Abstract
The presence of persistent contaminants in soils is of growing concern around the world. Contaminated soils can affect numerous ecological environments and lead to significant health risks to humans, affecting soil biodiversity, structure and geomechanical behaviour and agricultural sustainability. Additionally, soil contaminants can also leach into water flows, which is another concern. In general, soil contamination can be attributed to natural sources or to anthropogenic sources associated with human activity. Soil contaminants are usually classified in the following categories: biological, radioactive, organic and inorganic contaminants. State of the art information regarding some of the most common persistent soil contaminants, including possible sources and prevalence, and monitoring approaches and information about their effects on soil characteristics, including usability, as well as information on possible mobility to other environmental media is presented in this review paper. Finally, a comprehensive overview of remediation strategies which are being developed, including the more traditional ones as well as novel strategies that have been proposed lately by the scientific community, is provided. This includes physicochemical and biological technologies, as well as mixed remediation technologies aimed at enhancing remediation efficiency.
1. Introduction
The presence of toxic contaminants in soils has been a subject of increasing concern to scientist and humans in general, especially during the last two decades, since it can pose significant health risks to humans and affect other ecological systems. Additionally, soil biodiversity and agricultural sustainability are also negatively affected in the long term. According to the Food and Agriculture Organization (FAO) of the United Nations, concerns about soil contamination are growing around the world. In fact, soil contamination can result in a decrease in microbial activity, decrease in water retention in soil and increase in soil erosion [1,2]. Apart from the fact that the presence of contaminants in soil affects plant growth, the possibility of the uptake of contaminants from the soil by the plants, leading, eventually, to their transfer to animals and also to human beings [3,4] is of great concern. Moreover, soil contaminants can also leach into water flows, which is another source of worry. Although many times ignored, contaminants in soils also have an impact on a soil’s geomechanical behaviour as they can promote changes at the micro-structure level, with an impact on hydraulic conductivity [5,6]. However, the major concern associated with soil pollution comes from the fact that soil contamination can persist for many years. In fact, it is still possible to find elements of anthropogenic origin coming from the industrial revolution period [7]. In general, soil contaminants can be divided into either those resulting from natural sources or anthropogenic sources [4]. Natural sources may be related to earthquakes, volcanic eruptions, tsunamis, alterations of rainfall patterns, etc. [8]. On the other hand, anthropogenic causes are associated with human activity, namely industrial and agricultural activities that, together with municipal and domestic waste, are considered as the major anthropogenic sources of soil pollution [8,9].
Overall, soil contaminants can be classified into the following categories: biological, radioactive, organic and inorganic contaminants [4,10,11], as shown in Figure 1. The characteristics of the contaminants in these different categories will be discussed in more detail in the next section. In recent years, increasing attention has been given to soil contaminants of emerging concern (CECs), which include nano- and microplastics (NMPs), PFAs (polyfluoroalkyl substances), heavy metals (HMs) and additives such as biphenyls and phthalates [3], due to their increasing presence in soils and also to their negative effects on plants and animals, even at low concentrations. Some of the most common persistent soil contaminants and their major effects will be described in detail in Section 3.
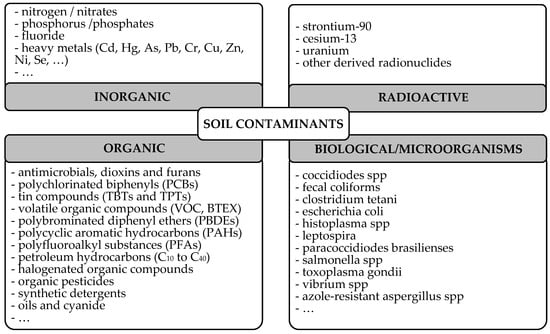
Figure 1.
Classification of soil contaminants.
The particle grain size distribution of soil affects the specific surface area available to adsorb contaminants. Fine-grained soils with a high proportion of clay particles (≤2 μm) exhibit a greater specific surface area and cation exchange capacity [12], enhancing the retention of heavy metal cations and other charged contaminants [13]. Conversely, coarse-grained sandy soils characterised by larger particle sizes with a lower specific surface area and cation exchange capacity, provide limited adsorption sites, thereby facilitating the mobility and leaching into groundwater of contaminants such as heavy metals, nitrates or PFAs compounds [14,15]. Microplastics, depending on their size and shape, can also become entrapped within the soil matrix, with finer particles potentially interacting more intimately with soil aggregates and influencing soil structure [16]. The mineralogical and chemical composition of soils significantly influences the reactivity and sorption of contaminants. Soils rich in clay minerals, such as smectite, vermiculite, illite and kaolinite, possess varying degrees of specific surface area and interlayer spaces, affecting their ability to adsorb and retain pollutants [17]. Soils rich in smectite and vermiculite are capable of trapping metal ions and organic molecules [17], while sorptive interactions are minimal in soils with low clay and oxide content, such as quartz-dominated sandy soils, allowing contaminants to percolate through the soil more readily. The chemical composition of a soil can also affect the degradation of organic contaminants, with certain minerals acting as catalysts in redox reactions [18]. For instance, the presence of iron and aluminium oxides and hydroxides can lead to adsorption of anions like phosphate and arsenate, as well as certain organic contaminants [19]. On the other hand, the presence of manganese oxides can significantly improve the adsorption of heavy metals [20]. Soil organic matter (OM) is another important factor influencing the behaviour of contaminants, namely the adsorption of both organic and inorganic contaminants. Indeed, the organic matter present in soils possesses a high specific surface area and a variety of weak acid and base functional groups, that can deprotonate, thereby becoming negatively charged, and subsequently adsorb cations (e.g., heavy metals type) through electrostatic interactions [21]. Furthermore, NMPs can interact with dissolved organic matter and co-adsorb other pollutants such as pesticides and PAHs, affecting their environmental fate [22].
Soil pH is a critical factor that affects a wide range of chemical and biological processes influencing the speciation, solubility, adsorption and fate of many contaminants [23]. In acidic conditions, the mobility of heavy metals increases due to increased solubility and reduced sorption, potentially leading to phytotoxicity and groundwater contamination [13]. Alkaline pH, in contrast, often promotes the precipitation of metals as hydroxides or carbonates, thereby reducing their mobility. The degradation of some pesticides is also highly pH-dependent, with hydrolytic degradation rates varying significantly across the pH spectrum [24].
Many remediation approaches for contaminated soils have been developed over the years. The most common remediation procedures already on-site involve excavation and removal of the soil to landfill sites [8], soil washing [4] and immobilisation technologies [8]. The first two techniques are expensive and pose environmental concerns. As for the immobilisation technologies, several strategies are available including containment technologies, solidification, stabilisation and vitrification [4,8,25], involving, in the last case, pyrolysis or oxidation, to melt and immobilise the contaminants [4,8,26]. The immobilisation technologies presently available cannot be applied to large contaminated sites; additionally, they limit the subsequent use of the soil and, in the case of vitrification technologies, significant energy consumption is required.
Other remediation strategies are being developed at present by the scientific community. These strategies include biological technologies, phytoremediation and nanoremediation strategies. In the case of biological technologies, the strategy is to use microorganisms to clean contaminated soils [8,27]. In the case of phytoremediation [4,8], the strategy is to use plants to remove contaminants from soils, namely heavy metals, taking advantage of the ability of plants to extract, accumulate or degrade soil contaminants [28,29,30]. These techniques have proved particularly useful to clean soils contaminated with heavy metals. Regarding the nanoremediation of soils, this involves using nanomaterials, like nanoparticles, for instance carbon nanotubes or nanocrystals, to immobilise or extract soil contaminants, such as heavy metals [8,31,32,33]. Nanomaterials have also been used to treat soils contaminated with hydrocarbons [8].
The different soil remediation approaches that have been developed over the years, some of them being already used on-site, will be described in detail in Section 4.
Before moving on to analyse in more detail the persistent soil contaminants and remediation techniques, it is important to provide an overview of the legal framework across the globe. Indeed, the regulatory framework for soil contamination and remediation varies significantly across different regions, reflecting different environmental priorities, economic conditions, industrial activities and the development of the legal system. While some regions have established comprehensive legislation and strict standards, others are still developing their regulatory approaches.
In Europe, soil contamination is primarily addressed through a collection of directives and national legislation rather than a unified European law. The Environmental Liability Directive (2004/35/EC) [34] establishes liability for environmental damage based on the “polluter pays” principle, while the Industrial Emissions Directive 2.0 (2024/1785/EU) [35] and the Sewage Sludge Directive (86/278/EEC) [36] contribute to pollution prevention and control concerning soil, water, and air. A significant recent development is the proposal of a Soil Health Law under the EU Soil Strategy for 2030 [37,38], the aim of which is to define soil health, implement monitoring systems, and encourage sustainable management and remediation practices. The European Environment Agency (EEA) supports these initiatives by providing scientific assessments and data to guide policy implementation. Despite the EU’s efforts to move towards harmonised standards, member states retain authority to establish their own soil contamination thresholds. Countries like the Netherlands and Germany have implemented detailed threshold values and soil quality standards to assess and manage soil pollution at the national level.
In North America, the United States maintains a well-developed legal and institutional framework to manage soil contamination. The main law is the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA or Superfund) [39], which empowers the Environmental Protection Agency (EPA) to identify and remediate hazardous waste sites and hold the responsible parties accountable. The EPA also issues Soil Screening Levels and Regional Screening Levels as guidance for site assessments and remediation planning.
Canada applies a shared governance model, with both federal and provincial authorities contributing to soil contamination management. The Canadian Environmental Protection Act (CEPA) [40] provides a federal framework for pollution control, while the Canadian Council of Ministers of the Environment develops national soil quality guidelines applicable to different land uses (e.g., residential, industrial, agricultural). Provinces implement region-specific standards, such as Ontario’s Soil, Ground Water and Sediment Standards [41]. Federally, Environment and Climate Change Canada manages contamination on federal lands through the Federal Contaminated Sites Action Plan.
In Asia, China has adopted comprehensive legislation to address its growing soil contamination challenges. The Soil Pollution Prevention and Control Law (2019) [42] establishes a national framework for risk assessment, monitoring, and site remediation. National standards, such as GB 15618-2018 [43] for agricultural land and GB 36600-2018 [44] for development land, set thresholds for contaminants. The Ministry of Ecology and Environment oversees these regulatory and remediation activities. Taiwan has advanced soil and groundwater remediation policies, led by the Environmental Management Administration under the Ministry of Environment. Several other Asian countries have developed sector-specific legislation. For example, Japan’s Act on Countermeasures against Soil Contamination [45] focuses on both prevention and remediation, while South Korea maintains detailed soil quality standards.
Australia, although geographically distinct, has a comparable system in place, governed by the National Environment Protection (Assessment of Site Contamination) Measure 1999 (amended in 2013) [46], with implementation delegated to the states and territories.
As it may be seen, the scope and sophistication of legal frameworks vary by country and region, even if the emerging trend is towards more integrated and comprehensive policies. Continued international collaboration and the harmonisation of guidelines are essential to advance global soil protection and remediation efforts.
The present review presents both an overview of the most common as well as contaminants of emerging concern in soils and also provides a comprehensive and critical synopsis of existing remediation strategies, including novel approaches. Thus, this review can be used as a tool for researchers and practitioners involved in soil remediation, to select the most adequate technique to be used for specific contamination situations.
2. Literature Review Methodology
A systematic and comprehensive literature review was employed in the present paper, which synthesises current knowledge on persistent soil contaminants, including sources and prevalence, monitoring approaches, their effects on soil characteristics, their environmental and health impacts, and the remediation strategies available. The primary databases used for the bibliographic search were the Web of Science© and Scopus©, chosen for their extensive and multidisciplinary coverage of peer-reviewed scientific papers on environmental science, soil science, toxicology, and related fields. The bibliographic search also included master’s and doctoral theses and books, as well as reports and legal documents relevant to the topic of this study.
A detailed search strategy was developed using a set of carefully chosen keywords combined with Boolean operators (“and”, “or”) to ensure a broad and relevant search. Keywords included “soil contaminants”, “persistent soil contaminants”, “heavy metals”, “petroleum hydrocarbons”, “micro-plastics” or “nano-plastics”, “persistent organic pollutants” or “POPs”, “remediation technologies” or “soil remediation”. These were integrated into structured search strings using logical combinations (e.g., “soil contaminants” or “persistent soil contaminants” and “soil remediation”).
Inclusion criteria focused on publications addressing persistent soil contaminants as the primary subject, particularly those reporting original research, systematic reviews, and authoritative or legal reports. Preference was given to studies published within the last two decades to ensure that the information is relevant and up to date, although foundational works were also included when appropriate. Studies unrelated to persistent soil contaminants as the main topic were excluded.
Documents identified through this process underwent a two-step screening: first by title, abstract and conclusions, followed by full-text analysis for those considered relevant. Key data were extracted concerning persistent soil contaminant types, sources, prevalence, and documented effects on soil quality, biota (plants, microorganisms, animals) and human health. In addition, information on monitoring methods and remediation strategies was systematically compiled.
