Decoding Salinization Dynamics in Mediterranean Coastal Aquifers: A Case Study from a Wetland in Southern Italy
Abstract
1. Introduction
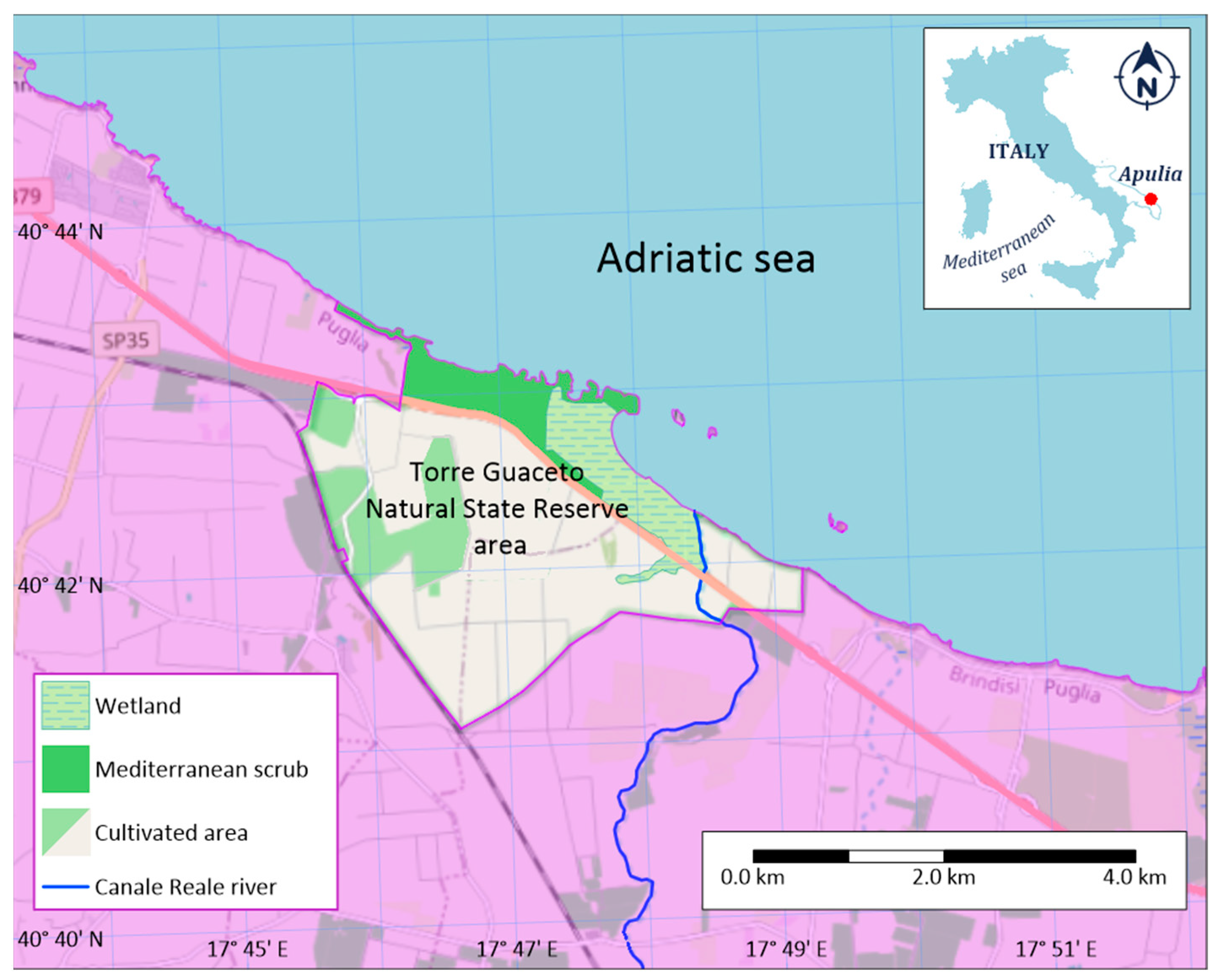
2. Study Area
2.1. Geographic Context
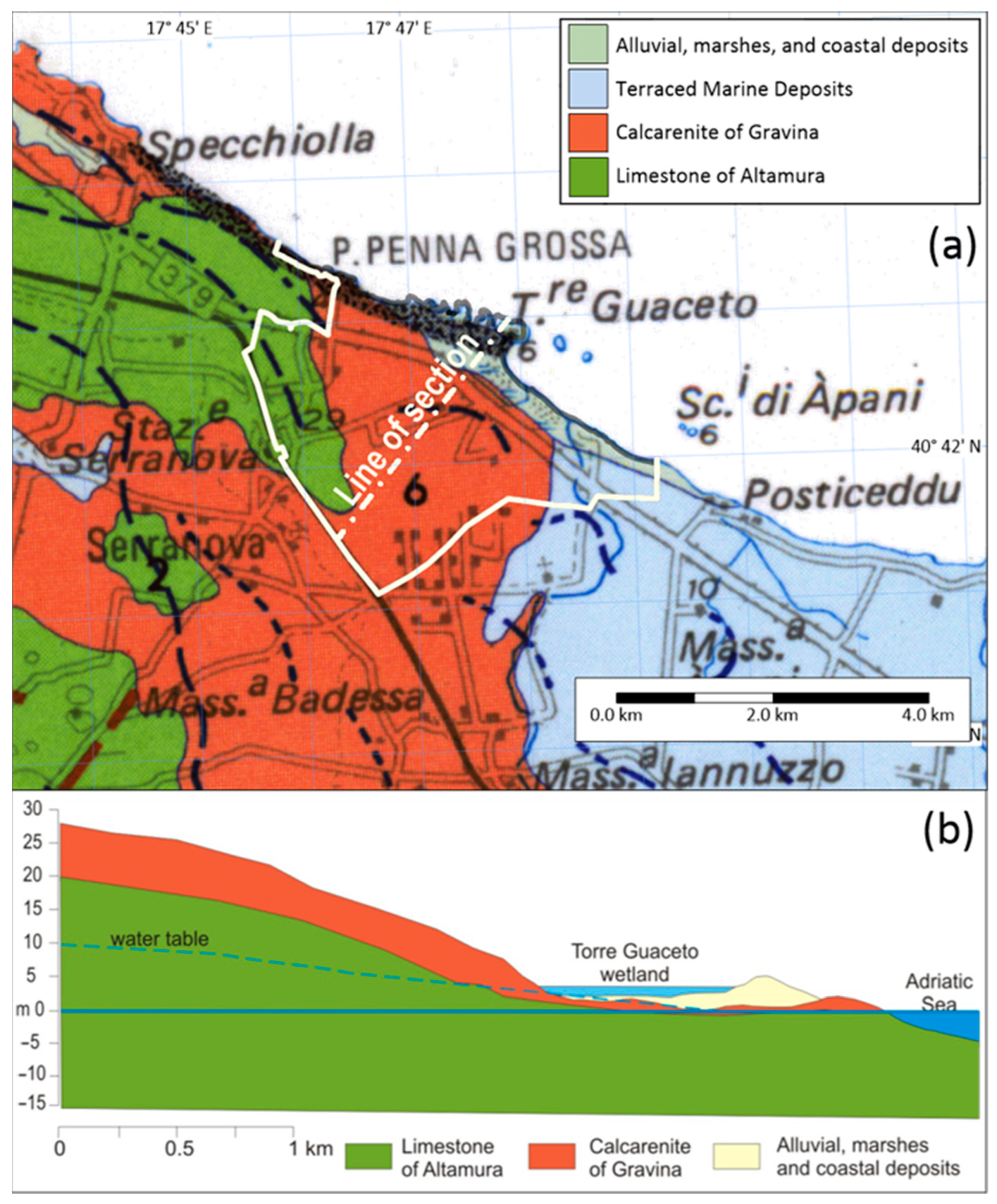
2.2. Geological and Hydrogeological Features
- Limestone of Altamura (Cretaceous), forming the carbonate basement, made up of calcareous and calcareous–dolomitic rocks, widely outcropping in the western part of the study area. Clayey sediments, known as terra rossa, are broadly interspersed into the carbonate formations;
- Calcarenite of Gravina (upper Pliocene—lower Pleistocene), consisting of calcarenite sediments having a variable cementation degree, outcropping in the central part of the study area, with a thickness not exceeding 20–30 m;
- Subapennine clays (lower Pleistocene) made of clay and sandy clay;
- Terraced marine deposits (middle-upper Pleistocene) characterized by considerable variations of facies but generally made up of yellow sands and a base level of marly clays, and outcropping in the eastern part of the study area with a thickness not exceeding 10–20 m;
- Alluvial, marshes, and coastal deposits (middle-upper Pleistocene—Holocene) with a small thickness and limited extension (Figure 2).
