Assessing Pharmaceuticals in Bivalves and Microbial Sewage Contamination in Hout Bay, Cape Town: Identifying Impact Zones in Coastal and Riverine Environments
Abstract
1. Introduction
2. Material and Methods
2.1. Site Background/Description
Sampling Sites
2.2. Microbiological Sampling and Analysis
2.3. Mussel Sample Collection, Handling, and Preservation
2.4. Reagents and Chemicals
2.5. Sample Preparation (Extraction and Clean-Up)
2.6. Instrumental Method
2.7. Quality Assurance
2.8. Statistical Analysis
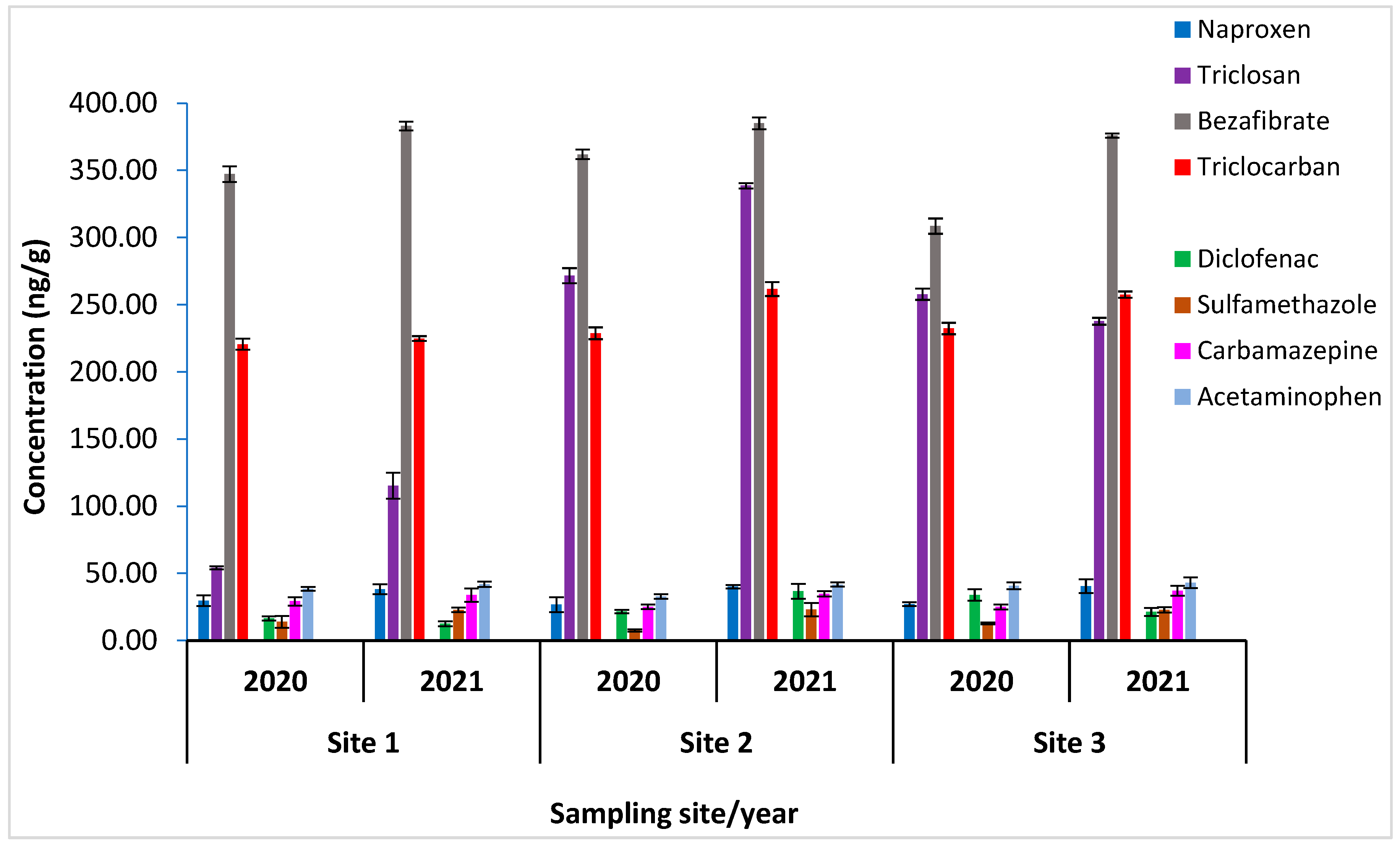
3. Results and Discussion
Addressing CECs in Hout Bay’s Marine Environment
4. Conclusions
5. Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burket, S.R.; White, M.; Ramirez, A.J.; Stanley, J.K.; Banks, K.E.; Waller, W.T.; Chambliss, C.K.; Brooks, B.W. Corbicula Fluminea Rapidly Accumulate Pharmaceuticals from an Effluent Dependent Urban Stream. Chemosphere 2019, 224, 873–883. [Google Scholar] [CrossRef]
- Brooks, B.W. Urbanization, Environment and Pharmaceuticals: Advancing Comparative Physiology, Pharmacology and Toxicology. Conserv. Physiol. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Kay, P.; Hughes, S.R.; Ault, J.R.; Ashcroft, A.E.; Brown, L.E. Widespread, Routine Occurrence of Pharmaceuticals in Sewage Effluent, Combined Sewer Overflows and Receiving Waters. Environ. Pollut. 2017, 220, 1447–1455. [Google Scholar] [CrossRef]
- Ghazal, H. Pharmaceuticals Contamination in the Environment. Environ. Toxicol. Pharmacol. 2023, 103, 104251. [Google Scholar] [CrossRef]
- Queirós, V.; Azeiteiro, U.M.; Soares, A.M.V.M.; Freitas, R. The Antineoplastic Drugs Cyclophosphamide and Cisplatin in the Aquatic Environment—Review. J. Hazard. Mater. 2021, 412, 125028. [Google Scholar] [CrossRef]
- Paut Kusturica, M.; Jevtic, M.; Ristovski, J.T. Minimizing the Environmental Impact of Unused Pharmaceuticals: Review Focused on Prevention. Front. Environ. Sci. 2022, 10, 1077974. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Occurrences, Levels and Risk Assessment Studies of Emerging Pollutants (Pharmaceuticals, Perfluoroalkyl and Endocrine Disrupting Compounds) in Fish Samples from Kalk Bay Harbour, South Africa. Environ. Pollut. 2019, 252, 562–572. [Google Scholar] [CrossRef]
- Feng, W.; Deng, Y.; Yang, F.; Miao, Q.; Ngien, S.K. Systematic Review of Contaminants of Emerging Concern (CECs): Distribution, Risks, and Implications for Water Quality and Health. Water 2023, 15, 3922. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A Review of Pharmaceuticals and Endocrine-Disrupting Compounds: Sources, Effects, Removal, and Detections. Water Air Soil. Pollut. 2013, 224, 1–29. [Google Scholar] [CrossRef]
- Sousa, H.; Sousa, C.A.; Simões, L.C.; Simões, M. Microalgal-Based Removal of Contaminants of Emerging Concern. J. Hazard. Mater. 2022, 423, 127153. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals in the Marine Environment: A Review. Environ. Rev. 2019, 27, 151–165. [Google Scholar] [CrossRef]