This systematic methodology provided a robust and comprehensive review, making it possible to identify prevailing trends, knowledge gaps and future research needs in the field of persistent soil contaminants.
3. Persistent Soil Contaminants and Effects
This section presents the state-of-the-art information on some of the most common persistent soil contaminants, namely heavy metals, petroleum hydrocarbons, nano- and microplastics and persistent organic pollutants. Each contaminant will be characterised in terms of potential sources and prevalence, monitoring approaches and its effects on soil characteristics, including usability, as well as its effects on plants, animals and humans. A summary of the impacts of these persistent soil contaminants is provided in Table 1, at the end of the section.

Table 1.
Sources and effects of persistent soil contaminants.
3.1. Heavy Metals as Soil Contaminants
Heavy metals (HMs), including elements such as lead (Pb), cadmium (Cd), copper (Cu), arsenic (As), mercury (Hg), zinc (Zn), nickel (Ni) and chromium (Cr), naturally occur in soils but their concentrations can be significantly enhanced due to industrial and military activities, mining operations, application of fertilisers and pesticides, disposal of metal-containing wastes, or even by forest fires [13,47]. The HMs are non-biodegradable and can accumulate in soil matrices, taken up by plants and living organisms, leading to phytotoxicity and bioaccumulation in the terrestrial food chain [48].
Heavy metals can negatively impact a soil’s microbial communities, reducing their diversity and activity, which are essential for nutrient cycling and decomposition of organic matter [49]. They can also alter soil enzyme activities, with an impact on microbial metabolism and soil nutrient cycling [50]. Plants can absorb heavy metals from contaminated soils or groundwater through their root systems. The accumulation of these HMs can inhibit plant growth and development, reduce the efficiency of photosynthesis, and lead to yield losses [51]. The toxicity varies depending on the heavy metal type and concentration and the plant species. Herbivorous animals can ingest heavy metals by consuming contaminated plants or soil. Thereafter, the HMs can accumulate in their tissues, leading to various physiological disfunctions, and potentially enter the food chain, posing risks to higher trophic levels [52,53,54]. Human exposure to heavy metals from contaminated soils can occur through the ingestion of contaminated food crops and animals, direct contact with soil, and inhalation of contaminated dust or vapours. Exposure to elevated levels of heavy metals has been linked to various health issues in humans, including neurological disorders, organ damage and cancer, depending on the specific heavy metal and exposure pathway [53,55,56].
HMs can also have an impact on a soil’s physical properties, such as aggregation and hydraulic conductivity. For instance, the presence of copper and zinc in loamy clay soils led to increased aggregation, which subsequently influenced pore generation and enhanced hydraulic conductivity. Conversely, sandy soils exhibited no significant changes in aggregation or hydraulic properties as a result of the presence of HMs [57].
Monitoring heavy metal contamination requires the prior extraction of analytes from the soil matrix before measurement, i.e., it typically involves systematic soil sampling and chemical analysis, using techniques such as atomic absorption spectrometry (AAS) or inductively coupled plasma mass spectrometry or atomic emission spectroscopy (ICP-MS or ICP-AES) to determine the concentration of various heavy metals or for the high-precision analysis of trace elements in soil samples, respectively [58,59]. X-ray fluorescence (XRF) is also used for simultaneous analysis of the concentrations of HMs in soil samples, offering rapid results [60]. The spatial distribution and variability of HM contamination can be assessed through grid or targeted sampling strategies combined with geostatistical analysis [61,62]. Biological monitoring, using indicator plants or soil organisms, can provide insights into the bioavailability and ecological impacts of heavy metal contamination [63,64].
Plants, aqueous solutions enhanced with chelating agents or surfactants, chemical amendments such as Portland cement or lime, clay minerals, nanoparticles and biochars, can be used to isolate, immobilise/stabilise and reduce the heavy metal solubility and mobility (toxicity reduction) or promote the physical separation or extraction of heavy metals from soil matrices [31,51,65,66,67,68,69]. More details about such strategies will be presented in Section 4.
3.2. Petroleum Hydrocarbons as Soil Contaminants
Petroleum-based hydrocarbon contamination of soils is a widespread environmental issue, predominantly originating from anthropogenic sources such as crude oil exploration, production, transportation, processing and refining, storage and accidental spills. Additional contributions stem from industrial discharges, improper waste disposal and leakage from storage tanks [70,71]. These activities result in the infiltration of various hydrocarbon compounds into terrestrial ecosystems, compromising soil and groundwater quality and ecological health.
Petroleum hydrocarbons naturally occur in geological deposits, in sedimentary rocks, existing in the form of gases (natural gas), semisolids (bitumen), solids (wax or asphaltite) and liquids, as petroleum crude oil, commonly known as fossil fuel [70]. Petroleum hydrocarbons (PHs) are complex mixtures primarily composed of aliphatic hydrocarbons (e.g., alkanes, alkenes), aromatic hydrocarbons (e.g., benzene, toluene, ethylbenzene, xylene—BTEX), and polycyclic aromatic hydrocarbons (PAHs) [72]. These compounds vary in molecular weight, volatility, solubility, and persistence in the environment, influencing their behaviour and toxicity in the soil matrix. PHs are mostly durable and stable, remaining for long periods in the environment and do not undergo degradation easily [73].
The contamination of soils with petroleum hydrocarbons adversely affects a soil’s physicochemical properties, including texture, porosity, soil resistance, water retention and nutrient availability [71]. Due to their hydrophobic nature, PHs can replace water molecules in soil pores, reducing oxygen availability and water infiltration and reducing nutrient availability [74]. They can also coat soil particles, altering their physical structure, affecting hydraulic conductivity, reducing soil shear strength and increasing the risk of landslides and structural failures [75].
The presence of PHs in soils disrupts microbial communities by reducing microbial biomass and enzymatic activities, which are essential for nutrient cycling and organic matter decomposition [70]. Furthermore, the PH compounds exert toxic effects on soil fauna and flora, inhibit seed germination, reduce plant growth, and lead to bioaccumulation in terrestrial animals [72]. Animals can be exposed to hydrocarbons through direct contact, ingestion of contaminated soil or plants and inhalation of volatile compounds. This can lead to various toxic effects, including organ damage, neurological impairment and reproductive problems [76]. The toxicity is primarily associated with the hydrophobic nature of hydrocarbons, which allows them to persist in the environment and interact with cellular membranes, disrupting biological functions.
Petroleum hydrocarbon contamination also represents a serious threat to human health. These compounds, derived from crude oil and its refined products, can enter the human body through various exposure pathways, including the inhalation of volatile compounds, ingestion of contaminated food or water and dermal contact with polluted soils or sediments [72]. Volatile organic compounds (VOCs) such as BTEX and PAHs, are among the most toxic constituents of petroleum hydrocarbons. These substances are known to be carcinogenic, mutagenic and teratogenic [70]. Acute exposure to petroleum hydrocarbons can cause neurological symptoms such as headaches, dizziness and confusion, as well as respiratory issues due to the inhalation of volatile compounds. Chronic exposure, particularly in occupational settings or in communities located near contaminated sites, has been associated with liver and kidney damage, hormonal disruption, immune system suppression and reproductive toxicity [71]. Furthermore, indirect human health risks arise from the bioaccumulation of PHs in plants and animals, which can then enter the human food chain. Contaminated groundwater used for drinking or irrigation further amplifies these risks, especially in low-income or rural communities with limited access to clean water sources [72].
Transport mechanisms of petroleum hydrocarbons in soils involve volatilization, adsorption to soil particles, leaching into deeper layers and lateral migration with surface runoff [71]. Their fate in soils is determined by both abiotic and biotic degradation processes. Biodegradation, often the most effective pathway for natural attenuation, is mediated by indigenous microorganisms capable of metabolising hydrocarbons under aerobic or anaerobic conditions [70]. However, factors such as oxygen availability, nutrient concentration, temperature and the chemical structure of the contaminant influence degradation rates.
Investigation of petroleum-based hydrocarbon contaminated sites typically involves soil and groundwater sampling followed by laboratory analysis using gas chromatography techniques (mass spectrometry type, GC-MS or flame ionisation detection type, GC-FID) to identify and quantify the specific hydrocarbon compounds present [77]. Soil vapour surveys can also be used to assess the extent of volatile hydrocarbon contamination [78]. Monitoring often involves tracking the migration of hydrocarbon plumes in groundwater and assessing the effectiveness of remediation efforts over time.
Several remediation techniques have been developed to address petroleum hydrocarbon contamination. These include in situ methods such as bioremediation, phytoremediation and soil vapour extraction, and ex situ techniques such as excavation and landfilling, soil washing and thermal desorption [72]. Bioremediation has gained prominence due to its cost-effectiveness and environmental compatibility, relying on the stimulation of microbial activity to degrade hydrocarbons [71]. The success of these techniques often depends on site-specific conditions and the physicochemical nature of the pollutants. More details about possible remediation techniques will be presented in Section 4.
3.3. Nano- and Microplastics as Soil Contaminants
Environmental pollution by nano- and microplastics (NMPs) is considered an anthropogenic contamination raising, presently, great concern. Globally, a very high quantity of plastics is disposed every year in the environment, either in landfills or dumped directly into aquatic or terrestrial environments. Plastic production has moved from 2 Mt in 1950 to 359 Mt in 2018 [79,80], with a continuous increase up to today. The cumulative production of plastics from 1950 to 2018 is estimated to be 9.6 billion tons [79,80]. As a consequence, soil is considered the major sink and carrier of NMPs.
Microplastic particles have sizes below 5 mm, while nanoplastics are particles below 1 µm, resulting usually from the degradation of larger pieces of plastic or those which have previously been used, already in that size range, in different types of formulations (health products, cosmetics, food, coatings, etc.). Plastic degradation is the result of several complementary mechanisms, which include chemical degradation, physical fragmentation and bio and photo-oxidation degradation [81,82,83]. Additionally, the type of degradation process may influence NMP toxicity [84,85].
Sources of NMPs in the soil environment originate mainly from landfills, soil additives, agricultural practices, irrigation and run-off and atmospheric deposition [86]. These anthropogenic activities act as sources of NMPs which can accumulate both in agricultural and urban soils.
Regarding agricultural activities, the application of sewage sludge and compost, including food waste, as soil fertilisers, can lead to the introduction of NMPs into agricultural soils [87,88]. Additionally, plastic residues from mulching, greenhouse films and wastewater irrigation practices are also significant sources of NMPs in the soil [84,88].
The main types of plastics found in agricultural soils are low-density polyethylene (LDPE), polyvinyl chloride (PVC), linear LDPE (LLDPE) and polyethylene terephthalate (PET) [89]. Agricultural soils can, potentially, store more NMPs than oceanic basins [90].
Another important issue is the fact that, once present in the soil, NMPs can easily migrate through the soil depth and eventually into water streams, rivers and marine environments [91].
In the case of agricultural soils, farming practices such as drilling, irrigation and harvesting, and also chemical and biological soil processes, contribute to the transportation of NMPs deeper into the soil [86,92]. Besides this, NMPs can be transferred horizontally across the soil, and to other environments, through the action of wind and water [89].
The presence of NMPs in soils can affect soil quality and fertility including their water holding capacity [89,93]. As a consequence, the quality of agricultural products and the growth and photosynthesis of plants can also be altered [89,94,95]. For instance, in a recent study, it was proved that the root biomass of plants can be affected by the presence of microplastics, while the dry biomass of onion bulbs is also affected by their presence [96]. Additionally, the health of soil organisms and their enzymatic activity can also be altered through NMP contamination [97]. In addition, the legacy of plastic use in agricultural soils is usually not reversible. So, the accumulation of NMPs in agricultural soils over time is a direct threat to agricultural productivity and safety [91]. The bioaccumulation of NMPs in soil and subsequent transfer to plants and other organisms may eventually reach humans through terrestrial food chains, which can adversely affect human health [83,84], even if this effect is not yet fully understood.
Regarding urban soil contamination by NMPs, there are several sources that can contribute to this pollution including tyre erosion, littering, disposal of medical wastes, use of treated wastewater in urban areas (gardens, parks, etc.) and atmospheric dust [98,99]. Similarly to agricultural soils, a higher abundance of NMPs is found in the surface layers of the soil but they migrate easily into deeper layers or disperse to the surrounding environment [83].
Industrial areas are also major contributors of the transfer of NMPs to soils. Plastic particles are mainly transferred through wastewater treatment plants (WWTPs), since the treated effluent is quite often used for irrigation, but also through the sludge collected which is usually deposited in landfills. Also, a large amount of plastic waste can be discarded from industrial and mining plants, ending up particularly in landfills, and then degrade to NMP size that eventually accumulate in soils in the long term [3]. Landfill leachates play an important role in soil contamination by NMPs, especially considering possible defects of, or damage to, landfill liners [100]. The main industries which have been identified as contributing to the release of NMPs cover a wide range of sectors, from the plastics production sector, petrochemical processes, cosmetics and pharmaceutical industries, paint production, textile sector, automotive, electronics and transportation industries [87,101,102,103], among others.
