Laminaria digitata Supplementation as a Climate-Smart Strategy to Counteract the Interactive Effects of Marine Heatwaves and Disease Outbreaks in Farmed Gilthead Seabream (Sparus aurata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Seaweed Extract and Experimental Diets
2.2. Modulation of Marine Heatwaves
2.3. Vibrio harveyi Bacterial Culture
2.4. Sparus aurata and Acclimation Period
2.5. Experimental Design and Fish-Rearing Conditions
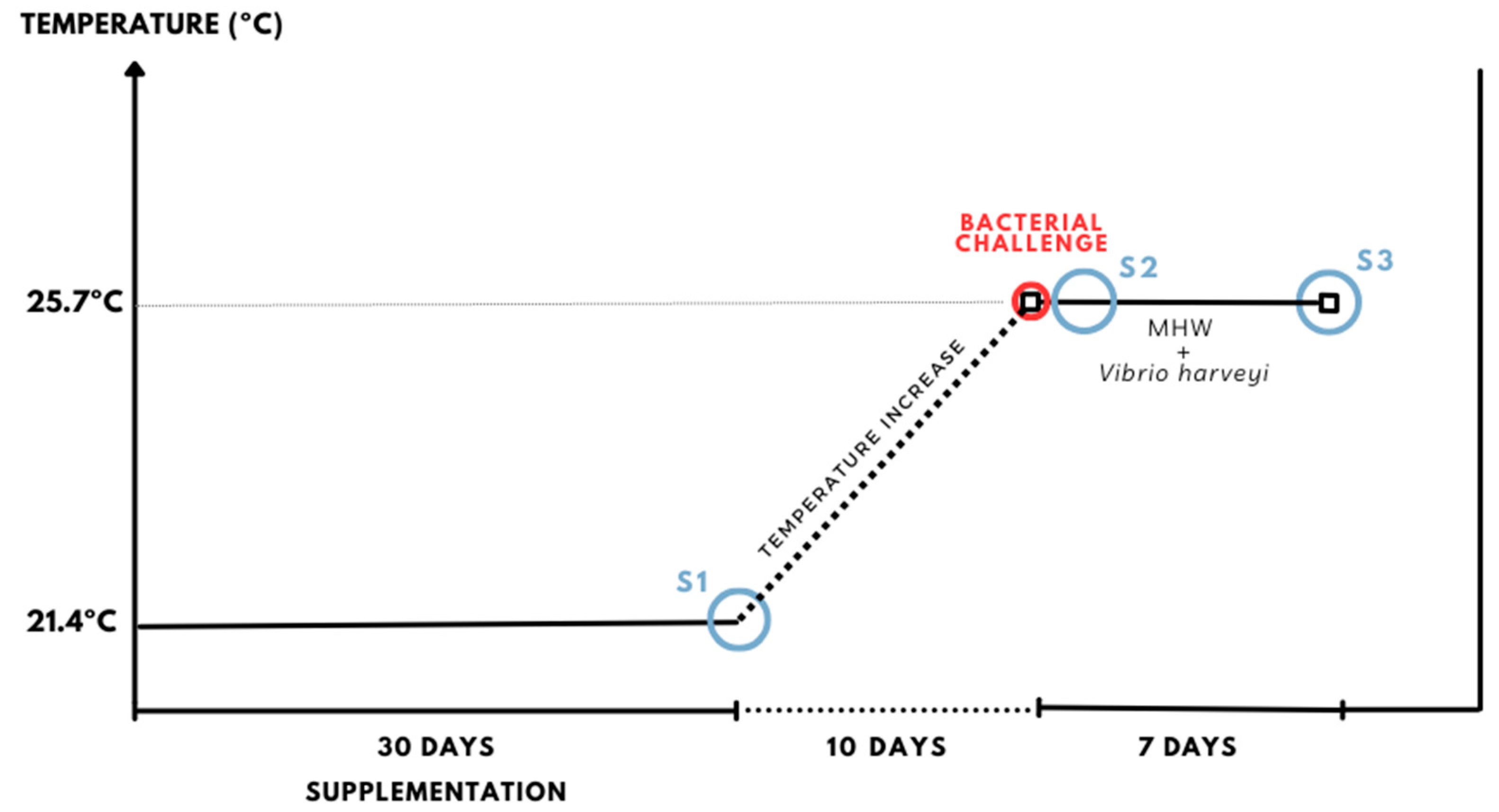
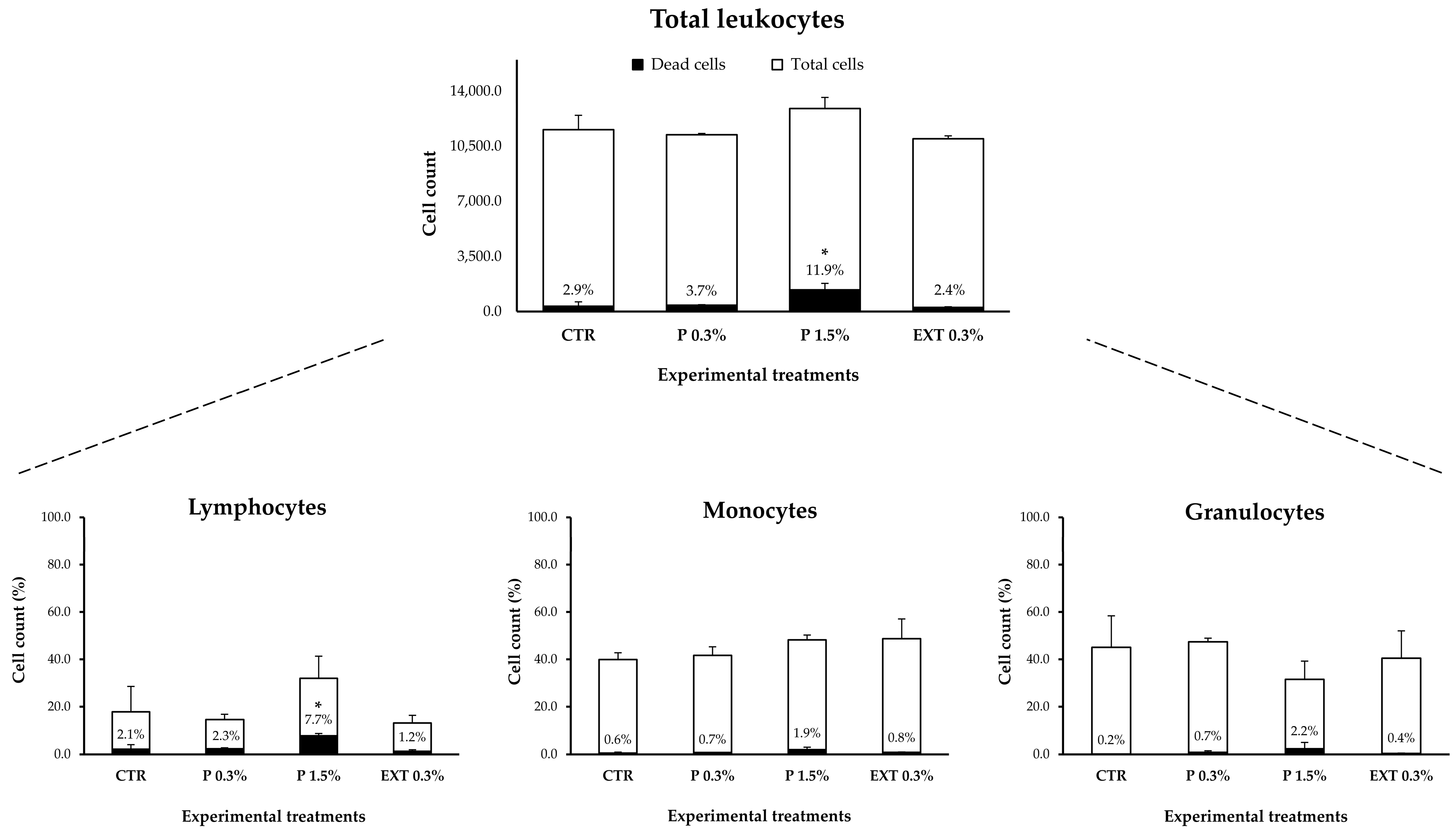
- S1 (prophylactic supplementation phase): For 30 days, fish were hand-fed twice daily (2% bw) with experimental diets under optimal temperature conditions (21.4 °C; Figure 1). Each treatment consisted of 27 fish divided into three replicates (9 fish/tank; Appendix A, Figure A1). The dietary treatments were as follows: (i) CTR (control): non-supplemented commercial diet for S. aurata juveniles; (ii) P 0.3%: diet supplemented with 0.3% L. digitata powder; (iii) P 1.5%: diet supplemented with 1.5% L. digitata powder; and iv) EXT 0.3%: diet supplemented with 0.3% L. digitata extract. After the 30-day supplementation period, 6 fish per treatment (2 fish/tank) were sampled (indicated by the blue tanks and “scissors” symbol in Appendix A, Figure A1). An equal number of fish were removed from the other treatments to ensure population density consistency.