- Swartz, C.D.; Genthe, B.; Chamier, J.; Petrik, L.; Tijani, J.; Adeleye, A.; Coomans, C.; Ohlin, A.; Falk, D.; Menge, J. Emerging Contaminants In Wastewater Treated For Direct Potable Re-Use: The Human Health Risk Priorities In South Africa. VOLUME III: Occurrence, Fate, Removal And Health Risk Assessment Of Chemicals Of Emerging Concern In Reclaimed Water For Potable Reuse; Water Research Commission: Gezina, South Africa, 2018. [Google Scholar]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, Impacts and Trends of Pharmaceuticals in the Marine and Coastal Environment. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130572. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. Wastewater Treatment Plants as Chemical Observatories to Forecast Ecological and Human Health Risks of Manmade Chemicals. Sci. Rep. 2014, 4, 3731. [Google Scholar] [CrossRef]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and Ecotoxicological Assessment of Pharmaceuticals: Is There a Risk for the Mediterranean Aquatic Environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef]
- AL Falahi, O.A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ewadh, H.M.; Kurniawan, S.B.; Imron, M.F. Occurrence of Pharmaceuticals and Personal Care Products in Domestic Wastewater, Available Treatment Technologies, and Potential Treatment Using Constructed Wetland: A Review. Process Saf. Environ. Prot. 2022, 168, 1067–1088. [Google Scholar] [CrossRef]
- Sangion, A.; Gramatica, P. PBT Assessment and Prioritization of Contaminants of Emerging Concern: Pharmaceuticals. Environ. Res. 2016, 147, 297–306. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on Efficiency of Various Techniques in Treatment of Waste and Sewage Water—A Comprehensive Review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Ismail, W.N.W.; Mokhtar, S.U.; Ismail, W.N.W.; Mokhtar, S.U. Various Methods for Removal, Treatment, and Detection of Emerging Water Contaminants. In Emerging Contaminants; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in Electrochemical Detection Methods for Measuring Contaminants of Emerging Concerns. Electrochem. Sci. Adv. 2022, 2, e2100184. [Google Scholar] [CrossRef]
- Okoye, C.O.; Okeke, E.S.; Okoye, K.C.; Echude, D.; Andong, F.A.; Chukwudozie, K.I.; Okoye, H.U.; Ezeonyejiaku, C.D. Occurrence and Fate of Pharmaceuticals, Personal Care Products (PPCPs) and Pesticides in African Water Systems: A Need for Timely Intervention. Heliyon 2022, 8, e09143. [Google Scholar] [CrossRef]
- Kalebaila, N.; Hlophe-Ginindza, S.; Kalebaila, N.; Hlophe-Ginindza, S. Evolution of Water Research in South Africa from Legacy Pollutants to Contaminants of Emerging Concern: Successes and Opportunities; Springer Nature: London, UK, 2025; pp. 131–158. [Google Scholar] [CrossRef]
- Huerta, B.; Rodríguez-Mozaz, S.; Barceló, D. Pharmaceuticals in Biota in the Aquatic Environment: Analytical Methods and Environmental Implications. Anal. Bioanal. Chem. 2012, 404, 2611–2624. [Google Scholar] [CrossRef]
- Wijsman, J.; Troost, K.; Fang, J.; Roncarati, A. Global Production of Marine Bivalves. Trends and Challenges; Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, Ø., Smaa, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-96775-2. [Google Scholar]
- Ojemaye, C.Y.; Pampanin, D.M.; Sydnes, M.O.; Green, L.; Petrik, L. The Burden of Emerging Contaminants upon an Atlantic Ocean Marine Protected Reserve Adjacent to Camps Bay, Cape Town, South Africa. Heliyon 2022, 8, e12625. [Google Scholar] [CrossRef]
- Núñez, M.; Borrull, F.; Pocurull, E.; Fontanals, N. Pressurized Liquid Extraction Followed by Liquid Chromatography with Tandem Mass Spectrometry to Determine Pharmaceuticals in Mussels. J. Sep. Sci. 2016, 39, 741–747. [Google Scholar] [CrossRef]
- Maskrey, B.H.; Dean, K.; Morrell, N.; Turner, A.D. A Simple and Rapid Ultra–High-Performance Liquid Chromatography–Tandem Mass Spectrometry Method for the Quantitation of Pharmaceuticals and Related Compounds in Mussels and Oysters. Environ. Toxicol. Chem. 2021, 40, 3263–3274. [Google Scholar] [CrossRef]
