Determination of 18 Elements Using ICP-MS Method and Their Statistical Analysis. J. Environ. Sci. Health Part A 2010, 45, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Chennappa, G.; Adkar-Purushothama, C.R.; Suraj, U.; Tamilvendan, K.; Sreenivasa, M.Y. Pesticide Tolerant Azotobacter Isolates from Paddy Growing Areas of Northern Karnataka, India. World J. Microbiol. Biotechnol. 2014, 30, 1–7. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Huygens, D.; Boeckx, P.; Kuyper, T.W.; Lubbers, I.M.; Rütting, T.; Groffman, P.M. The Soil n Cycle: New Insights and Key Challenges. SOIL 2015, 1, 235–256. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Romero, E.; Fernández-Bayo, J.; Díaz, J.M.C.; Nogales, R. Enzyme Activities and Diuron Persistence in Soil Amended with Vermicompost Derived from Spent Grape Marc and Treated with Urea. Appl. Soil Ecol. 2010, 44, 198–204. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Karas, P.A.; Baguelin, C.; Pertile, G.; Papadopoulou, E.S.; Nikolaki, S.; Storck, V.; Ferrari, F.; Trevisan, M.; Ferrarini, A.; Fornasier, F.; et al. Assessment of the Impact of Three Pesticides on Microbial Dynamics and Functions in a Lab-to-Field Experimental Approach. Sci. Total Environ. 2018, 637–638, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Brözel, V.S. Phylogeny of Nitrogenase Structural and Assembly Components Reveals New Insights into the Origin and Distribution of Nitrogen Fixation across Bacteria and Archaea. Microorganisms 2021, 9, 1662. [Google Scholar] [CrossRef]
- Su, Z.-c.; Zhang, H.-w.; Li, X.-y.; Zhang, Q.; Zhang, C.-g. Toxic Effects of Acetochlor, Methamidophos and Their Combination on NifH Gene in Soil. Sci. Total Environ. 2007, 19, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Effects of Trifluralin on the Soil Microbial Community and Functional Groups Involved in Nitrogen Cycling. J. Hazard. Mater. 2018, 353, 204–213. [Google Scholar] [CrossRef]
- Nestor, S.L.; Bancroft, J.D. Enzyme Histochemistry and Its Diagnostic Applications. In Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: London, UK, 2008; pp. 405–432. [Google Scholar] [CrossRef]
- Schneider, K.; Turrion, M.B.; Grierson, P.F.; Gallardo, J.F. Phosphatase Activity, Microbial Phosphorus, and Fine Root Growth in Forest Soils in the Sierra de Gata, Western Central Spain. Biol. Fertil. Soils 2001, 34, 151–155. [Google Scholar] [CrossRef]
- De Cesare, F.; Garzillo, A.M.V.; Buonocore, V.; Badalucco, L. Use of Sonication for Measuring Acid Phosphatase Activity in Soil. Soil Biol. Biochem. 2000, 32, 825–832. [Google Scholar] [CrossRef]
- Yan, H.; Wang, D.; Dong, B.; Tang, F.; Wang, B.; Fang, H.; Yu, Y. Dissipation of Carbendazim and Chloramphenicol Alone and in Combination and Their Effects on Soil Fungal: Bacterial Ratios and Soil Enzyme Activities. Chemosphere 2011, 84, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Reshi, Z.A. Effect of the Fungicide Mancozeb at Different Application Rates on Enzyme Activities in a Silt Loam Soil of the Kashmir Himalaya, India. Trop. Ecol. 2010, 51, 199–205. [Google Scholar]
- Zhang, W. Eco-Sustainability Assessment of Integrated Pest Management (IPM): Indicator System and Calculator. Comput. Ecol. Softw. 2025, 15, 99–113. [Google Scholar]
- Baćmaga, M.; Boros, E.; Kucharski, J.; Wyszkowska, J. Enzymatic activity in soil contaminated with the Aurora 40 WG herbicide. Environ. Prot. Eng. 2012, 38, 91–102. [Google Scholar]