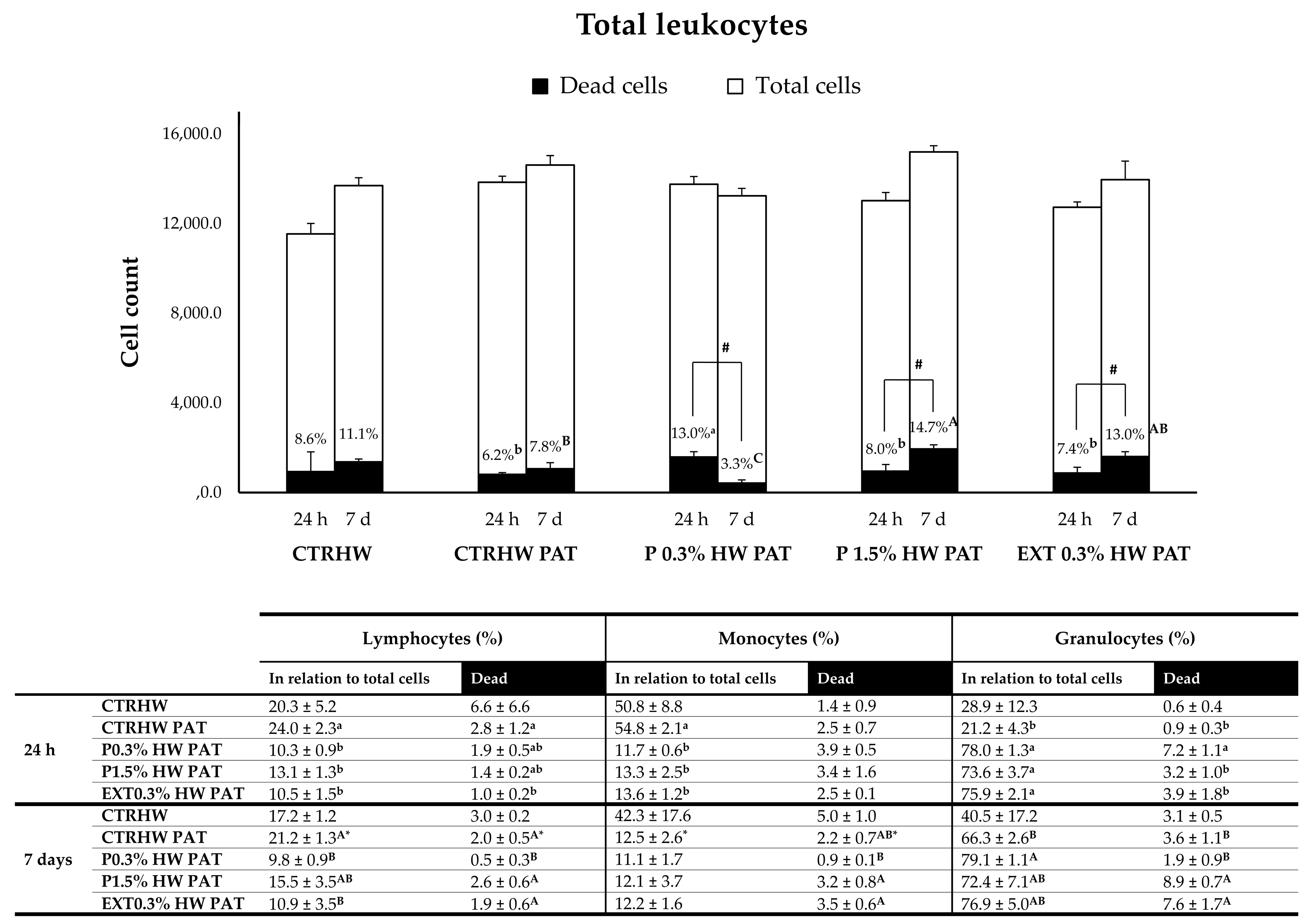
- S2 (temperature-increase ramp and bacterial challenge): A gradual MHW ramp was initiated over 10 days (0.5 °C/day; Figure 1), except for the CTR group, which was kept at optimal conditions throughout the experiment. The feeding regime and diets remained the same as in S1. At this stage, each treatment consisted of 21 fish (7 fish/tank; Appendix A, Figure A1). Once the temperature reached 25.7 °C, the treatments CTRHW PAT, P 0.3% HW PAT, P 1.5% HW PAT, and EXT 0.3% HW PAT were challenged with V. harveyi (Figure 1). Anesthetized fish were intraperitoneally injected with 100 µL of a bacterial suspension (4.9 × 108 CFU mL−1). The other groups (CTR, CTRHW, P 0.3% HW, P 1.5% HW, and EXT 0.3% HW) were injected with 100 µL of sterile PBS. At 24 h post-challenge, 6 fish per treatment (2 fish/tank) from the pathogen-challenged groups and the CTRHW group were sampled (represented by the blue tanks and “scissors” symbol in Appendix A, Figure A1). An equal number of fish were removed from the other treatments to maintain a consistent population density.
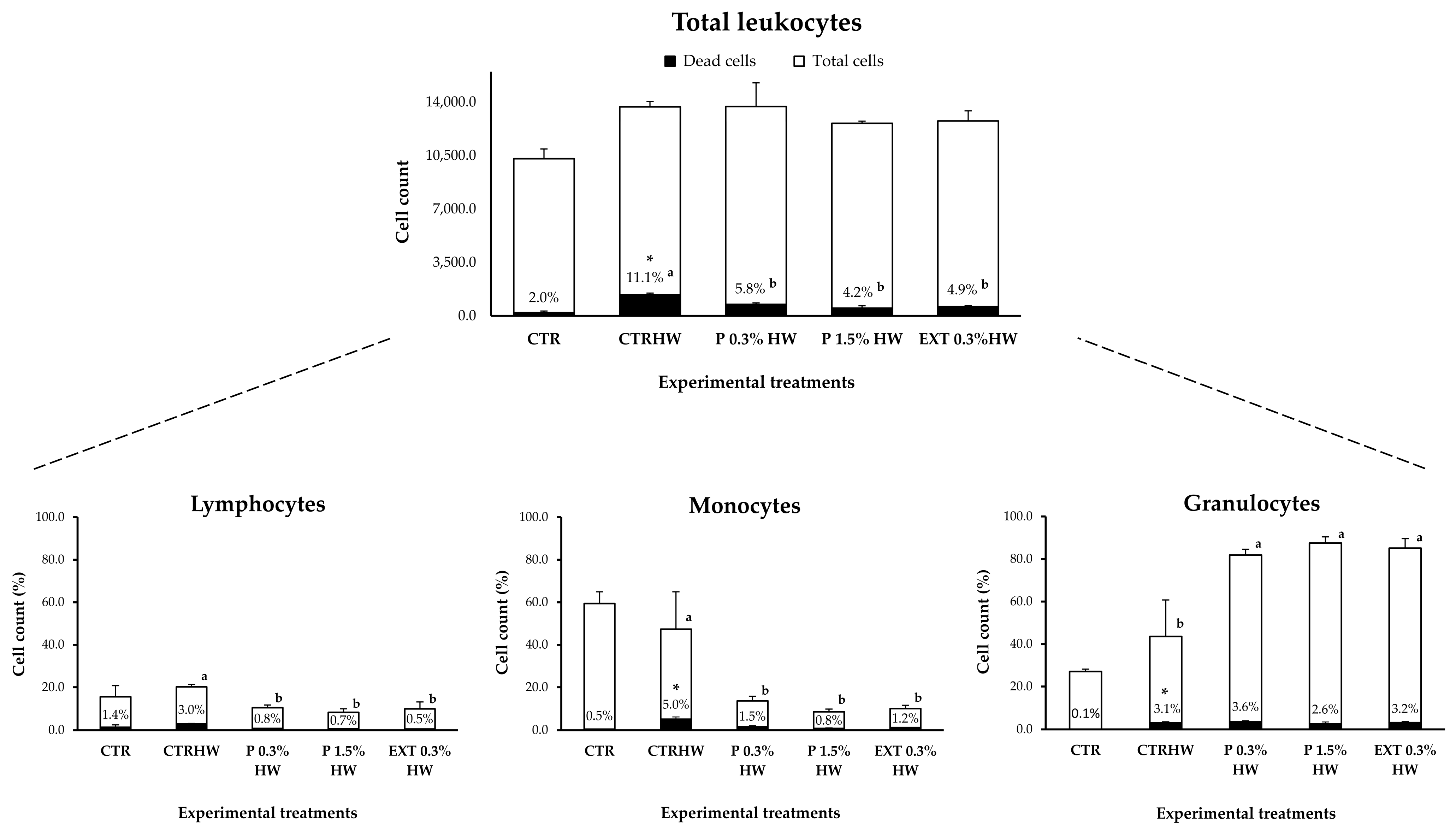
- S3 (exposure to MHW and pathogen): During a 7-day period, fish were exposed to either an MHW (25.7 °C) or a combination of an MHW and V. harveyi challenge (Figure 1). The feeding regime and diets remained the same as in S1 and S2. Each treatment consisted of 15 fish (5 fish/tank; Appendix A, Figure A1). After the 7-day MHW exposure, 6 fish per treatment (2 fish/tank) were sampled for further analysis (Appendix A, Figure A1).