2.3. Ecological Significance
2.4. Socioeconomic Context
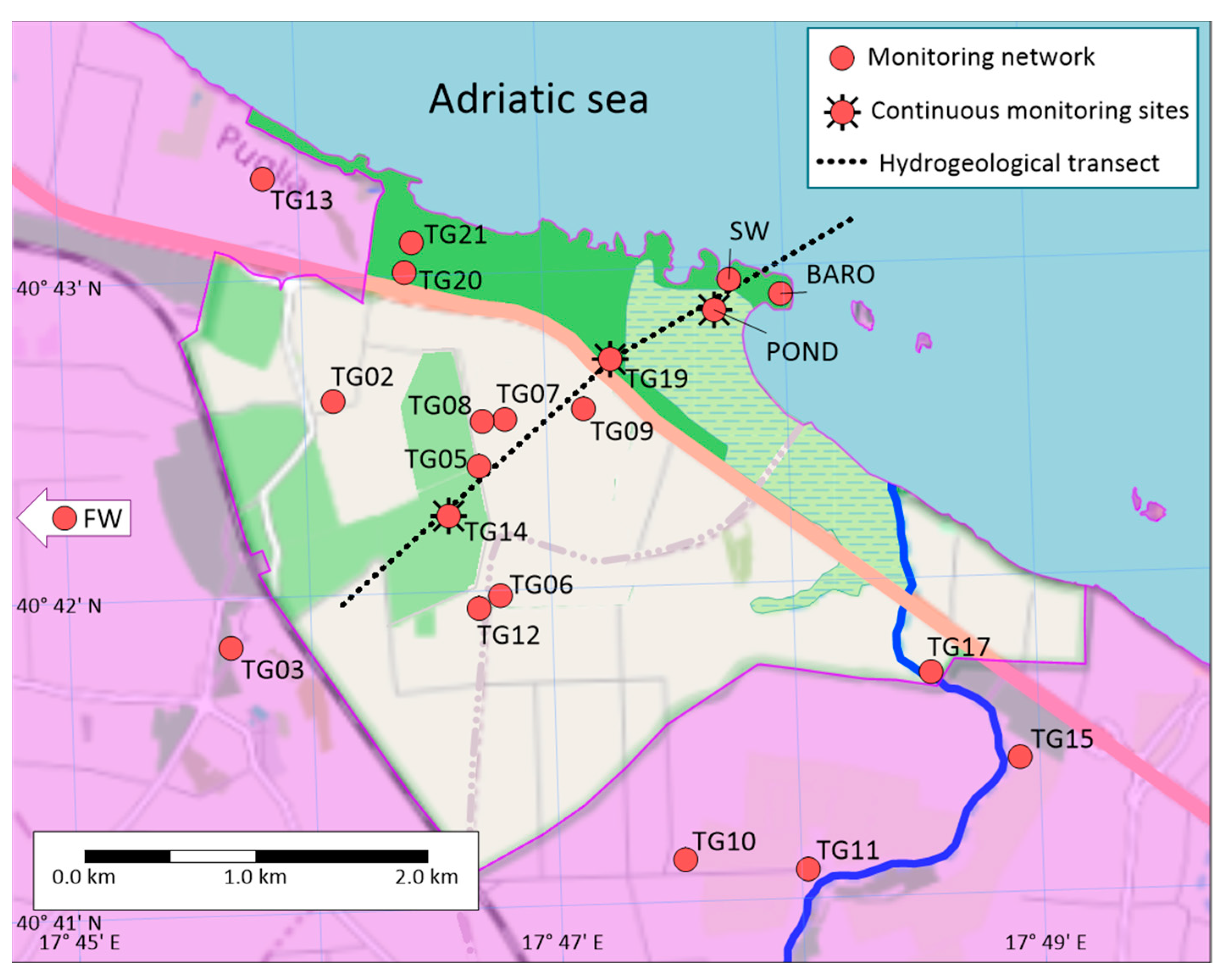
3. Materials and Methods
3.1. Monitoring Network and Data Collection
3.2. Geochemical and Isotopic Analyses
3.3. Integration of Meteorological Data
4. Results and Discussion
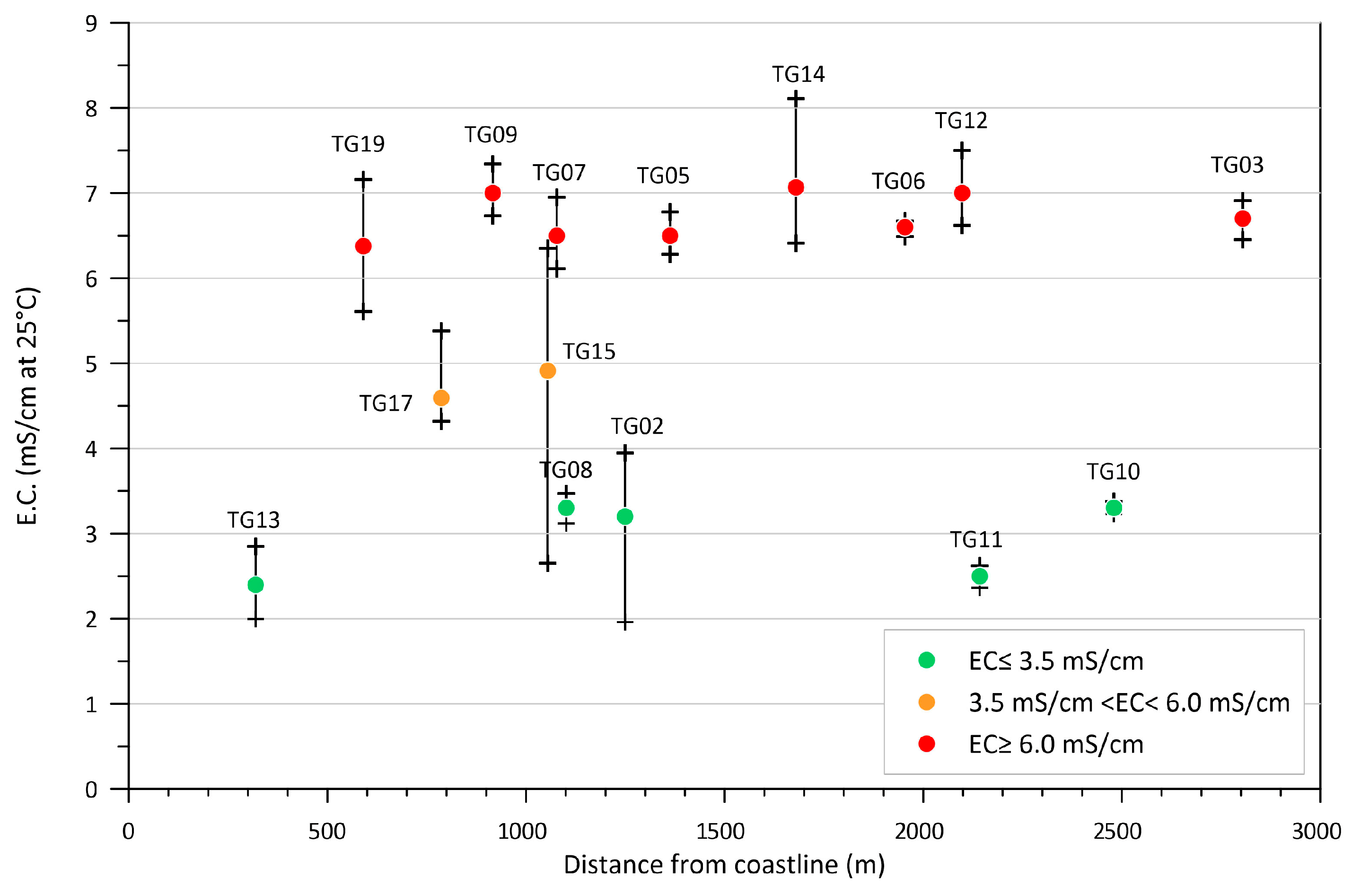
4.1. Hydrodynamic and Physicochemical Characterization
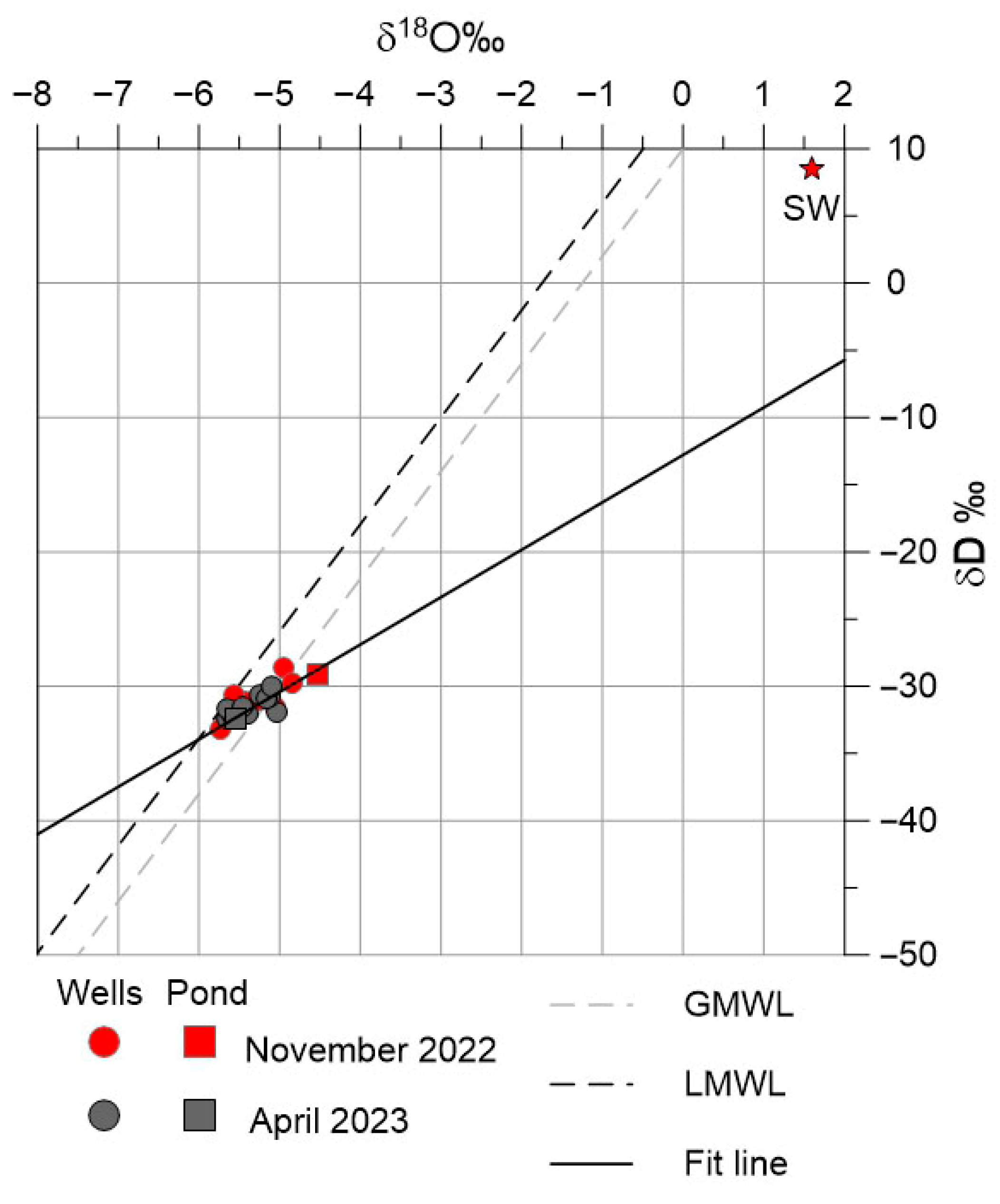
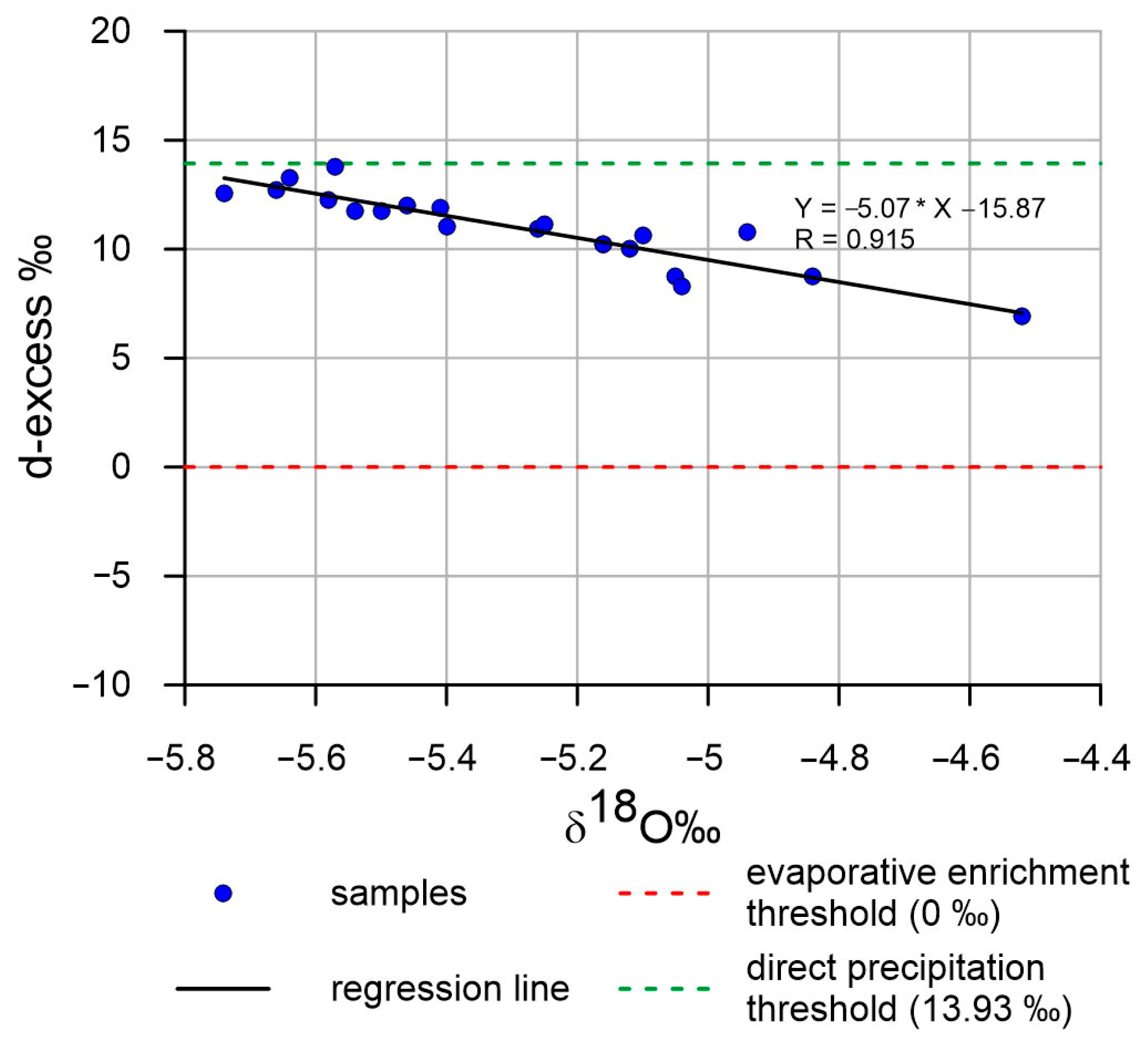
4.2. Geochemical and Isotopic Characterization
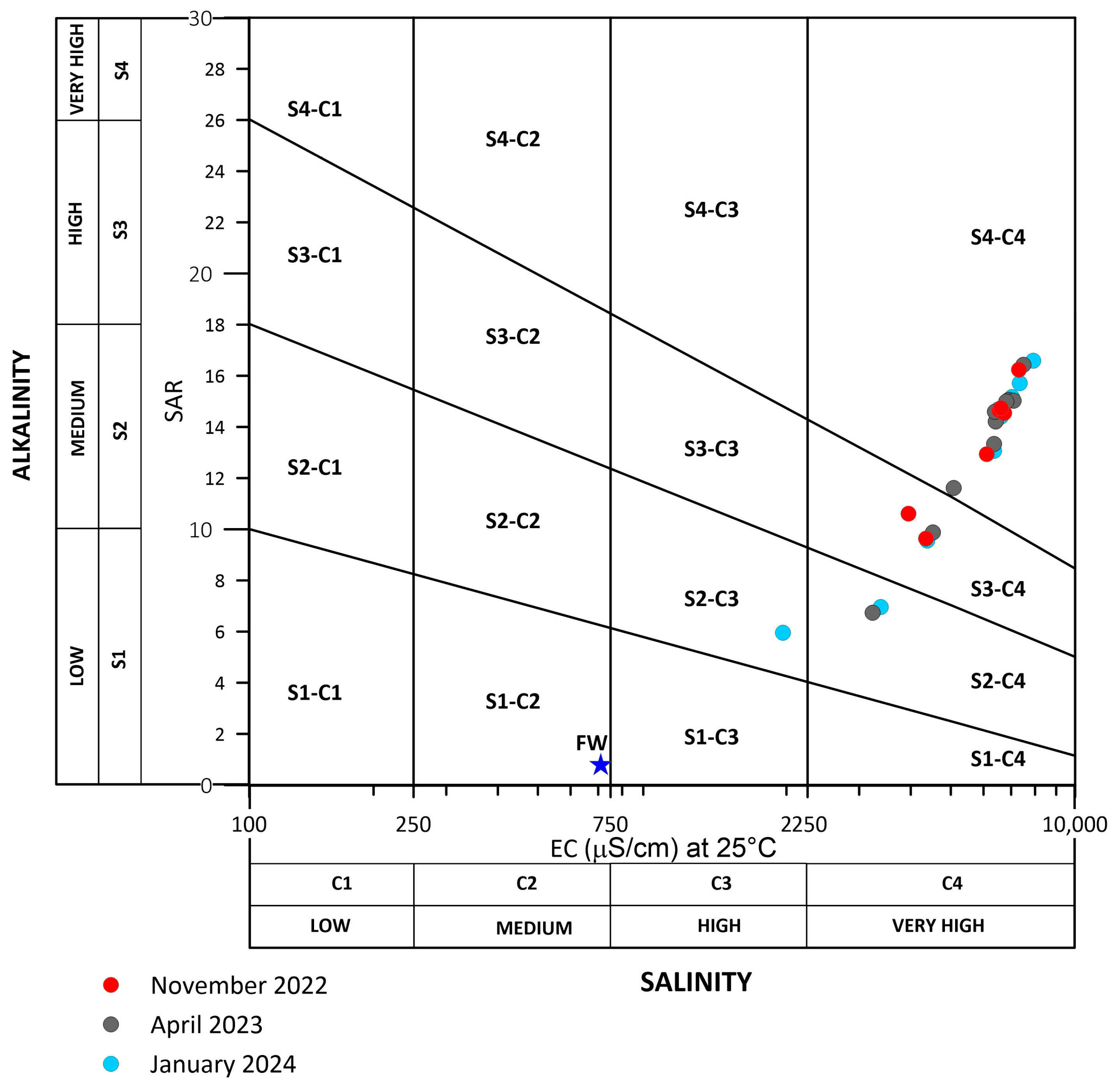
4.3. Water Quality for Irrigation
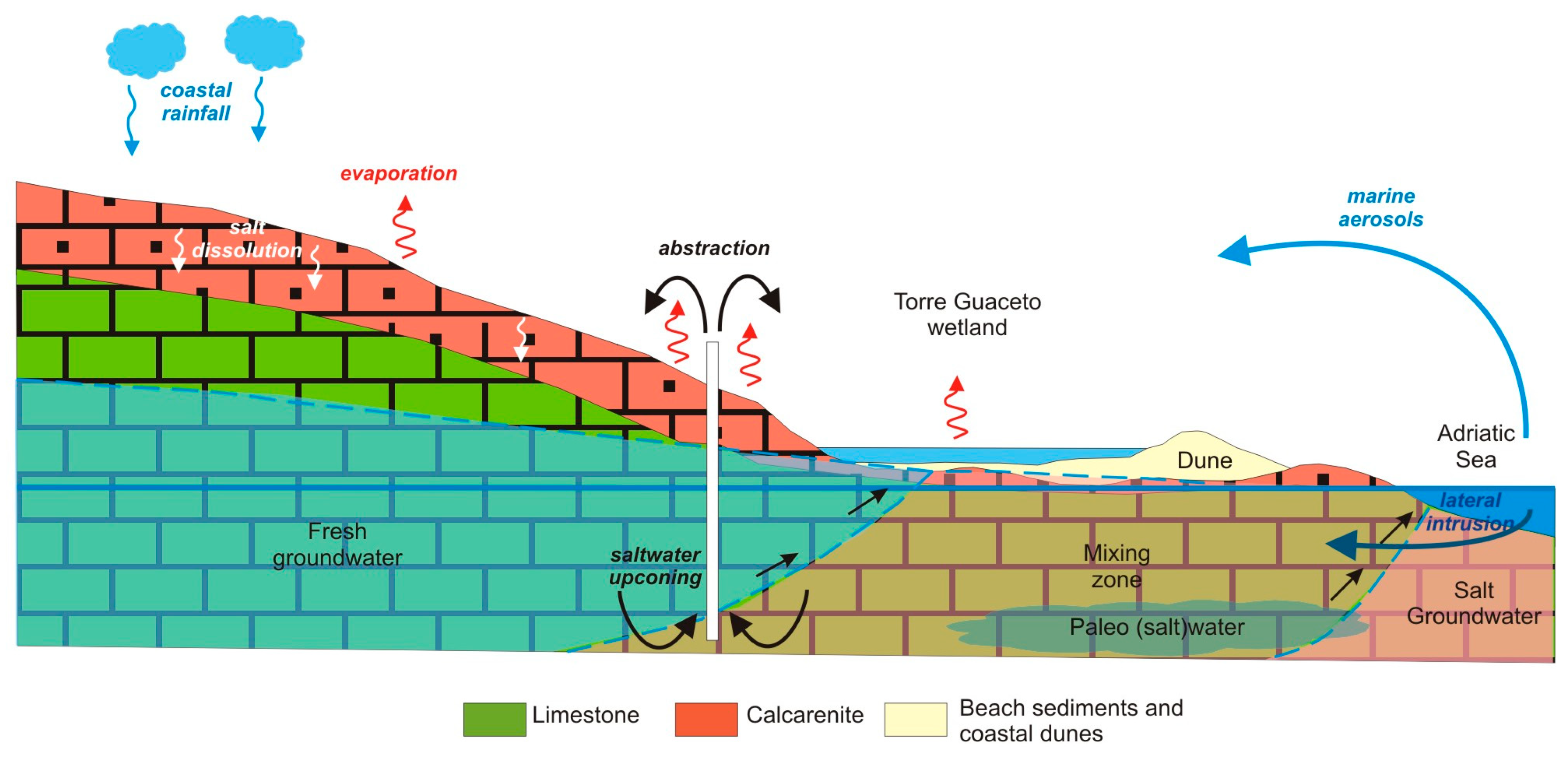
4.4. Conceptual Model, Discussion, and Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNR | National Research Council of Italy |
| IRSA | Water Research Institute (Istituto di Ricerca Sulle Acque) |
| IGG | Institute of Geosciences and Earth Resources (Istituto di Geoscienze e Georisorse) |
| EC | Electrical Conductivity |
| FW | Freshwater End-Member |
| SW | Seawater End-Member |
| ASL | Above Sea Level |
| BGL | Below Ground Level |
| GMWL | Global Meteoric Water Line |
| LMWL | Local Meteoric Water Line |
| SAR | Sodium Adsorption Ratio |
References
- Barbieri, M. Groundwater salinity: Origin, impact, and potential remedial measures and management solutions. Front. Water. 2023, 5, 1202576. [Google Scholar] [CrossRef]
- Pappa, D.; Kaliampakos, D. Unveiling the Non-Market Value of a Fragile Coastal Wetland: A CVM Approach in the Amvrakikos Gulf, Greece. Environments 2025, 12, 59. [Google Scholar] [CrossRef]
- Middleton, B.A.; Boudell, J. Salinification of coastal wetlands and freshwater management to support resilience. Ecosyst. Health Sustain. 2023, 9, 0083. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Sun, G.; King, J.S. Coastal wetland resilience to climate variability: A hydrologic perspective. J. Hydrol. 2019, 568, 275–284. [Google Scholar] [CrossRef]
- Agbasi, J.C.; Abu, M.; Pande, C.B.; Uwajingba, H.C.; Abba, S.I.; Egbueri, J.C. Groundwater Salinization in Coastal Regions and the Control Mechanisms: Insights for Sustainable Groundwater Development and Management. In Sustainable Groundwater and Environment: Challenges and Solutions; Li, P., He, X., Wu, J., Elumalai, V., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 165–191. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Colombani, N. The issue of groundwater salinization in coastal areas of the Mediterranean region: A review. Water 2021, 13, 90. [Google Scholar] [CrossRef]
