Mercury Concentration and Distribution in Remiges, Rectrices, and Contour Feathers of the Barn Swallow Hirundo rustica
Abstract
1. Introduction
2. Materials and Methods
2.1. Area and Study Species
2.2. Chemical Analysis
2.3. Statistical Analyses
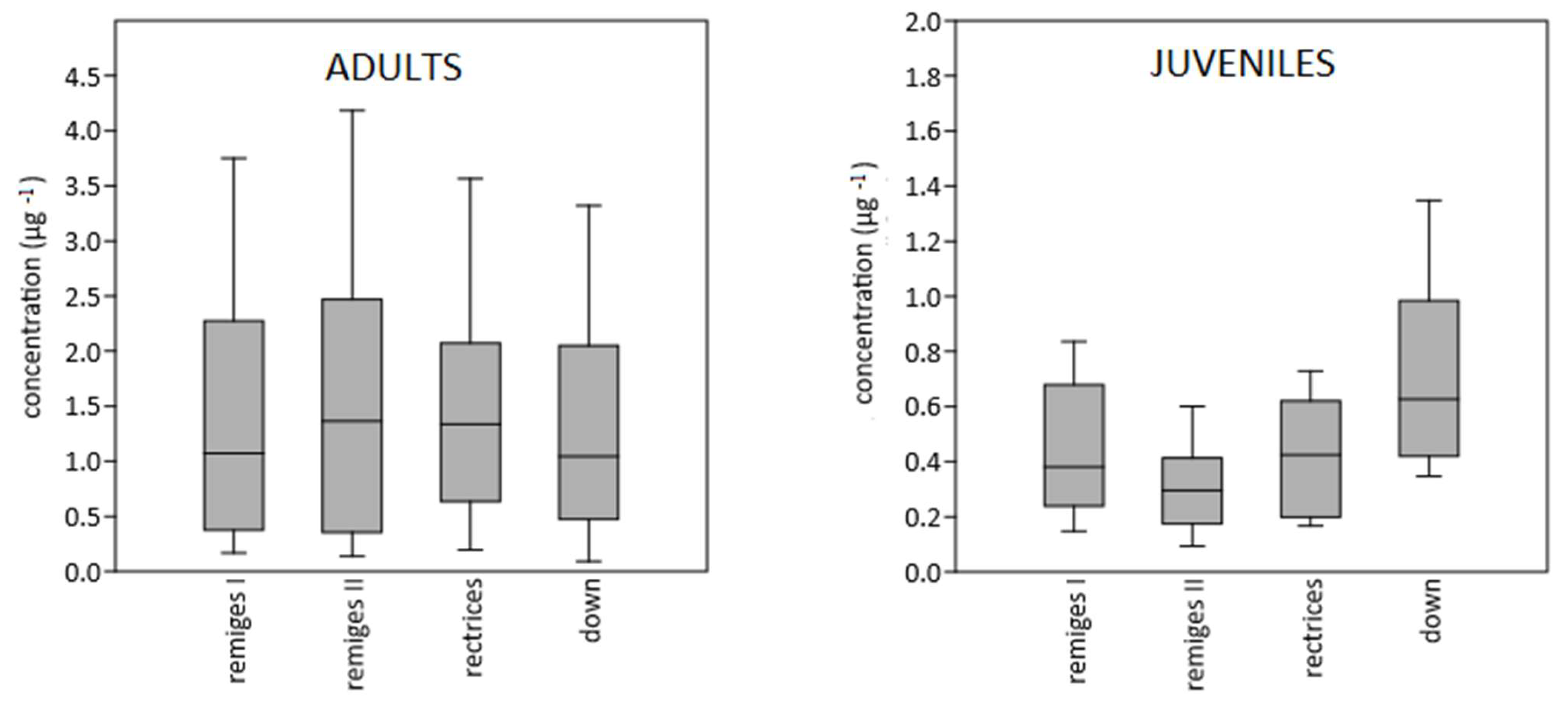
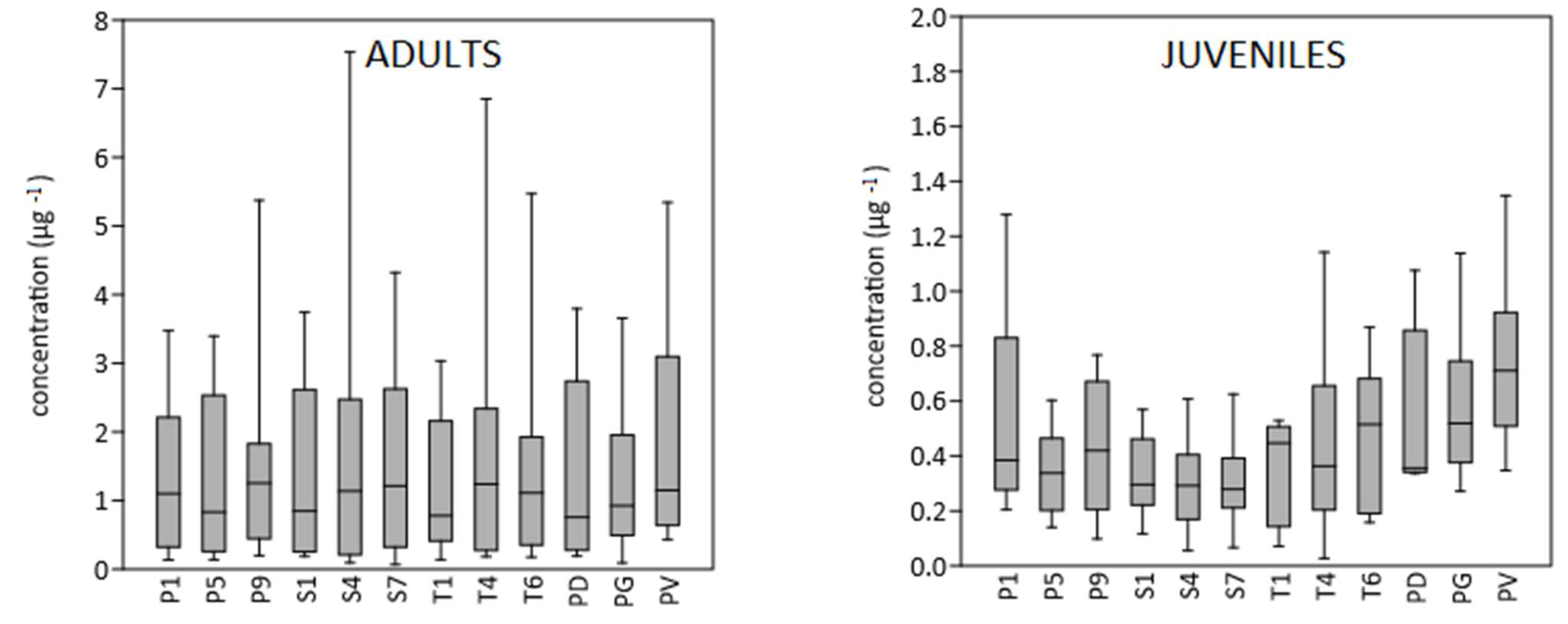
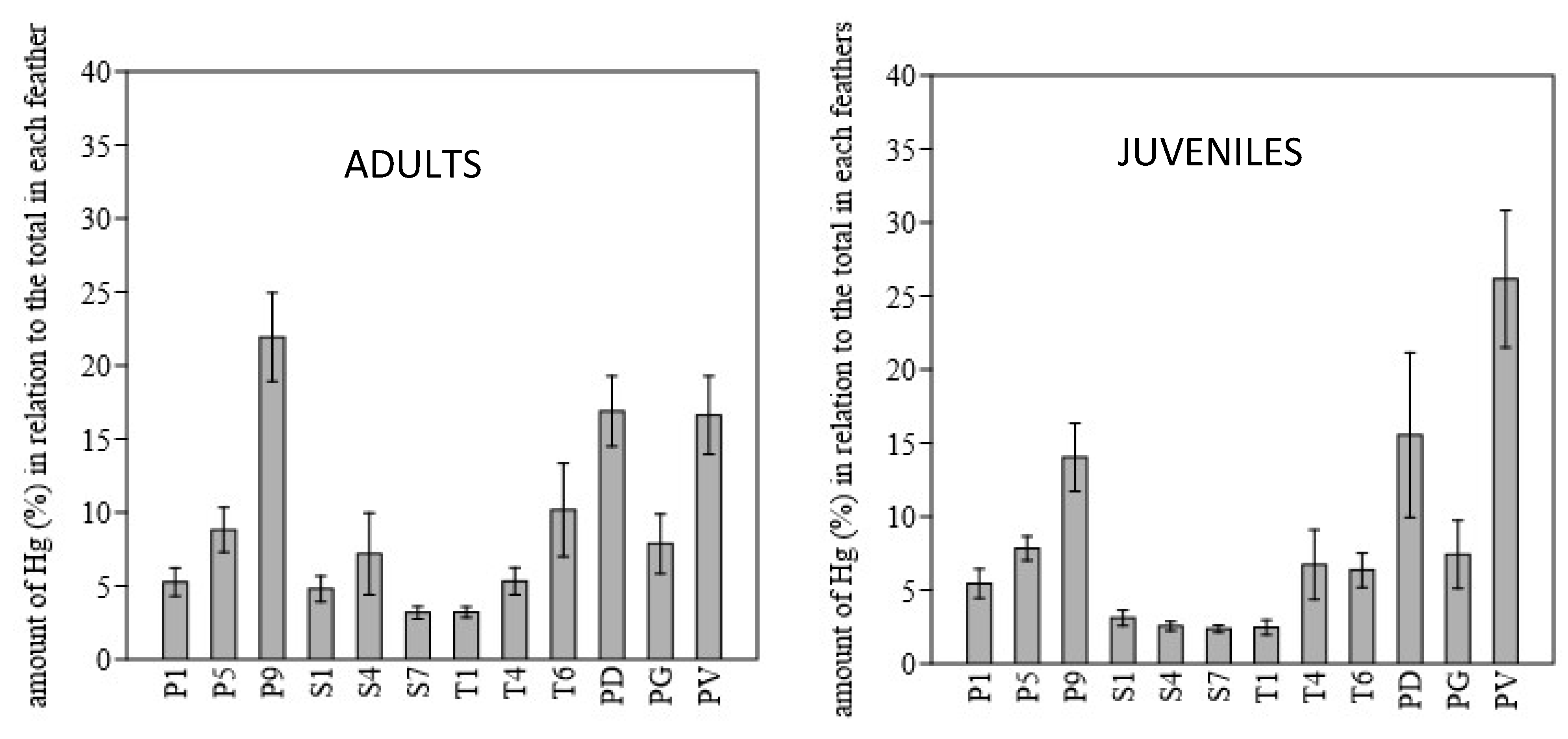
3. Results
4. Discussion
4.1. Mercury Contents in Barn Swallow Feathers
4.2. Prediction of Individual Mercury Exposure by Specific Feathers and Feather Categories
4.3. The Issue of External Contamination
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [Hg] | mercury concentration |
References
- Allyson, K.J.; Evers, D.C.; Folsom, S.B.; Condon, A.M.; Diener, J.; Goodrick, L.F.; McGann, A.J.; Schmerfeld, J.; Cristol, D.A. Mercury exposure in terrestrial birds far downstream of an historical point source. Environ. Pollut. 2011, 159, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Hobson, K.; Branfireun, B. Rapidly increasing methyl mercury in endangered ivory gull (Pagophila eburnea) feathers over a 130 year record. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150032. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Jacob, D.J.; Lu, Z.; Levin, L.; Ter Schure, A.F.; Sunderland, E.M. Total Mercury Released to the Environment by Human Activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef]
- Weiner, J.G.; Krabbemhoft, D.P.; Heinz, G.H.; Scheuhammer, A.M. Ecotoxicology of mercury. In Handbook of Ecotoxicology, 2nd ed.; Hoffman, D.J., Rattner, B.A., Burton, G.A., Cairns, J., Eds.; CRC Press: New York, NY, USA, 2003; pp. 409–463. [Google Scholar]
- Cherel, Y.; Barbraud, C.; Lahournat, M.; Jaeger, A.; Jaquemet, S.; Ross, M.; Wanless, R.; Phillips, A.; Thompson, D.R.; Bustamante, P. Accumulate or eliminate? Seasonal mercury dynamics in albatrosses, the most contaminated family of birds. Environ. Pollut. 2018, 241, 124–135. [Google Scholar] [CrossRef]