- Jastrzębska, E. The effect of chlorpyrifos and teflubenzuron on the enzymatic activity of soil. Pol. J. Environ. Stud. 2011, 20, 903–910. [Google Scholar]
- Yao, X.-h.; Min, H.; Lü, Z.-h.; Yuan, H. ping Influence of Acetamiprid on Soil Enzymatic Activities and Respiration. Eur. J. Soil Biol. 2006, 42, 120–126. [Google Scholar] [CrossRef]
- Ramani, V. Effect of Pesticides on Phosphate Solubilization by Bacillus sphaericus and Pseudomonas cepacia. Pestic. Biochem. Physiol. 2011, 99, 232–236. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Effects of Insecticides on Plant-Growth-Promoting Activities of Phosphate-Solubilizing Rhizobacterium Klebsiella sp. Strain PS19. Pestic. Biochem. Physiol. 2011, 100, 51–56. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Effect of Fungicides on Plant Growth Promoting Activities of Phosphate Solubilizing Pseudomonas putida Isolated from Mustard (Brassica compestris) Rhizosphere. Chemosphere 2012, 86, 945–950. [Google Scholar] [CrossRef]
- Das, A.C.; Mukherjee, D. Insecticidal Effects on Soil Microorganisms and Their Biochemical Processes Related to Soil Fertility. World J. Microbiol. Biotechnol. 1998, 14, 903–909. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, C.Y.; Son, H.J. Mechanism of Insoluble Phosphate Solubilization by Pseudomonas Fluorescens RAF15 Isolated from Ginseng Rhizosphere and Its Plant Growth-Promoting Activities. Lett. Appl. Microbiol. 2009, 49, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sashidhar, B.; Podile, A.R. Mineral Phosphate Solubilization by Rhizosphere Bacteria and Scope for Manipulation of the Direct Oxidation Pathway Involving Glucose Dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12. [Google Scholar] [CrossRef]
- Rajasankar, R.; Manju Gayathry, G.; Sathiavelu, A.; Ramalingam, C.; Saravanan, V.S. Pesticide Tolerant and Phosphorus Solubilizing Pseudomonas sp. Strain SGRAJ09 Isolated from Pesticides Treated Achillea clavennae Rhizosphere Soil. Ecotoxicology 2013, 22, 707–717. [Google Scholar] [CrossRef]
- Babar, S.; Baloch, A.; Qasim, M.; Wang, J.; Wang, X.; Li, Y.; Khalid, S.; Jiang, C. Unearthing the Soil-Bacteria Nexus to Enhance Potassium Bioavailability for Global Sustainable Agriculture: A Mechanistic Preview. Microbiol. Res. 2024, 288, 127885. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate Effects on Plant Mineral Nutrition, Crop Rhizosphere Microbiota, and Plant Disease in Glyphosate-Resistant Crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, F.; Ou, J. Global Pesticide Consumption and Pollution: With China as a Focus. Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125. [Google Scholar]
- Carriger, J.F.; Rand, G.M.; Gardinali, P.R.; Perry, W.B.; Tompkins, M.S.; Fernandez, A.M. Pesticides of Potential Ecological Concern in Sediment from South Florida Canals: An Ecological Risk Prioritization for Aquatic Arthropods. Soil Sediment Contam. 2006, 15, 21–45. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, P.; Mahapatra, S.C.; Mithun, S.K. Modelling Impacts of Chemical Fertilizer on Agricultural Production: A Case Study on Hooghly District, West Bengal, India. Model. Earth Syst. Environ. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Virk, A.L.; Shakoor, A.; Abdullah, A.; Chang, S.X.; Cai, Y. Pesticide Effects on Crop Physiology, Production and Soil Biological Functions. Adv. Agron. 2024, 187, 171–212. [Google Scholar] [CrossRef]
- Jyot, G.; Mandal, K.; Singh, B. Effect of Dehydrogenase, Phosphatase and Urease Activity in Cotton Soil after Applying Thiamethoxam as Seed Treatment. Environ. Monit. Assess. 2015, 187, 298. [Google Scholar] [CrossRef]