- Huang, G.; Li, L. Groundwater Chemistry and Quality in Coastal Aquifers. Water 2024, 16, 2041. [Google Scholar] [CrossRef]
- Zaccaria, D.; Passarella, G.; D’Agostino, D.; Giordano, R.; Solis, S.S. Risk Assessment of Aquifer Salinization in a Large-Scale Coastal Irrigation Scheme, Italy. Clean-Soil Air Water 2016, 44, 371–382. [Google Scholar] [CrossRef]
- Passarella, G.; Bruno, D.; Lay-Ekuakille, A.; Maggi, S.; Masciale, R.; Zaccaria, D. Spatial and temporal classification of coastal regions using bioclimatic indices in a Mediterranean environment. Sci. Total Environ. 2020, 700, 134415. [Google Scholar] [CrossRef]
- Doglioni, A.; Simeone, V. Effects of climatic changes on groundwater availability in a semi-arid Mediterranean region. In IAEG/AEG Annual Meeting Proceedings, San Francisco, California, 2018; Shakoor, A., Cato, K., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 4, pp. 105–110. [Google Scholar] [CrossRef]
- Kreis, M.B.; Taupin, J.D.; Patris, N.; Lachassagne, P.; Vergnaud-Ayraud, V.; Burte, J.D.P.; Leduc, C.; Martins, E.S.P.R. Multidisciplinary approach to understand the salinization of fractured crystalline aquifers in semi-arid region. Proc. IAHS 2024, 385, 393–398. [Google Scholar] [CrossRef]
- Martínez-Santos, P.; Sánchez-Alcón, A. Assessment of groundwater–surface water interactions in a Mediterranean wetland using hydrological and isotopic tools. Water 2022, 14, 345. [Google Scholar] [CrossRef]
- Passarella, G.; Barca, E.; Sollitto, D.; Masciale, R.; Bruno, D.E. Cross-calibration of two independent groundwater balance models and evaluation of unknown terms: The case of the shallow aquifer of “Tavoliere di Puglia” (South Italy). Water Resour. Manag. 2017, 31, 327–340. [Google Scholar] [CrossRef]
- Frollini, E.; Parrone, D.; Ghergo, S.; Masciale, R.; Passarella, G.; Pennisi, M.; Preziosi, E. An integrated approach for investigating the salinity evolution in a Mediterranean coastal karst aquifer. Water 2022, 14, 1725. [Google Scholar] [CrossRef]
- Polemio, M. Monitoring and management of karstic coastal aquifers in Apulia (southern Italy). Water 2016, 8, 148. [Google Scholar] [CrossRef]
- Portoghese, I.; Brigida, S.; Masciale, R.; Passarella, G. Assessing transmission losses through ephemeral streams: A methodological approach based on the infiltration of treated effluents released into streams. Water 2022, 14, 3758. [Google Scholar] [CrossRef]