- de Solla, S.R.; Gilroy, È.A.M.; Klinck, J.S.; King, L.E.; McInnis, R.; Struger, J.; Backus, S.M.; Gillis, P.L. Bioaccumulation of Pharmaceuticals and Personal Care Products in the Unionid Mussel Lasmigona Costata in a River Receiving Wastewater Effluent. Chemosphere 2016, 146, 486–496. [Google Scholar] [CrossRef]
- Republic of South Africa Government Gazette: The National Water Act (Act 36 of 1998). 1988. Available online: https://www.gov.za/documents/national-water-act (accessed on 10 July 2025).
- Republic of South Africa Government Gazette: Act No. 27 of 2014: National Water Amendment Act, 2014. 2014. Available online: https://www.gov.za/documents/national-water-amendment-act-0 (accessed on 10 July 2025).
- Republic of South Africa Government Gazatte: National Environmental Management Laws Amendment Act 2 of 2022. 2022. Available online: https://www.gov.za/documents/acts/national-environmental-management-laws-amendment-act-2-2022-english-afrikaans-24-jun (accessed on 10 July 2025).
- Republic of South Africa Government Gazatte: National Environmental Management Act 107 of 1998. 1998. Available online: https://www.gov.za/documents/national-environmental-management-act (accessed on 10 July 2025).
- Republic of South Africa Government Gazette: Water Services Amendment Act 30 of 2004. 2005. Available online: https://www.gov.za/documents/water-services-amendment-act (accessed on 10 July 2025).
- Republic of South Africa Government Gazette: Water Services Act 108 of 1997. 1997. Available online: https://www.gov.za/documents/water-services-act (accessed on 10 July 2025).
- Thatcher, A.; Biyela, P.; Field, T.L.; Hildebrandt, D.; Kidd, M.; Nadan, S.; Petrik, L.; Sheridan, C.; Topkin, J. Contextualising Urban Sanitation Solutions through Complex Systems Thinking: A Case Study of the South African Sanitation System. J. Clean. Prod. 2024, 451, 142084. [Google Scholar] [CrossRef]
- Grindley, S. Estuaries of the Cape—Report No.29: Hout Bay (CW27); CSIR Research Space: Pretoria, South Africa, 1988. [Google Scholar]
- Pearce, M.W. Assessment of Factors Influencing the Quality of Surface and Ground Water in the Hout Bay River Catchment; Rhodes University, Faculty of Science: Grahamstown, South Africa, 1989. [Google Scholar]
- CSIR. Cape Town Outfalls Monitoring Programme: Surveys Made in 2015/2016. CSIR Report CSIR/NRE/ECOS/IR/2017/0035/B; CSIR: Cape Town, South Africa, 2017. [Google Scholar]
- Botes, W. Water Research Commission Dilution Studies on Large Offshore Pipelines; Water Research Commission: Pretoria, South Africa, 1994. [Google Scholar]
- Beukes, A. A Sea of Contested Evidence: Disputes Over Coastal Pollution in Hout Bay, Cape Town, South Africa; Faculty of Humanities, School of African and Gender Study, Anth and Ling, 2022; Available online: http://hdl.handle.net/11427/36558 (accessed on 12 June 2024).
- Capeetc. Ongoing Sewage Crisis Raises Public Health Concerns in Hout Bay. Available online: https://www.capetownetc.com/news/ongoing-sewage-crisis-raises-public-health-concerns-in-hout-bay/ (accessed on 18 June 2025).
- CoCT, (City of Cape Town) Water Services and the Cape Town Urban Water Cycle. 2018; pp. 1–41. Available online: https://resource.capetown.gov.za/documentcentre/Documents/Graphics%20and%20educational%20material/Water%20Services%20and%20Urban%20Water%20Cycle.pdf (accessed on 20 August 2020).