In summary, the presence of NMPs in the soil poses environmental threats, changing the soil’s characteristics, affecting soil structure, namely porosity, which affects the infiltration capacity of soil for rainwater and adversely affects soil water capacity [104,105,106]. Additionally, soil biodiversity including microbial activity can decrease, affecting the ability to fix carbon dioxide and organic matter [107], destroying soil fauna and affecting plant growth [108,109].
Another important aspect related to soil contamination by NMPs is the fact that, quite often, the plastic materials being discarded are doped with a range of additives, used in the preparation of plastic products, like phthalates and polychlorinated biphenyls (PCBs). These are highly toxic, and can also migrate through the soil structure, affecting microbial communities and the soil enzymes they produce, and can even migrate to plants [3].
Furthermore, the NMP particles themselves can adsorb and concentrate toxic chemicals, like heavy metals, glyphosate and chlorpyrifos, which are persistent organic pollutants (POPs), and antibiotics and pathogenic microbes, during their (NMP particles) stay in landfills, eventually, in the end, transferring them to soils and, through plants, to the food chain [110,111,112].
3.4. Persistent Organic Soil Contaminants
Persistent organic pollutants (POPs) are usually halogenated chemicals and are persistent, bio-accumulative and toxic in the environment, with a high potential for long-range transport. The most common POPs are polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) [113]. Also, polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) should be mentioned, considering their prevalence in the environment [114,115]. POPs are quite common in soils, especially in intensively industrialised areas. A few large-scale studies have investigated POPs in surface soils to better understand their distribution and fate [116,117,118,119,120].
The four major processes that transfer POPs from air to soil are diffusion, rain and capture in snow, for gaseous POPs and dry deposition for particulate POPs [116]. The principal sinks of POPs in the environment include agricultural soils, urban surface run off and discharges from sewage treatment plants [3]. Reports on the mobility and deposition of POPs are available for different regions in the world, Australia, Africa, Europe, Latin America, Asia and North America [3,117,118,119,120].
In general, the sources of POPs can be divided into primary and secondary sources. While primary sources involve the direct application or emission of POPs into the environment during their use and production, secondary sources involve the redistribution of POPs among air and soil or water. After the initial release of POPs, they disperse through the air and accumulate on the surface of the soil through wet or dry depositions [116].
Regarding primary sources, the contamination of soils by POPs is linked to several industrial activities including oil refining plants, oil burning, waste incineration, waste dumping, engine oil production and oil spills. In agricultural areas, the use of organic chemicals such as pesticides leads to widespread contamination. Motor vehicle emissions also contribute to soil contamination by POPs [4].
In fact, petrochemical industries have been identified as significant sources of contamination by POPs, including volatile organic compounds but also PAHs and PCBs. PCBs and PAHs have been identified as deriving from the incomplete combustion of organic materials, such as fossil fuels, coal and biomass in energy plants. PAH emissions are strongly correlated with thermal energy generation and consumption [113,121]. The PAHs released in particulate and/or gaseous phases are then transported and deposited on vegetation, water bodies and soil [122]. Moreover, there is a high probability that PAHs remain in the soil considering their capacity to attach to soil particles tightly. Additionally, soils can be re-emission sources of PAHs through volatilization, degradation and leaching [121]. In the case of OCPs, they include low-cost pesticides like DDTs (dechlorinated diphenyl-trichloroethane) and HCHs (hexachlorohexane), which have been widely used for public health, agricultural and industrial activities [121]. Even if these pesticides have been forbidden or restricted in many countries for many years [123], their long-range atmospheric transport and very low degradability results in the fact that they can still be found in many regions, including the Artic region [124]. They are known for their persistence and bio-accumulative properties, still causing chronic and acute effects in soils [121].
PCBs and their derivatives have been used in the production of pesticides, pharmaceutical products, wood preservation, flame retardants and industrial production of plastics, paints, insulators for electronic transporters, capacitors, etc. As a consequence, they can later enter the environment through several anthropogenic sources, including industrial activities and domestic effluents. Their sorption to organic matter leads to persistent soil contamination [113,121], besides the contamination of other environmental systems (air and water). High concentrations of less chlorinated PCBs (tri and tetra-Cl PCBs) in rural areas are probably due to dry or wet atmospheric deposition [113]. PCBs have been found in urban area soils (Canada and Sweden) [116,125,126], a relation with the presence of flame retardants in the air has been found. In China, high molecular weight PCBs were detected in urban soils, while low molecular weight PCBs were detected in higher levels in agricultural soils [116,127].
Another important factor associated with soil contamination by POPs is related to the fact that these chemicals are known to degrade only slowly, like PAHs, while some are highly persistent, like dioxins and organochlorines [128]. In the case of PAHs, molecular weight can be an important parameter influencing their possible accumulation in soils. Lower molecular weight PAHs are more soluble in water, and thus can more easily migrate through the soil, while higher molecular weight PAHs are more slowly desorbed from the soil matrix, accumulating more easily in the soil for very long periods [128].
Due to their lyophilic character, POPs tend to bio-accumulate and increase easily in the terrestrial food chain, with harmful consequences for animals and human beings [113,129]. POPs are absorbed by plants through their roots and leaves. For example, plant roots can absorb PCBs and herbicides if these contaminants come in direct contact with plants, through liquid or vapour [130]. PCBs are of particular concern since they are resistant to degradation and tend to concentrate in animal fat and animal products (e.g., milk). Additionally, PCBs have been considered as endocrine disruptive chemicals, affecting the endocrine system even at low concentrations, with effects on human health [3].
4. Remediation Strategies for Contaminated Soils
There are many remediation technologies for soil treatment, which can generally be classified based on the technological process involved such as physicochemical treatments or biological treatments [8,131]. The physicochemical treatments encompass a range of methodologies involving physical processes, chemical or electrochemical reactions, thermal and heat processes, as well as acoustic and ultrasonic methods, while the biological treatments rely on natural processes carried out by microorganisms or plants to degrade or immobilise contaminants. In general, the physicochemical treatments present a high speed of remediation and efficiency but several of them condition the future use of the soil and are expensive [131,132]. Biological treatments are usually eco-friendly, but they frequently require longer periods to remove the contaminants from the soil [133,134,135]. The technologies that are biological have received greater social acceptance. The remediation technologies can also be classified based on the conceptual model of contamination, targeting the source, transport path or receptor. The source-oriented remediation technologies are the most common and, in principle, should be favoured over others, unless it proves impossible to tackle the pollution at the source. Additionally, the remediation technologies can be classified based on the location of application as in situ and ex situ technologies [8,131]. In situ remediation techniques are applied directly at the contamination site without soil excavation. Ex situ remediation techniques involve removal by excavation (soil) or pumping (water) and transport to a treatment site. The treatment itself can be carried out on-site (if the treatment takes place close to the contaminated area) or off-site (when the contaminated material is treated at a different location, or taken to landfill, after excavation and transport). It must also be re-mentioned that, sometimes, higher removal efficiency can be achieved by combining physicochemical and biological treatments [131], i.e., mixed remediation technologies.
In general, the remediation treatments are able to contain, sequester, separate, extract, remove, destroy, transform and/or mineralise pollutants in the contaminated soil into less harmful, non-hazardous and less reactive forms [136]. The selection of a certain type of remediation technology is usually conditioned by several factors such as, type and concentration of pollutants, type of soil including its future use, distribution of the pollutants and extension of the contaminated area, accessibility of the affected region and cost of the remediation process, among others [4]. It is also important to take into consideration the nature and sources of pollutants [110].
A summary of the remediation techniques, including their main advantages and limitations, as well as their applications based on contaminant type, is presented in Table 2, at the end of this section.
4.1. Physicochemical Technologies
Physicochemical treatments are pivotal in addressing soil contamination, especially when dealing with persistent pollutants such as heavy metals, petroleum hydrocarbons and pesticides. These treatments encompass a range of physical, chemical, electrochemical, thermal and acoustic processes designed to remove, immobilise or degrade contaminants. The selection of an appropriate treatment depends on factors such as pollutant type and concentration, soil characteristics, site conditions and remediation goals. Combining these treatments can often enhance remediation efficiency and effectiveness.
Without being exhaustive, some of the most common and widely available physicochemical treatments for remediating contaminated soils are briefly described below.
4.1.1. Excavation and Landfilling
Excavation and disposal in landfills represents one of the most straightforward and widely implemented ex situ soil remediation techniques. This method involves the mechanical removal/excavation of contaminated soil from a site, followed by its transportation to a designated landfill facility for secure disposal. It is particularly effective for sites with high concentrations of pollutants existing near the surface or where rapid risk mitigation is necessary.
The exact depth and extent of the area to be treated must be evaluated using geological prospecting, chemical analyses and risk assessment. Transport to an appropriate and licenced landfill is generally performed in containers or lorries, provided with suitable protection against possible spillages. The sites from which the soil has been removed can be backfilled with new, uncontaminated soil, or with the same soil after treatment. This technique is often chosen for its ability to provide immediate risk reduction and its applicability across various soil types and contamination scenarios. However, it is essential to consider the associated costs and potential environmental impacts, such as greenhouse gas emissions from transportation and the loss of soil structure and biodiversity at the excavation site. Indeed, it provides immediate treatment of the polluted site, but it simply transfers the problem to another location [137]. It must be stressed that excavation and landfilling should be at the bottom of the contaminated soil treatment hierarchy, and many environmental agencies and regulatory entities are promoting the principles of a circular economy and imposing taxes on landfills to encourage more sustainable management of polluted soils [138].
4.1.2. Soil Washing or Soil Flushing
This treatment is applicable in situ and uses injected aqueous solutions to dissolve and recover pollutants from the soil. This treatment involves extracting contaminants from the soil by passing water or an aqueous solution through the contaminated soil layers using an injection and pumping process. The contaminants are removed or transported to the groundwater table and then the groundwater is pumped out and treated. There is at least one injection well and one pumping well or trench to collect the contaminated fluid. The contaminated fluid is treated on the surface to remove contaminants using a water treatment plant. The water may then be returned to the aquifer (possibly after being conditioned) and discharged to the ground or to a sewer, complying with regulatory requirements [139]. In soils with low hydraulic conductivity (clayey soils) this treatment may have limited effectiveness.
The process removes organic (e.g., petroleum hydrocarbons) and inorganic contaminants (e.g., heavy metals) from soils either by physical and/or chemical means. The aqueous solutions are enriched with additives that include acids, alkalis, chelating agents, surfactants and organic solvents [138,140]. An optimal chemical agent should ideally (i) exhibit minimal interaction with the soil matrix relative to the contaminants targeted, (ii) improve the mobility and solubility of these contaminant and (iii) be environmentally non-toxic and biodegradable [141]. Although acid-based additives can be highly effective, their use often leads to significant dissolution of soil minerals and organic matter, degrades soil structure and increases soil acidity. Chelating additives are capable of extracting metals with less impact on soils. One of the best-known chelating additives is EDTA (ethylenediaminetetraacetic acid), while in recent years, biodegradable chelating additives have also been studied, such as EDDS (ethylenediaminedisuccinic acid), IDSA (iminodisuccinic acid) and MGDA (methylglycinediacetic acid) [142]. The efficiency of removing heavy metals from contaminated soils using this procedure can range from 50 to 100%, depending on the heavy metal, soil properties and chemical additive [140].
4.1.3. Soil Vapour Extraction and Air Sparging
Soil vapour extraction is an in situ soil contaminated treatment where an appropriate gas or air (which may be heated) flows through the polluted soil to volatilise the volatile organic compounds and semi-volatile contaminants. This method uses vacuum pumps and extraction wells to create a gas or air pressure gradient in the unsaturated vadose layer, promoting the transport of the contaminated soil vapour to the extraction wells, followed by a treatment process of the contaminants above the ground [143]. In air sparging, the gas or air is injected below groundwater level and the contaminants are transferred from the soil and groundwater to gas or air bubbles, which migrate upwards into the vadose zone [138].
As the objective of both these treatments is to volatilise the contaminants, the gas- or air-flow rates tend to be high. Although the focus is on extraction, the presence of gas or air can facilitate aerobic biodegradation of the contaminants as a secondary beneficial effect. These processes are most effective in soils with high porosity but are non-effective in clayey soils [138]. It has been shown in a pilot scale study that soil vapour extraction can reach a removal efficiency of 89% for semi-volatile organic compounds [144].
4.1.4. Physical Barriers, Hydraulic Control/Containment and Permeable Reactive Barrier
These treatment methods are applicable in situ and employ both physical and engineered barriers to control and limit the movement of pollutants within the soil environment. The aim is to restrict lateral and vertical migration, reduce permeability, and minimise leaching into soils, sediments and groundwater. Additionally, it serves to contain the pollutants and prevent their dispersion into surface waters, thereby protecting ecologically sensitive areas [145]. The approach typically involves the construction of barriers (such as cut-off walls, steel sheet pile walls, bentonite or cement-based barriers, slurry trench walls, geomembrane barriers, grout curtains, vitrified barriers, asphalt layers or concrete retaining systems) to isolate polluted soils and manage groundwater flow. When it deals with hydraulic control/containment, the treatment is accomplished by installing pumping wells, combined or not with a subsurface drainage system [139].