2.6. Fish Sampling Procedures
2.7. Confirmation of Vibrio harveyi Infection
2.7.1. DNA Extraction
2.7.2. Pathogen Detection Using PCR Amplification
2.8. Hematological Parameters
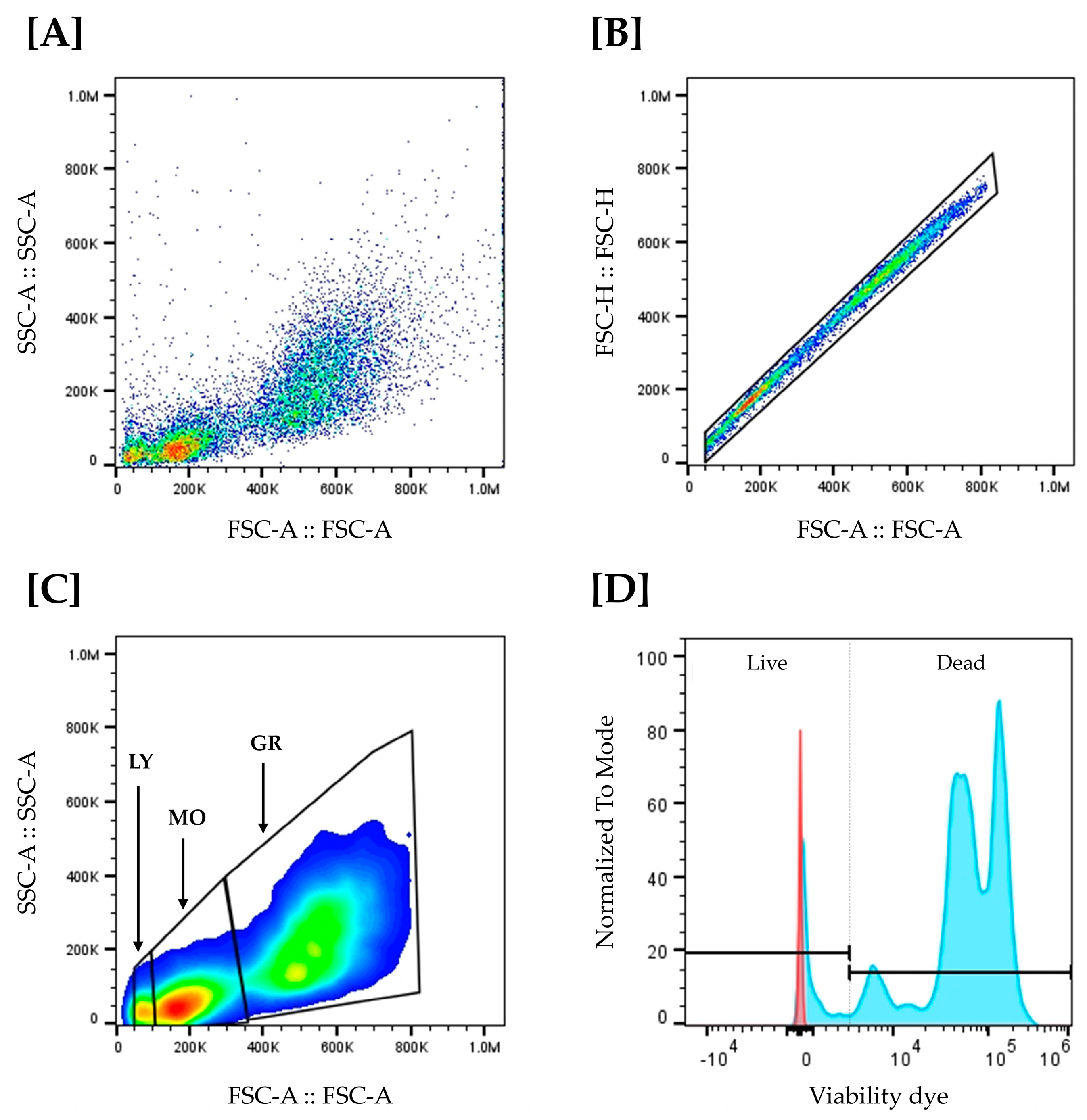
2.9. Head Kidney Leucocyte Isolation and Flow Cytometry Analysis
2.10. Innate Humoral Parameters
2.11. Statistical Analysis
3. Results
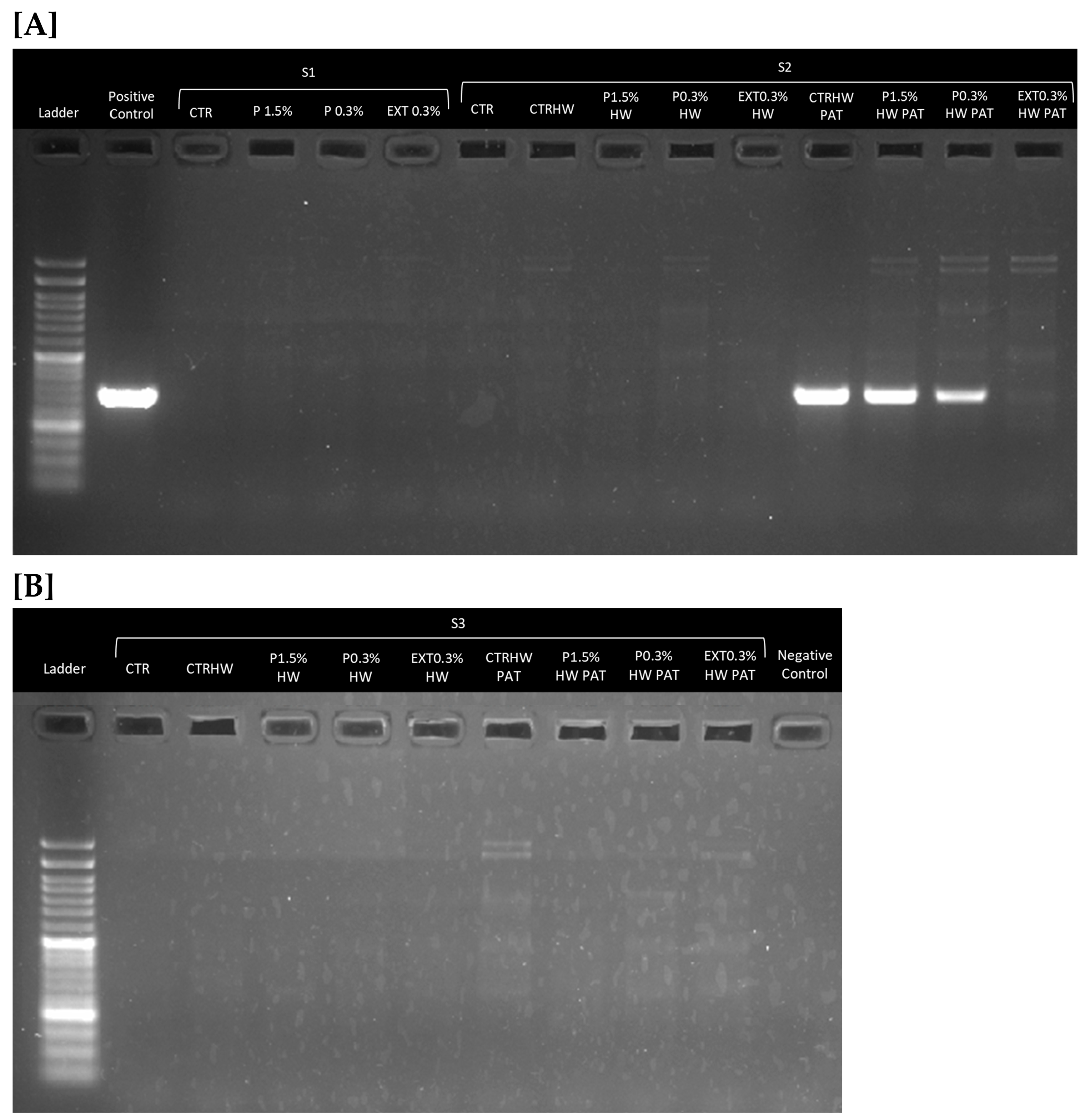
3.1. Confirmation of Vibrio harveyi Infection
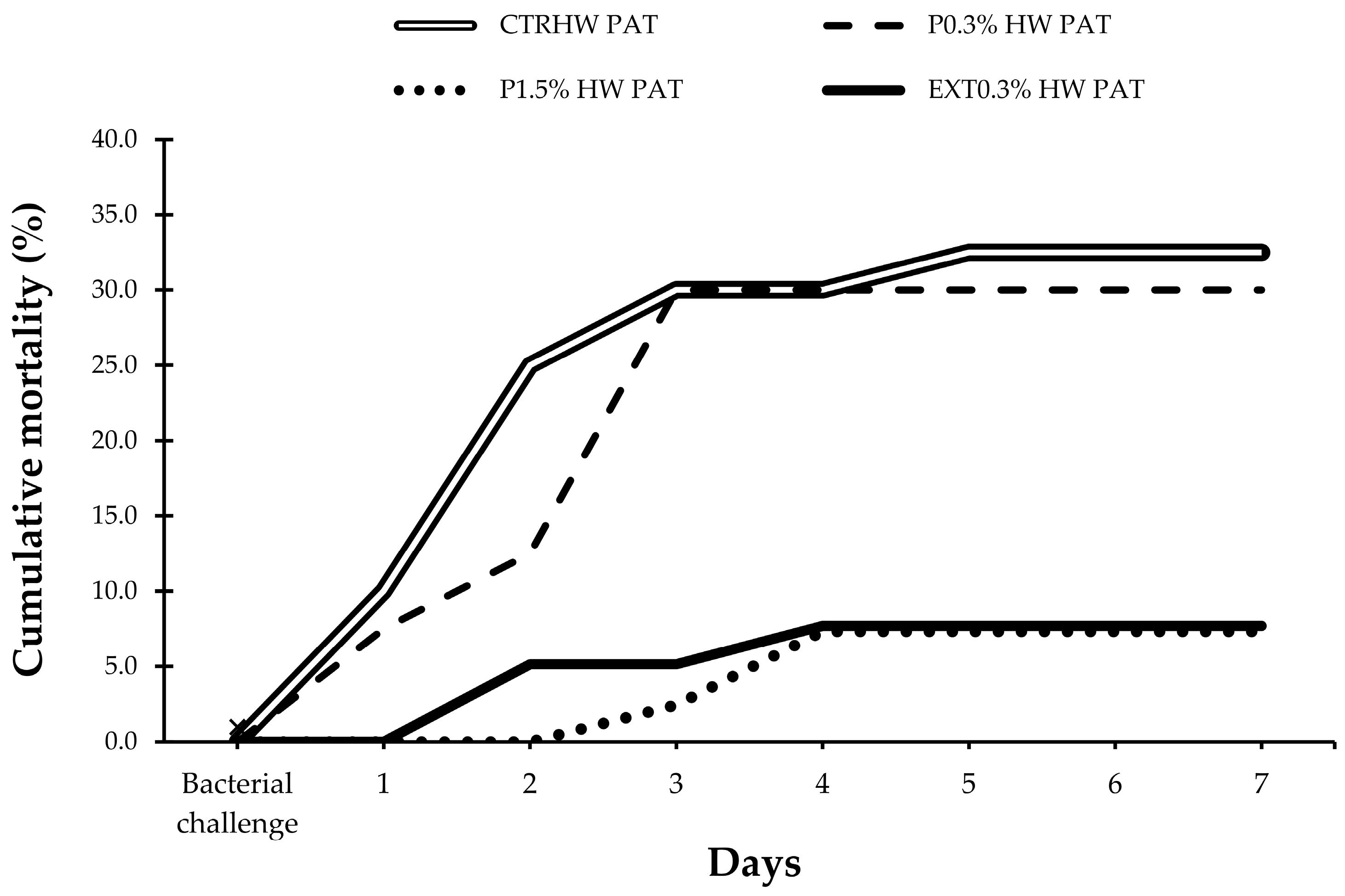
3.2. Mortality Rates After Vibrio harveyi Challenge
3.3. Hematological Parameters
3.3.1. Effects of Seaweed Supplementation in Non-Infected Fish Under Optimal (S1) and Marine Heatwave (S3) Conditions
3.3.2. Effect of Seaweed Supplementation on Fish Responses upon 24 h (S2) and 7 Days (S3) of Vibrio harveyi Challenge
3.4. Leukocyte Abundance and Viability
3.4.1. Effects of Seaweed Supplementation on Non-Infected Fish Under Optimal Conditions (S1)
3.4.2. Effects of Seaweed Supplementation on Non-Infected Fish Under Marine Heatwave Conditions (S3)
3.4.3. Effect of Seaweed Supplementation on Fish Responses upon 24 h (S2) and 7 Days (S3) of Vibrio harveyi Challenge
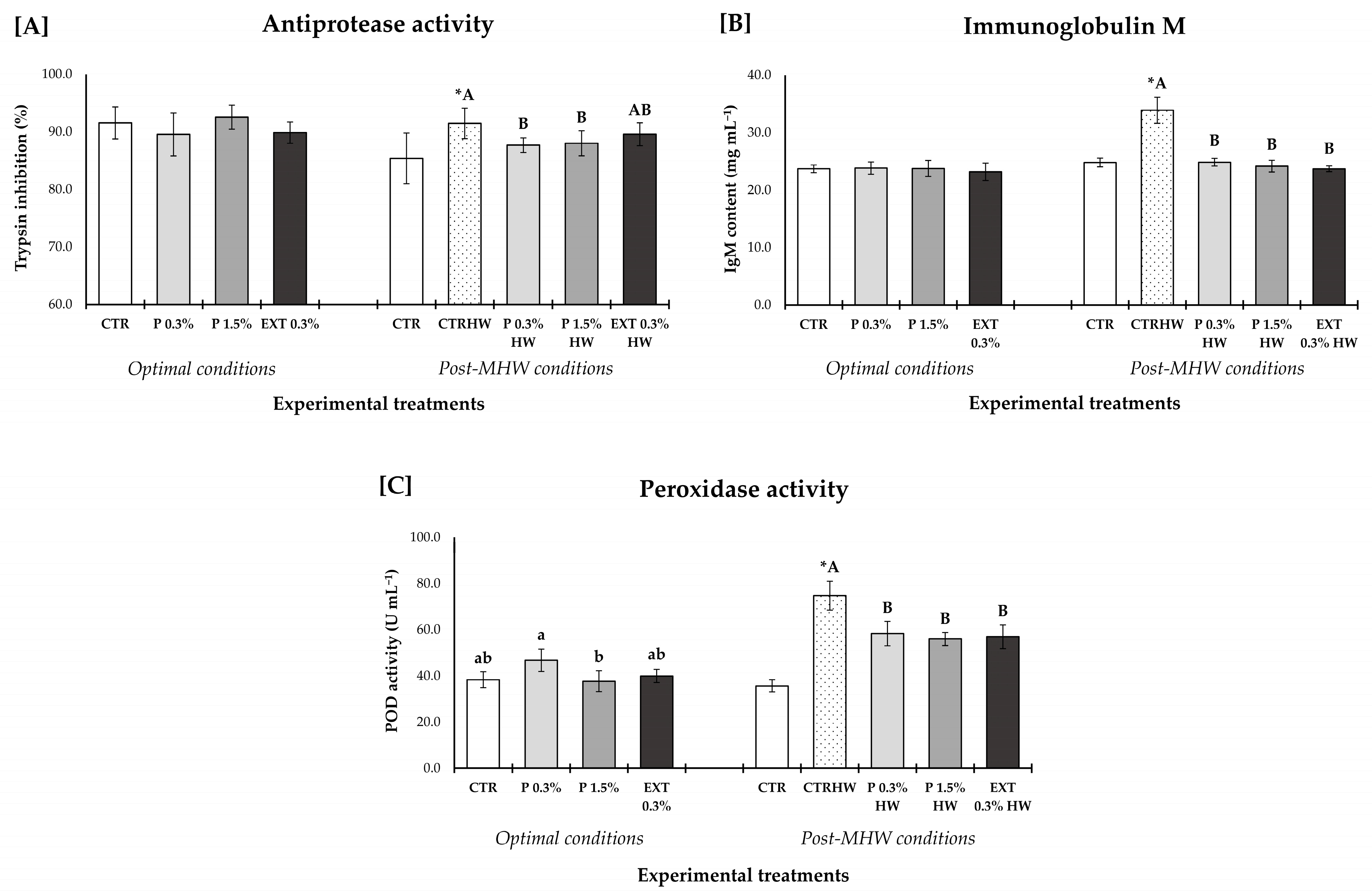
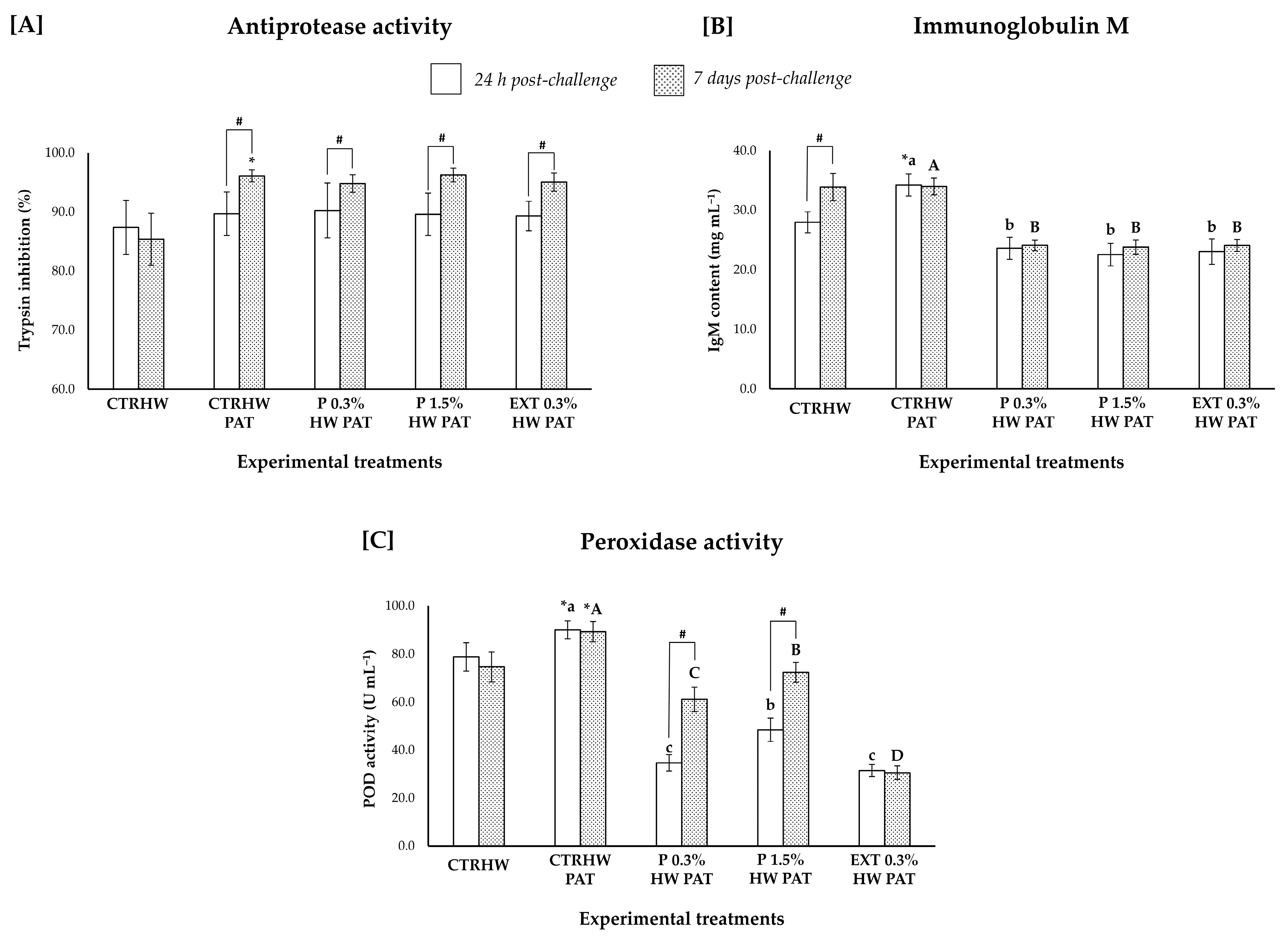
3.5. Innate Humoral Parameters
3.5.1. Effects of Seaweed Supplementation on Non-Infected Fish Under Optimal (S1) and Marine Heatwave (S3) Conditions
3.5.2. Effect of Seaweed Supplementation on Fish Responses to Vibrio harveyi upon 24 h (S2) and 7 Days (S3) of Challenge
4. Discussion
4.1. Effects of Laminaria digitata Supplementation Under Optimal Conditions
4.2. Effects of Laminaria digitata Supplementation Under Marine Heatwave Conditions
4.3. Effects of Laminaria digitata Supplementation on the Interaction Between Marine Heatwaves and Vibrio harveyi Outbreaks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AP | Antiprotease activity |
| bw | Body weight |
| CTR | Control |
| EXT0.3% | Diet with 0.3% extract |
| Hb | Hemoglobin |
| Ht | Hematocrit |
| IgM | Immunoglobulin M |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| MHW | Marine heatwave |
| p | p-value |
| P0.3% | Diet with 0.3% powder |
| P1.5% | Diet with 1.5% powder |
| PCR | Polymerase chain reaction |
| POD | Peroxidase activity |
| RBC | Red blood cells |
| SD | Standard deviation |
| WBC | White blood cells |
Appendix A
Appendix A.1. Detailed Feed Composition of the Experimental Diets
| Ingredients (%) | CTR | P 0.3% | P 1.5% | EXT 0.3% |
|---|---|---|---|---|
| Fishmeal Super Prime 1 | 17.0 | 17.0 | 17.0 | 17.0 |
| Fish protein hydrolysate 2 | 2.0 | 2.0 | 2.0 | 2.0 |
| Poultry meal 3 | 7.0 | 7.0 | 7.0 | 7.0 |
| Soy protein concentrate 4 | 6.0 | 6.0 | 6.0 | 6.0 |
| Wheat gluten 5 | 10.0 | 10.0 | 10.0 | 10.0 |
| Corn gluten meal 6 | 11.0 | 11.0 | 11.0 | 11.0 |
| Soybean meal (Hipro) 7 | 12.8 | 12.8 | 12.8 | 12.8 |
| Wheat meal 8 | 12.0 | 11.7 | 10.5 | 11.7 |
| Whole peas 9 | 6.0 | 6.0 | 6.0 | 6.0 |
| Vitamin and mineral premix 10 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride 50% 11 | 0.2 | 0.2 | 0.2 | 0.2 |
| Monosodium phosphate 12 | 1.0 | 1.0 | 1.0 | 1.0 |
| Fish oil 13 | 6.0 | 6.0 | 6.0 | 6.0 |
| Salmon oil 14 | 8.0 | 8.0 | 8.0 | 8.0 |
| Macroalga Laminaria digitata 15 | - | 0.3 | 1.5 | - |
| Macroalga Extract (Laminaria digitata 15) | - | - | - | 0.3 |
| Proximate composition (%) | ||||
| Crude protein, %DM | 46.0 | 46.0 | 45.9 | 46.0 |
| Crude fat, %DM | 16.0 | 16.0 | 16.0 | 16.0 |
| Fiber, %DM | 1.4 | 1.4 | 1.4 | 1.4 |
| Starch, %DM | 13.5 | 13.3 | 12.5 | 13.3 |
| Ash, %DM | 7.4 | 7.4 | 7.7 | 7.4 |
| Gross energy, MJ kg−1 | 20.5 | 20.5 | 20.4 | 20.5 |