- Tozzi, M. Assetto tettonico dell’avampaese apulo meridionale (Murge meridionali—Salento) sulla base di dati strutturali. Geol. Romana 1993, 29, 95–111. [Google Scholar]
- Ciaranfi, N.; Pieri, P.; Ricchetti, G. Note alla carta geologica delle Murge e del Salento (Puglia centromeridionale). Mem. Soc. Geol. Ital. 1988, 41, 449–460. [Google Scholar]
- Fidelibus, M.D.; Tulipano, L.; D’Amelio, P. Convective thermal field reconstruction by ordinary kriging in karstic aquifers (Puglia, Italy): Geostatistical analysis of anisotropy. In Advances in Research in Karst Media; Carrasco, F., Valsero, J.J.D., LaMoreaux, J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 203–208. Available online: https://www.researchgate.net/publication/227131699_Convective_Thermal_Field_Reconstruction_by_Ordinary_Kriging_in_Karstic_Aquifers_Puglia_Italy_Geostatistical_Analysis_of_Anisotropy (accessed on 20 June 2025).
- Maggiore, M.; Pagliarulo, P. Circolazione idrica ed equilibri idrogeologici negli acquiferi della Puglia. Geol. Territ. 2004, 1, 13–35. [Google Scholar]
- Cotecchia, V.; Grassi, D.; Polemio, M. Carbonate aquifers in Apulia and seawater intrusion. G. Geol. Appl. 2005, 1, 219–231. [Google Scholar]
- Basset, A.; Sangiorgio, F.; Pinna, M. Monitoring with benthic macroinvertebrates: Advantages and disadvantages of body size descriptors. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, S43–S58. [Google Scholar] [CrossRef]
- Lapietra, I.; Lisco, S.; Mastronuzzi, G.; Milli, S.; Pierri, C.; Sabatier, F.; Scardino, G.; Moretti, M. Morpho-sedimentary dynamics of Torre Guaceto beach (Southern Adriatic Sea, Italy). J. Earth Syst. Sci. 2022, 131, 64. [Google Scholar] [CrossRef]
- Milnes, E.; Renard, P. The problem of salt recycling and seawater intrusion in coastal irrigated plains: An example from the Kiti aquifer (Southern Cyprus). J. Hydrol. 2004, 288, 327–343. [Google Scholar] [CrossRef]
- Manca, F.; Capelli, G.; Tuccimei, P. Sea salt aerosol groundwater salinization in the Litorale Romano Natural Reserve (Rome, Central Italy). Environ. Earth Sci. 2015, 73, 4179–4190. [Google Scholar] [CrossRef]
- Chebotarev, I.I. Metamorphism of natural waters in the crust of weathering. Geochim. Cosmochim. Acta 1955, 8, 22–48. [Google Scholar] [CrossRef]