- Eisler, R. Mercury: Hazards to Living Organisms; Taylor and Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ackerman, J.T.; Peterson, S.; Herzog, M.; Yee, J. Methylmercury Effects on Birds: A Review, Meta-Analysis, and Development of Toxicity Reference Values for Injury Assessment Based on Tissue Residues and Diet. Environ. Toxicol. Chem. 2024, 43, 1195–1241. [Google Scholar] [CrossRef]
- Outridge, P.M.; Scheuhammer, A.M. Bioaccumulation and toxicology of nickel: Implications for wild mammals and birds. Environ. Rev. 1993, 1, 172–197. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.; Qiu, G. Methylmercury exposure and health effects from rice and fish consumption: A review. Int. J. Environ. Res. Public Health 2010, 7, 2666–2691. [Google Scholar] [CrossRef]
- Wu, Y.S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 22, 5100–5126. [Google Scholar] [CrossRef]
- Lodenius, M.; Solonen, T. The use of feathers of birds of prey as indicators of metal pollution. Ecotoxicology 2013, 22, 1319–1334. [Google Scholar] [CrossRef]
- Varela, Z.; García-Seoane, R.; Fernández, J.A.; Carballeira, A.; Aboal, J.R. Study of temporal trends in mercury concentrations in the primary flight feathers of Strix aluco. Ecotox. Environ. Saf. 2016, 130, 199–206. [Google Scholar] [CrossRef]
- Monteiro, L.R.; Furness, R.W. Kinetics, dose–response, and excretion of methylmercury in free-living adult Cory’s shearwaters. Environ. Sci. Technol. 2001, 35, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, T.; Bervoets, L.; Pinxten, R.; Blust, R.; Eens, M. Variation of heavy metals within and among feathers of birds of prey: Effects of molt and external contamination. Environ. Pollut. 2003, 124, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Veerle, L.; Jaspers, B.; Covaci, A.; Herzke, D.; Eulaers, I.; Eens, M. Bird feathers as a biomonitor for environmental pollutants: Prospects and pitfalls. TrAC Trends Anal. Chem. 2019, 118, 223–226. [Google Scholar] [CrossRef]
- Honda, K.; Min, B.Y.; Tatsukawa, R. Distribution of heavy metals and their age-related changes in the eastern great white egret Egretta alba modesta, in Korea. Arch. Environ. Contam. Toxicol. 1986, 15, 185–197. [Google Scholar] [CrossRef]