- Baćmaga, M.; Kucharski, J.; Wyszkowska, J. Microbial and Enzymatic Activity of Soil Contaminated with Azoxystrobin. Environ. Monit. Assess. 2015, 187, 615. [Google Scholar] [CrossRef]
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-Enabled Pesticides for Sustainable Agriculture and Global Food Security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Zhou, W.; Arcot, Y.; Medina, R.F.; Bernal, J.; Cisneros-Zevallos, L.; Akbulut, M.E.S. Integrated Pest Management: An Update on the Sustainability Approach to Crop Protection. ACS Omega 2024, 9, 41130–41147. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, Pesticides, Food Security and Food Safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Peritore, A.F.; Gugliandolo, E.; Cuzzocrea, S.; Crupi, R.; Britti, D. Current Review of Increasing Animal Health Threat of Per- and Polyfluoroalkyl Substances (PFAS): Harms, Limitations, and Alternatives to Manage Their Toxicity. Int. J. Mol. Sci. 2023, 24, 11707. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Fathima, J.; Varghese, S.; Chatterjee, P.; Gadhamshetty, V. Advances in Bioremediation Strategies for PFAS-Contaminated Water and Soil. Soil Environ. Health 2025, 3, 100126. [Google Scholar] [CrossRef]
- Abdul Halim, N.S.; Abdullah, R.; Karsani, S.A.; Osman, N.; Panhwar, Q.A.; Ishak, C.F. Influence of Soil Amendments on the Growth and Yield of Rice in Acidic Soil. Agronomy 2018, 8, 165. [Google Scholar] [CrossRef]
- De Corato, U. Agricultural Waste Recycling in Horticultural Intensive Farming Systems by On-Farm Composting and Compost-Based Tea Application Improves Soil Quality and Plant Health: A Review under the Perspective of a Circular Economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.S.; Kuldeep, D.K.; Chouhan, M.; Prajapati, R.; Singh, S.K. A Review on Eco-Friendly Pesticides and Their Rising Importance in Sustainable Plant Protection Practices. Int. J. Plant Soil. Sci. 2023, 35, 200–214. [Google Scholar] [CrossRef]
- Singh, D.K. Biodegradation and Bioremediation of Pesticide in Soil: Concept, Method, and Recent Developments. Indian J. Microbiol. 2008, 48, 35–40. [Google Scholar] [CrossRef]
- Whitford, R.; Gilbert, M.; Langridge, P. Biotechnology in Agriculture. In Climate Change and Crop Production; CABI Publishing: Wallingford, UK, 2010; pp. 219–244. ISBN 9781845936334. [Google Scholar]
- Sarker, A.; Shin, W.S.; Al Masud, M.A.; Nandi, R.; Islam, T. A Critical Review of Sustainable Pesticide Remediation in Contaminated Sites: Research Challenges and Mechanistic Insights. Environ. Pollut. 2024, 341, 122940. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.C.; Jiang, J.; Huang, G.X.; Yu, H.Q. Sludge Biochar-Based Catalysts for Improved Pollutant Degradation by Activating Peroxymonosulfate. J. Mater. Chem. A Mater. 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. Biochar Efficiency in Pesticides Sorption as a Function of Production Variables—A Review. Environ. Sci. Pollut. Res. 2015, 22, 13824–13841. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zongo, K.F.; Coulibaly, A.; Guebre, D.; Naba, A.; Nandkangre, H.; Sanon, A.; Hien, E. Effects of Agro-Ecological Practices on the Productivity of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam) and Soil Fertility in the Sudano-Sahelian Zone of Burkina Faso. Agric. Sci. 2023, 14, 1624–1642. [Google Scholar] [CrossRef]