- CLS South Africa. Environmental Summary Report on Modelling and Measurement Programmes: Hout Bay Outfall. Reference: CLS-SA-21-51 SPR HB-V3.0-26/08/2022; CLS Southern Africa: Cape Town, South Africa, 2022. [Google Scholar]
- Laird, M. Marine Specialist Report Marine Environmental Impact Assessment for the Proposed Desalination Plants Around the Cape Peninsula, South Africa; Anchor Research & Monitoring (Pty) Ltd.: Tokai, South Africa, 2017. [Google Scholar]
- Statistics South Africa Main Place Statistics South Africa. Statistics South Africa 2011. Available online: https://www.statssa.gov.za/?page_id=4286&id=332 (accessed on 22 June 2023).
- Toussaint, H. Anchoring: Imizamo Yethu, Hout Bay 2010. Available online: https://open.uct.ac.za/items/21fa070e-018f-418e-8af6-16639ff2d0c5 (accessed on 23 June 2023).
- CityFacts Hout Bay—Population Trends and Demographics. Available online: https://www.city-facts.com/hout-bay/population (accessed on 22 April 2024).
- SAHO Imizamo Yethu, Hout Bay. Available online: https://www.sahistory.org.za/place/imizamo-yethu-hout-bay (accessed on 22 April 2024).
- Le Roux, M. Hout Bay Land Inequality a “Microcosm” of SA. Mail. Guard. Online. 2007. Available online: https://mg.co.za/article/2007-05-21-hout-bay-land-inequality-a-microcosm-of-sa/ (accessed on 26 June 2023).
- Gara, F. Living with Water: An Ethnographic Study Relating to Water and Infrastructure Entanglements, in the Hout Bay Suburb of Cape Town, for a Water Sensitive Designed “Liveable”. Neighbourhood. 2021. Available online: http://hdl.handle.net/11427/35735 (accessed on 23 June 2023).
- Hout Bay Ratepayers Association A Timeline View of the History of IY. Available online: https://houtbayratepayers.co.za/timeline/ (accessed on 24 April 2024).
- Smith, H.M. The Relationship between Settlement Density and Informal Settlement Fires: Case Study of Imizamo Yethu, Hout Bay and Joe Slovo, Cape Town Metropolis. Geo-Inf. Disaster Manag. 2005, 1333–1355. [Google Scholar] [CrossRef]
- Schmiedeke, L. Sewerage in Storm Water Systems Hout Bay; Hout Bay Ratepayers Association: Cape Town, South Africa, 2021. [Google Scholar]
- City of Cape Town Llandudno Beach Reopened, Hout Bay Beach and Dalebrook to Kalk Bay Updates. Available online: https://www.capetown.gov.za/Media-and-news/Llandudno%20beach%20reopened,%20Hout%20Bay%20beach%20and%20Dalebrook%20to%20Kalk%20Bay%20updates%2011%20january%202023 (accessed on 24 April 2024).
- Metelerkamp, T. Latest Cape Town Beach Sewage Closures Hit Hout Bay and Dalebrook Tidal Pool. Available online: https://www.dailymaverick.co.za/article/2023-01-18-latest-round-of-cape-town-coastal-closures-affects-hout-bay-and-dalebrook-tidal-pool/ (accessed on 24 April 2024).
- City of Cape Town; Carter, R.; Holton, L. Environmental Summary Report on Modelling and Measurement Programmes: Hout Bay Outfall. Reference: CLS-SA-21-51 SPR HB. 2022. Available online: https://resource.capetown.gov.za/documentcentre/Documents/City%20research%20reports%20and%20review/Hout_Bay_Marine_Outfall_Environmental_Summary_Report.pdf (accessed on 10 July 2025).
- Baird, R.B.; Eaton, A.D.; Rice, E.W.; Bridgewater, L.; American Water Works Association, W.E.F. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- SANS, (South African National Bureau of Standards) South African National Standard (SANS)241-1. 2015. Available online: https://gwd.org.za/sites/default/files/2022-05/SANS241ED7_TC147_DSS_1%20-%20draft%20May%202022.pdf (accessed on 25 April 2024).
- Bio-Rad Laboratories RAPID’E.ColiAgar for Water Testing. Available online: https://www.bio-rad.com/sites/default/files/webroot/web/pdf/fsd/literature/Bulletin_6944.pdf (accessed on 1 October 2024).
- Bio-Rad Laboratories RAPID’Enterococcus Agar for Water Testing. Available online: https://www.bio-rad.com/en-za/product/rapidenterococcus-agar-for-water-testing?ID=LS5HKWH4E (accessed on 1 October 2024).
