Enhancement in Lithium Recovery from Spent Lithium Batteries by Nanofiltration Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Mass and NF Samples Preparation
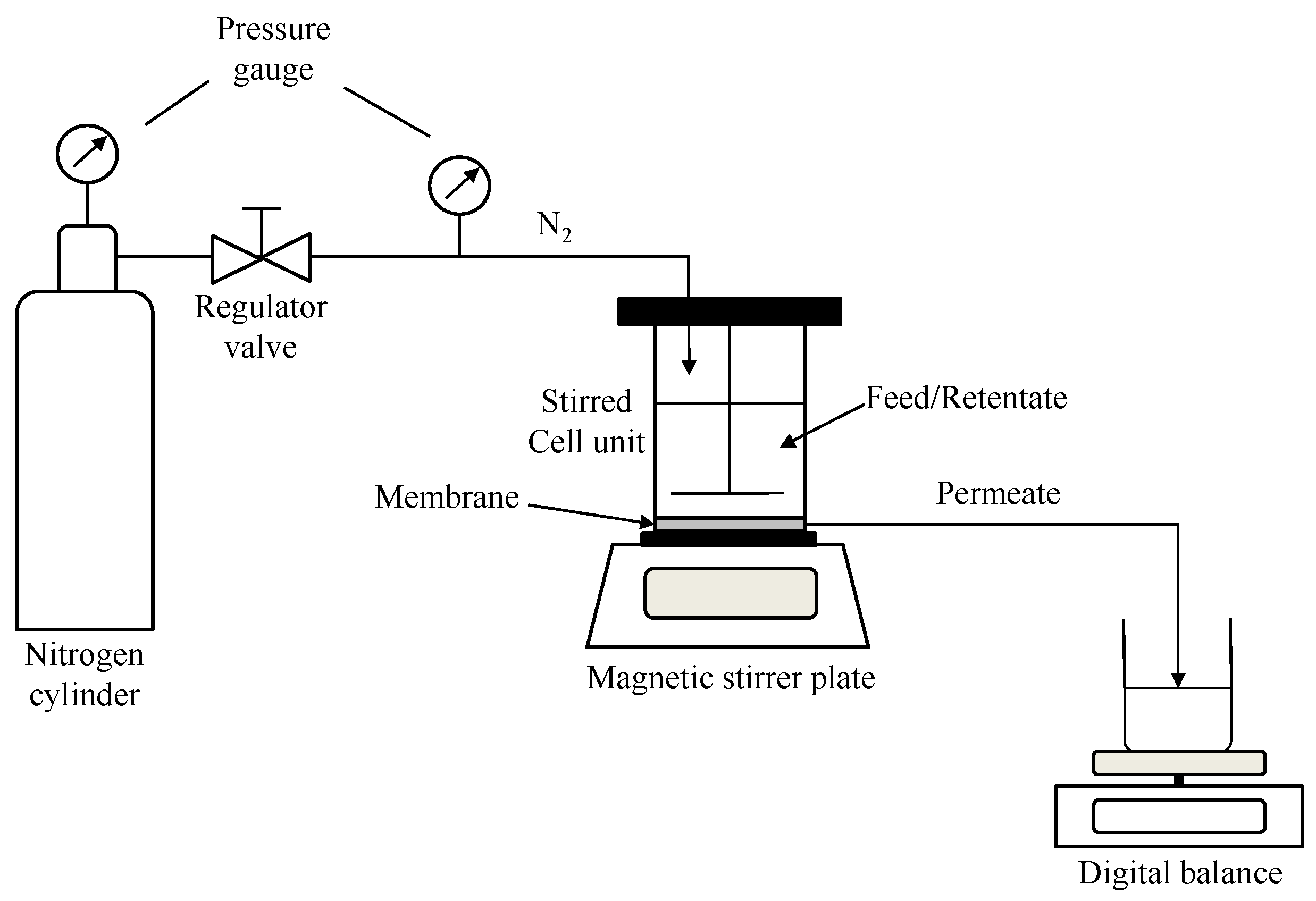
2.2. Nanofiltration Set-Up
2.3. ICP-MS Analysis
3. Results and Discussion
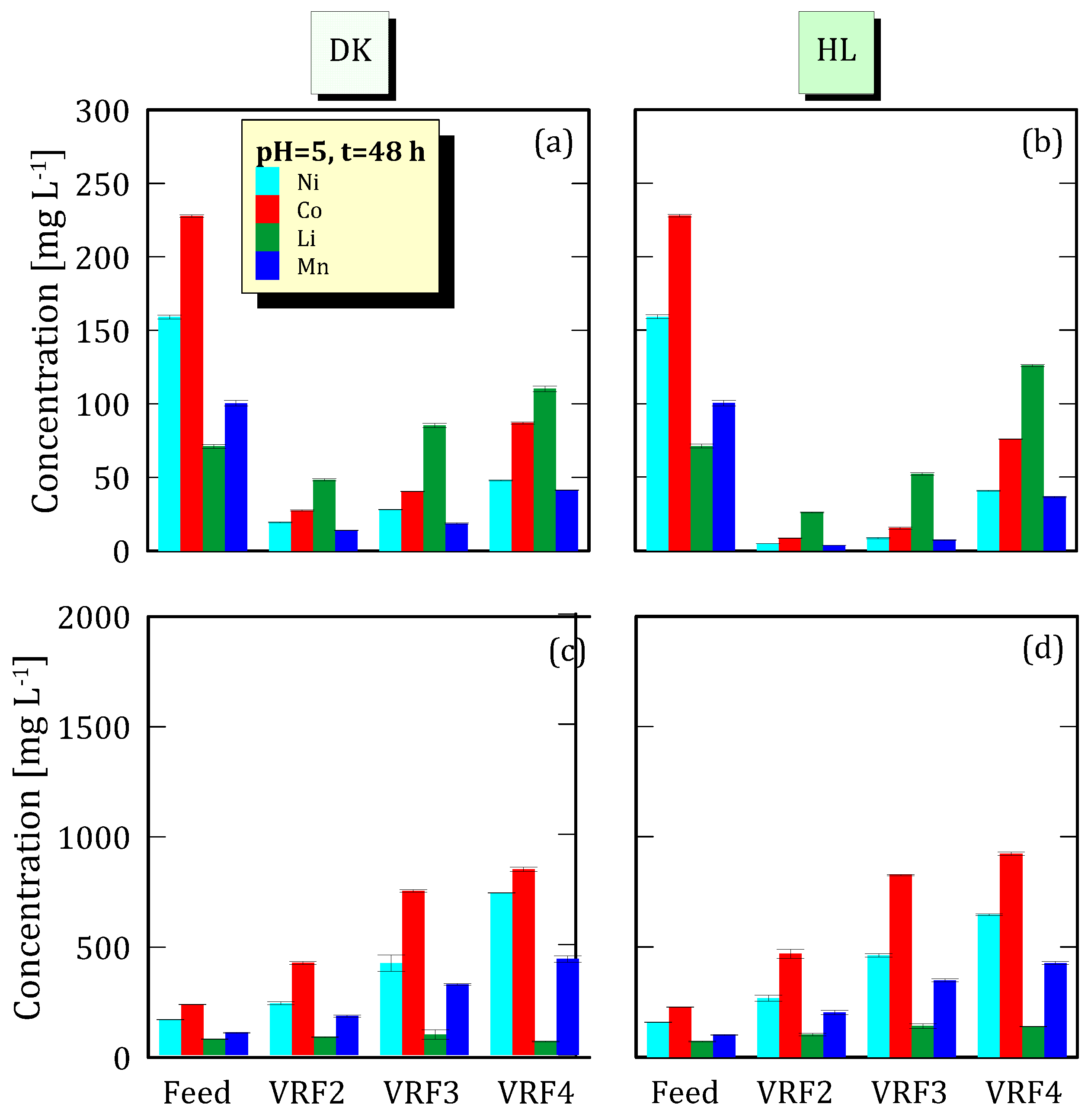
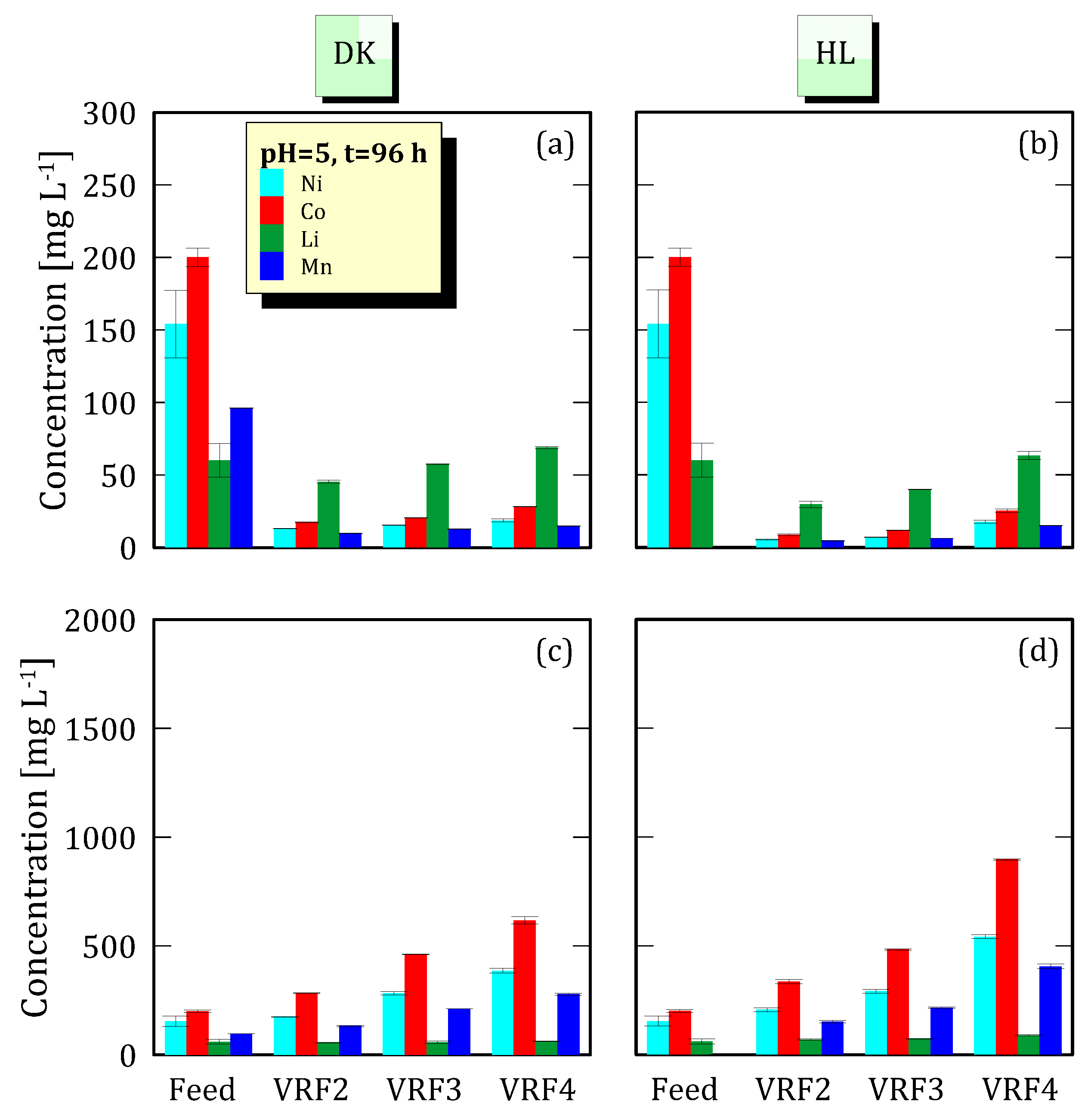
3.1. Analyses of NF Feed
3.2. Analyses of Permeate Flux
3.3. Rejection Performance
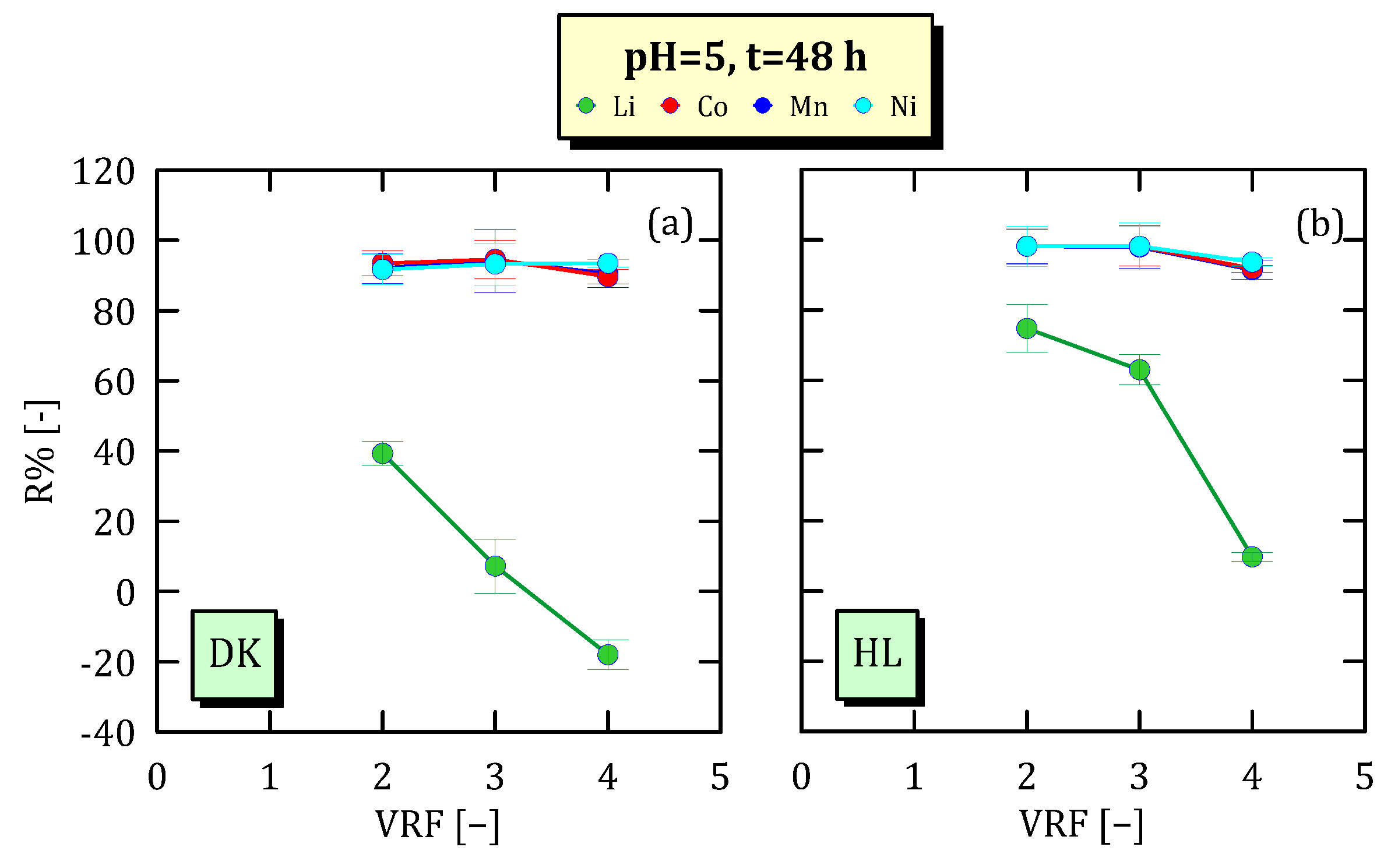
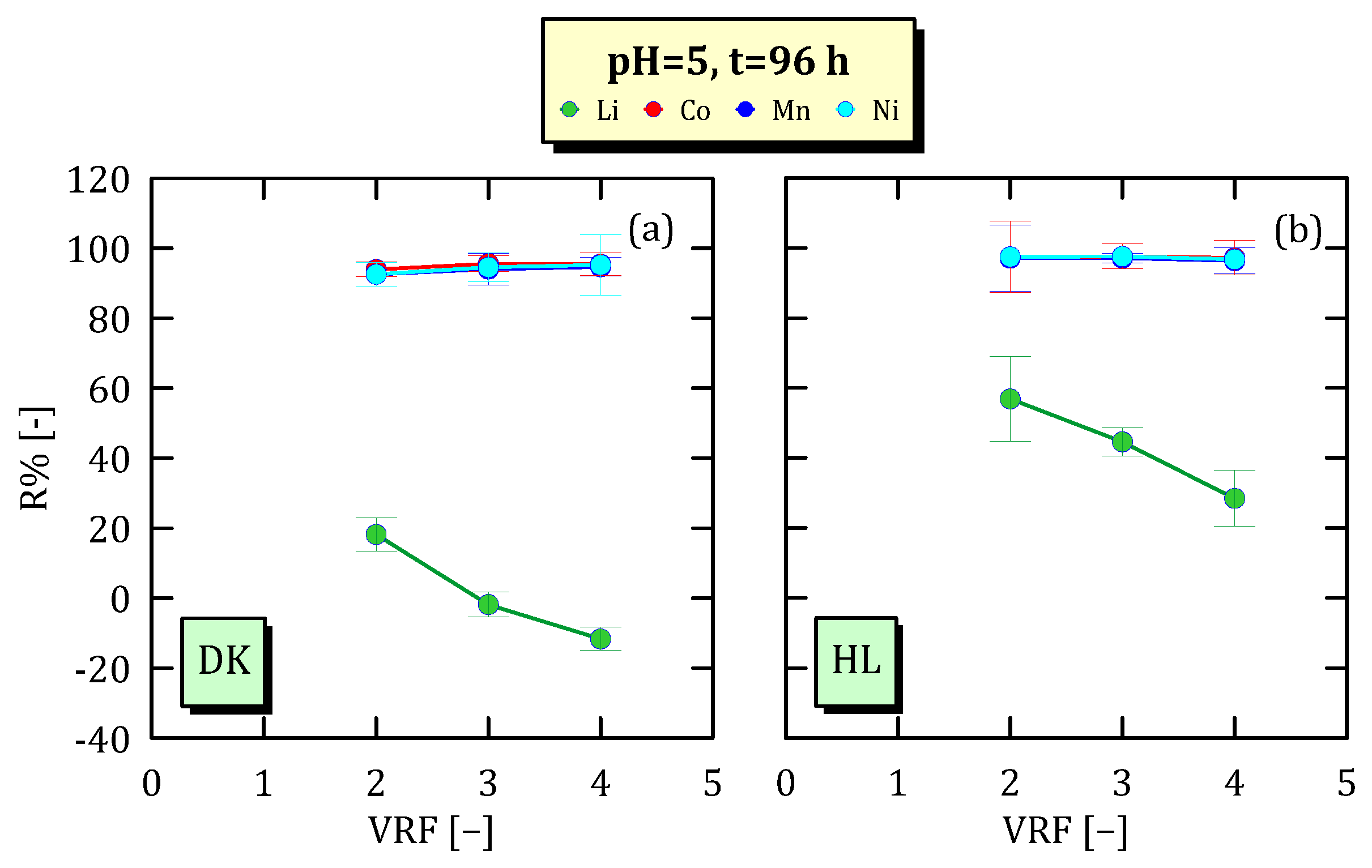
3.4. Effect of VRF on Ion Concentration
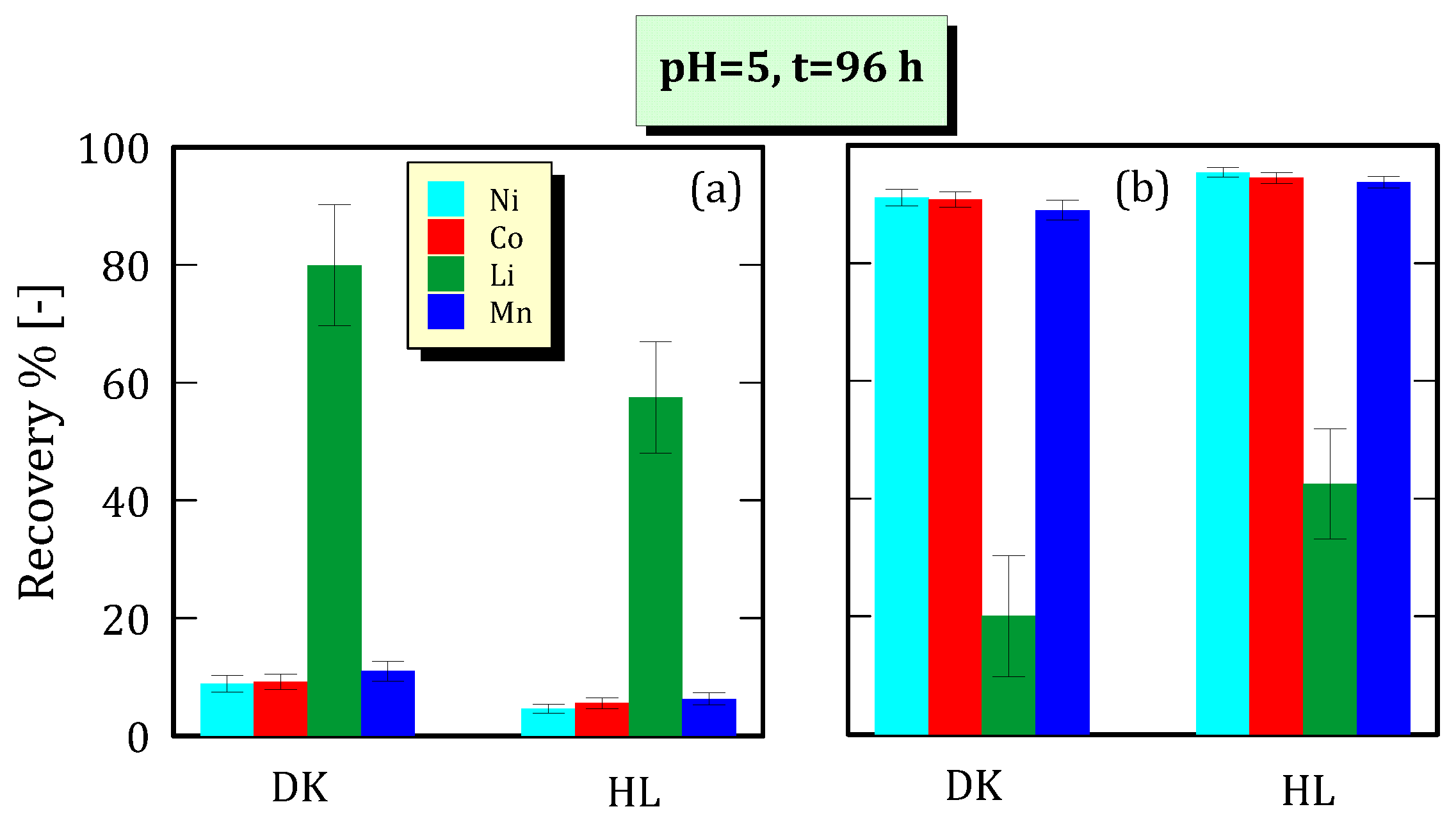
3.5. Ion Recovery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Chen, Z.; Yildizbasi, A.; Wang, Y.; Sarkis, J. Safety in lithium-ion battery circularity activities: A framework and evaluation methodology. Resour. Conserv. Recycl. 2023, 193, 106962. [Google Scholar] [CrossRef]