Appendix A.2. Experimental Design

References
- Pastor, F.; Khodayar, S. Marine Heat Waves: Characterizing a Major Climate Impact in the Mediterranean. Sci. Total Environ. 2023, 861, 160621. [Google Scholar] [CrossRef] [PubMed]
- De Luzinais, V.G.; Gascuel, D.; Reygondeau, G.; Cheung, W.W.L. Large Potential Impacts of Marine Heatwaves on Ecosystem Functioning. Glob. Change Biol. 2024, 30, e17437. [Google Scholar] [CrossRef]
- Brayden, W.C.; Noblet, C.L.; Evans, K.S.; Rickard, L. Consumer Preferences for Seafood Attributes of Wild-Harvested and Farm-Raised Products. Aquacult. Econ. Manag. 2018, 22, 362–382. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic Temperature Fluctuations and Their Effect on Aquaculture: A Comprehensive Review. Aquacult. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Marmelo, I.; Lourenço-Marques, C.; Silva, I.A.L.; Soares, F.; Pousão-Ferreira, P.; Mata, L.; Marques, A.; Diniz, M.S.; Maulvault, A.L. Eco-Innovative Aquafeeds Biofortified with Asparagopsis taxiformis to Improve the Resilience of Farmed White Seabream (Diplodus sargus) to Marine Heatwave Events. Heliyon 2024, 10, e35135. [Google Scholar] [CrossRef]
- Cascarano, M.C.; Stavrakidis-Zachou, O.; Mladineo, I.; Thompson, K.D.; Papandroulakis, N.; Katharios, P. Mediterranean Aquaculture in a Changing Climate: Temperature Effects on Pathogens and Diseases of Three Farmed Fish Species. Pathogens 2021, 10, 1205. [Google Scholar] [CrossRef]
- Atalah, J.; Ibañez, S.; Aixalà, L.; Barber, X.; Sánchez-Jerez, P. Marine Heatwaves in the Western Mediterranean: Considerations for Coastal Aquaculture Adaptation. Aquaculture 2024, 588, 740917. [Google Scholar] [CrossRef]
- Rowley, A.F.; Baker-Austin, C.; Boerlage, A.S.; Caillon, C.; Davies, C.E.; Duperret, L.; Martin, S.A.M.; Mitta, G.; Pernet, F.; Pratoomyot, J.; et al. Diseases of Marine Fish and Shellfish in an Age of Rapid Climate Change. iScience 2024, 27, 110838. [Google Scholar] [CrossRef]
- Suzzi, A.L.; Stat, M.; Gaston, T.F.; Siboni, N.; Williams, N.L.R.; Seymour, J.R.; Huggett, M.J. Elevated Estuary Water Temperature Drives Fish Gut Dysbiosis and Increased Loads of Pathogenic Vibrionaceae. Environ. Res. 2023, 219, 115144. [Google Scholar] [CrossRef]
- Zhang, X.-H.; He, X.; Austin, B. Vibrio harveyi: A Serious Pathogen of Fish and Invertebrates in Mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Zamri Saad, M.; Nasruddin, N.S.; Al-saari, N.; Mino, S.; Sawabe, T. Vibriosis in Cultured Marine Fishes: A Review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Sanches-Fernandes, G.M.M.; Sá-Correia, I.; Costa, R. Vibriosis Outbreaks in Aquaculture: Addressing Environmental and Public Health Concerns and Preventive Therapies Using Gilthead Seabream Farming as a Model System. Front. Microbiol. 2022, 13, 904815. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev. Aquacult. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of Alternatives to Antibiotic Use in Aquaculture. Rev. Aquacult. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Hegde, A.; Kabra, S.; Basawa, R.M.; Khile, D.A.; Abbu, R.U.F.; Thomas, N.A.; Manickam, N.B.; Raval, R. Bacterial Diseases in Marine Fish Species: Current Trends and Future Prospects in Disease Management. World J. Microbiol. Biotechnol. 2023, 39, 317. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial Roles of Feed Additives as Immunostimulants in Aquaculture: A Review. Rev. Aquacult. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food Safety Impacts of Antimicrobial Use and Their Residues in Aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Gonzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant Effects of Seaweeds and Their Active Compounds on Animal Health and Production—A Review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Francis, P.; Rohani, M.F.; Azam, M.S.; Mock, T.S.; Francis, D.S. Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants 2023, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Marmelo, I.; Dias, M.; Grade, A.; Pousão-Ferreira, P.; Diniz, M.S.; Marques, A.; Maulvault, A.L. Immunomodulatory and Antioxidant Effects of Functional Aquafeeds Biofortified with Whole Laminaria digitata in Juvenile Gilthead Seabream (Sparus aurata). Front. Mar. Sci. 2024, 11, 1325244. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Harikrishnan, R.; Devi, G.; Bhat, E.A.; Paray, B.A. Stimulatory Effects of Seaweed Laminaria digitata Polysaccharides Additives on Growth, Immune-Antioxidant Potency and Related Genes Induction in Rohu Carp (Labeo rohita) during Flavobacterium columnare Infection. Aquaculture 2024, 579, 740253. [Google Scholar] [CrossRef]
- Pereira, A.; Marmelo, I.; Dias, M.; Anacleto, P.; Pires, C.; Batista, I.; Marques, A.; Maulvault, A.L. Antioxidant, Metabolic and Digestive Biomarker Responses of Farmed Sparus aurata Supplemented with Laminaria digitata. Aquaculture 2025, 598, 741984. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The Seasonal Variation in the Chemical Composition of the Kelp Species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Bonfim-Mendonça, P.; Capoci, I.; Tobaldini-Valerio, F.; Negri, M.; Svidzinski, T. Overview of β-Glucans from Laminaria Spp.: Immunomodulation Properties and Applications on Biologic Models. Int. J. Mol. Sci. 2017, 18, 1629. [Google Scholar] [CrossRef]
- Vissers, A.M.; Caligiani, A.; Sforza, S.; Vincken, J.-P.; Gruppen, H. Phlorotannin Composition of Laminaria digitata. Phytochem. Anal. 2017, 28, 487–495. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine Algal Flavonoids and Phlorotannins; an Intriguing Frontier of Biofunctional Secondary Metabolites. Crit. Rev. Biotechnol. 2022, 42, 23–45. [Google Scholar] [CrossRef]
- Pérez, R.; Barbaroux, O. Ces Algues Qui Nous Entourent: Conception Actuelle, Rôle Dans La Biosphère, Utilisations, Culture; Ifremer: Plouzané, France, 1997. [Google Scholar]
- Schlegel, R.W.; Smit, A.J. HeatwaveR: A Central Algorithm for the Detection of Heatwaves and Cold-Spells. J. Open Source Softw. 2018, 3, 821. [Google Scholar] [CrossRef]
- Hobday, A.; Oliver, E.; Sen Gupta, A.; Benthuysen, J.; Burrows, M.; Donat, M.; Holbrook, N.; Moore, P.; Thomsen, M.; Wernberg, P.; et al. Categorizing and Naming Marine Heatwaves. Oceanog 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric Studies. J. Biol. Chem. 1935, 112, 51–65. [Google Scholar] [CrossRef]
- Marmelo, I.; Silva, Z.; Bolotas, D.; Alves, R.N.; Videira, P.A.; Marques, A.; Sousa, M.; Maulvault, A.L. Isolation, Fixation and Characterization of Juvenile Gilthead Seabream Head Kidney Leukocytes by Flow Cytometry. J. Vis. Exp. 2025, 219, e67978. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Bakopoulos, V.; Dimitriadis, G.J. Maternal Transfer of Humoral Specific and Non-Specific Immune Parameters to Sea Bream (Sparus aurata) Larvae. Fish Shellfish Immunol. 2004, 17, 411–435. [Google Scholar] [CrossRef]