It can also be used to cap the surface to isolate polluted soil from potential receptors (usually, human and animals) and to limit rainfall infiltration [65]. A typical surface cap is usually made of high-density polyethylene (HDPE) liners, geomembranes, asphalt, a cement-based barrier or low permeability soil, which has a layer of vegetative cover applied on top to reduce soil erosion.
In specific cases, involving the treatment of contaminated groundwater, a permeable reactive barrier (PRB) can be used. The use of different reactive media within the reactive zone allows the treatment of a wide variety of groundwater contaminants, including acid spills, heavy metals, radionuclides and volatile organic compounds [139]. Reactive media could include zero-valent metals, chelators, sorbents, nanoparticles, biochars or microbes [31,66,67,68,139,146]. The mechanisms involved may be sorption, oxidation/reduction, precipitation, fixation and biodegradation. The performance of the permeable reactive barrier may be enhanced by the construction of gravel or sand trenches and reaction vessels.
These treatments are relatively easy to maintain and monitor and can be a solution for inaccessible or dispersed pollutants. However, their applicability may be limited by the depth of the pollutants below the surface, and their performance may be compromised over time due to erosion or the action of terrestrial animals that can cause holes in capping systems or even due to clogging or the loss of permeability of permeable reactive barriers.
4.1.5. Solidification/Stabilisation and Immobilisation
These techniques are applicable in situ with the intention of restricting and preventing the mobility of the contaminants by locking them into a durable matrix, or by the conversion of the contaminants to chemically stable forms, through the addition of cement-based additives into the contaminated medium to produce an immobile monolithic block that is non-leachable. The performance of the treatment relies on efficient mixing of the additives with the contaminated soil, and for that, mechanical mixing procedures (deep mixing, mass stabilising or jet-grouting equipment) are usually employed. It is good practice to perform laboratory and some pilot tests to design the mixing procedure of additives and contaminated soils for each application [139].
In these treatment methods, the mobility of organic and inorganic contaminants can be reduced through a series of processes such as precipitation, complexation and adsorption. However, better results are obtained with inorganic contaminants, like heavy metals [139,147].
The solidifying/stabilising additives can range from a single additive to a multi-component additive. The commonly used solidifying/stabilising additives include Portland cement, lime, gypsum, silicates, zeolites, phosphates, sulphur-based binders and organo-clays [70,138,148]. If the aim is to reduce the contaminant solubility, the use of chemical additives such as diammonium phosphate may be useful [70]. In both cases, the soil is unlikely to be suitable for agricultural use and the site will require some long-term monitoring to ensure that the contaminants remain stable or immobile [138]. Activated carbon and biochar can also be used to reduce the bioavailability of organic contaminants and can assist in returning polluted soil to agricultural use [149]. The effectiveness of biochar depends on the biochar feedstock type and properties, concentration and mixing depth; it decreases with time due to the effects of ageing [138,146]. The addition of carbon to the soil also contributes to carbon sequestering as a mitigation measure against climate change and improves the soil’s fertility [150]. Clay minerals, natural zeolites and nanoparticles can also be applied to immobilise soil contaminants like heavy metals due to their high specific surface area and surface charge, which promotes adsorption of the heavy metals [31,67,68,147,151].
There is a secondary beneficial effect, related to the physical properties of the soils that are often improved (e.g., increased strength, lower permeability), during these treatment methods. However, these treatments do not destroy or remove the contaminants, may require long-term maintenance and/or long-term monitoring and may result in an overall increase in the volume of the material [139].
4.1.6. Chemical Oxidation–Reduction
This soil treatment involves in situ injection of liquid or gaseous oxidising–reducing agents (or oxidants–reductants) into the contaminated soil or groundwater to promote the subsequent oxidation, reduction and/or degradation of organic compounds into non-hazardous and less toxic compounds. Some of the typical oxidants–reductants include permanganate (MnO4−), hydroxyl radical, sulphate radical, ozone (O3), ferrous iron (Fe2+), sodium persulfate (Na2S2O8), hydrogen peroxide (H2O2), zero valent iron and polysulphides [70,130,150].
Successful application of chemical oxidation–reduction treatment depends on the contact between the oxidising agents and the contaminants. Implementation demands a complete site assessment to evaluate the physical and chemical characteristics of the contaminated soil, including organic matter content, pH of the soil and hydraulic conductivity, groundwater gradient and hydraulic conductivity of the unsaturated vadose zone and both electrical conductivity and resistivity of groundwater, as well as the contaminants properties and concentrations. Inadequate evaluation of the contaminated soil’s characteristics can lead to ineffective remediation due, for instance, to poor oxidant distribution or competitive consumption of oxidants by the soil’s organic matter [152].
In situ chemical oxidation–reduction treatment can rapidly and effectively degrade various organic contaminants, often achieving over 90% removal within minutes (e.g., removal of unsaturated aliphatic or aromatic compounds like benzene). This process is more efficient for pollutants in the highly permeable zones of contaminated soils with varied permeability, while those in less permeable regions may remain untreated. With time, contaminants can migrate from the regions of low permeability, a phenomenon known as “rebound”, which may require multiple treatments to maintain remediation performance [150].
In situ chemical oxidation–reduction treatments may be applied to a wide range of organic contaminants and use oxidising–reducing agents that are considered low-cost and easily delivered to contaminated soils. However, the treatment may require large volumes of oxidising–reducing agents, which themselves may pose some environmental concerns, the contact between the oxidising–reducing agents and the contaminants may be difficult. In addition, toxic intermediate products may be formed during the oxidation–reduction reactions, and some precipitation may be reversible with changes in redox conditions over time [139].
4.1.7. Thermal/Heat Treatment and Vitrification
The thermal treatment methods involve the use of heating systems (steam of hot air, electrical resistance, electromagnetic heating, thermal conductive medium) to increase the temperature of the contaminated soil to enhance the removal of the contaminant. One of the following methods may be used: increasing volatilisation; reducing viscosity; increasing solubility; decreasing adsorption; drying the soil, which can increase its permeability to air and contaminant extraction; acceleration of chemical reactions with the destruction of contaminants [139,150].
Thermal treatment methods increase the temperature of the contaminated soil from 100 °C up to 800 °C depending on the in situ heating system. Electrical resistance is based on an electrical current that passes through electrodes inserted in the contaminated soil area, generating temperatures of 100 °C. Steam or hot air with temperatures of up to 170 °C may be injected into the contaminated soil area via a series of injection wells, promoting the movement of contaminants towards the extraction wells. Radiofrequency and microwaves emitted from electrodes or antennae can heat the contaminated area to over 300 °C. Using metal rods installed within cased wells allows conductive heating of the soil’s contaminated area up to 800 °C. In situ vitrification uses extremely high temperatures (typically 1400 to 2000 °C) to melt the soil in the contaminated area [139]. In any case, the surface of the contaminated area is covered to ensure all the vapours released are captured by the extraction system and to provide thermal insulation [150].
The thermal treatment methods have differing ranges of applicability for contaminants, soil and groundwater conditions, treatment efficiencies and cost. Therefore, they should not necessarily be compared by their ability to attain a specific temperature, as it may not be an efficient form of heating under a particular set of conditions. Indeed, thermal desorption of contaminants is achieved through multiple mechanisms such as oxidation, incineration, thermal cracking or pyrolytic reactions and depends on the distribution of temperature and oxygen [153]. Thermal desorption is suitable for volatile and semi-volatile contaminants such as PAHs, petroleum hydrocarbons (PHs), and polychlorinated biphenyls (PCBs). Thermal desorption of hydrocarbons starts at temperatures of between 100 and 300 °C, but in the presence of high molecular hydrocarbons, it is necessary to attain temperatures between 300 and 500 °C. Thermal cracking of hydrocarbons occurs at higher temperatures, in the range of 800 to 900 °C [70]. In the case of in situ vitrification, the high temperatures cause the thermal or chemical destruction of contaminants, or they can be incorporated within the vitrification product [139].
The main objective of the thermal treatment methods is to increase the efficiency of removing volatile and semi-volatile contaminants by heating the contaminated soil. The increased temperatures can stimulate the chemical and biological degradation of some contaminants. Vapour extraction and ex situ recovery or destruction of the mobilised contaminants and their degradation products is an integral part of these technologies [150,153,154]. Both low-temperature and high-temperature thermal desorption can yield removal efficiencies up to 99% for in situ thermal treatments, but the treatment time may vary due to process configuration and contaminant composition [70,153].
Thermal treatment methods may be applicable to a wide range of soil types and can also be applicable to difficult dense non-aqueous phase (DNAPL) contaminants. However, the high temperatures used in thermal treatment methods may induce operational problems in buried utilities, affect fauna and flora, impact on groundwater quality, degrade the soil structure, chemical composition and biological community, rendering it difficult to return it to agricultural use. Moreover, the thermal treatments involving high and very high temperatures are expensive and energy intensive [139,150].
4.1.8. Electrokinetic Remediation
Electrokinetic remediation is an in situ technique that applies a low-voltage direct current across electrodes placed in low hydraulic conductivity soils (e.g., clay-rich soils) to mobilise and remove pollutants such as heavy metals, organic substances, and radionuclides from contaminated soils and groundwater. The technique relies on applying an electrical potential gradient across pairs of electrodes inducing water flow and contaminant movement toward electrodes of opposite charges, through mechanisms including electroosmosis, electromigration, electrophoresis, and electrolysis [155]. Positively charged organic compounds, heavy metal ions and ammonium ions move toward the cathode, while negatively charged organic compounds and anions such as chloride move toward the anode [139,156].
The contaminants are transported through the soil towards one electrode or the other by three mechanisms: electromigration, electroosmosis and electrophoresis. In electromigration the ions are transported towards an electrode. In electroosmosis, it involves the movement of a liquid (normally water) containing ions relative to a stationary charged surface. In electrophoresis, charged particles, together with the contaminants, are electrostatically attracted (moved) to one electrode and repelled from the other [70,139].
The electrokinetic system may include electrolyte reservoirs to maintain conductivity and pH levels, enhancing contaminant mobilisation. Adjustments of voltage gradients, electrode spacing, and treatment duration are made based on site-specific conditions to optimise remediation efficiency. The removal efficiency of heavy metals can range from 52 to 90% depending on site conditions and the type and concentration of heavy metals [157,158].
The electrokinetic treatment method is effective in fine-grained low-permeability soils and is capable of targeting a wide range of contaminants simultaneously. On the other hand, a soil with a water content higher than 10% is required to be effective. The treatment may involve high energy consumption especially for large-scale applications. Buried utilities may have operating problems, and the treatment may cause damage to soil flora and fauna [139,159].
4.2. Biological Technologies
Biological treatments are effective tools to clean the environment, particularly contaminated soil. Using biological components, including microorganisms already present in the soil, to degrade or eliminate and extract pollutants from the soil can be a cost-effective method to clean and regenerate the soil [131,135].
Biological techniques possess a number of advantages, depending on the soil and pollutants’ characteristics, as follows [131,160]:
- Complete degradation of pollutants;
- Prevent the transfer of pollutants from one matrix to another;
- Safety and minimum health risks;
- Easy to apply and low installation and operation costs;
- Flexible and easy to adapt to different environments;
- Possibility of in situ application.
Biological techniques or bioremediation procedures, as they are usually referred to, can be carried out by using different organisms such as bacteria, fungi, plants and even animals [131,160], or through combinations of them [131,161].
4.2.1. Natural Attenuation
In the case of natural attenuation techniques, soil remediation is carried out by native populations of microbes existing in the contaminated area that easily interact with soil particles and contaminants. It is the simplest bioremediation strategy, with minimum waste production. It includes aerobic and anaerobic biodegradation of pollutants and biochemical stabilisation, and also physical phenomena such as diffusion, advection, volatilization and sorption/desorption, while chemical reactions can also occur [131]. Microorganisms metabolise organic contaminants into inorganic chemicals such as carbon dioxide, methane, water and inorganic salts [162].
Several factors can affect the degradation process of contaminants, including the native characteristics of the microbial population, the soil’s physicochemical properties and the concentration and chemical nature of the contaminants [8,131]. This procedure is not always successful and usually requires long-term treatment. Generally, it is more successful for low contaminant levels [74]. It has been reported that around 25% of petroleum-contaminated lands have been remediated by natural attenuation [163]. Agnello et al. [164] reported the removal of 37% of total petroleum hydrocarbons through natural attenuation, even if the efficiency in the case of more recalcitrant contaminants is quite low, especially for aged contaminants [165].
Composting of organic matter can be used together with natural attenuation to increase and supplement natural nutrients and, in this way, increase the metabolic capacity of natural microorganisms to improve the biodegradation of contaminants [166]. Bioventing is also another complimentary technique to improve in situ natural remediation, by providing air or oxygen to the existing soil microorganisms. Simultaneously, nutrients can also be added to the contaminated soil. Bioventing wells may be mounted horizontally or vertically, depending on the soil’s geology [167].