- Braune, B.M.; Gaskin, D.E. Mercury levels in Bonaparte’s gulls (Larus Philadelphia) during autumn molt in the Quoddy region, New Brunswick, Canada. Arch. Environ. Contam. Toxicol. 1987, 16, 539–549. [Google Scholar] [CrossRef]
- Lewis, S.A.; Furness, R.W. Mercury accumulation and excretion in laboratory reared black-headed gull Larus ridibundus chicks. Arch. Environ. Contam. Toxicol. 1991, 21, 316–320. [Google Scholar] [CrossRef]
- Rutkowska, M.; Płotka-Wasylka, J.; Lubinska-Szczygeł, M.; Różańska, A.; Możejko-Ciesielska, J.; Namieśnik, J. Birds’ feathers—Suitable samples for determination of environmental pollutants. TrAC Trends Anal. Chem. 2018, 109, 97–115. [Google Scholar] [CrossRef]
- Kenow, K.P.; Meyer, M.W.; Hines, R.K.; Karasov, W.H. Distribution and accumulation of mercury in tissues of captive-reared common loon (Gavia immer) chicks. Environ. Toxicol. Chem. 2007, 26, 1047–1055. [Google Scholar] [CrossRef]
- Bottini, C.J.; Scott, A.; MacDougall-Shackleton, B.; Branfireun, K.; Hobson, A. Feathers accurately reflect blood mercury at time of feather growth in a songbird. Sci. Total Environ. 2021, 775, 145739. [Google Scholar] [CrossRef]
- Evers, D.C.; Kaplan, J.D.; Meyer, M.W.; Reaman, P.S.; Braselton, W.E.; Major, A.; Burgess NScheuhammer, A.M. Geographic trend in mercury measured in common loon feathers and blood. Environ. Toxicol. Chem. 1998, 17, 173–183. [Google Scholar] [CrossRef]
- Kahle, S.; Becker, P.H. Bird blood as bioindicator for mercury in the environment. Chemosphere 1999, 39, 2451–2457. [Google Scholar] [CrossRef]
- Rimmer, C.C.; McFarland, K.P.; Evers, D.C.; Miller, E.K.; Aubry, Y.; Busby, D.; Taylor, R.J. Mercury Concentrations in Bicknell’s Thrush and Other Insectivorous Passerines in Montane Forests of Northeastern North America. Ecotoxicology 2005, 14, 223–240. [Google Scholar] [CrossRef]
- Eagles-Smith, C.A.; Ackerman, J.T.; Terrence, L.; Adelsbach, J.; Takekawa, A.; Miles, K.; Keiste, R.A. Mercury correlations among six tissues for four waterbird species breeding in San Francisco Bay, California, USA. Environ. Toxicol. Chem. 2008, 27, 2136–2153. [Google Scholar] [CrossRef]