- Cuesta, A.; Meseguer, J.; Esteban, M.A. Total Serum Immunoglobulin M Levels Are Affected by Immunomodulators in Seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 2004, 101, 203–210. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A Rapid, Direct Assay to Measure Degranulation of Bovine Neutrophil Primary Granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Araújo, M.; Rema, P.; Sousa-Pinto, I.; Cunha, L.M.; Peixoto, M.J.; Pires, M.A.; Seixas, F.; Brotas, V.; Beltrán, C.; Valente, L.M.P. Dietary Inclusion of IMTA-Cultivated Gracilaria vermiculophylla in Rainbow Trout (Oncorhynchus mykiss) Diets: Effects on Growth, Intestinal Morphology, Tissue Pigmentation, and Immunological Response. J. Appl. Phycol. 2016, 28, 679–689. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Chisti, Y. Seaweed-Based Diets Lead to Normal Growth, Improved Fillet Color but a down-Regulated Expression of Somatotropic Axis Genes in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2022, 554, 738183. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Galafat, A.; Galindo-Melero, R.; Perera, E.; Casal-Porras, I.; Zubía, E.; Vega, J.; Figueroa, F.L.; Martínez, T.F.; et al. Effects of Feeding European Seabass (Dicentrarchus labrax) Juveniles with Crude, Hydrolysed and Fermented Biomass of the Invasive Macroalga Rugulopteryx okamurae (Ochrophyta). Aquacult. Rep. 2024, 34, 101877. [Google Scholar] [CrossRef]
- Lay, P.A.; Baldwin, J. What Determines the Size of Teleost Erythrocytes? Correlations with Oxygen Transport and Nuclear Volume. Fish Physiol. Biochem. 1999, 20, 31–35. [Google Scholar] [CrossRef]
- Mokhtar, D.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.; Sayed, R. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, I.; Ahmad, A.; Usmani, N.; Ahmad, M. Studies on the Alterations in Haematological Indices, Micronuclei Induction and Pathological Marker Enzyme Activities in Channa punctatus (Spotted Snakehead) Perciformes, Channidae Exposed to Thermal Power Plant Effluent. SpringerPlus 2016, 5, 761. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, A.J. Fish Granulocytes: Morphology, Distribution, and Function. Annu. Rev. Fish. Dis. 1992, 2, 123–148. [Google Scholar] [CrossRef]
- Pereira, A.; Marmelo, I.; Dias, M.; Silva, A.C.; Grade, A.C.; Barata, M.; Pousão-Ferreira, P.; Dias, J.; Anacleto, P.; Marques, A.; et al. Asparagopsis taxiformis as a Novel Antioxidant Ingredient for Climate-Smart Aquaculture: Antioxidant, Metabolic and Digestive Modulation in Juvenile White Seabream (Diplodus sargus) Exposed to a Marine Heatwave. Antioxidants 2024, 13, 949. [Google Scholar] [CrossRef]
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown Seaweed (AquaArom) Supplementation Increases Food Intake and Improves Growth, Antioxidant Status and Resistance to Temperature Stress in Atlantic Salmon, Salmo salar. PLoS ONE 2019, 14, e0219792. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Ghafarifarsani, H.; Hoseinifar, S.H.; Ahani, S.; Chou, C.-C. Use of Algae in Aquaculture: A Review. Fishes 2024, 9, 63. [Google Scholar] [CrossRef]
- Muñoz, N.J.; Farrell, A.P.; Heath, J.W.; Neff, B.D. Hematocrit Is Associated with Thermal Tolerance and Modulated by Developmental Temperature in Juvenile Chinook Salmon. Physiol. Biochem. Zool. 2018, 91, 757–762. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and Immune System in Fish. In Biology and Physiology of Freshwater Neotropical Fish; Elsevier: London, UK, 2020; pp. 93–114. ISBN 978-0-12-815872-2. [Google Scholar]
- Manchanayake, T.; Salleh, A.; Amal, M.N.A.; Yasin, I.S.M.; Zamri-Saad, M. Pathology and Pathogenesis of Vibrio Infection in Fish: A Review. Aquacult. Rep. 2023, 28, 101459. [Google Scholar] [CrossRef]