4.2.2. Biostimulation
Biostimulation procedures involve the modification of environmental conditions to stimulate the biodegradation of the contaminants. The efficiency of the process is enhanced by stimulating the degrading capacity of the natural microbial populations by providing essential nutrients (carbon, nitrogen, phosphorus), for instance by adding suitable fertilisers, organic waste and biochars [167], oxygen, moisture and increasing temperature [8,131,168].
Biostimulation is influenced by the soil’s properties and is more efficient in mineral soils than in organic soils [131]. Biostimulation has proved an efficient methodology in the degradation of PAHs in contaminated soils. For instance, Lau et al. [169] proved that biostimulation with the addition of mushroom compost led to an 82% efficiency in the degradation of PAHs. Also, Breedvelt and Sparrevik [170] proved that it was possible to stimulate microbial growth and improve PAH degradation in a creosote-contaminated soil in Norway by adding nitrogen and phosphorus [162,170]. Biostimulation has also been used effectively in crude oil-contaminated soils [162]. The same has been proved in the remediation of pesticide-contaminated soils, namely a soil contaminated with DDT, after biostimulation with potassium phosphate and urea [131,171]. Caldeira et al. [172] showed the potential of biostimulation to reduce the leaching of inorganic contaminants (aluminium, arsenic, titanium, tungsten, etc.) from the residue from a tungsten mine. Biostimulation can be considered a cost-effective technique which is already being used for on-site remediation of contaminated soils.
4.2.3. Bioaugmentation
Bioaugmentation procedures in soil remediation involve inoculation of specific microbial strains into the soil, which have the ability to degrade targeted contaminants [8]. Bioaugmentation procedures are quite often necessary to enhance the performance of natural microbial populations in in situ remediation procedures. The selected microbial strains can be injected individually or as a consortium, to improve the biodegradation efficiency [173] so that the microorganisms combine their metabolic activities, complementing each other in a more effective bioremediation of the soil [173,174,175]. Consortium injection is in fact more frequent than individual microbial inoculation, and is currently being used commercially, even if it may involve higher costs than natural attenuation or biostimulation strategies.
Recent studies reported the use of a living mono fungus or mixed fungal cultures, fungal–bacteria consortium or fungal–yeast cultures to enhance the biodegradation of soils contaminated with a high concentration of oil [162]. The bioremediation of soils contaminated with high molecular weight PAHs, usually requires the use of more than one species in the bioremediation process [162].
In general, bioaugmentation has proved effective in the bioremediation of soils contaminated with hydrocarbons from different sources, alkanes and aromatic compounds [8,176]. The genetic modification of certain bacterial strains, to improve their performance when biodegrading some pollutants, has also been studied [173,177], with positive results. Still, this strategy raises some concerns, including health and safety issues, and has not yet been adopted in real scenarios.
4.2.4. Vermiremediation
Vermiremediation involves the use of earthworms for the removal, in situ, of contaminants from the soil [173,177]. Earthworms can increase the interaction between a soil’s microbial communities and contaminants, making biodegradation easier. Some studies have investigated the interaction between earthworms and metal contaminants [173], even if vermiremediation has been more commonly used for the removal of organic contaminants (PCBs and PAHs and petroleum derived hydrocarbons) [178,179]. Earthworms can use organic compounds in their own metabolism and can accumulate, extract, transform or degrade contaminants existing in the soil.
Successful vermiremediation is dependent on several factors, which include the soil’s characteristics, the nutritional requirements of the earthworms and the nature of the contaminants. Earthworms can be used to remediate topsoil or be injected into biopiles constructed off-situ, which are being subjected to remediation. This is a procedure that is already being used in the field [173]. Vermiremediation is only applicable in slightly or moderately contaminated soils that allow the earthworms’ survival [131].
4.2.5. Phytoremediation
Phytoremediation is an in situ remediation approach which uses green plants and the associated microorganisms to remove, contain or reduce and degrade contaminants in soils, sediments, surface or ground water [162]. It uses plant interactions (physical, biochemical, biological, chemical and microbiological) with contaminants to mitigate the toxic effects of pollutants. It is an attractive and cost-effective technology because it is non-invasive and may deliver a biologically active soil to the environment. The most common plant species used in phytoremediation are willows, poplar, sunflowers and different types of grasses [180]. This technology involves growing plants in contaminated areas for the period required to remove the contaminants or facilitate mobilisation or degradation of the contaminants. Plants are selected taking into consideration the nature and concentration of the contaminants, soil characteristics and local climate parameters.
Plants act according to different mechanisms to remove the contaminants from the polluted soils, depending on pollutant type and concentration and soil characteristics. The pollutants transport through the plant tissues (from roots to other tissues), where they can be metabolised by plant enzymes, accumulated or volatilised [180].
There are several processes for phytoremediation, depending on the environmental characteristics and type of contaminant: phytovolatilisation, phytoextraction, phytostabilisation and phytodegradation, as described below [162].
- Phytovolatilisation: plants are used to transform contaminants into volatile molecules moving them, through the leaves, and together with vapour to the atmosphere [29].
- Phytoextraction: the uptake, adsorption, concentration and precipitation of contaminants from soil by plant roots. These plants are called hyperaccumulators and are particularly suited to remove heavy metals from soils permanently [30,162].
- Phytostabilisation: this process leads to the mobilisation of contaminants through adsorption by roots, absorption and accumulation in the plant root zone, reducing the mobility and bioavailability of the contaminants. It does not generate secondary contaminated wastes and is particularly suited for removing heavy metals from soil, even if it can also be used to remove some organic contaminants [162].
- Phytodegradation: the degradation of organic pollutants, such as PAHs and pesticides, through the enzymatic activity of plants released by the roots, producing simple non-toxic or less toxic molecules, which are incorporated into the plant tissue [162].
On-site phytoremediation can be improved by a combination with common agricultural practices (use of fertilisers and irrigation). This allows for a higher availability of nutrients for both plants and microbial communities [180,181].
Phytoremediation has been applied with success in soils contaminated with hydrocarbon, heavy metals and persistent organic pollutants [162,180]. This remediation technique, which has been used on-site for many years, is particularly effective in the treatment of large areas of soils contaminated at shallow depths [162]. Plant selection is crucial for a successful remediation [131].
Phytoremediation has several advantages when compared to other techniques, which are related to the use of natural resources making it less expensive, and also to the ability to immobilise the pollutants in the rhizosphere, preventing the runoff of pollutants to nearby water bodies. The main disadvantage of this technique is related to the time required for an effective soil remediation, which is dependent on the type of soil, characteristics and concentration of pollutants and also on the plants’ attributes. This time can easily extend to three or four years to reach an effective soil remediation.
4.3. Mixed Remediation Technologies
Quite often, especially when soils present a mixed contamination due to the presence of both inorganic and organic pollutants, a single remediation technology is not effective enough; a combination of remediation technologies is required to achieve an appropriate de-contamination of the soil, as together they may achieve a much higher level of efficiency.
For instance, the presence of metals can cause toxic effects in soil microbial communities and reduce their capacity to degrade organic contaminants [173,182]. In these cases, a combination of techniques is required; for instance, bioattenuation supplemented by bioaugmentation has proved successful in the remediation of soils contaminated with Cr(VI) and organic compounds [173,183].
An approach combining biological degradation, chemical oxidation and subsequent biological treatment has shown enhanced remediation efficiency for crude oil-contaminated soils, achieving a 68.3% removal rate, outperforming standalone bioremediation or chemical oxidation methods [184]. The incorporation of biochar amendments into bioremediation processes enhances the degradation of petroleum hydrocarbons (PHs) and the adsorption and degradation of heavy metals (HMs), by increasing microbial activity and contributing to the adsorbtion of the contaminants [185].
Furthermore, the integration of biological techniques with phytoremediation, using plants to support microbial activity, has shown promising results in treating complex contaminated soils [132,177]. Microbial species can also promote plant nutrition, supplying nitrogen, phosphorus or iron to the plants, and thus increasing plant growth, which results in a more effective phytoremediation [131,186]. Furthermore, combining electrokinetic remediation with phytoremediation leverages the strengths of both methods. Electrokinetics mobilises heavy metals, making them more bioavailable for plant uptake, as it was demonstrated by the removal of chromium and cadmium from a contaminated soil [187,188].
Factors such as type of soil, including its geological characteristics, chemical properties of the contaminants and the biological species selected for the remediation can strongly affect the efficiency of the remediation process. Making use of mixed remediation techniques offers a broad approach to addressing complex soil contamination scenarios. By combining physicochemical and biological remediation treatments, it is possible to enhance contaminant removal, restore soil health and mitigate environmental risks more efficiently. Continued research and field trials are essential to optimise these integrated strategies for various contaminants and site conditions.

Table 2.
Remediation methods with their advantages, limitations and applications.
Table 2.
Remediation methods with their advantages, limitations and applications.
| Remediation Technique | Brief Description | Main Advantages | Main Limitations | Applications (Contaminant Type) | Refs. |
|---|---|---|---|---|---|
| Excavation and Landfilling | Physical removal of contaminated soil and its disposal in a waste landfill. | Rapid removal of contaminants; effective and simple to implement. | Expensive; risk of secondary contamination at the landfill. | Broad range of contaminants: petroleum hydrocarbons, heavy metals, POPs. | [137,138] |
| Soil Washing and Flushing | Use of aqueous solutions (possibly with surfactants or chelating agents) to wash out contaminants. | Effective for a wide range of contaminants; applicable in situ or ex situ; relatively fast. | May generate secondary waste; less effective for fine-grained soils; may alter soil structure. | Heavy metals, petroleum hydrocarbons, nano- and microplastics. | [138,139,140,141,142] |
| Soil Vapour Extraction and Air Sparging | Removes volatile contaminants from unsaturated soils using vacuum extraction and air injection. | Effective in soils with porosity; in situ application; minimise soil disturbance. | Not effective for non-volatile contaminants; requires soil permeability; requires off-gas treatment. | Volatile organic compounds (VOCs), such as BTEX and PAHs, POPs. | [138,143,144] |
| Physical Barriers and Hydraulic Control/Permeable Reactive Barriers | In situ construction of impermeable or reactive barriers to contain or treat groundwater contaminants. | Minimises contaminant migration; PRBs can treat water passively; can be a long-term solution. | High installation cost; effectiveness depends on subsurface conditions; does not remove contaminants; potential of PRBs clogging. | Broad range of contaminants: heavy metals, petroleum hydrocarbons, POPs. | [139,145,146] |
| Solidification/ Stabilisation and Immobilisation | Addition of binders to soil to reduce contaminant mobility by altering physical/chemical properties. | Reduces contaminant mobility and bioavailability; can improve soil geotechnical properties. | Does not remove contaminants; long-term monitoring needed; require maintenance costs; limits the use of soil. | Heavy metals, POPs, nano- and microplastics, radionuclides. | [139,147,148,149,150,151] |
| Chemical Oxidation–reduction | In situ injection of oxidants or reduction agents to chemically transform contaminants into less harmful compounds. | Rapid degradation of contaminants; adaptable to site needs; minimises excavation and off-site disposal. | Ineffective in heterogeneous soils; potential for non-targeted reactions; cost of oxidants or reduction agents. | Petroleum hydrocarbons, POPs, PFAS. | [139,150,152] |
| Thermal/Heat Treatment and Vitrification | Application of heat to volatilise, destroy, or vitrify contaminants, often in situ. | Destroys contaminants; highly effective for organic contaminants; effective for inorganic contaminants. | High energy costs; may damage soil structure; normally combined with other remediation techniques. | Persistent organics (e.g., PCBs), petroleum hydrocarbons. | [139,153,154] |
| Electrokinetic | In situ application of low-intensity electric current to mobilise and remove contaminants. | Suitable for low-permeability soils; relatively low energy consumption. | Low efficiency in heterogeneous soils; applicable in soils with water content > 10%. | Effective for heavy metals and POPs. | [155,156,157,158,159] |
| Natural attenuation | In situ technique that uses natural processes to reduce concentration of contaminants. Relies on the use of indigenous microorganisms. | Use of indigenous microorganisms. Low cost. In situ technique. | Requires long term treatment. Adequate only for low levels of contaminants. | Petroleum contaminated soils; degradation of organic contaminants. | [74,131,161,164,165] |
| Biostimulation | Involves adjusting environmental parameters to stimulate the growth of microorganisms enhancing contaminant degradation. | Natural technique more efficient than natural attenuation. Can be used for POPs. Low cost. In situ technique. | Influenced by soil properties. More efficient in mineral than organic soils. | PAHs and oil contaminate soils. Degradation of pesticides in soils. | [131,167,168,169,170] |
| Bioaugmentation | Involves enhancing the performance of microorganisms by inoculating specific microbial strains to degrade targeted contaminants. | Can be used for high contaminant concentrations. Soil is made available for further use without limitations. In situ technique. | Higher cost. | High concentration of oil-contaminated soils; high molecular weight PAHs. | [161,172,173,174] |
| Vermiremediation | Uses earthworms for on-site removal of contaminants. Earthworms increase interactions between soil and microorganisms. | Natural technique. Low cost. | Soil characteristics and presence of earthworm nutrients. Only efficient in low or moderate contaminated soils. | Removal of metal and organic contaminants including hydrocarbons | [131,172,176,177,178] |
| Phytoremediation | In situ remediation strategy using plants to remove or degrade soil contaminants. | In situ green technique. Delivers a biologically active soil to environment. Low cost. Treatment of large areas. | Long time required for the remediation; dependence on soil characteristics. | Hydrocarbon or heavy metal contaminated soils. POCs removal. Used to treat large areas. | [131,161,179,180] |
5. Final Remarks
This review paper provides a comprehensive review of the different types of persistent soil contaminants and the existing remediation techniques. The objective is to provide a tool for researchers and practitioners involved in soil remediation to be able to select the best technique for the specific case of contamination being dealt with and also to have an overview of the remediation strategies available.