- Fidelibus, M.D.; Balacco, G.; Alfio, M.R.; Arfaoui, M.; Bassukas, D.; Güler, C.; Tziritis, E. A chloride threshold to identify the onset of seawater/saltwater intrusion and a novel categorization of groundwater in coastal aquifers. J. Hydrol. 2025, 653, 132775. [Google Scholar] [CrossRef]
- Salvadori, M.; Pennisi, M.; Masciale, R.; Fidelibus, M.D.; Frollini, E.; Ghergo, S.; Parrone, D.; Preziosi, E.; Passarella, G. Isotopic study for evaluating complex groundwater circulation patterns, hydrogeological zoning, and water-rock interaction in a Mediterranean coastal karst environment. Sci. Total Environ. 2024, 955, 176850. [Google Scholar] [CrossRef] [PubMed]
- Giménez, E.; Morell, I. Hydrogeochemical analysis of salinization processes in the coastal aquifer of Oropesa (Castellón, Spain). Environ. Geol. 1997, 29, 118–131. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pisciotta, A.; De Maio, M. Evaluation of groundwater salinization and pollution level on Favignana Island, Italy. Environ. Pollut. 2019, 249, 969–981. [Google Scholar] [CrossRef]
- Abu-Alnaeem, M.F.; Yusoff, I.; Ng, T.F.; Alias, Y.; Raksmey, M. Assessment of groundwater salinity and quality in Gaza coastal aquifer, Gaza Strip, Palestine: An integrated statistical, geostatistical and hydrogeochemical approaches study. Sci. Total Environ. 2018, 615, 972–989. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Casellas, E.; Latron, J.; Cayuela, C.; Bech, J.; Udina, M.; Sola, Y.; Llorens, P. Moisture origin and characteristics of the isotopic signature of rainfall in a Mediterranean mountain catchment (Vallcebre, eastern Pyrenees). J. Hydrol. 2019, 575, 767–779. [Google Scholar] [CrossRef]
- Kendall, C.; McDonnell, J.J. (Eds.) Isotope Tracers in Catchment Hydrology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Liu, F.; Hu, X.; Zhen, P.; Zou, J.; Zhang, J. Characterizing groundwater recharge sources within reduced pumped aquifers in the Heilonggang region, North China Plain. J. Geochem. Explor. 2023, 247, 107176. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Benettin, P.; Volkmann, T.H.M.; von Freyberg, J.; Frentress, J.; Penna, D.; Dawson, T.E.; Kirchner, J.W. Effects of climatic seasonality on the isotopic composition of evaporating soil waters. Hydrol. Earth Syst. Sci. 2018, 22, 2881–2890. [Google Scholar] [CrossRef]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil salinization in agriculture: Mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955; Circular No. 969; 19p.