- Li, L.; Deshmane, V.G.; Paranthaman, M.P.; Bhave, R.; Moyer, B.A.; Harrison, S. Lithium recovery from aqueous resources and batteries: A brief review. Johns. Matthey Technol. Rev. 2018, 62, 161–176. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Del Campo, F.J.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Ojanen, S.; Lundström, M.; Santasalo-Aarnio, A.; Serna-Guerrero, R. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling. Waste Manag. 2018, 76, 242–249. [Google Scholar] [CrossRef]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Bargeman, G. Recent developments in the preparation of improved nanofiltration membranes for extreme pH conditions. Sep. Purif. Technol. 2021, 279, 119725. [Google Scholar]
- Van der Bruggen, B.; Manttari, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Yadav, D.; Karki, S.; Ingole, P.G. Nanofiltration (NF) Membrane processing in the food industry. Food Eng. Rev. 2022, 14, 579–595. [Google Scholar]
- Natarajan, S. Resource recovery from dye wastewaters using nanofiltration systems. In Resource Recovery in Industrial Waste Waters, 1st ed.; Khadir, A., Gurung, K., Sillanpää, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 229–252. [Google Scholar]
- Vives, M.B.; Thuvander, J.; Arkell, A.; Lipnizki, F. Low-molecular-weight lignin recovery with nanofiltration in the Kraft pulping process. Membranes 2022, 12, 310. [Google Scholar]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.H.; Moon, S.J.; Jung, J.T.; Wang, H.H.; Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E.; Lee, Y.M. Lithium recovery from artificial brine using energy-efficient membrane distillation and nanofiltration. J. Membr. Sci. 2020, 598, 117683. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Kentish, S.; Nghiem, L.D.; Hai, F.I. Lithium enrichment from a simulated salt lake brine using an integrated nanofiltration-membrane distillation process. J. Environ. Chem. Eng. 2019, 7, 103395. [Google Scholar]
- Pramanik, B.K.; Asif, M.B.; Roychand, R.; Shu, L.; Jegatheesan, V.; Bhuiyan, M.; Hai, F.I. Lithium recovery from salt-lake brine: Impact of competing cations, pretreatment and preconcentration. Chemosphere 2020, 260, 127623. [Google Scholar] [PubMed]