In summary, the interaction between soil contaminants and soil properties is multifaceted and context-dependent. Fine-textured, mineralogically diverse and chemically active soils tend to exhibit a higher contaminant retention tendency, while coarse, poorly reactive soils are more prone to pollutant leaching. Understanding these dynamics is crucial not only for risk assessment, but also for appropriate site-specific soil management and selection and optimisation of the best remediation approaches.
Besides the more traditional techniques already being implemented onsite, such as physicochemical technologies including excavation and landfilling, soil washing and soil stabilisation, new, more eco-friendly technologies are being developed, namely biological technologies which include natural remediation, biostimulation and phytoremediation, some of which are also already implemented onsite.
It is also important to stress that new pollutants are being transported and captured in soils, namely nano- and microplastics and also other persistent pollutants such as PAHs and PCBs and also radionuclides, which may require the use of mixed remediation procedures to be effective and further research. The same applies to situations where soils present a mixed contamination. Another issue of social concern is related to the fact that some contaminants may be present in the soils for very long periods and, again, further research is still necessary at this level.
Finally, it must be mentioned that the regulatory framework for soil contamination and remediation across the globe varies significantly, depending on the region, reflecting different environmental priorities, economic conditions and the development of the legal system. While some regions have established comprehensive legislation and strict standards, making remediation procedures compulsory, others are still developing their regulatory approaches and, again, research in the field can warn of the harmful effects of soil contaminants and contribute to bringing novel cost-efficient procedures to the field.
Author Contributions
Conceptualization, A.A.S.C. and M.G.R.; methodology, A.A.S.C. and M.G.R.; validation, A.A.S.C. and M.G.R.; formal analysis, A.A.S.C. and M.G.R.; resources, A.A.S.C. and M.G.R.; data curation, A.A.S.C. and M.G.R.; writing—original draft preparation, A.A.S.C. and M.G.R.; writing—review and editing, A.A.S.C. and M.G.R.; visualisation, A.A.S.C. and M.G.R.; funding acquisition, A.A.S.C. and M.G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge the support given by FCT, Fundação para a Ciência e a Tecnologia, Portugal, under the project UIDB/00102/2020 (DOI: 10.54499/UIDB/00102/2020 and DOI: 10.54499/UIDP/00102/2020).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. (Eds.) Soil and pollution: An introduction to the main issues, chapter 1. In Soil Pollution from Monitoring to Remediation; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Muhlbachova, G.; Sagova-Mareckova, M.; Omelka, M. The influence of soil organic carbon on interactions between microbial parameters and metal concentrations at a long term contaminated site. Sci. Total Environ. 2015, 502, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Maddela, R.M.; Ramakrishnan, B.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Major contaminants of emerging concern in soils: A perspective on potential health risks. RSC Adv. 2022, 12, 12396–12415. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Murtaza, B.; Niazi, N.K.; Sabir, M. Soil contaminants: Sources, effects and approaches for remediation, Chapter 7. In Improvement of Crops in the Era of Climate Changes; Ahmad, P., Vani, M.R., Azooz, M.M., Tran, L.P., Eds.; Springer: New York, NY, USA, 2014; Volume 2, pp. 171–196. [Google Scholar]
- Rajabi, H.; Sharifipour, M. Geotechnical properties of hydrocarbon-contaminated soils: A comprehensive review. Bull. Eng. Geol. Environ. 2019, 78, 3685–3717. [Google Scholar] [CrossRef]
- Osia, S.; VandenBerge, D. Effects of Pollutants on Soil Shear Strength Parameters and Slope Stability. In Geotechnical Frontiers; ASCE 2025; pp. 28–37. [CrossRef]
- Azoury, S.; Tronczynski, J.; Chiffoleau, J.F. Historical records of mercury, lead and polycyclic aromatic hydrocarbons deposition in a dated sediment from the eastern Mediteraanean. Environ. Sci. Technol. 2013, 47, 7101–7109. [Google Scholar] [CrossRef]
- Sethi, S.; Gupta, P. Soil contamination: A menace to life, chapter 15, In Soil Contamination: Threats and Sustainable Solutions; Larramendy, M.L., Soloneski, S., Eds.; Intechopen: London, UK, 2021; pp. 1–23. [Google Scholar]
- Batterman, S. Findings on Assessment of Small Scale Incinerators for Health-Care Waste, Wastewater, Sanitation and Health Protection of the Human Environment; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- National Environmental and Health Action Plan. EP Soil and Sediment Activity Report 2009/2011. 2012. Available online: https://www.insa.min-saude.pt/wp-content/uploads/2017/10/RelatorioActividadesEPSoloSedimentos.pdf (accessed on 19 May 2025). (In Portuguese).
- Yuan, X. Current Problems and Countermeasures of Soil Pollution Management. E3S Web Conf. 2023, 424, 04017. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Fetter, C.W. Applied Hydrogeology, 4th ed.; Prentice Hall: Denver, CO, USA, 2001. [Google Scholar]
- Askeland, M.; Clarke, B.O.; Cheema, S.A.; Mendez, A.; Gascó, G.; Paz-Ferreiro, J. Biochar sorption of PFOS, PFOA, PFHxS and PFHxA in two soils with contrasting texture. Sci. Total Environ. 2020, 718, 137341. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil. Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical Redox Processes and Their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- McKenzie, R.M. The adsorption of lead and other heavy metals on oxides of manganese and iron. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Strawn, D.G. Sorption Mechanisms of Chemicals in Soils. Soils 2021, 5, 13. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, S.; Hendershot, W.; Allen, H.E. Solid-Solution Partitioning of Metals in Contaminated Soils: Dependence on pH, Total Metal Burden, and Organic Matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Racke, K.D.; Steele, K.P.; Yoder, R.N.; Dick, W.A.; Avidov, E. Factors Affecting the Hydrolytic Degradation of Chlorpyrifos in Soil. J. Agric. Food Chem. 1996, 44, 1582–1592. [Google Scholar] [CrossRef]
- Wilk, C. Solidification/Stabilization Treatment: Principles and Practice; Air and Waste Management Association’s Magazine for Environmental Managers: Pittsburgh, PA, USA, 2003; pp. 31–37. [Google Scholar]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef]
- Lloyd, J.R. Bioremediation of metals: The application of microorganisms that make and brake minerals. Microbiol. Today 2002, 29, 67–69. [Google Scholar]
- Alkorta, I.; Hernandez-Allica, J.; Becerril, J.; Amezaga, I.; Albizu, I.; Onaindia, M.; Garbisu, C. Chelate-enhanced phytoremediation of soils polluted with heavy metals. Rev. Environ. Sci. Biotechnol. 2004, 3, 55–70. [Google Scholar] [CrossRef]
- Raskin, I.; Smith, R.D.; Salt, D.E. Phytoremediation of metals: Using plants to remove pollutants from the environment. Curr. Opin. Biotechnol. 1997, 8, 221–226. [Google Scholar] [CrossRef]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Bioavaliability of weathered hydrocarbons in engine oil-contaminated soil: Impact of bioaugmentation mediated by Pseudomonas spp. on bioremediation. Sci. Total Environ. 2018, 636, 968–974. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Rasteiro, M.G. Effect of multiwall carbon nanotubes and surfactants characteristics on immobilization of heavy metals in contaminated soils. Discov. Appl. Sci. 2024, 6, 205. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tai, Y.T. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere 2010, 78, 1200–1206. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental application of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- EC. Directive 2004/35/EC. Environmental Liability Directive. 2004. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02004L0035-20130718 (accessed on 5 April 2025).
- EC. Directive 2024/1785/EU of the European Parliament and of the Council of 24 April 2024 “Industrial and Livestock Rearing Emissions Directive (IED 2.0)”, Off. J. Eur. Union, L 2024/1784 from 15/07/2024. 2024. Available online: https://environment.ec.europa.eu/topics/industrial-emissions-and-safety/industrial-and-livestock-rearing-emissions-directive-ied-20_en (accessed on 19 May 2025).
- EC. Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. Off. J. Eur. Union, L 181. 1986. Available online: https://eur-lex.europa.eu/eli/dir/1986/278/oj/eng (accessed on 19 May 2025).
- Communication from the Commission to the Council; the European Parliament; the European Economic and Social Committee; the Committee of the Regions. EU Soil Strategy for 2030 Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate; Farm Europe: Bruxelles, Belgium, 2021. [Google Scholar]
- EU. Soil Health Law: Legal Principles Underpinning the Framework. ClientEarth.org 2022. Available online: https://www.clientearth.org/media/uoenhrtn/eu-soil-health-law_legal-principles-underpinning-the-framework.pdf (accessed on 5 April 2025).
- USEPA—United States Environmental Protection Agency. Summary of the Comprehensive Environmental Response, Compensation, and Liability Act (Superfund). Available online: https://www.epa.gov/laws-regulations/summary-comprehensive-environmental-response-compensation-and-liability-act (accessed on 29 April 2025).
- Canadian Environmental Protection Act, 1999 (S.C. 1999, c. 33), Government of Canada. Available online: https://laws-lois.justice.gc.ca/eng/acts/c-15.31/ (accessed on 5 April 2025).
- Ministry of Environment. Soil, Ground Water and Sediment Standards for Use Under Part XV.1 of the Environmental Protection Act. 2011. Available online: https://www.ontario.ca/page/soil-ground-water-and-sediment-standards-use-under-part-xv1-environmental-protection-act (accessed on 5 April 2025).
- Ministry of Ecology and Environment. Law of the People’s Republic of China on Prevention and Control of Soil Contamination. 2019. Available online: https://english.mee.gov.cn/Resources/laws/environmental_laws/202011/t20201113_807786.shtml (accessed on 5 April 2025).
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. National Standard of the People’s Republic of China, Ministry of Ecology and Environment: Beijing, China, 2018.
- GB 36600-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Development Land. National Standard of the People’s Republic of China, Ministry of Ecology and Environment: Beijing, China, 2018.
- Act No. 53, Soil Contamination Countermeasures Act. Act No. 53 of May 29, 2002. Ministry of the Environment of Japan. 2017. Available online: https://www.env.go.jp/en/laws/water/sccact.pdf (accessed on 5 April 2025).
- ASC NEPM, National Environment Protection (Assessment of Site Contamination) Measure 1999 (Amended 2013). Department of Climate Change, Energy, the Environment and Water, Australia. 2013. Available online: https://faolex.fao.org/docs/pdf/aus197207.pdf (accessed on 5 April 2025).