- Mullen, R.A.; Wigginton, K.R.; Noe-Hays, A.; Nace, K.; Love, N.G.; Bott, C.B.; Aga, D.S. Optimizing Extraction and Analysis of Pharmaceuticals in Human Urine, Struvite, Food Crops, Soil, and Lysimeter Water by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Methods 2017, 9, 5952–5962. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals and Personal Care Products in the Marine Environment Around False Bay, Cape Town, South Africa: Occurrence and Risk-Assessment Study. Environ. Toxicol. Chem. 2022, 41, 614–634. [Google Scholar] [CrossRef]
- Prichard, E.; Granek, E.F. Effects of Pharmaceuticals and Personal Care Products on Marine Organisms: From Single-Species Studies to an Ecosystem-Based Approach. Environ. Sci. Pollut. Res. 2016, 23, 22365–22384. [Google Scholar] [CrossRef]
- Peters, J.R.; Granek, E.F. Long-Term Exposure to Fluoxetine Reduces Growth and Reproductive Potential in the Dominant Rocky Intertidal Mussel, Mytilus Californianus. Sci. Total Environ. 2016, 545–546, 621–628. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Rodrigues, H.; Meisel, L.M.; Lino, C.M.; Pena, A. SSRIs Antidepressants in Marine Mussels from Atlantic Coastal Areas and Human Risk Assessment. Sci. Total Environ. 2017, 603–604, 118–125. [Google Scholar] [CrossRef]
- Mezzelani, M.; Fattorini, D.; Gorbi, S.; Nigro, M.; Regoli, F. Human Pharmaceuticals in Marine Mussels: Evidence of Sneaky Environmental Hazard along Italian Coasts. Mar. Environ. Res. 2020, 162, 105137. [Google Scholar] [CrossRef]
- Wolecki, D.; Caban, M.; Pazdro, K.; Mulkiewicz, E.; Stepnowski, P.; Kumirska, J. Simultaneous Determination of Non-Steroidal Anti-Inflammatory Drugs and Natural Estrogens in the Mussels Mytilus Edulis Trossulus. Talanta 2019, 200, 316–323. [Google Scholar] [CrossRef]
- Cunha, S.C.; Pena, A.; Fernandes, J.O. Mussels as Bioindicators of Diclofenac Contamination in Coastal Environments. Environ. Pollut. 2017, 225, 354–360. [Google Scholar] [CrossRef]
- Wille, K.; Kiebooms, J.A.L.; Claessens, M.; Rappé, K.; Vanden Bussche, J.; Noppe, H.; Van Praet, N.; De Wulf, E.; Van Caeter, P.; Janssen, C.R.; et al. Development of Analytical Strategies Using U-HPLC-MS/MS and LC-ToF-MS for the Quantification of Micropollutants in Marine Organisms. Anal. Bioanal. Chem. 2011, 400, 1459–1472. [Google Scholar] [CrossRef]
- Mello, F.V.; Cunha, S.C.; Fogaça, F.H.S.; Alonso, M.B.; Torres, J.P.M.; Fernandes, J.O. Occurrence of Pharmaceuticals in Seafood from Two Brazilian Coastal Areas: Implication for Human Risk Assessment. Sci. Total Environ. 2022, 803, 149744. [Google Scholar] [CrossRef]
- Klosterhaus, S.L.; Grace, R.; Hamilton, M.C.; Yee, D. Method Validation and Reconnaissance of Pharmaceuticals, Personal Care Products, and Alkylphenols in Surface Waters, Sediments, and Mussels in an Urban Estuary. Environ. Int. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Serra-Compte, A.; Maulvault, A.L.; Camacho, C.; Álvarez-Muñoz, D.; Barceló, D.; Rodríguez-Mozaz, S.; Marques, A. Effects of Water Warming and Acidification on Bioconcentration, Metabolization and Depuration of Pharmaceuticals and Endocrine Disrupting Compounds in Marine Mussels (Mytilus Galloprovincialis). Environ. Pollut. 2018, 236, 824–834. [Google Scholar] [CrossRef]
- Alvarez-Muñoz, D.; Huerta, B.; Fernandez-Tejedor, M.; Rodríguez-Mozaz, S.; Barceló, D. Multi-Residue Method for the Analysis of Pharmaceuticals and Some of Their Metabolites in Bivalves. Talanta 2015, 136, 174–182. [Google Scholar] [CrossRef]
- Interino, N.; Comito, R.; Simoni, P.; Franzellitti, S.; Palladino, G.; Rampelli, S.; Mosendz, A.; Gotti, R.; Roda, A.; Candela, M.; et al. Extraction Method for the Multiresidue Analysis of Legacy and Emerging Pollutants in Marine Mussels from the Adriatic Sea. Food Chem. 2023, 425, 136453. [Google Scholar] [CrossRef]
- Serra-Compte, A.; Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Barceló, D. Multi-Residue Method for the Determination of Antibiotics and Some of Their Metabolites in Seafood. Food Chem. Toxicol. 2017, 104, 3–13. [Google Scholar] [CrossRef]
- Bayen, S.; Estrada, E.S.; Juhel, G.; Kelly, B.C. Direct Injection of Tissue Extracts in Liquid Chromatography/ Tandem Mass Spectrometry for the Determination of Pharmaceuticals and Other Contaminants of Emerging Concern in Mollusks. Anal. Bioanal. Chem. 2015, 407, 5553–5558. [Google Scholar] [CrossRef]
- Capolupo, M.; Franzellitti, S.; Kiwan, A.; Valbonesi, P.; Dinelli, E.; Pignotti, E.; Birke, M.; Fabbri, E. A Comprehensive Evaluation of the Environmental Quality of a Coastal Lagoon (Ravenna, Italy): Integrating Chemical and Physiological Analyses in Mussels as a Biomonitoring Strategy. Sci. Total Environ. 2017, 598, 146–159. [Google Scholar] [CrossRef]
- Castaño-Ortiz, J.M.; Courant, F.; Gomez, E.; García-Pimentel, M.M.; León, V.M.; Campillo, J.A.; Santos, L.H.M.L.M.; Barceló, D.; Rodríguez-Mozaz, S. Combined Exposure of the Bivalve Mytilus Galloprovincialis to Polyethylene Microplastics and Two Pharmaceuticals (Citalopram and Bezafibrate): Bioaccumulation and Metabolomic Studies. J. Hazard. Mater. 2023, 458, 131904. [Google Scholar] [CrossRef]
- Edwards, M.A.; Kimbrough, K.; Fuller, N.; Davenport, E.; Rider, M.; Freitag, A.; Regan, S.; Leight, A.K.; Burkart, H.; Jacob, A.; et al. An Assessment and Characterization of Pharmaceuticals and Personal Care Products (PPCPs) within the Great Lakes Basin: Mussel Watch Program (2013–2018). Environ. Monit. Assess. 2024, 196, 345. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological Aspects Related to the Presence of Pharmaceuticals in the Aquatic Environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Seiler, J.P. Pharmacodynamic Activity of Drugs and Ecotoxicology—Can the Two Be Connected? Toxicol. Lett. 2002, 131, 105–115. [Google Scholar] [CrossRef]
- Republic of South Africa, Department of Environmental Affairs. South African Water Quality Guidelines for Coastal Marine Waters. Volume 2: Guideline For recreational Use; 2012. Available online: https://www.dffe.gov.za/sites/default/files/docs/strategy.framework/oceans/waterqualityguideline2012recreationaluse.pdf (accessed on 4 July 2025).