- Raygoza-Viera, J.R.; Ruiz-Fernández, A.C.; Ruelas-Inzunza, J. The Use of Blood in Anas clypeata as an Efficient and Non-lethal Method for the Biomonitoring of Mercury. Bull. Environ. Contam. Toxicol. 2013, 91, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.T.; Eagles-Smith, C.A.; Takekawa, J.Y.; Bluso, J.D.; Adelsbach, T.L. Mercury concentrations in blood and feathers of prebreeding Foster’s terns in relation to space use of San Francisco Bay, California, USA, habitats. Environ. Toxicol. Chem. 2008, 27, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, G. Flaws and pitfalls in the chemical analysis of feathers: Bad news-good news for avian chemoecology and toxicology. Ecol. Appl. 2010, 20, 1766–1774. [Google Scholar] [CrossRef]
- Furness, R.W.; Muirhead, S.J.; Woodburn, M. Using bird feathers to measure mercury in the environment: Relationships between mercury content and moult. Mar. Pollut. Bull. 1986, 17, 27–30. [Google Scholar] [CrossRef]
- Martínez, A.; Crespo, D.; Fernández, J.; Aboal, J.; Carballeira, A. Selection of flight feathers from Buteo buteo and Accipiter gentilis for use in biomonitoring heavy metal contamination. Sci. Total Environ. 2012, 425, 254–261. [Google Scholar] [CrossRef]
- Peterson, S.H.; Ackerman, J.T.; Toney, M.; Herzog, M.P. Mercury concentrations vary within and among individual bird feathers: A critical evaluation and guidelines for feather use in mercury monitoring programs. Environ. Toxicol. Chem. 2019, 38, 1164–1187. [Google Scholar] [CrossRef]
- Debén García, S.; Fernández, J.; Aboal, J.; Carballeira, J. Evaluation of different contour feather types for biomonitoring lead exposure in Northern goshawk (Accipiter gentilis) and tawny owl (Strix aluco). Ecotoxicol. Environ. Saf. 2012, 85, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Militao, T.; Gonzales-Solis, J.; Ruiz, X. Moulting strategies of a long-distance migratory seabird, the Mediterranean Cory’s Shearwater Calonectris diomedea diomedea. IBIS 2009, 151, 151–159. [Google Scholar] [CrossRef]