- Odigie, K.O.; Flegal, A.R. Trace metal inventories and lead isotopic composition chronicle a forest fire’s remobilization of industrial contaminants deposited in the Angeles National Forest. PLoS ONE 2014, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Witter, E.; McGrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance in plants. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Robert, N. Heavy Metal Accumulation in Food Chains: Ecological and Health Implications. J. Heavy Met. Toxic. Dis. 2024, 9. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Shetty, B.R.; Jagadeesha, P.B.; Salmataj, S.A. Heavy metal contamination and its impact on the food chain: Exposure, bioaccumulation, and risk assessment. CyTA—J. Food 2025, 23, 2438726. [Google Scholar] [CrossRef]
- Acharya, A.; Perez, E.; Maddox-Mandolini, M.; De La Fuente, H. The Status and Prospects of Phytoremediation of Heavy Metals, Quantitative Biology—Tissues and Organs. arXiv 2023, arXiv:2312.14288. [Google Scholar]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2015, 157, 141–161. [Google Scholar] [CrossRef]
- Bourazanis, G.; Kallioras, A.; Pliakas, F. Assessment of Contamination Management Caused by Copper and Zinc Cations Leaching and Their Impact on the Hydraulic Properties of a Sandy and a Loamy Clay Soil. Land 2022, 11, 290. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring Techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Pan, F.; Yu, Y.; Yu, L.; Lin, H.; Wang, Y.; Zhang, L.; Pan, D.; Zhu, R. Quantitative assessment on soil concentration of heavy metal–contaminated soil with various sample pretreatment techniques and detection methods. Environ. Monit. Assess 2020, 192, 800. [Google Scholar] [CrossRef]
- Qu, M.; Guang, X.; Liu, H.; Zhao, Y.; Huang, B. Additional sampling using in-situ portable X-ray fluorescence (PXRF) for rapid and high-precision investigation of soil heavy metals at a regional scale. Environ. Pollut. 2022, 292, 118324. [Google Scholar] [CrossRef]
- Webster, R.; Oliver, M.A. Geostatistics for Environmental Scientists, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Ersoy, A.; Yünsel, T.Y. The assessment of soil contamination by heavy metals using geostatistical sequential Gaussian simulation method. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 2142–2161. [Google Scholar] [CrossRef]
- Markert, B.; Breure, A.M.; Zechmeister, H.G. Chapter 1: Definitions, strategies and principles for bioindication/biomonitoring of the environment. In Bioindicators and Biomonitors; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Nahmani, J.; Hodson, M.E.; Black, S. A review of studies performed to assess metal uptake by earthworms. Environ. Pollut. 2007, 145, 402–424. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Correia, A.A.S.; Rasteiro, M.G. Heavy Metals Removal from Aqueous Solutions by Multiwall Carbon Nanotubes: Effect of MWCNTs Dispersion. Nanomaterials 2021, 11, 2082. [Google Scholar] [CrossRef]
- Matos, M.P.S.R.; Correia, A.A.S.; Rasteiro, M.G. Application of carbon nanotubes to immobilize heavy metals in contaminated soils. J. Nanoparticle Res. 2017, 19, 126. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Matos, M.P.S.R.; Gomes, A.R.; Rasteiro, M.G. Immobilization of Heavy Metals in Contaminated Soils—Performance Assessment in Conditions Similar to a Real Scenario. Appl. Sci. 2020, 10, 7950. [Google Scholar] [CrossRef]
- Ghandali, M.; Safarzadeh, S.; Ghasemi-Fasaei, R.; Zeinali, S. Heavy metals immobilization and bioavailability in multi-metal contaminated soil under ryegrass cultivation as affected by ZnO and MnO2 nanoparticle-modified biochar. Sci. Rep. 2024, 14, 61270. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Majeed, B.K.; Shwan, D.M.S.; Rashid, K.A. A review on environmental contamination of petroleum hydrocarbons, its effects and remediation approaches. Environ. Sci. Process. Impacts 2025, 27, 526–548. [Google Scholar] [CrossRef]
- Elijah, A.A. A Review of the Petroleum Hydrocarbons Contamination of Soil, Water and Air and the Available Remediation Techniques, Taking into Consideration the Sustainable Development Goals. Earthline J. Chem. Sci. 2022, 7, 97–113. [Google Scholar] [CrossRef]
- Gennadiev, A.N.; Pikovskii, Y.I.; Tsibart, A.S.; Smirnova, M.A. Hydrocarbons in soils: Origin, composition, and behavior (Review). Eurasian Soil Sci. 2015, 48, 1076–1089. [Google Scholar] [CrossRef]
- Daâssi, D.; Almaghribi, F.Q. Petroleum-contaminated soil: Environmental occurrence and remediation strategies. 3 Biotech 2022, 12, 139. [Google Scholar] [CrossRef]
- Khodaparast, M.; Khoshgoftar, A. A Review of the Effect of Hydrocarbon Contamination on Soil Resistance Parameters; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Mostrom, M.S. Petroleum Product Poisoning in Animals. MSD Veterinary Manual. 2021. Available online: https://www.msdvetmanual.com/toxicology/petroleum-product-poisoning/petroleum-product-poisoning-in-animals (accessed on 5 April 2025).
- Al-Mebayedh, H.; Niu, A.; Lin, C. Petroleum Hydrocarbon Composition of Oily Sludge and Contaminated Soils in a Decommissioned Oilfield Waste Pit under Desert Conditions. Appl. Sci. 2022, 12, 1355. [Google Scholar] [CrossRef]
- Hamamin, D.F. Passive soil gas technique for investigating soil and groundwater plume emanating from volatile organic hydrocarbon at Bazian oil refinery site. Sci. Total Environ. 2018, 622–623, 1485–1498. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782–e1700787. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe, Association of Plastics Manufacturers: Brussels, Belgium, 2019. [Google Scholar]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic production. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Karbalaei, S.; Maselli, V.; Walker, T.R. Micro(nano) plastic sources, fate and effects: What we know after 10 years of research. J. Hazardous Mater. Adv. 2022, 6, 100057–100069. [Google Scholar] [CrossRef]
- Magalhães, S.; Paciência, D.; Rodrigues, J.M.M.; Lindman, B.; Alves, L.; Medronho, B.; Rasteiro, M.G. Insights on Microplastic Contamination from Municipal andTextile Industry Effluents and Their Removal Using a Cellulose-Based Approach. Polymers 2024, 16, 2803. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicity of microplastics in soil. Environ. Int. 2020, 137, 105263–105276. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Xia, M.; Jiang, K.; Cao, Z.; Zhang, X.; Hu, X. Photo-oxidative degradation mitigated the developmental toxicity of polyamide microplastics to zebra fish larvae by modulating macrophage-triggered proinflammatory responses and apoptosis. Environ. Sci. Technol. 2020, 54, 13888–13898. [Google Scholar] [CrossRef]
- Blasing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks, Track Trends in Analytical. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Kim, Y.N.; Yoon, J.H.; Kim, K.H. Microplastic contamination in soil environment—A review. Soil Sci. Annu. 2020, 71, 300–308. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 3–11. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Gregory, A.S.; Storkey, J.; Stevens, C.J. Agricultural fertilisers contribute substantially to microplastics concentrations in UK soils. Commun. Earth Environ. 2024, 5, 1–5. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, S.; Ma, Q.; Lu, Y. Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ. Exp. Botany 2021, 192, 104635–104644. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, S.; Pelaez, A.M.; Huerta-Lavanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro and micro plastics in soil plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Yang, M.; Huang, D.; Tian, Y.; Zhang, Q.; Zhu, H.; Xu, C. Influences of different source microplastics with different particle sizes and application rates on soil properties and growth of Chinese cabbage (Brassica chinensis L.). Ecotoxcol. Environ. Saf. 2021, 222, 112480–112488. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.H.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Gorlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Boughattas, I.; Hattab, S.; Zitouni, N.; Mkhinini, M.; Missawi, O.; Bousseehine, N.; Bannin, M. Assesing the presence of microplasticsin Tunisian agricultural soils and their potential toxicity effects using Eisenia Andrei as bioindicator. Sci. Total Environ. 2021, 796, 148959–148969. [Google Scholar] [CrossRef]
- Grbic, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623–115633. [Google Scholar] [CrossRef]
- Wright, S.I.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in a urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411–105418. [Google Scholar] [CrossRef]
- Monkul, M.M.; Ozhan, H. Microplastic contamination in soils: A review from geotechnical engineering view. Polymers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Karakurt, O.; Altuntas, O.; Simsek, I.; Hatinoglu, D.; Sanin, F.D. Microplastics from industrial sources: A known but overlooked problem. J. Water Proc. Eng. 2025, 72, 107487–107498. [Google Scholar] [CrossRef]
- Deng, I.; Xi, H.; Wan, C.; Fu, L.; Wang, Y.; Wu, C. Is the petrochemical industry an overlooked critical source of environmental microplastics. J. Hazard. Mater. 2023, 451, 131199–131208. [Google Scholar] [CrossRef] [PubMed]
- Kilic, B.I.; Ustin, G.E.; Can, T. Characteristics and seasonal change of microplastics in organized industrial zone wastewater treatment plant. J. Environ. Chem. Eng. 2025, 13, 115516–115526. [Google Scholar] [CrossRef]
- Liu, J.; Bu, L.; Zhu, L.; Luo, S.; Chen, X.; Li, S. Optimizing plant density and plastic film mulch to increase maize and water use efficiency in semiarid areas. Agron. J. 2014, 106, 1138–1146. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. White revolution to white pollution—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001–091004. [Google Scholar] [CrossRef]
- Liu, X.E.; Li, X.G.; Hai, I.; Wang, Y.P.; Fu, T.T.; Turner, N.C.; Li, F.M. Film mulch ridge-furrow management increases maize productivity and sustains soil organic carbon in a dryland cropping system. J. Soil Sci. Soc. Am. 2014, 78, 1434–1441. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Hu, W.; Qin, J.; Zheng, Y.; Wang, J.; Wang, Q.; Xu, Y.; Guo, G.; Hu, S. Effects of plastic mulch film residues on soil-microbe-plant systems under different soil pH conditions. Chemosphere 2021, 267, 128901–128911. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Lu, R.; Zhou, Q.; Peijnenburg, W.J.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Cai, Y.B. Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020, 257, 113570–113582. [Google Scholar] [CrossRef]
- Saijad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408–102433. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption and transfer to biota. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Lima, A.R.A.; Costa, M.F. Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South America estuaries. Sci. Total Environ. 2019, 651, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Panescu, V.A.; Bintintan, V.B.; Herghelegiu, M.C.; Coman, R.T.; Berg, V.; Lyche, J.V.; Belden-Galea, M.S. Pollution assessment with persistent organic pollutants in upper soil of a series of rural Roma communities in Transylvania, Romania, its sources apportionment, and the associated risk on human health. Sustainability 2024, 16, 232. [Google Scholar] [CrossRef]
- Grassi, P.; Fattore, E.; Generoso, C.; Fanelli, R.; Arvati, M.; Zuccato, E. Polychlorobiphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in fruit and vegetables from an industrial area in northern Italy. Chemosphere 2010, 79, 292–298. [Google Scholar] [CrossRef]
- Dreyer, A.; Minkos, A. Polychlorobiphenyls (PCBs), polychlorinated dibenzo-para-dioxins and dibenzofurans (PCDD/F) in ambient and depositions in German background. Environ. Pollut. 2023, 316, 120511–120522. [Google Scholar] [CrossRef]
- Li, Y.F.; Hao, S.; Ma, W.L.; Yand, P.F.; Li, W.L.; Zhang, Z.F.; Liu, L.Y.; Macdonald, R.W. Persistent organic pollutants in global surface soils: Distributions and fractionations. Environ. Sci. Ecothech. 2024, 18, 100311–100326. [Google Scholar] [CrossRef]
- K’Oreje, K.O.; Okoth, M.; Van Langenhove, H.; Demeestere, J. Occurrence and treatment of contaminants of emerging concern in the African aquatic environment: Literature review and a look ahead. J. Environ. Manag. 2020, 254, 109752–109770. [Google Scholar] [CrossRef]
- Kroon, F.J.; Berry, K.L.E.; Brinkman, D.L.; Kookana, R.; Leusch, D.L.; Melvin, S.D.; Neale, P.A.; Negri, A.P.; Puotinen, M.; Tsang, J.J.; et al. Sources, presence and potential effects of contaminants of emerging concern in the marine environments of the Great Barrier Reef and Torres Strait, Australia. Sci. Total Environ. 2020, 719, 135140–135161. [Google Scholar] [CrossRef]
- Vandermeersch, G.; Lourenço, H.M.; Alvarez-Munoz, D.; Cunha, S.; Diogène, J.; Cano-Sancho, G.; Sloth, J.J.; Kwadijk, C.; Barcelo, D.; Allegaert, W.; et al. Environmental contaminants of emerging concern in seafood—European database on contaminant levels. Environ. Res. 2015, 143, 29–45. [Google Scholar] [CrossRef]
- Reichert, G.; Hilgert, S.; Fuchs, S.; Azevedo, J.C.R. Emerging contaminants and antibiotic resistance in the different environmental matrices of Latin America. Environ. Pollut. 2019, 235, 113140–113153. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, V.K.; Mishra, M.; Piyush; Kakkar, V.; Tiwari, A.; Kumar, S.; Yadav, V.P.; Gargava, P. Assessment of persistent organic pollutants in soil ans sediments from an urbanized flood plain area. Environ. Geochem. Health 2021, 43, 3375–3392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, X.; Na, G.; Lin, Z.; Ding, Q.; Yao, Z. Correlations between physicochemical properties of PAHs and their distribution in soil, moss and reindeer dung at Ny-Alesund of the Artic. Environ. Pollut. 2009, 157, 3132–3136. [Google Scholar] [CrossRef] [PubMed]
- UNEP (United Nations Environmental Programme). Revised Text and Annexes. 2017. Available online: http://www.pops.int/Home/tabid/2121/Default.aspx (accessed on 5 April 2025).