- Pérez-Martín, M.Á.; Pulido-Velazquez, M. Comparative analysis of runoff and evaporation assessment methods to evaluate wetland–groundwater interaction in Mediterranean evaporitic-karst aquatic ecosystem. Water 2021, 13, 1482. [Google Scholar] [CrossRef]
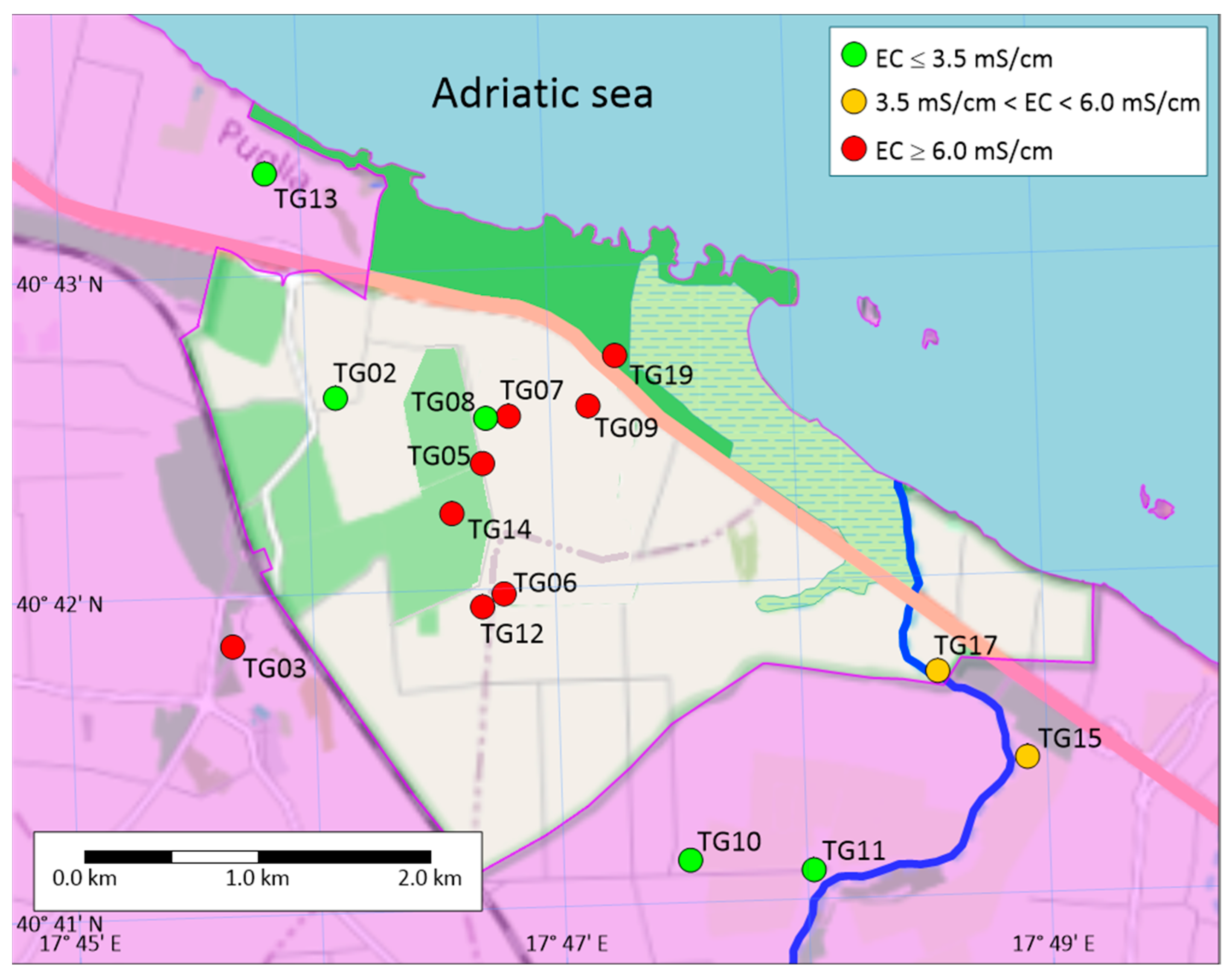


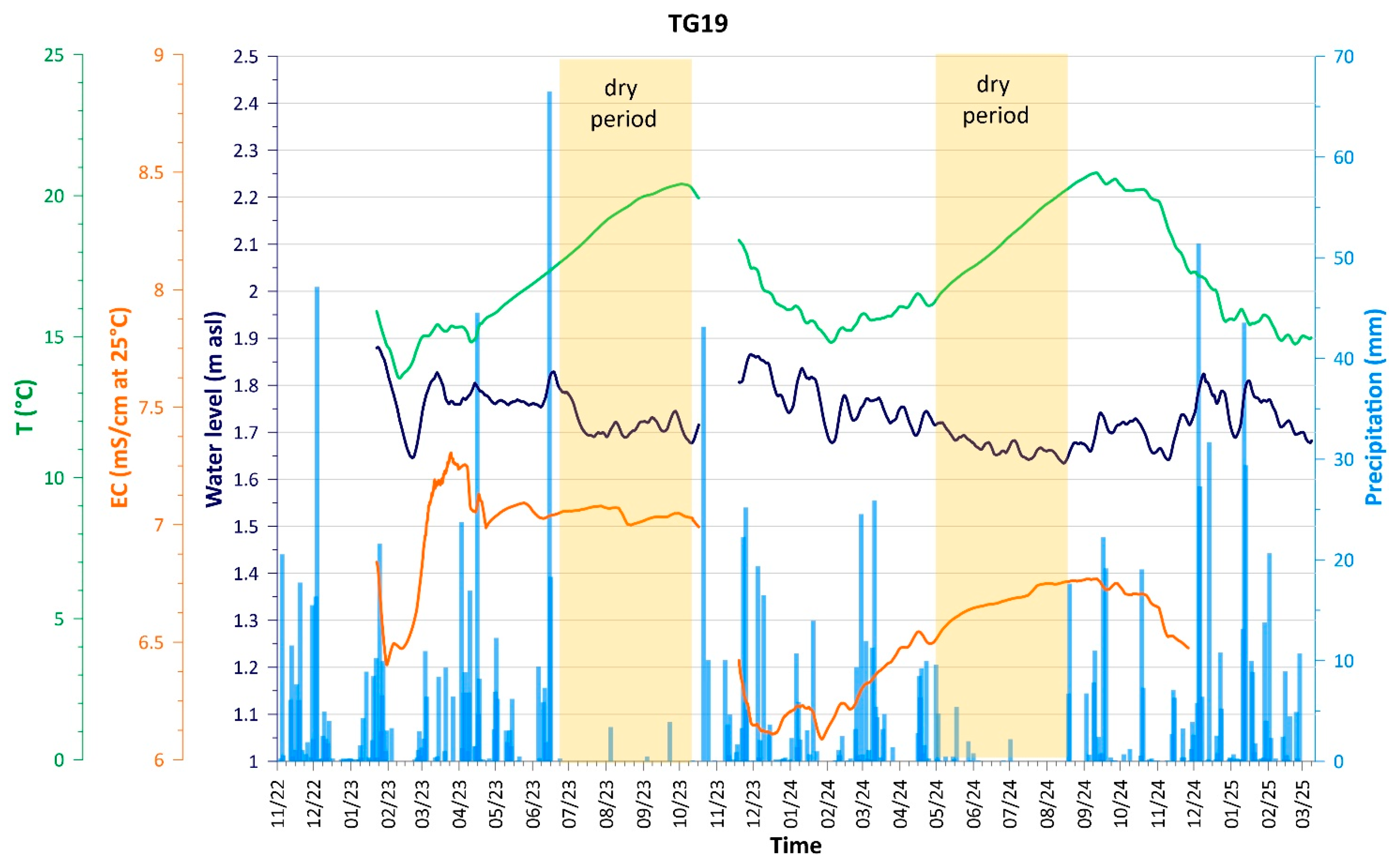
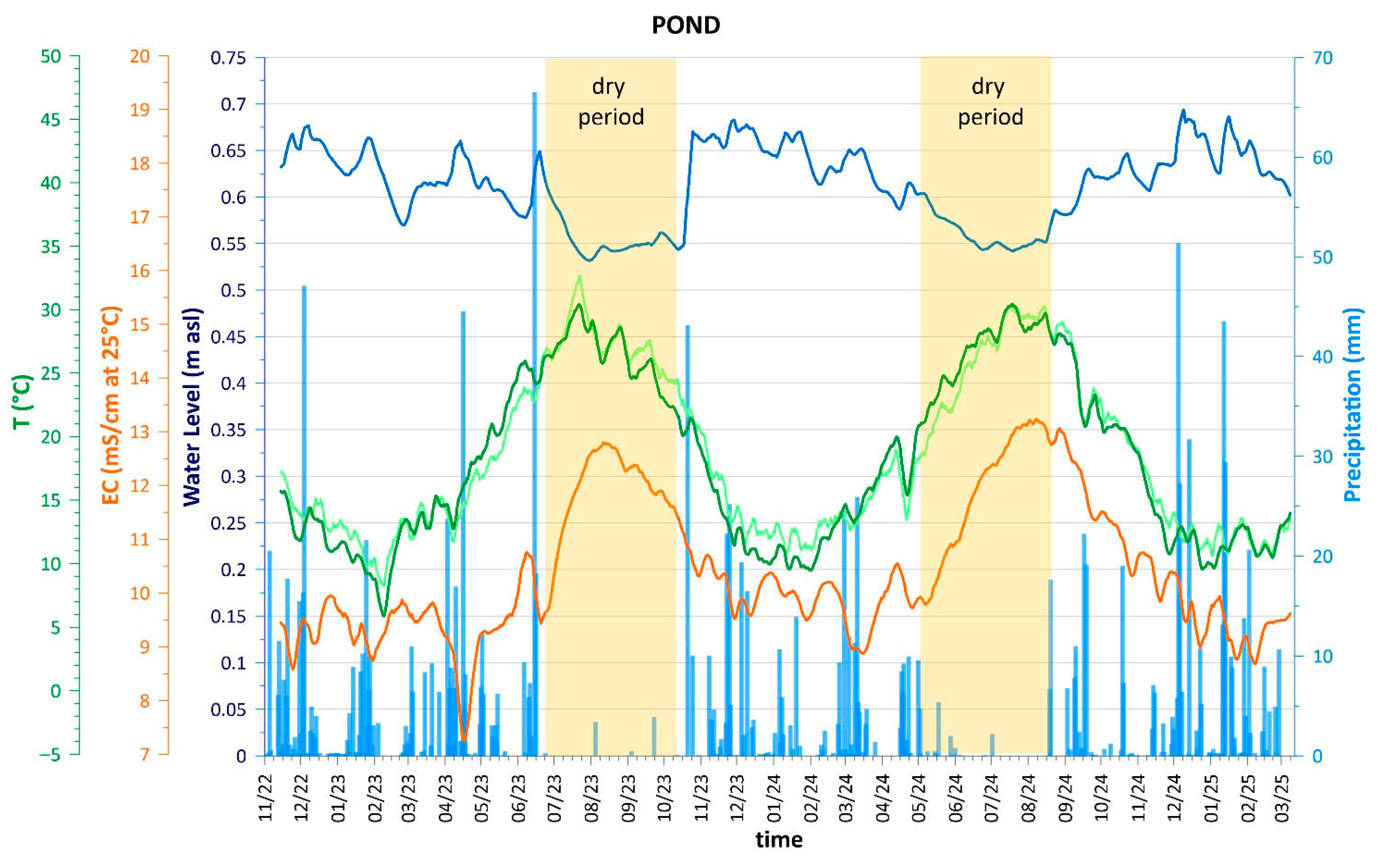

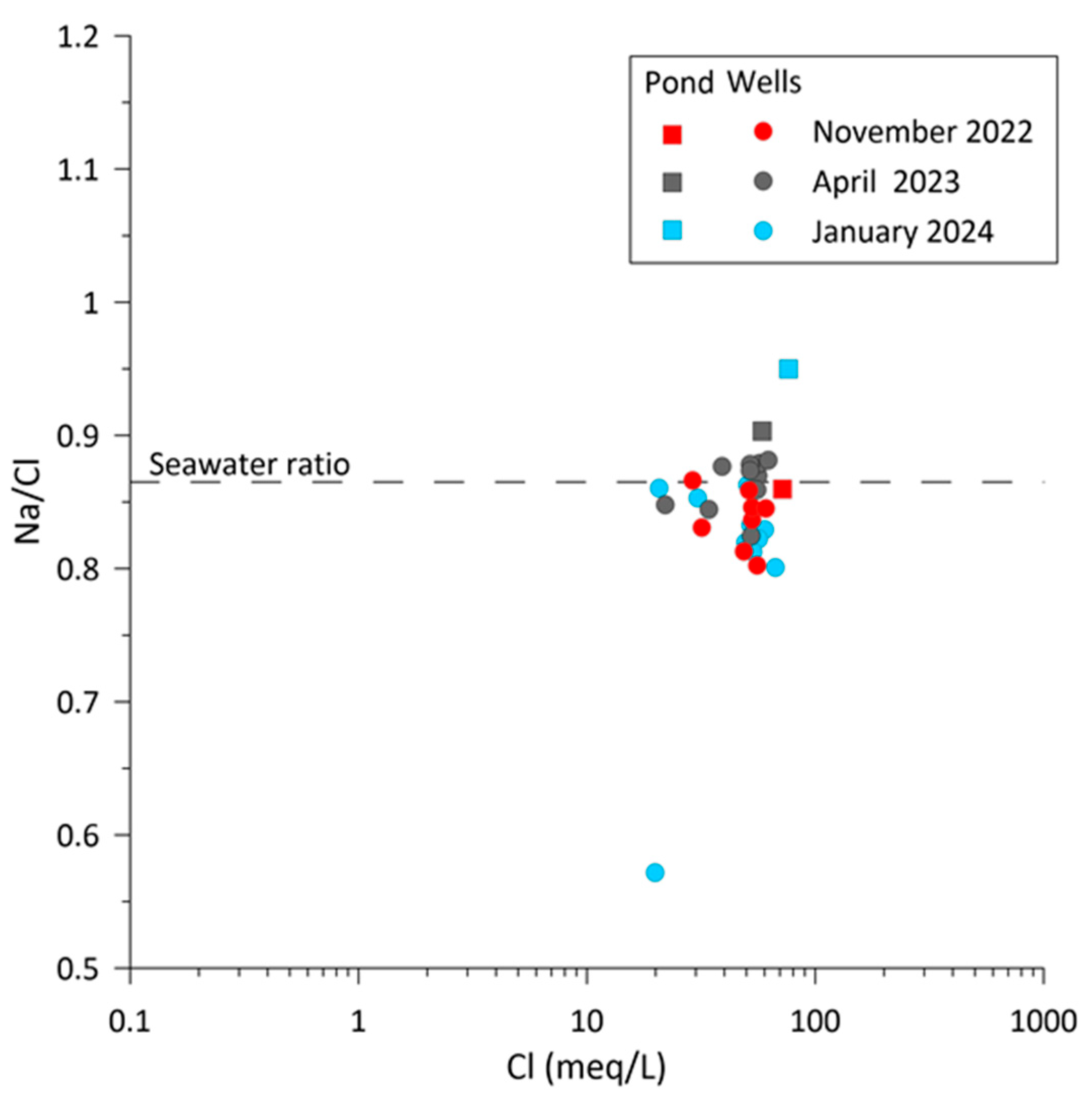
| Name | Type of Well | Sampling | Coordinate X | Coordinate Y | Ground Elevation | Distance from Coastline | Reference Point Elevation | Water Table | Well Depth * |
|---|---|---|---|---|---|---|---|---|---|
| DD | DD | m ASL | m | m ASL | m ASL | m BGL | |||
| TG02 | Drilled | Pump | 17.76868 | 40.71023 | 15.0 | 1249.11 | - | - | 20–25 |
| TG03 | Drilled | Pump | 17.76114 | 40.69743 | 45.0 | 2803.94 | - | - | 20–25 |
| TG05 | Drilled | Pump | 17.77870 | 40.70656 | 12.0 | 1362.88 | 12.54 | 1.97 | 20–25 |
| TG06 | Drilled | Pump | 17.77981 | 40.69978 | 19.0 | 1954.23 | - | - | 20–25 |
| TG07 | Drilled | Pump | 17.78053 | 40.70902 | 11.0 | 1077.18 | - | - | 20–25 |
| TG08 | Dug | Bailer | 17.77895 | 40.70864 | 11.0 | 1101.18 | 11.00 | 0.99 | 20–25 |
| TG09 | Drilled | Pump | 17.78948 | 40.70561 | 8.0 | 916.69 | - | - | 20–25 |
| TG10 | Drilled | Pump | 17.79202 | 40.68557 | 21.0 | 2479.80 | - | - | 20–25 |
| TG11 | Drilled | Pump | 17.80045 | 40.68493 | 20.0 | 2142.64 | - | - | 20–25 |
| TG12 | Drilled | Pump | 17.77833 | 40.69913 | 19.0 | 2097.45 | - | - | 20–25 |
| TG13 | Dug | Bailer | 17.76430 | 40.72195 | 13.0 | 319.52 | 13.48 | 3.17 | 20–25 |
| TG14 | Drilled | Pump | 17.77399 | 40.70342 | 15.0 | 1679.38 | 15.70 | 2.67 | 25.0 |
| TG15 | Drilled | Pump | 17.81530 | 40.69040 | 10.0 | 1054.38 | - | - | 20–25 |