- Farmaha, B.S.; Sekaran, U.; Franzluebbers, A.J. Cover Cropping and Conservation Tillage Improve Soil Health in the Southeastern United States. Agron. J. 2022, 114, 296–316. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential Benefits and Risks for Soil Health Derived from the Use of Organic Amendments in Agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for Soil Applications-Sustainability Aspects, Challenges and Future Prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Marcińska-Mazur, L.; Jarosz, R.; Mierzwa–Hersztek, M. Effect of Organic/Inorganic Composites as Soil Amendments on the Biomass Productivity and Root Architecture of Spring Wheat and Rapeseed. J. Environ. Manag. 2023, 344, 118628. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Lan, T.; Müller, K.; Niazi, N.K.; Chen, X.; Xu, S.; Zheng, L.; Chu, Y.; Li, J.; et al. Unraveling Sorption of Lead in Aqueous Solutions by Chemically Modified Biochar Derived from Coconut Fiber: A Microscopic and Spectroscopic Investigation. Sci. Total Environ. 2017, 576, 766–774. [Google Scholar] [CrossRef]
- Belousova, M.E.; Malovichko, Y.V.; Shikov, A.E.; Nizhnikov, A.A.; Antonets, K.S. Dissecting the Environmental Consequences of Bacillus Thuringiensis Application for Natural Ecosystems. Toxins 2021, 13, 355. [Google Scholar] [CrossRef]
- Saroj, A.; Srivastava, A.K.; Kumar Nayak, A.; Chanotiya, C.S.; Samad, A. Essential oils in pest control and disease management. In Phytochemistry; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 341–368. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Integrated Pest Management for Sustainable Intensification of Agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef]
- Ehler, L.E. Integrated Pest Management (IPM): Definition, Historical Development and Implementation, and the Other IPM. Pest. Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Rao, A.; Bradleigh Vinson, S. Biological Control in China: Past, Present and Future—An Introduction to This Special Issue. Biol. Control 2014, 68, 1–5. [Google Scholar] [CrossRef]
- Payne, W.; Tapsoba, H.; Baoua, I.B.; Malick, B.N.; N’Diaye, M.; Dabire-Binso, C. On-Farm Biological Control of the Pearl Millet Head Miner: Realization of 35 Years of Unsteady Progress in Mali, Burkina Faso and Niger. Int. J. Agric. Sustain. 2011, 9, 186–193. [Google Scholar] [CrossRef]
- Rösch, A.; Beck, B.; Hollender, J.; Singer, H. Picogram per Liter Quantification of Pyrethroid and Organophosphate Insecticides in Surface Waters: A Result of Large Enrichment with Liquid–Liquid Extraction and Gas Chromatography Coupled to Mass Spectrometry Using Atmospheric Pressure Chemical Ionization. Anal. Bioanal. Chem. 2019, 411, 3151–3164. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of Synthetic Fertilizers and Pesticides on Soil Health and Soil Microbiology. In Agrochemicals Detection, Treatment and Remediation: Pesticides and Chemical Fertilizers; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar] [CrossRef]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Chapter 5 Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Sharma, A.; Zhang, W.; Mishra, S.; Chen, S. Biotechnological Basis of Microbial Consortia for the Removal of Pesticides from the Environment. Crit. Rev. Biotechnol. 2021, 41, 317–338. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sharma, B.; Hill, R.T.; Shukla, P. Bioremediation through Microbes: Systems Biology and Metabolic Engineering Approach. Crit. Rev. Biotechnol. 2019, 39, 79–98. [Google Scholar] [CrossRef]