- Nadal, J.; Ponz, C.; Margalida, A. The end of primary moult as an indicator of global warming effects in the Red-legged Partridge Alectoris rufa, a medium sized, sedentary species. Ecol. Indic. 2021, 122, 107287. [Google Scholar] [CrossRef]
- Parolini, M.; Sturini, M.; Maraschi, F.; Profumo, A.; Costanzo, A.; Caprioli, M.; Rubolini, D.; Ambrosini, R.; Canova, L. Trace Elements Fingerprint of Feathers Differs between Breeding and Non-Breeding Areas in an Afro-Palearctic Migratory Bird, the barn swallow (Hirundo rustica). Environ. Sci. Pollut. Res. 2021, 28, 15828–15837. [Google Scholar] [CrossRef]
- González-Olvera, M.; Hernandez-Colina, A.; Chantrey, J.; Allen, S.; Lopez, J. A non-invasive feather-based methodology for the detection of blood parasites (Haemosporida). Sci. Rep. 2023, 13, 16712. [Google Scholar] [CrossRef]
- Fuentes, E.F.; Moity, N.; Ramírez-González, J.; Andrade-Vera, S.; Hardisson, A.; Rubio, C.; Paz, S.; González-Weller, D.; Rubio, C.; Gutiérrez, A.J. Analysis of metals and metalloid in commercial fish species from the Galapagos Marine Reserve: Toxicological and nutritional assessment. Mar. Pollut. Bull. 2023, 189, 114739. [Google Scholar] [CrossRef]
- Ambrosini, R.; Bolzern, A.M.; Canova, L.; Arieni, S.; Møller, A.P.; Saino, N. The distribution and colony size of barn swallows in relation to agricultural land use. J. Appl. Ecol. 2002, 39, 524–534. [Google Scholar] [CrossRef]
- Møller, A.P.; Gregersen, J. Sexual Selection and the Barn Swallow; Oxford University Press: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Pancerasa, M.; Ambrosini, R.; Romano, A.; Rubolini, D.; Winkler, D.W.; Casagrandi, R. Across the deserts and sea: Inter-individual variation in migration routes of south-central European barn swallows (Hirundo rustica). Mov. Ecol. 2022, 10, 51. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol. Rev. 2010, 85, 935–956. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 13 April 2025).