- Somrani, A.; Hamzaoui, A.H.; Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 2013, 317, 184–192. [Google Scholar]
- Sun, S.Y.; Cai, L.J.; Nie, X.Y.; Song, X.; Yu, J.G. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Wen, X.; Ma, P.; Zhu, C.; He, Q.; Deng, X. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar] [CrossRef]
- Zhai, J.; Balogun, A.; Bhattacharjee, S.; Vogler, R.J.; Khare, R.; Malmali, M.; Deonarine, A.; Shen, Y.X. Nanofiltration as pretreatment for lithium recovery from salt lake brine. J. Membr. Sci. 2024, 710, 123150. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.; Tang, H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Wang, R.Y.; Alghanayem, R.; Lin, S.H. Multipass nanofiltration for lithium separation with high selectivity and recovery. Environ. Sci. Technol. 2023, 57, 14464–14471. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, C.; Ha, G.S.; Park, Y.K.; Khan, M.A.; Jang, M.; Kim, S.H.; Amin, M.A.; Gacem, A.; Jeon, B.H. Downstream recovery of Li and value-added metals (Ni, Co, and Mn) from leach liquor of spent lithium-ion batteries using a membrane-integrated hybrid system. Chem. Eng. J. 2022, 447, 137507. [Google Scholar] [CrossRef]
- Gao, S.L.; Qin, Z.X.; Wang, B.F.; Huang, J.; Xu, Z.L.; Tang, Y.J. Lithium recovery from the spent lithium-ion batteries by commercial acid-resistant nanofiltration membranes: A comparative study. Desalination 2024, 572, 117142. [Google Scholar] [CrossRef]
- Benitez, F.J.; Acero, J.L.; Leal, A.I.; Gonzalez, M. The use of ultrafiltration and nanofiltration for the purification of cork processing wastewater. J. Hazard. Mater. 2009, 162, 1438–1445. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Heijman, S.G.J.; Cornelissen, E.R.; Amy, G.; Van der Bruggen, B.; Van Dijk, J.C. Nanofiltration as a treatment method for the removal of pesticides from groundwater. Water Res. 2007, 41, 139–147. [Google Scholar]
- Simonič, M. Compost leachate treatment using polyaluminium chloride and nanofiltration. Open Chem. 2017, 15, 123–128. [Google Scholar] [CrossRef]