- Health and Ecological Criteria Division, Office of Science and Technology.; United States (U.S.) Environmental Protection Agency (EPA). Recreational Water Quality Criteria; 2012. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/rwqc2012.pdf (accessed on 4 July 2025).
- Weissman, G.; Rumpler, J. Safe for Swimming? Water Quality at Our Beaches. 2019. Available online: https://publicinterestnetwork.org/wp-content/uploads/2019/08/OHE-Safe-for-Swimming-Jul19web-rev1.1.pdf (accessed on 28 August 2023).
- City of Cape Town. Coastal Water Quality Dashboard. 2024. Available online: https://www.capetown.gov.za/Explore%20and%20enjoy/nature-and-outdoors/our-precious-biodiversity/coastal-water-quality (accessed on 4 July 2025).
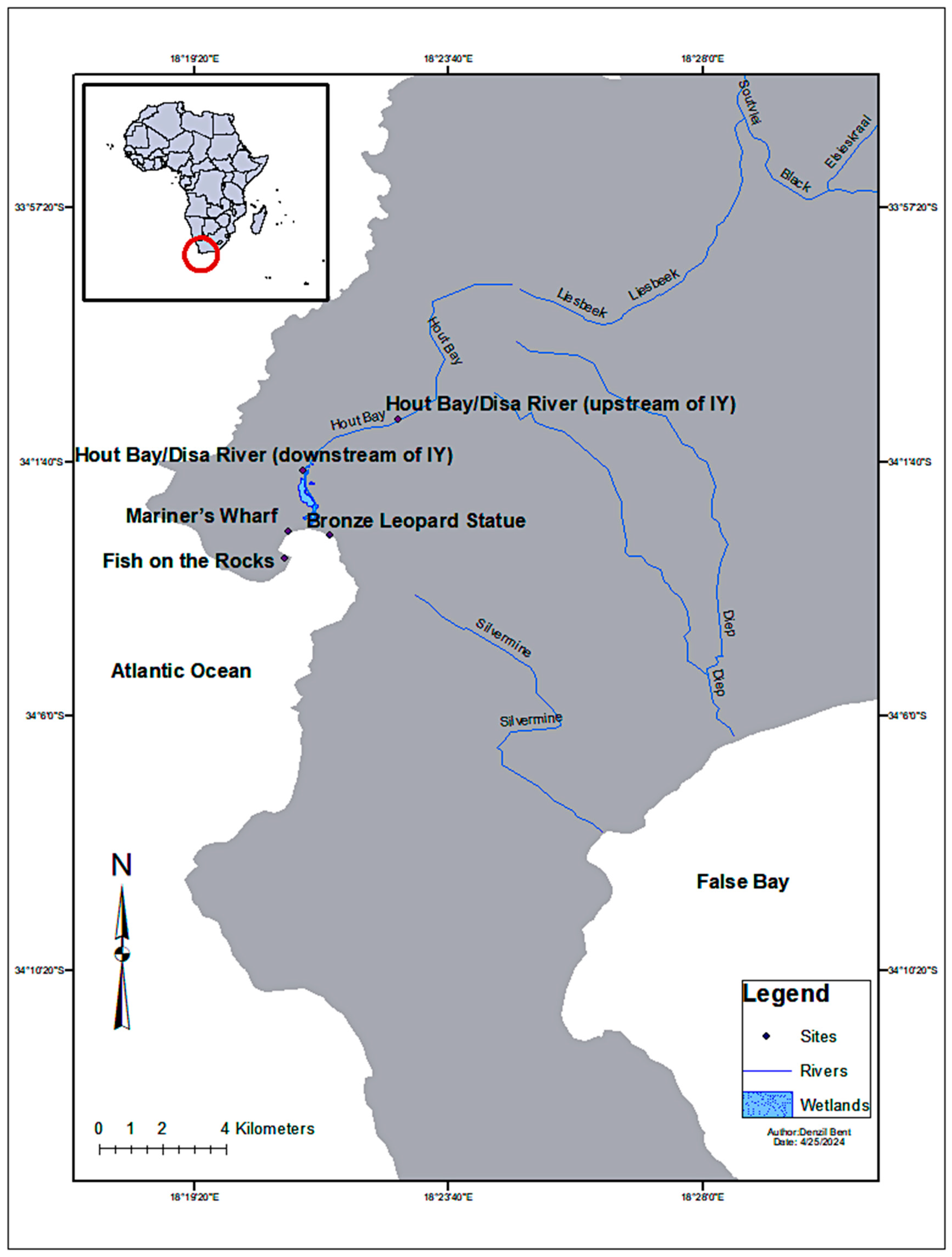
| Sampling Site/Name | Longitude and Latitude | Time | Wind/Speed Direction | ||
|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | ||
| Site 1 | 34°03′18.8″ S 18°20′52.2″ E | 7:35 a.m. | 6:54 a.m. | 7 km/h S | 10 km/h S |
| Site 2 | 34°02′51.4″ S 18°20′56.0″ E | 8:30 a.m. | 8:00 a.m. | 7 km/h S | 10 km/h S |
| Site 3 | 34°02′55.6″ S 18°21′38.8″ E | 9:15 a.m. | 9:10 a.m. | 7 km/h S | 10 km/h S |
| Compound Name | Molecular Weight (g/mol) | Molecular Structure | Log Kow | RT (min) | Ion Transition (m/z) | Ionisation Mode | CE (eV) | LOD (ng/g) | LOQ (ng/g) | % Recovery | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetaminophen | 151.16 | 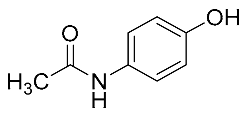 | 1.10 | 3.22 | 152 > 93 | 152 > 110 | ES+ | 24 | 0.43 | 1.30 | 98.5 |
| Carbamazepine | 236.27 | 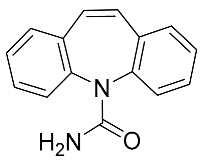 | 2.67 | 6.43 | 237 > 135 237 > 179 | 237 > 194 | ES+ | 10 | 0.2 | 0.5 | 99.2 |
| Sulfamethoxazole | 253.28 | 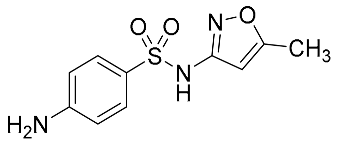 | 1.31 | 4.50 | 254 > 147 | 254 > 156 | ES+ | 25 | 0.3 | 1 | 98.9 |
| Diclofenac | 296.15 | 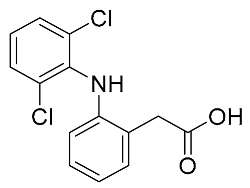 | 4.06 | 9.12 | 296 > 215 | 296 > 250 | ES+ | 15 | 0.7 | 2 | 99.1 |
| Triclocarban | 315.58 | 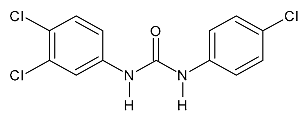 | 5.28 | 8.76 | 313 > 126 | 313 > 160 | ES- | 20 | 0.6 | 1.9 | 97.2 |