- Odsjö, T.; Roos, A.; Johnels, A. The Tail Feathers of Osprey Nestlings (Pandion haliaetus L.) as Indicators of Change in Mercury Load in the Environment of Southern Sweden (1969–1998): Case Study with a Note on the Simultaneous Intake of Selenium. Ambio 2004, 33, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, G.; Esmaili-Sari, A.; Ghasempouri, S.M.; Kiabi, B.H. Examination of mercury concentration in the feathers of 18 species of birds in southwest Iran. Environ. Res. 2007, 104, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Cardiel, I.E.; Taggart, M.A.; Mateo, R. Using Pb–Al ratios to discriminate between internal and external deposition of Pb in feathers. Ecotoxicol. Environ. Saf. 2011, 74, 911–917. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Comparison of arsenic, cadmium; chromium; lead; manganese, mercury and selenium in feathers in bald eagle (Haliaeetus leucocephalus), and comparison with common eider (Somateria mollissima), glaucous-winged gull (Larus glaucescens), pigeon guillemot (Cepphus columba), and tufted puffin (Fratercula cirrhata) from the Aleutian Chain of Alaska. Environ. Monit. Assess. 2009, 152, 357–367. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Jeitner, C. Mercury and other metals in eggs and feathers of glaucous-winged gulls (Larus glaucescens) in the Aleutians. Environ. Monit. Assess. 2009, 152, 179–194. [Google Scholar] [CrossRef]
- Stenhouse, I.J.; Adams, E.M.; Phillips, L.M.; Weidensaul, S.; McIntyre, C.L. A preliminary assessment of mercury in the feathers of migratory songbirds breeding in the North American subarctic. Ecotoxicology 2020, 29, 1221–1228. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, Y.; Yu, R.; Zheng, X. A Pilot Study on Bioaccumulation and Tissue Distribution of Mercury in barn swallow (Hirundo rustica). Toxics 2024, 12, 206. [Google Scholar] [CrossRef]
- Kardynal, K.J.; Jardine, T.D.; Génier, C.S.V. Mercury exposure to swallows breeding in Canada inferred from feathers grown on breeding and non-breeding grounds. Ecotoxicology 2020, 29, 876–891. [Google Scholar] [CrossRef]
- Costanzo, A.; Sturini, M.; Maraschi, F.; Caprioli, M.; Romano, A.; Vanni, S.; Parolini, M.; Profumo, A.; Rubolini, D.; Ambrosini, R.; et al. Local Variability of Trace Element Concentration in barn swallow (Hirundo rustica) Nestlings from the Po Plain (Northern Italy). Environments 2023, 10, 145. [Google Scholar] [CrossRef]
- Jenni, L.; Winkler, R. Moult and Ageing of European Passerines; Bloomsbury Publishing: New York, NY, USA, 2020. [Google Scholar]
- Rubolini, D.; Massi, A.; Spina, F. Replacement of body feathers is associated with low pre-migratory energy stores in a long-distance migratory bird, the barn swallow Hirundo rustica. J. Zool. 2002, 258, 441–447. [Google Scholar] [CrossRef]
- Lindström, A.; Visser, G.H.; Daan, S. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 1993, 66, 490–510. [Google Scholar] [CrossRef]
- Cyr, N.E.; Wikelski, M.; Romero, L.M. Increased energy expenditure but decreased stress responsiveness during moult. Physiol. Biochem. Zool. 2008, 81, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E. Energetics and nutrition in molt. In Avian Energetics and Nutritional Ecology; Carey, C., Ed.; Chapman & Hall: New York, NY, USA, 1996; pp. 158–198. [Google Scholar]
- Saino, N.; Romano, M.; Caprioli, M.; Lardelli, R.; Micheloni, P.; Scandolara, C.; Rubolini, D. Molt, feather growth rate and body condition of male and female barn swallows. J. Ornithol. 2012, 154, 537–547. [Google Scholar] [CrossRef]
- Goodale, W.; David, E.; Mierzykowski, S.; Bond, A.; Burgess, N.; Otorowski, C.; Welch, L.; Hall, C.; Ellis, J.; Allen, R.; et al. Marine Foraging Birds As Bioindicators of Mercury in the Gulf of Maine. EcoHealth 2008, 5, 409–425. [Google Scholar] [CrossRef]
- Canova, L.; Sturini, M.; Profumo, A.; Maraschi, F. Evidence of Low-Habitat Contamination Using Feathers of Three Heron Species as a Biomonitor of Inorganic Elemental Pollution. Int. J. Environ. Res. Public Health 2020, 17, 7776. [Google Scholar] [CrossRef]