- Firmino, J.; Furones, M.D.; Andree, K.B.; Sarasquete, C.; Ortiz-Delgado, J.B.; Asencio-Alcudia, G.; Gisbert, E. Contrasting Outcomes of Vibrio harveyi Pathogenicity in Gilthead Seabream, Sparus aurata and European Seabass, Dicentrachus labrax. Aquaculture 2019, 511, 734210. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.-S.; Balasundaram, C.; Heo, M.-S. Protection of Vibrio harveyi Infection through Dietary Administration of Pueraria thunbergiana in Kelp Grouper, Epinephelus bruneus. Aquaculture 2012, 324–325, 27–32. [Google Scholar] [CrossRef]
- Rashidah, A.R.; Shariff, M.; Yusoff, F.M.; Ismail, I.S. Dietary Supplementation of Polygonum chinense Improves the Immunity of Asian Seabass, Lates calcarifer (Bloch, 1790) against Vibrio harveyi Infection. Fish Shellfish Immunol. Rep. 2023, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, N.; Khalil, R.H.; Afifi, A.A.M.; Alkhuriji, A.F.; Metwally, D.M. Nutritional Innovation Using Green Seaweed (Ulva Sp.) and Garlic Powder Extracts for White-Leg Shrimp (Litopenaeus vannamei) Challenged by Vibrio harveyi. Vet. Med. Sci. 2024, 10, e70052. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, I.; Rossi, A.G. Granulocyte Apoptosis: Who Would Work with a ‘Real’ Inflammatory Cell? Biochem. Soc. Trans. 2004, 32, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef]
- Santos, R.A.; Mariz-Ponte, N.; Martins, N.; Magalhães, R.; Jerusik, R.; Saavedra, M.J.; Peres, H.; Oliva-Teles, A.; Serra, C.R. In vitro Modulation of Gilthead Seabream (Sparus aurata L.) Leukocytes by Bacillus Spp. Extracellular Molecules upon Bacterial Challenge. Fish Shellfish Immunol. 2022, 121, 285–294. [Google Scholar] [CrossRef]
- Fuentes-Appelgren, P.; Opazo, R.; Barros, L.; Feijoó, C.G.; Urzúa, V.; Romero, J. Effect of the Dietary Inclusion of Soybean Components on the Innate Immune System in Zebrafish. Zebrafish 2014, 11, 41–49. [Google Scholar] [CrossRef]
| Effect of Supplementation (No MHW) | Effect of MHW (No Supplementation) | Effect of Supplementation Under MHW Conditions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparisons Against CTR at S1 (Fold-Change) | Comparisons Against CTR at S3(Fold-Change) | Comparisons Against CTRHW at S3 (Fold-Change) | |||||||
| RBCs (µL−1) | S1 | P 0.3% | n.s. | S3 | CTRHW | n.s. | S3 | P 0.3% HW | n.s. |
| P 1.5% | n.s. | P 1.5% HW | n.s. | ||||||
| EXT 0.3% | n.s. | EXT 0.3% HW | n.s. | ||||||
| WBCs (µL−1) | S1 | P 0.3% | n.s. | S3 | CTRHW | n.s. | S3 | P 0.3% HW | n.s. |
| P 1.5% | n.s. | P 1.5% HW | n.s. | ||||||
| EXT 0.3% | n.s. | EXT 0.3% HW | n.s. | ||||||
| MCV (µm3) | S1 | P 0.3% | ↑ 0.5-fold | S3 | CTRHW | n.s. | S3 | P 0.3% HW | n.s. |
| P 1.5% | ↑ 0.4-fold | P 1.5% HW | n.s. | ||||||
| EXT 0.3% | ↑ 0.4-fold | EXT 0.3% HW | n.s. | ||||||
| MCH (pg cell−1) | S1 | P 0.3% | ↑ 0.4-fold | S3 | CTRHW | ↓ 0.3-fold | S3 | P 0.3% HW | ↑ 0.3-fold |
| P 1.5% | n.s. | P 1.5% HW | n.s. | ||||||
| EXT 0.3% | n.s. | EXT 0.3% HW | ↑ 0.4-fold | ||||||
| MCHC (g 100 mL−1) | S1 | P 0.3% | n.s. | S3 | CTRHW | n.s. | S3 | P 0.3% HW | ↑ 0.2-fold |
| P 1.5% | n.s. | P 1.5% HW | ↑ 0.2-fold | ||||||
| EXT 0.3% | n.s. | EXT 0.3% HW | n.s. | ||||||
| Hb (g dL−1) | S1 | P 0.3% | n.s. | S3 | CTRHW | n.s. | S3 | P 0.3% HW | ↑ 0.4-fold |
| P 1.5% | n.s. | P 1.5% HW | ↑ 0.3-fold | ||||||
| EXT 0.3% | n.s. | EXT 0.3% HW | ↑ 0.2-fold | ||||||
| Ht (%) | S1 | P 0.3% | n.s. | S3 | CTRHW | n.s. | S3 | P 0.3% HW | n.s. |
| P 1.5% | n.s. | P 1.5% HW | n.s. | ||||||
| EXT 0.3% | n.s. | EXT 0.3% HW | n.s. | ||||||
| Effect of V. harveyi Under MHW Conditions (No Supplementation) | Effect of Supplementation Under MHW and V. harveyi Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparisons Against CTRHW(Fold-Change) | Comparisons Against CTRHW PAT at S2 (Fold-Change) | Comparisons Against CTRHW PAT at S3 (Fold-Change) | |||||||