| Efavirenz | 315.67 | 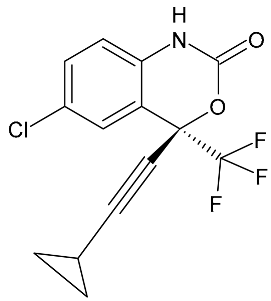 | 4.84 | 7.11 | 316 > 168 | 316 > 244 | ES+ | 30 | 9.9 | 30 | 90.4 |
| Naproxen | 230.26 | 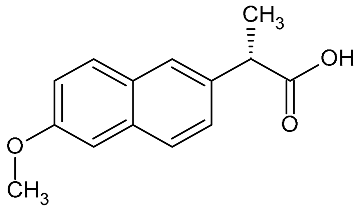 | 3.00 | 7.68 | 231 >149 | 231 > 185 | ES+ | 20 | 0.2 | 0.5 | 98.5 |
| Bezafibrate | 361.82 | 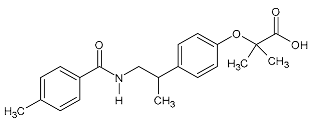 | 3.81 | 9.06 | 360.12 > 273.9 | 360.12 > 273.9 | ES- | 20 | 0.8 | 2.5 | 98.9 |
| Aspirin | 180.16 | 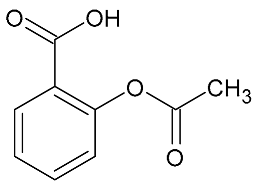 | 1.19 | 9.2 | 136.7 > 64.6 | 136.7 > 92.7 | ES- | 20 | 4.9 | 15 | 90.1 |
| Irbesartan | 428.53 | 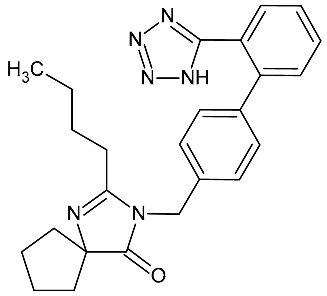 | 4.50 | 9.80 | 429.1 > 206.9 | 429.1 > 206.9 | ES+ | 20 | 0.2 | 0.5 | 98.9 |
| Beta-estradiol | 272.4 |  | 4.13 | 9.25 | 271 > 183 | 271 > 183 | ES- | 20 | 9.9 | 30 | 96.2 |
| Triclosan | 289.54 |  | 5.17 | 9.20 | 289 > 34.8 | 289 > 35 | ES+ | 55 | 9.9 | 30 | 97.5 |
| Location | Compounds | Concentration | References |
|---|---|---|---|
| San Francisco Bay | Carbamazepine Sulfamethoxazole Triclocarban | 1.3–5.3 ng/g ww <LOD <LOD–1.5 ng/g ww | [74] |
| Ebro Delta, Tarragona, Spain | Triclosan Carbamazepine Sulfamethoxazole | 1106.44 ng/g dw 453.2 ng/g dw 81.3 ng/g dw | [75] |
| Ebro Delta (Spain) | Sulfamethoxazole | <LOQ | [76] |
| False Bay, South Africa | Acetaminophen Sulfamethoxazole Carbamazepine Diclofenac | 46.7–85.5 ng/g dw 36–272 ng/g dw 26.06–66.00 ng/g dw 67.67–232.33 ng/g dw | [65] |
| Northwestern Adriatic Sea | Carbamazepine sulfamethoxazole | 2 ng/g 43 ng/g | [77] |
| Ebro Delta, Tarragona, Europe | Sulfamethoxazole | nd–<LOQ | [78] |
| Singapore | Carbamazepine | <LOQ–0.1 ng/g | [79] |
| Italian coast | Diclofenac carbamazepine | <1.4–171.1 ng/g dw <1.0–299.7 ng/g dw | [69] |
| Coastal lagoon (Ravenna, Italy) | Diclofenac | 2.1–4.6 ng/g ww | [80] |
| Brazilian coasts | Naproxen Carbamazepine Diclofenac Bezafibrate | nd–1.6 ng/g ww nd–0.9 ng/g ww nd–3.0 ng/g ww nd–5 ng/g ww | [73] |
| Benalmádena (S Spain) | Bezafibrate | <LOQ–2.7 ng/g dw | [81] |
| Gulf of Gdansk (southern Baltic Sea) | Diclofenac Naproxen | 560 ng/g dw 473 ng/g dw | [70] |
| USA | Triclocarban | 1.02–46.2 ng/g ww | [82] |
| Sampling Site—Name | Source | Results cfu/100 mL | |
|---|---|---|---|