- Carravieri, A.; Bustamante, P.; Tartu, S.; Meillère, A.; Labadie, P.; Budzinski, H.; Peluhet, L.; Barbraud, C.; Weimerskirch, H.; Chastel, O.; et al. Wandering albatrosses document latitudinal variations in the transfer of persistent organic pollutants and mercury to southern ocean predators. Environ. Sci. Technol. 2014, 48, 14746–14755. [Google Scholar] [CrossRef]
- Bighetti, G.B.; Padilha, J.A.; Cunha, L.S.T.; Kasper DMalm, O.; Mancini, P.L. Bioaccumulation of mercury is equal between sexes but different by age in seabird (Sula leucogaster) population from southeast coast of Brazil. Environ. Pollut. 2021, 285, 117222. [Google Scholar] [CrossRef]
- Vizuete, J.; Hernández-Moreno, D.; López-Beceiro, A. Heavy metals and metalloid levels in the tissues of yellow-legged gulls (Larus michahellis) from Spain: Sex, age, and geographical location differences. Environ. Sci. Pollut. Res. 2022, 29, 54292–54308. [Google Scholar] [CrossRef]
- Sebastiano MBustamante, P.; Costantini, D.; Eulaers, G.; Malarvannan, G.; Mendez-Fernandez, P.; Churlaud, C.; Blévin, C.; Hauselmann, A.; Dell’Omo, G.; Covaci, A.; et al. High levels of mercury and low levels of persistent organic pollutants in a tropical seabird in French Guiana, the Magnificent frigatebird, Fregata magnificens. Environ. Pollut. 2016, 214, 384–393. [Google Scholar] [CrossRef]
- Turner, A.K. Optimal foraging by the swallow (Hirundo rustica, L): Prey size selection. Anim. Behav. 1982, 30, 862–872. [Google Scholar] [CrossRef]
- Orłowski, G.; Karg, J. Diet of nestling barn swallows Hirundo rustica in rural areas of Poland. Open Life Sci. 2011, 6, 1023–1035. [Google Scholar] [CrossRef]
- Grigolo, C.P.; Sicurella, B.; Musitelli, F.; Romano, A.; Caprioli, M.; Rubolini, D.; Ambrosini, R.; Gobbi, M. Diet heterogeneity and antioxidant defence in barn swallow Hirundo rustica nestlings. Avocetta 2019, 43, 127–137. [Google Scholar] [CrossRef]
- Turner, A.K. The Barn Swallow; T & AD Poyser: London, UK, 2006. [Google Scholar]
- Sommer, N.O.; Luttinen, A.; Lehikoinen, A. Intra- and interspecific variation in trace element concentrations in feathers of north European trans-African migrants. J. Avian Biol. 2023, 2023, e03106. [Google Scholar] [CrossRef]
- Szép, T.; Hobson, K.A.; Vallner, J.; Piper, S.E.; Kovács, B.; Szabó, D.Z.; Møller, A.P. Comparison of trace element and stable isotope approaches to the study of migratory connectivity: An example using two hirundine species breeding in Europe and wintering in Africa. J. Ornithol. 2009, 150, 621–636. [Google Scholar] [CrossRef]
- Óvári, M.; Laczi, M.; Török, J.; Mihucz, V.G.; Záray, G. Elemental composition in feathers of a migratory passerine for differentiation of sex, age, and molting areas. Environ. Sci. Pollut. Res. 2018, 25, 2021–2034. [Google Scholar] [CrossRef]
- Jaspers, V.L.; Covaci, A.; Van den Steen, E.; Eens, M. Is external contamination with organic pollutants important for concentrations measured in bird feathers? Environ. Int. 2007, 33, 766–772. [Google Scholar] [CrossRef]
- Monteiro, L.R.; Granadeiro, J.P.; Furness, R.W. Relationship between mercury levels and diet in Azores seabirds. Mar. Ecol. Prog. Ser. 1998, 166, 259–265. [Google Scholar] [CrossRef]
- Thompson, D.; Bearhop, S.; Speakman, J.R.; Furness, R.W. Feathers as a means of monitoring mercury in seabirds: Insights from stable isotope analysis. Environ. Pollut. 1998, 101, 258–265. [Google Scholar] [CrossRef]
- Ackerman, J.T.; Collin, A.; Eagles-Smith, M.P.; Herzog, C.; Hartman, A.; Peterson, S.H.; Evers, D.; Jackson, A.K.; Elliott, J.E.; Vander Pol, S.J.; et al. Avian mercury exposure and toxicological risk across western North America: A synthesis. Sci. Total Environ. 2016, 568, 749–769. [Google Scholar] [CrossRef]