- Singh, R.P.; Manchanda, G.; Li, Z.F.; Rai, A.R. Insight of Proteomics and Genomics in Environmental Bioremediation. In Handbook of Research on Inventive Bioremediation Techniques; IGI Global: Hershey, PA, USA, 2017; pp. 46–69. ISBN 9781522523260. [Google Scholar]
- Schäfer, R.B.; Liess, M.; Altenburger, R.; Filser, J.; Hollert, H.; Roß-Nickoll, M.; Schäffer, A.; Scheringer, M. Future Pesticide Risk Assessment: Narrowing the Gap between Intention and Reality. Environ. Sci. Eur. 2019, 31, 21. [Google Scholar] [CrossRef]
- Welch, S.A.; Lane, T.; Desrousseaux, A.O.S.; van Dijk, J.; Mangold-Döring, A.; Gajraj, R.; Hader, J.D.; Hermann, M.; Parvathi Ayillyath Kutteyeri, A.; Mentzel, S.; et al. ECORISK2050: An Innovative Training Network for Predicting the Effects of Global Change on the Emission, Fate, Effects, and Risks of Chemicals in Aquatic Ecosystems. Open Res. Eur. 2021, 1, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Niu, S. Modeling Pesticides in Global Surface Soils: Evaluating Spatiotemporal Patterns for USEtox-Based Steady-State Concentrations. Sci. Total Environ. 2021, 791, 148412. [Google Scholar] [CrossRef]
- Pullan, S.P.; Whelan, M.J.; Rettino, J.; Filby, K.; Eyre, S.; Holman, I.P. Development and Application of a Catchment Scale Pesticide Fate and Transport Model for Use in Drinking Water Risk Assessment. Sci. Total Environ. 2016, 563–564, 434–447. [Google Scholar] [CrossRef]
- Cantoni, B.; Penserini, L.; Vries, D.; Dingemans, M.M.L.; Bokkers, B.G.H.; Turolla, A.; Smeets, P.W.M.H.; Antonelli, M. Development of a Quantitative Chemical Risk Assessment (QCRA) Procedure for Contaminants of Emerging Concern in Drinking Water Supply. Water Res. 2021, 194, 116911. [Google Scholar] [CrossRef] [PubMed]
- Focks, A.; ter Horst, M.; van den Berg, E.; Baveco, H.; van den Brink, P.J. Integrating Chemical Fate and Population-Level Effect Models for Pesticides at Landscape Scale: New Options for Risk Assessment. Ecol. Model. 2014, 280, 102–116. [Google Scholar] [CrossRef]
- Bolan, S.; Padhye, L.P.; Jasemizad, T.; Govarthanan, M.; Karmegam, N.; Wijesekara, H.; Amarasiri, D.; Hou, D.; Zhou, P.; Biswal, B.K.; et al. Impacts of Climate Change on the Fate of Contaminants through Extreme Weather Events. Sci. Total Environ. 2024, 909, 168388. [Google Scholar] [CrossRef]
- Oldenkamp, R.; Benestad, R.E.; Hader, J.D.; Mentzel, S.; Nathan, R.; Madsen, A.L.; Jannicke Moe, S. Incorporating Climate Projections in the Environmental Risk Assessment of Pesticides in Aquatic Ecosystems. Integr. Environ. Assess. Manag. 2024, 20, 384–400. [Google Scholar] [CrossRef]
- Rodríguez, A.; Castrejón-Godínez, M.L.; Salazar-Bustamante, E.; Gama-Martínez, Y.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Omics Approaches to Pesticide Biodegradation. Curr. Microbiol. 2020, 77, 545–56261. [Google Scholar] [CrossRef]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef]
- Banerjee, D.; Adhikary, S.; Bhattacharya, S.; Chakraborty, A.; Dutta, S.; Chatterjee, S.; Ganguly, A.; Nanda, S.; Rajak, P. Breaking Boundaries: Artificial Intelligence for Pesticide Detection and Eco-Friendly Degradation. Environ. Res. 2024, 241, 117601. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Kiely, G. Modeling the Change in Soil Organic Carbon of Grassland in Response to Climate Change: Effects of Measured versus Modelled Carbon Pools for Initializing the Rothamsted Carbon Model. Agric. Ecosyst. Environ. 2011, 140, 372–381. [Google Scholar] [CrossRef]