| RBCs (µL−1) | S2 | CTRHW PAT | n.s. | S2 | P 0.3% HW PAT | n.s. | S3 | P 0.3% HW PAT | n.s. |
| P 1.5% HW PAT | n.s. | P 1.5% HW PAT | n.s. | ||||||
| S3 | CTRHW PAT | n.s. | EXT 0.3% HW PAT | n.s. | EXT 0.3% HW PAT | n.s. | |||
| WBCs (µL−1) | S2 | CTRHW PAT | n.s. | S2 | P 0.3% HW PAT | n.s. | S3 | P 0.3% HW PAT | ↑ 3.4-fold |
| P 1.5% HW PAT | n.s. | P 1.5% HW PAT | n.s. | ||||||
| S3 | CTRHW PAT | ↓ 0.8-fold | EXT 0.3% HW PAT | n.s. | EXT 0.3% HW PAT | ↑ 2.3-fold | |||
| MCV (µm3) | S2 | CTRHW PAT | n.s. | S2 | P 0.3% HW PAT | n.s. | S3 | P 0.3% HW PAT | ↓ 0.5-fold |
| P 1.5% HW PAT | n.s. | P 1.5% HW PAT | n.s. | ||||||
| S3 | CTRHW PAT | ↑ 0.8-fold | EXT 0.3% HW PAT | n.s. | EXT 0.3% HW PAT | n.s. | |||
| MCH (pg cell−1) | S2 | CTRHW PAT | ↓ 0.3-fold | S2 | P 0.3% HW PAT | ↑ 0.5-fold | S3 | P 0.3% HW PAT | ↓ 0.3-fold |
| P 1.5% HW PAT | ↑ 0.5-fold | P 1.5% HW PAT | n.s. | ||||||
| S3 | CTRHW PAT | ↑ 0.4-fold | EXT 0.3% HW PAT | ↑ 0.5-fold | EXT 0.3% HW PAT | n.s. | |||
| MCHC (g 100 mL−1) | S2 | CTRHW PAT | ↓ 0.3-fold | S2 | P 0.3% HW PAT | n.s. | S3 | P 0.3% HW PAT | ↑ 0.2-fold |
| P 1.5% HW PAT | ↑ 0.4-fold | P 1.5% HW PAT | n.s. | ||||||
| S3 | CTRHW PAT | ↓ 0.2-fold | EXT 0.3% HW PAT | n.s. | EXT 0.3% HW PAT | n.s. | |||
| Hb (g dL−1) | S2 | CTRHW PAT | n.s. | S2 | P 0.3% HW PAT | ↑ 0.4-fold | S3 | P 0.3% HW PAT | ↑ 0.2-fold |
| P 1.5% HW PAT | ↑ 0.4-fold | P 1.5% HW PAT | ↑ 0.2-fold | ||||||
| S3 | CTRHW PAT | ↓ 0.2-fold | EXT 0.3% HW PAT | n.s. | EXT 0.3% HW PAT | ↑ 0.2-fold | |||
| Ht (%) | S2 | CTRHW PAT | ↑ 0.7-fold | S2 | P 0.3% HW PAT | n.s. | S3 | P 0.3% HW PAT | n.s. |
| P 1.5% HW PAT | n.s. | P 1.5% HW PAT | n.s. | ||||||
| S3 | CTRHW PAT | n.s. | EXT 0.3% HW PAT | n.s. | EXT 0.3% HW PAT | n.s. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmelo, I.; Chainho, T.; Bolotas, D.; Pereira, A.; Özkan, B.; Marques, C.; Silva, I.A.L.; Soares, F.; Pousão-Ferreira, P.; Vieira, E.F.; et al. Laminaria digitata Supplementation as a Climate-Smart Strategy to Counteract the Interactive Effects of Marine Heatwaves and Disease Outbreaks in Farmed Gilthead Seabream (Sparus aurata). Environments 2025, 12, 226. https://doi.org/10.3390/environments12070226
Marmelo I, Chainho T, Bolotas D, Pereira A, Özkan B, Marques C, Silva IAL, Soares F, Pousão-Ferreira P, Vieira EF, et al. Laminaria digitata Supplementation as a Climate-Smart Strategy to Counteract the Interactive Effects of Marine Heatwaves and Disease Outbreaks in Farmed Gilthead Seabream (Sparus aurata). Environments. 2025; 12(7):226. https://doi.org/10.3390/environments12070226
Chicago/Turabian StyleMarmelo, Isa, Tomás Chainho, Daniel Bolotas, Alícia Pereira, Busenur Özkan, Cátia Marques, Iris A. L. Silva, Florbela Soares, Pedro Pousão-Ferreira, Elsa F. Vieira, and et al. 2025. "Laminaria digitata Supplementation as a Climate-Smart Strategy to Counteract the Interactive Effects of Marine Heatwaves and Disease Outbreaks in Farmed Gilthead Seabream (Sparus aurata)" Environments 12, no. 7: 226. https://doi.org/10.3390/environments12070226
APA StyleMarmelo, I., Chainho, T., Bolotas, D., Pereira, A., Özkan, B., Marques, C., Silva, I. A. L., Soares, F., Pousão-Ferreira, P., Vieira, E. F., Delerue-Matos, C., Silva, Z., Videira, P. A., Repolho, T., Diniz, M. S., Marques, A., & Maulvault, A. L. (2025). Laminaria digitata Supplementation as a Climate-Smart Strategy to Counteract the Interactive Effects of Marine Heatwaves and Disease Outbreaks in Farmed Gilthead Seabream (Sparus aurata). Environments, 12(7), 226. https://doi.org/10.3390/environments12070226