- Bajracharya, S.S.; Zahor, D.L.; Glynn, K.J.; Gratz, L.E.; Cornelius, J.M. Feather mercury concentrations in omnivorous and granivorous terrestrial songbirds in Southeast Michigan. Ecotoxicology 2022, 31, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Cramp, S. The Complete Birds of the WESTERN Palearctic on CD-ROM; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Jaspers, V.; Dauwe, T.; Pinxten, R.; Bervoets, L.; Blust, R.; Eens, M. The importance of exogenous contamination on heavy metal levels in bird feathers, A field experiment with free-living great tits, Parus major. J. Environ. Monit. 2004, 6, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, F.; Migani, F.; Andreotti, A.; Baccetti, N.; Bianchi, N.; Birke, M.; Dinelli, E. Metals and trace elements in feathers: A geochemical approach to avoid misinterpretation of analytical responses. Sci. Total Environ. 2016, 544, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Chételat, J.; Hickey, M.B.C.; Poulain, A.J.; Dastoor, A.; Ryjkov, A.; McAlpine, D.; Vanderwolf, K.; Jung, T.S.; Hale, L.; Cooke, E.L.; et al. Spatial variation of mercury bioaccumulation in bats of Canada linked to atmospheric mercury deposition. Sci. Total Environ. 2018, 626, 668–677. [Google Scholar] [CrossRef]
- Aloupi, M.; Ferentinou, E.; Zaharaki, O.M.; Akriotis, T. Does dilute nitric acid improve the removal of exogenous heavy metals from feathers? A comparative study towards the optimization of the cleaning procedure of feather samples prior to metal analysis. Ecotoxicol. Environ. Saf. 2020, 200, 110759. [Google Scholar] [CrossRef]
- Keute, J.; Rizzo, J.; Giunta, F.; Hernout, B.V. Evaluating washing techniques to eliminate external contamination of trace elements in bat fur and bird feathers. Ecotoxicol. Environ. Saf. 2024, 283, 116819. [Google Scholar] [CrossRef]
- Onjia, A.; Xin, H.; Trujillo González, J.M.; Egbueri Johnbosco, C. Chemometric approach to distribution, source apportionment, ecological and health risk of trace pollutants. Front. Environ. Sci. 2022, 10, 1107465. [Google Scholar] [CrossRef]

| Adults | Juveniles | Adults | Juveniles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (µg g−1) | Amount (µg) | |||||||||||
| Header | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max |
| P1 | 1.10 | 0.14 | 3.47 | 0.38 | 0.20 | 1.28 | 0.010 | 0.010 | 0.030 | 0.002 | 0.002 | 0.010 |
| P5 | 0.83 | 0.14 | 3.40 | 0.34 | 0.14 | 0.60 | 0.012 | 0.002 | 0.063 | 0.004 | 0.002 | 0.012 |
| P9 | 1.25 | 0.20 | 5.37 | 0.42 | 0.10 | 0.77 | 0.042 | 0.006 | 0.180 | 0.008 | 0.002 | 0.024 |
| PD | 0.76 | 0.19 | 3.79 | 0.36 | 0.34 | 1.08 | 0.029 | 0.007 | 0.132 | 0.021 | 0.011 | 0.034 |
| PG | 0.92 | 0.09 | 3.66 | 0.52 | 0.27 | 1.14 | 0.013 | 0.001 | 0.042 | 0.007 | 0.004 | 0.016 |
| PV | 1.30 | 0.43 | 6.58 | 0.71 | 0.35 | 1.35 | 0.017 | 0.012 | 0.126 | 0.017 | 0.006 | 0.031 |
| S1 | 0.85 | 0.19 | 3.74 | 0.30 | 0.12 | 0.57 | 0.007 | 0.001 | 0.033 | 0.002 | 0.001 | 0.006 |
| S4 | 1.14 | 0.09 | 7.53 | 0.29 | 0.06 | 0.61 | 0.007 | 0.001 | 0.156 | 0.002 | <0.001 | 0.004 |
| S7 | 1.21 | 0.07 | 4.32 | 0.28 | 0.07 | 0.62 | 0.006 | <0.001 | 0.027 | 0.001 | <0.001 | 0.003 |
| T1 | 0.78 | 0.14 | 3.04 | 0.45 | 0.07 | 0.53 | 0.005 | 0.001 | 0.028 | 0.002 | <0.001 | 0.003 |
| T4 | 1.24 | 0.18 | 6.85 | 0.34 | 0.03 | 1.14 | 0.009 | 0.001 | 0.062 | 0.004 | <0.001 | 0.011 |
| T6 | 1.11 | 0.17 | 5.47 | 0.52 | 0.16 | 0.87 | 0.014 | 0.002 | 0.065 | 0.003 | 0.001 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canova, L.; Maraschi, F.; Ambrosini, R.; Costanzo, A.; Parolini, M.; Profumo, A.; Romano, A.; Rubolini, D.; Sturini, M. Mercury Concentration and Distribution in Remiges, Rectrices, and Contour Feathers of the Barn Swallow Hirundo rustica. Environments 2025, 12, 249. https://doi.org/10.3390/environments12070249
Canova L, Maraschi F, Ambrosini R, Costanzo A, Parolini M, Profumo A, Romano A, Rubolini D, Sturini M. Mercury Concentration and Distribution in Remiges, Rectrices, and Contour Feathers of the Barn Swallow Hirundo rustica. Environments. 2025; 12(7):249. https://doi.org/10.3390/environments12070249
Chicago/Turabian StyleCanova, Luca, Federica Maraschi, Roberto Ambrosini, Alessandra Costanzo, Marco Parolini, Antonella Profumo, Andrea Romano, Diego Rubolini, and Michela Sturini. 2025. "Mercury Concentration and Distribution in Remiges, Rectrices, and Contour Feathers of the Barn Swallow Hirundo rustica" Environments 12, no. 7: 249. https://doi.org/10.3390/environments12070249
APA StyleCanova, L., Maraschi, F., Ambrosini, R., Costanzo, A., Parolini, M., Profumo, A., Romano, A., Rubolini, D., & Sturini, M. (2025). Mercury Concentration and Distribution in Remiges, Rectrices, and Contour Feathers of the Barn Swallow Hirundo rustica. Environments, 12(7), 249. https://doi.org/10.3390/environments12070249