| Chemical Groups | Pesticides | Observed Effects | Reference |
|---|---|---|---|
| Fungicides and bactericidal | Captan | Declined fungal growth, specifically reduced fungal hyphae, density, and lowered carbon and nitrogen content. | [30] |
| Captan and chlorothalonil | Inhibited microbial respiration | [31] | |
| Chlorothalonil and azoxystrobin | Fungal toxin | [48] | |
| Metalaxyl and mefenoxam | Toxic to nitrogen-fixing bacteria | [24] | |
| Mancozeb and dimethomorph | Suppressed bacterial and fungal growth | [50] | |
| Diazinon and linuron | Decreased microbial colony-forming units | [50] | |
| Insecticides | DDT | It affected bacteria and fungi, but showed a limited effect on fungi | [51] |
| DDT and arsenic | Significant reduction in the carbon biomass, including bacterial and fungal biomass | [52] | |
| Chlorpyrifos and dimethoate | Had adverse effects on the collembolan density | [53] | |
| Cypermethrin and thiomethoxam | Detrimental to the soil microorganisms | [54] | |
| Carbofuran and methamidophos | Increased microbial growth | [23] | |
| Fenamiphos | Detrimental to microbial growth | [55] | |
| Herbicides | Glyphosate | Considerable reduction in bacterial and fungal population. Increased in actinomycetes activity and microbial activity by 9–19% | [32] |
| Atrazine and Metolachlor | Reduction in microbial growth and changed the composition and diversity | [25] | |
| Butachlor | Suppressive effect on microbial growth | [33] | |
| Diuron and chlorotoluron | Influenced microbial development | [33] | |
| Pendimethalin | A decline in the population of rhizobia and nematodes in the soil was observed | [35] | |
| Heavy metals | Copper | Negatively influencing soil microbial biomass and impairing soil microbiota | [35] |
| Arsenic | Suppressed microbial biomass, enzymatic activity, and respiration of soil | [34] |
| Pesticides | Enzymes | Observed Effects | Reference |
|---|---|---|---|
| Captam and thiram | Nitrogenase in Azospirillum brasilense | Suppressed enzymatic functions | [69] |
| Fenvalerate and cuprosan | Nitrogenase | Observed inhibition | [36] |
| Profenophos | Nitrate reductase | Enzyme activity reduced | [37] |
| Terbutryn, Simazine, and Prometryn | Nitrogenase | Affected nitrogen fixation activity | [38] |
| Glyphosate | Dehydrogenases | Temporarily inhibited | [70] |
| Brominal and Selecron | Cellulase | Affected the activity | [71] |
| Carbendazim, imazetapyr, and thiram | Nitrogenase | Activity reduced | [72] |
| Oxafun, Funaben, and Baytan | Nitrogenase | Higher concentration decreased the activity | [73] |
| Metalaxyl | Urease and phosphatase | Inhibited urease phosphatase activities; increased initially and then decreased | [74] |
| Methabenzthiazurn and Terbutryn | Nitrogenase | Inhibited nodulation and impaired enzymatic activity | [75] |
| Atrazine and Northrin | Dehydrogenases | Stimulated at low doses and inhibited at higher concentrations | [76] |
| Azoxystrobin, Tebuconazol, and Chlorothalonil | Dehydrogenases | Less activity in the soil with low organic matter was reported, and no effect on the organic matter in the soil was observed | [77] |
| Validamycin | Phosphatase and urease | Temporary inhibition and activity recovered | [72] |
| Fenamiphos | Dehydrogenase and urease | No significant toxicity was observed | [78] |
| Endosulfan | Dehydrogenases | Increased activity | [41] |
| Diuron | Urease | No effect detected | [79] |
| Propiconazole | Cellulase | Activity declined by 5–40% | [80] |