- Religa, P.; Kowalik-Klimczak, A.; Gierycz, P. Study on the behavior of nanofiltration membranes using for chromium(III) recovery from salt mixture solution. Desalination 2013, 315, 115–123. [Google Scholar] [CrossRef]
- Starzak, M.E. Membranes, Synthetic (Chemistry). In Encyclopedia of Physical Science and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 345–354. [Google Scholar]
- Yaroshchuk, A.E. Negative rejection of ions in pressure-driven membrane processes. Adv. Colloid Interface Sci. 2008, 139, 150–173. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, P.; Qin, Y.; Bai, C.; Gu, Z.; Yu, S. Negative rejection phenomenon in the mixed salt nanofiltration: Law and mechanism. Desalination 2024, 583, 117667. [Google Scholar] [CrossRef]










| Membrane Type | DK | HL |
|---|---|---|
| Manufacturer | GE Water & Process Technologies | GE Water & Process Technologies |
| Membrane material | PA-TFC | PA-TFC |
| Configuration | flat-sheet | flat-sheet |
| Nominal MWCO (Da) | 150–300 | 150–300 |
| pH operating range | 2–10 | 3–9 |
| Max. operating temperature (°C) | 80 | 50 |
| Max. operating pressure (bar) | 40 | 40 |
| MgSO4 rejection * (%) | 98 | 95 |
| Water permeability (L m−2 h−1 bar−1) | 5.4 | 9.6 |
| Contact angle (°) | 41 a | 41 b |
| N. Sample | Extraction Conditions | Li (mg L−1) | Co (mg L−1) | Mn (mg L−1) | Ni (mg L−1) |
|---|---|---|---|---|---|
| 1 | 48 h, pH 5 | 71 ± 1 | 228 ± 1 | 100 ± 1 | 159 ± 1 |
| 2 | 96 h, pH 2.5 | 72 ± 3 | 232 ± 5 | 107 ± 6 | 162 ± 2 |
| 3 | 96 h, pH 5 | 69 ± 2 | 231 ± 5 | 98 ± 3 | 154 ± 3 |
| 4 | 168 h, pH 2.5 | 76 ± 3 | 265 ± 5 | 141 ± 6 | 167 ± 3 |
| Species | Atomic Radius (Å) | Van der Waals Radius (Å) | Charge |
|---|---|---|---|
| Lithium | 1.45 | 1.82 | +1 |
| Cobalt | 1.35 | 2.00 | +2 |
| Manganese | 1.40 | 2.20 | +2 |
| Nickel | 1.35 | 1.63 | +2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prenesti, G.; Tagarelli, A.; Elliani, R.; Napoli, A.; Caravella, A.; Tocci, E.; Cappuccino, G.; Cassano, A. Enhancement in Lithium Recovery from Spent Lithium Batteries by Nanofiltration Membranes. Environments 2025, 12, 186. https://doi.org/10.3390/environments12060186
Prenesti G, Tagarelli A, Elliani R, Napoli A, Caravella A, Tocci E, Cappuccino G, Cassano A. Enhancement in Lithium Recovery from Spent Lithium Batteries by Nanofiltration Membranes. Environments. 2025; 12(6):186. https://doi.org/10.3390/environments12060186
Chicago/Turabian StylePrenesti, Giuseppe, Antonio Tagarelli, Rosangela Elliani, Anna Napoli, Alessio Caravella, Elena Tocci, Gregorio Cappuccino, and Alfredo Cassano. 2025. "Enhancement in Lithium Recovery from Spent Lithium Batteries by Nanofiltration Membranes" Environments 12, no. 6: 186. https://doi.org/10.3390/environments12060186
APA StylePrenesti, G., Tagarelli, A., Elliani, R., Napoli, A., Caravella, A., Tocci, E., Cappuccino, G., & Cassano, A. (2025). Enhancement in Lithium Recovery from Spent Lithium Batteries by Nanofiltration Membranes. Environments, 12(6), 186. https://doi.org/10.3390/environments12060186