| TG17 | Drilled | Pump | 17.80936 | 40.69502 | 5.0 | 786.46 | - | - | 20–25 |
| TG19 | Dug | Bailer | 17.78787 | 40.71197 | 3.0 | 590.20 | 3.35 | 1.75 | 4.0 |
| Pond | Pond | Bailer | 17.79516 | 40.71439 | 1.2 | 180.00 | - | 0.61 | - |
| FW | Drilled | Pump | 17.65795 | 40.69329 | 135.0 | 8286.00 | - | - | - |
| SW | Sea | Bailer | 17.79625 | 40.71597 | 0 | 0.00 | - | - | - |
| BARO | Air | - | 17.79978 | 40.71514 | - | - | - | - | - |
| Campaign | Period | Type of Monitoring | |
|---|---|---|---|
| On-Site | Sampling | ||
| I | April 2022 | x | |
| II | June–July 2022 | x | |
| III | October–November 2022 | x | x |
| IV | January 2023 | x | |
| V | April 2023 | x | x |
| VI | June 2023 | x | |
| VII | October–November 2023 | x | |
| VIII | January 2024 | x | x |
| IX | July 2024 | x | |
| Monitoring Site | pH | T (°C) | EC (mS/cm at 25 °C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | min | max | n | Mean | min | max | n | Mean | min | max | |
| TG02 * | 2 | 7.2 | 7.1 | 7.2 | 5 | 18.9 | 18.5 | 19.2 | 5 | 3.2 (3.5) | 2.0 | 4.0 |
| TG03 * | 3 | 7.1 | 7.1 | 7.2 | 5 | 18.9 | 18.4 | 19.8 | 5 | 6.7 (6.8) | 6.5 | 6.9 |
| TG05 | - | - | - | - | 3 | 19.7 | 19.3 | 20.0 | 3 | 6.5 (7.0) | 6.3 | 6.8 |
| TG06 | - | - | - | - | 2 | 19.1 | 19.0 | 19.1 | 2 | 6.6 (7.0) | 6.5 | 6.7 |
| TG07 * | 3 | 7.1 | 7.0 | 7.2 | 5 | 18.9 | 18.0 | 19.4 | 5 | 6.5 (6.7) | 6.1 | 7.0 |
| TG08 | - | - | - | - | 2 | 17.5 | 17.3 | 17.6 | 2 | 3.3 (3.5) | 3.1 | 3.5 |
| TG09 * | 3 | 7.1 | 7.0 | 7.1 | 4 | 19.1 | 17.9 | 20.0 | 4 | 7.0 (6.4) | 6.7 | 7.3 |
| TG10 * | 2 | 7.1 | 7.0 | 7.2 | 4 | 16.8 | 10.7 | 19.2 | 4 | 3.3 (3.6) | 3.2 | 3.4 |
| TG11 | - | - | - | - | 2 | 20.7 | 20.2 | 21.1 | 2 | 2.5 (2.4) | 2.4 | 2.6 |
| TG12 * | 3 | 7.0 | 7.0 | 7.1 | 5 | 19.0 | 18.7 | 19.5 | 5 | 7.0 (7.3) | 6.6 | 7.5 |