| Thiamethoxam | Urease, phosphatase, and dehydrogenases | Dehydrogenases and phosphatases were inhibited | [81] |
| Azoxystrobin | Dehydrogenase, urease, acid and alkaline phosphatases, and catalase | Dehydrogenases showed resistance, with no effect on alkaline phosphatases, and others were inhibited | [82] |
| Dimethomorph | Dehydrogenase, urease, invertase, and alkaline phosphatases. | Dehydrogenase activity declined, and invertase activity was enhanced without affecting others | [83] |
| Pesticides Groups | Pesticides | Biochemical Process | Observed Effects | Reference |
|---|---|---|---|---|
| Insecticides | BHC and fenvelerate | Carbon mineralization | Increase mineralization by stimulating microbial activity | [93] |
| Cyfluthrin and imidacloprid | Nitrification, sulfur oxidation, and denitrification. | Stimulated sulfur oxidation, inhibited nitrification, and denitrification. | ||
| Chlorpyrifos and Quinalphos | Ammonification | Suppressed ammonification | [94] | |
| Acetamiprid | Microbial respiration | Suppressed microbial respiration activity | [95] | |
| Imidacloprid (with glyphosate and hexaconazole) | Nitrogen fixation | Toxic to Bradyrhizobium sp. | [96] | |
| Fenamiphos | Nitrification | Inhibited nitrifying bacteria | [97] | |
| Fungicides | Captan, benomyl, chlorothalonil, and anilazine | Nitrogen mineralization | Mineralization of organic nitrogen was elevated | [48] |
| Mancozeb, prosulfuron, and chlorothalonil | Nitrification | Suppressed nitrification | [52] | |
| Metalaxyl and mefenoxam | Ammonification and nitrification | Improved nitrification efficiency by enhanced conversion of organic nitrogen | [24] | |
| Herbicides | Terbutryn, simazine, premteryn, and benzoate | Nitrogen fixation | Suppressed symbiotic nitrogen fixation via decreased nodulation and nitrogen assimilation | [52] |
| Butachlor | Nitrogen fixation | Temporary enhanced N-fixation followed by a decline in nodulation activity | [98] | |
| Glyphosate, imidacloprid and hexaconazole | Nitrogen fixation | Demonstrated toxic effect on beneficial nitrogen-fixing bacteria | [89] | |
| Bensulfuron-methyl | Nitrogen mineralization | Resulted in diminished nitrogen release from the nitrogen source | [99] | |
| Imazetapyr with carbendazim and thiram | Nitrogen fixation | Suppressed formation of root nodules, impairing biological nitrogen assimilation | [100] | |
| Nematicides | Fenamiphos | Nitrification | Inhibited activity of ammonia oxidizing, impairing the nitrogen pathway | [78] |
| Organochlorines | DDT | Nitrogen fixation | Reduced nodulation in legume, limiting atmospheric nitrogen incorporation | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasir, M.; Hossain, A.; Pratap-Singh, A. Pesticide Degradation: Impacts on Soil Fertility and Nutrient Cycling. Environments 2025, 12, 272. https://doi.org/10.3390/environments12080272
Yasir M, Hossain A, Pratap-Singh A. Pesticide Degradation: Impacts on Soil Fertility and Nutrient Cycling. Environments. 2025; 12(8):272. https://doi.org/10.3390/environments12080272
Chicago/Turabian StyleYasir, Muhammad, Abul Hossain, and Anubhav Pratap-Singh. 2025. "Pesticide Degradation: Impacts on Soil Fertility and Nutrient Cycling" Environments 12, no. 8: 272. https://doi.org/10.3390/environments12080272
APA StyleYasir, M., Hossain, A., & Pratap-Singh, A. (2025). Pesticide Degradation: Impacts on Soil Fertility and Nutrient Cycling. Environments, 12(8), 272. https://doi.org/10.3390/environments12080272