- Becker, B.; Halshall, C.J.; Tych, W.; Kallenborn, R.; Schiabch, M.; Mano, S. Changing sources and environmental factors reduce the rates of decline of organochlorine pesticides in the Arctic atmosphere. Atmos. Chem. Phys. Dis. 2009, 9, 515–540. [Google Scholar] [CrossRef]
- Harner, T.; Shoeib, M.; Diamond, M.; Stern, G.; Rosenberg, B. Using passive air samplers to assess urban-rural trends for persistent organic pollutants. 1. Polychlorinated biphenyls and organochlorine pesticides. Environ. Sci. Technol. 2004, 38, 4474–4483. [Google Scholar] [CrossRef]
- Newton, S.; Sellstroem, U.; Wit, C. Emerging flame retardants, PBDEs, and HBCDDs in indoor and outdoor media in Stockholm, Sweden. Environ. Sci. Technol. 2015, 49, 2912–2920. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Li, Y.F.; Wang, D.; Jia, H.; Harner, T.; Sverko, E.; Wan, X.; Xu, D.; Ren, N.; et al. Analysis of polychlorinated biphenyls in concurrently sampled Chinese air and surface soil. Environ. Sci. Technol. 2008, 42, 6514–6518. [Google Scholar] [CrossRef]
- Haynes, R.; Murtaza, G.; Naidu, R. Inorganic and organic constituents and contaminants of biosolids: Implications for land application. Adv. Agron. 2009, 104, 165–267. [Google Scholar]
- Albanese, S.; Guarino, A. Assessing contamination sources and environmental hazards for potentially toxic elements and organic compounds in the soils of a heavily anthropized area: The case study of the Acerra plain (Southern Italy). Geosciences 2010, 8, 552–578. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.-W.; Chang, H.-Q.; Li, Z.-J.; Xue, J.-M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Raimondo, E.E.; Saez, J.M.; Costa-Gutierrez, S.B.; Álvarez, A.; Benimeli, C.S.; Polti, M.A. The current approach to soil remediation: A review of physicochemical and biological technologies, and the potential of their strategic combination. J. Environ. Chem. Eng. 2022, 10, 107141. [Google Scholar] [CrossRef]
- Santos, M.; Rebola, S.; Evtuguin, D.V. Soil Remediation: Current Approaches and Emerging Bio-Based Trends. Soil Syst. 2025, 9, 35. [Google Scholar] [CrossRef]
- Singh, A.; Kuhad, R.C.; Ward, O.P. (Eds.) Advances in applied bioremediation. In Soil Biology; Springer: Berlin, Germany, 2009; Volume 17, pp. 1–19. [Google Scholar]
- Babu, A.G.; Reja, S.I.; Akhtar, N.; Sultana, M.; Deore, P.S.; Ali, F.I. Bioremediation of polycyclic aromatic hydrocarbons (PAHs): Current practices and outlook. In Microbial Metabolism of Xenobiotic Compounds; Arora, N.K., Ed.; Springer: New York, NY, USA, 2019; pp. 189–216. [Google Scholar]
- Van Dillewijn, P.; Caballero, A.; Paz, J.A.; González-Pérez, M.M.; Oliva, J.M.; Ramos, J.L. Bioremediation of 2,4,6-trinitrotoluene under field conditions. Environ. Sci. Technol. 2007, 41, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Maletic, S.P.; Dalmacija, B.D.; Roncevic, S.D. Petroleum Hydrocarbon Biodegradability in Soil—Implications for Bioremediation. In Hydrocarbon; Kutcherov, V., Kolesnikov, A., Eds.; Intech: London, UK, 2013; Volume 3, pp. 43–64. [Google Scholar]
- National Research Council. Innovations in Ground Water and Soil Cleanup: From Concept to Commercialization; The National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- FAO; UNEP. Global Assessment of Soil Pollution: Report; FAO: Rome, Italy, 2021.
- CLAIRE. Contaminated Land Remediation, Defra Research Project SP1001 Final Report; CLAIRE: London, UK, 2010. [Google Scholar]
- Karthika, N.; Jananee, K.; Murugaiyan, V. Remediation of contaminated soil using soil washing—A review. Int. J. Eng. Res. Appl. 2016, 16, 13–18. [Google Scholar]
- Vulava, V.M.; Seaman, J.C. Mobilization of lead from highly weathered porous material by extracting agents. Environ. Sci. Technol. 2000, 34, 4828–4834. [Google Scholar] [CrossRef]
- Nowack, B.; VanBriesen, J.M. (Eds.) Biogeochemistry of Chelating Agents. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2005; Volume 910. [Google Scholar]
- Simpanen, S. Evaluation of In-Situ Remediation Methods in Soils Contaminated with Organic Pollutants. Ph.D. Thesis, University of Helsinki, Lahti, Finland, 2016. [Google Scholar]
- Zhang, C.; Chen, X.; Tan, Y.; Feng, Y.; Zhong, Z. Soil vapour extraction removal of semi volatile organic compounds in soil: A pilot-scale study. In Proceedings of the International Conference on Sustainable Energy and Environmental Engineering, Bangkok, Thailand, 25–26 October 2015; pp. 180–182. [Google Scholar]
- Jankaite, A.; Vasarevičius, S. Remediation technologies for soils contaminated with heavy metals. J. Environ. Eng. Land. Manag. 2005, 13, 109a–113a. [Google Scholar] [CrossRef]
- Agante, A.C.C. Remediation of Soils Contaminated by Heavy Metals Using Biochars. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2021. (In Portuguese). [Google Scholar]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/stabilization of soil heavy metals by alkaline industrial wastes: A critical review. Environ. Pollut. 2022, 312, 120094–120109. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Innovative Site Remediation Technology: Volume 4, Design and Application, Stabilization/Solidification; EPA 542/B-97/007; Office of Solid Waste and Emergency Response: Washington, DC, USA, 1997.
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- FAO. Recarbonization of Global Soils; Global Soil partnership; FAO: Roma, Italy, 2019. [Google Scholar]
- Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture 2022, 12, 321. [Google Scholar] [CrossRef]
- ITRC. Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater; Interstate Technology and Regulatory Cooperation Work Group In Situ Chemical Oxidation Work Team: 2001. Available online: https://apps.dtic.mil/sti/tr/pdf/ADA492437.pdf (accessed on 5 April 2025).
- Vidonish, J.E.; Zygourakis, K.; Masiello, C.A.; Sabadell, G.; Alvarez, P.J.J. Thermal Treatment of Hydrocarbon-Impacted Soils: A Review of Technology Innovation for Sustainable Remediation. Engineering 2016, 2, 426–437. [Google Scholar] [CrossRef]
- EPA. CLU-IN—Technologies—Remediation. Available online: https://clu-in.org/techfocus/default.focus/sec/Thermal_Treatment%3A_In_Situ/cat/Overview/ (accessed on 20 May 2025).
- Istrate, I.A.; Cocârtă, D.M.; Wu, Z.; Stoian, M.A. Minimizing the health risks from hydrocarbon-contaminated soils by using electric-field based treatment for soil remediation. Sustainability 2018, 10, 253. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Zhao, M.; Rong, H.; Zhang, K.; Chen, Q. Comparison of bioloeaching and electrokinetic remediation processes for removal of heavy metals from wastewater treatment sludge. Chemosphere 2016, 168, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Tan, W.; Gong, J.; Wei, G. Electrokinetic Remediation of Zn-Polluted Soft Clay Using a Novel Electrolyte Chamber Configuration. Toxics 2023, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, R.; Altaee, A.; Zhou, J.L.; Karbassiyazdi, E.; Ganbat, N. Effective remediation of heavy metals in contaminated soil by electrokinetic technology incorporating reactive filter media. Sci. Total Environ. 2021, 794, 148668–148679. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic remediation for the removal of heavy metals in soil: Limitations, solutions and prospection. Sci. Total Environ. 2023, 903, 165970–165992. [Google Scholar] [CrossRef]
- Song, B.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y.; et al. Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ. Int. 2017, 105, 43–55. [Google Scholar] [CrossRef]
- Polti, M.A.; Atjian, M.C.; Amoroso, M.J.; Abate, C.M. Soil chromium bioremediation: Synergic activity of actinobacteria and plants. Int. Biodeterior. Biodegrad. 2011, 65, 1175–1181. [Google Scholar] [CrossRef]
- Verma, A. Bioremediation Techniques for Soil Pollution: An Introduction. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, F.K., Nogueira de Sousa, R., Mielke, K.C., Eds.; Intech Open: London, UK, 2021; pp. 1–14. [Google Scholar]
- Stroud, J.; Paton, G.; Semple, K.T. Microbe-aliphatic hydrocarbon interactions in soil: Implications for biodegradation and bioremediation. J Appl Microbiol. 2007, 102, 1239–1253. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef]
- Leech, C.; Tighe, M.K.; Pereg, L.; Winter, G.; McMillan, M.; Esmaeili, A.; Wilson, S.C. Bioaccessibility constrains the co-composting bioremediation of field aged PAH contaminated soils. Int. Biodeterior. Biodegrad. 2020, 149, 104922–104934. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Dashti, N.; Khanafer, M.; Al-Awadhi, H.; Radwan. Bioremediation of soils saturated with spilled crude oil. Sci. Rep. 2020, 10, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.L.; Tsang, Y.Y.; Chiu, S.W. Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere 2003, 52, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, G.D.; Sparrevik, M. Nutrient-limited biodegradation of PAH in various soil strata at a creosote contaminated site. Biodegradation 2000, 11, 391–399. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Pino, N.J.; Cardona, S.; Peñuela, G.A. Evaluation of biostimulation and Tween 80 addition for the bioremediation of long-term DDT-contaminated soil. J. Environ. Sci. 2015, 28, 101–109. [Google Scholar] [CrossRef]
- Caldeira, J.B.; Correia, A.A.; Branco, R.; Morais, P.V. The effect of biopolymer stabilisation on biostimulated or bioaugmented mine residue for potential technosol production. Sci. Rep. 2024, 14, 25583. [Google Scholar] [CrossRef]
- Lacalle, R.G.; Becerril, J.M.; Garbisu, C. Biological methods of polluted soil remediation for an effective economically-optimal recovery of soil health and ecosystem services. J. Environ. Sci. Public Health 2020, 4, 112–133. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Raimondo, E.E.; Amoroso, M.J.; Benimeli, C.S. Removal of a mixture of pesticides by a Streptomyces consortium: Influence of different soil systems. Chemosphere 2017, 173, 359–367. [Google Scholar] [CrossRef]
- Shen, T.; Pi, Y.; Bao, M.; Xu, N.; Li, Y.; Lu, J. Biodegradation of different petroleum hydrocarbons by free and immobilized microbial consortia. Environ. Sci. Proc. Impacts 2015, 17, 2022–2033. [Google Scholar] [CrossRef]
- Bento, F.M.; Camargo, F.O.A.; Okeke, B.C.; Frankenberger, W.T. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresource Tech. 2005, 96, 1049–1055. [Google Scholar] [CrossRef]
- Patel, V.P.; Desai, S.A.; Thakur, S. Mechanistic approach of genetically modified organisms for detoxification of xe-nobiotic substances. In Microbiome-Assisted Bioremediation; Patel, V.P., Li, W.-J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 241–255. [Google Scholar]
- Schaefer, M.; Juliane, F. The influence of earthworms and organic additives on the biodegradation of oil contaminated soil. Appl. Soil Ecol. 2007, 36, 53–62. [Google Scholar] [CrossRef]
- Chachina, S.B.; Voronkova, N.A.; Baklanova, O.N. Biological remediation of the petroleum and diesel contaminated soil with earthworms Eisenia fetida. Procedia Eng. 2016, 152, 122–133. [Google Scholar] [CrossRef]
- Peter, O. Biological Remediation of Hydrocarbon and Heavy Metals Contaminated Soil. Chapter 7. In Soil Contamination; Pascucci, S., Ed.; InTech Open: London, UK, 2011; pp. 127–142. [Google Scholar]
- Farrell, R.E.; Germida, J.J. Phytotechnologies: Plant-based systems for remediation of oil impacted soils. In Proceedings of the Remediation Technologies Symposium, Banff, Canada, 16–18 October 2002. [Google Scholar]
- Sandrin, T.R.; Maier, R.M. Impact of metals on the biodegradation of organic pollutants. Environ. Health Perspect. 2003, 111, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, L.; Ge, S.; Liu, X.; Zhang, X.; Chen, X. Remediation potential of immobilized bacterial consortium with biochar as carrier in pyrene-Cr(VI) co-contaminated soil. Environ. Technol. 2019, 40, 2345–2353. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Li, S.; Huang, X.; Cheng, X.; Pan, W. Remediation of crude oil contaminated soil through an integrated biological-chemical-biological strategy. Sci. Total Environ. 2024, 919, 170756. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Gao, J.; Wang, F.; Hou, Y.; Cai, Q.; Liu, Q. Biochar assisted bioremediation of soils with combined contamination of petroleum hydrocarbons and heavy metals: A review. Appl. Soil Ecol. 2024, 204, 105720. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Adikesavan, S.; Aruliah, R.; Jayaraman, T.; Azhagesan, A.; Kuppusamy, S.; Jagannathan, M.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery from Various Environments—A Review. Front. Environ. Sci. 2019, 7, 1–15. [Google Scholar]
- Bhargavi, V.L.N.; Sudha, P.N. Removal of heavy metal ions from soil by electrokinetic assisted phytoremediation method. Int. J. Chem. Technol. Res. 2015, 8, 192–202. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