| E. coli | Enterococcus | ||
| Disa River at Disa River Street bridge | River | 100 | 727 |
| Disa River at Victoria Street bridge | River | 1400 | 2420 |
| Fish on the Rocks | Sea | 100 | 150 |
| Mariner’s Wharf | Sea | 18 | 24 |
| Bronze Leopard Statue | Sea | 16 | 24 |
| * South Africa Limit | - | ≤500 | ≤185 |
| * USEPA Limit (freshwater) | - | 126 | 33 |
| * USEPA Limit (marine water) | - | - | 35 |
| Sampling Site—Name | Type | E. coli (cfu/100 mL) |
|---|---|---|
| Hout Bay top of valley (weir below cascade) | River | None detected |
| At Victoria Street Bridge | River | 300 |
| At the join of stormwater from Imizamo Yethu | River | 8,300,000 |
| Estuary on beach, short distance from sea | Estuary (semi-saline) | 50,000 |
| * South Africa Limit | ≤500 | |
| * USEPA Limit | 126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojemaye, C.Y.; Beukes, A.; Moser, J.; Gara, F.; Barnes, J.; Petrik, L.; Green, L. Assessing Pharmaceuticals in Bivalves and Microbial Sewage Contamination in Hout Bay, Cape Town: Identifying Impact Zones in Coastal and Riverine Environments. Environments 2025, 12, 257. https://doi.org/10.3390/environments12080257
Ojemaye CY, Beukes A, Moser J, Gara F, Barnes J, Petrik L, Green L. Assessing Pharmaceuticals in Bivalves and Microbial Sewage Contamination in Hout Bay, Cape Town: Identifying Impact Zones in Coastal and Riverine Environments. Environments. 2025; 12(8):257. https://doi.org/10.3390/environments12080257
Chicago/Turabian StyleOjemaye, Cecilia Y., Amy Beukes, Justin Moser, Faith Gara, Jo Barnes, Lesley Petrik, and Lesley Green. 2025. "Assessing Pharmaceuticals in Bivalves and Microbial Sewage Contamination in Hout Bay, Cape Town: Identifying Impact Zones in Coastal and Riverine Environments" Environments 12, no. 8: 257. https://doi.org/10.3390/environments12080257
APA StyleOjemaye, C. Y., Beukes, A., Moser, J., Gara, F., Barnes, J., Petrik, L., & Green, L. (2025). Assessing Pharmaceuticals in Bivalves and Microbial Sewage Contamination in Hout Bay, Cape Town: Identifying Impact Zones in Coastal and Riverine Environments. Environments, 12(8), 257. https://doi.org/10.3390/environments12080257