| TG13 | - | - | - | - | 3 | 18.3 | 17.6 | 18.7 | 3 | 2.4 (3.1) | 2.0 | 2.9 |
| TG14 * | 3 | 7.2 | 7.1 | 7.4 | 4 | 18.7 | 17.4 | 20.1 | 4 | 7.1 | 6.4 | 8.1 |
| TG15 | - | - | - | - | 3 | 18.7 | 18.4 | 19.0 | 3 | 4.9 | 2.7 | 6.4 |
| TG17 * | 3 | 7.1 | 7.0 | 7.3 | 8 | 18.3 | 14.0 | 20.1 | 8 | 4.6 | 4.3 | 5.4 |
| TG19 * | 3 | 7.1 | 6.9 | 7.3 | 5 | 17.7 | 14.4 | 23.3 | 5 | 6.4 | 5.6 | 7.2 |
| Groundwater | - | 7.1 | 6.9 | 7.4 | - | 18.7 | 10.7 | 23.3 | - | 5.2 | 2.0 | 8.1 |
| Pond * | 3 | 7.5 | 7.4 | 7.5 | 3 | 14.9 | 9.7 | 18.6 | 3 | 8.5 | 7.1 | 9.7 |
| FW * | 1 | 6.9 | - | - | 1 | 16.0 | - | - | 1 | 0.7 | - | - |
| SW * | 1 | 8.1 | - | - | 1 | 18.5 | - | - | 1 | 52.6 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passarella, G.; Masciale, R.; Menichini, M.; Doveri, M.; Portoghese, I. Decoding Salinization Dynamics in Mediterranean Coastal Aquifers: A Case Study from a Wetland in Southern Italy. Environments 2025, 12, 227. https://doi.org/10.3390/environments12070227
Passarella G, Masciale R, Menichini M, Doveri M, Portoghese I. Decoding Salinization Dynamics in Mediterranean Coastal Aquifers: A Case Study from a Wetland in Southern Italy. Environments. 2025; 12(7):227. https://doi.org/10.3390/environments12070227
Chicago/Turabian StylePassarella, Giuseppe, Rita Masciale, Matia Menichini, Marco Doveri, and Ivan Portoghese. 2025. "Decoding Salinization Dynamics in Mediterranean Coastal Aquifers: A Case Study from a Wetland in Southern Italy" Environments 12, no. 7: 227. https://doi.org/10.3390/environments12070227
APA StylePassarella, G., Masciale, R., Menichini, M., Doveri, M., & Portoghese, I. (2025). Decoding Salinization Dynamics in Mediterranean Coastal Aquifers: A Case Study from a Wetland in Southern Italy. Environments, 12(7), 227. https://doi.org/10.3390/environments12070227









