Pesticide Pollution Provokes Histopathological Alterations in Apis mellifera (Linnaeus, 1758) Drone Gonads
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Chemical Analysis
2.3. Histopathological Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. International COLOSS Questionnaire
3.2. Chemical Levels
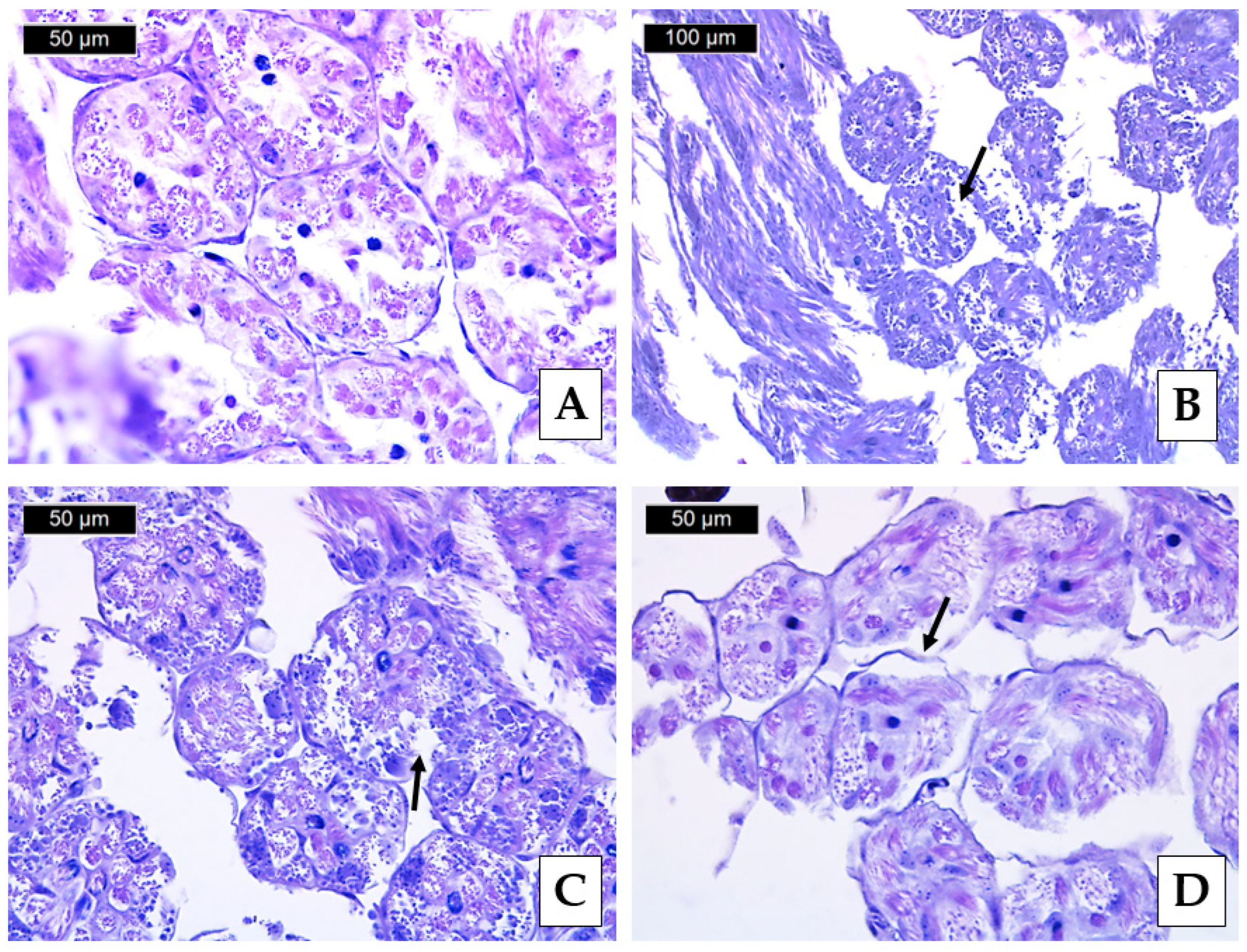
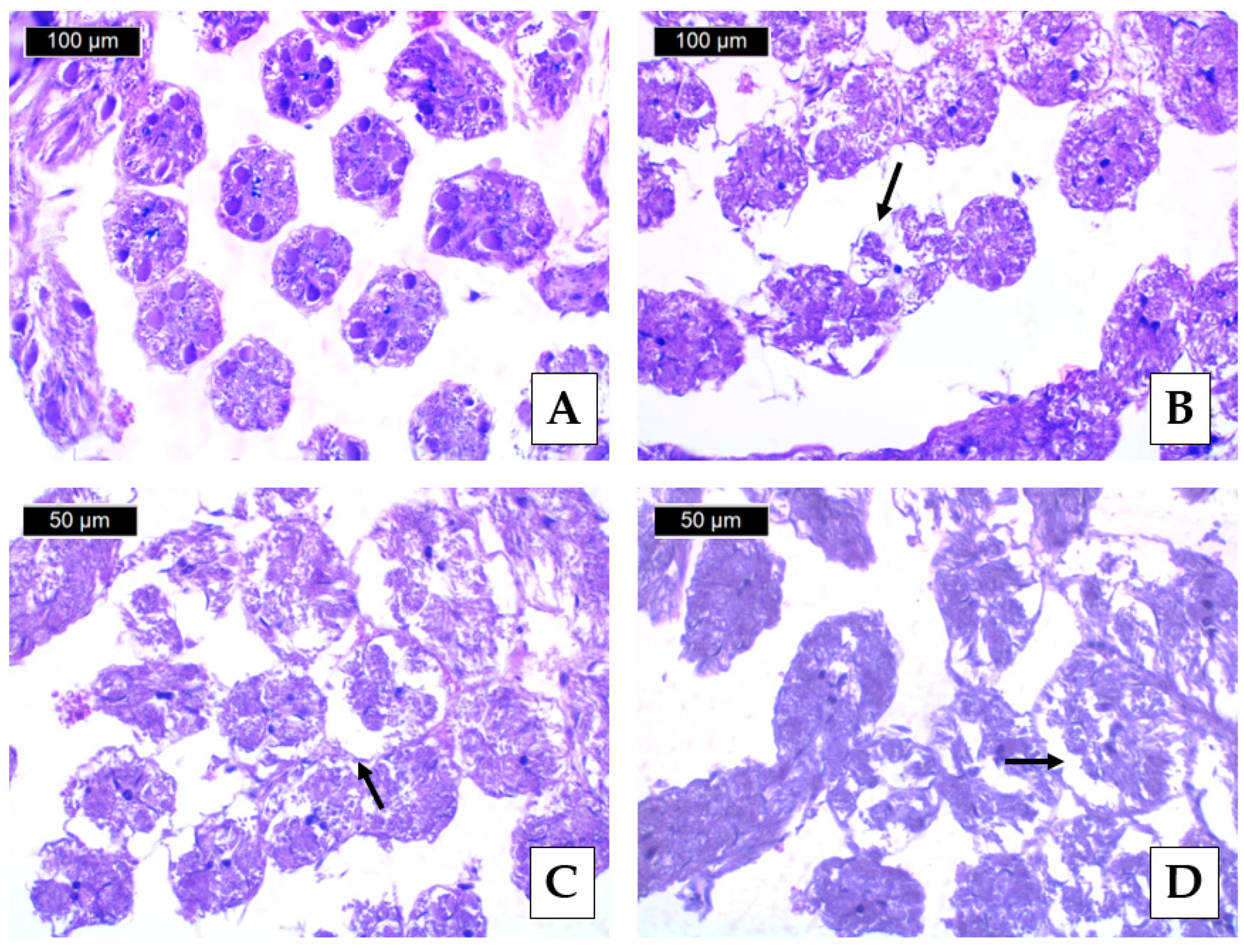
3.3. Histopathological Lesions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Kaila, L.; Ketola, J.; Toivonen, M.; Loukola, O.; Hakala, K.; Raiskio, S.; Hurme, T.; Jalli, M. Pesticide residues in honey bee-collected pollen: Does the EU regulation protect honey bees from pesticides? Environ. Sci. Pollut. Res. 2022, 29, 18225–18244. [Google Scholar] [CrossRef]
- Martinello, M.; Manzinello, C.; Dainese, N.; Giuliato, I.; Gallina, A.; Mutinelli, F. The Honey Bee: An Active Biosampler of Environmental Pollution and a Possible Warning Biomarker for Human Health. Appl. Sci. 2021, 11, 6481. [Google Scholar] [CrossRef]
- Klein, C.D.; Kozii, I.V.; Wood, S.C.; Koziy, R.V.; Zabrodski, M.W.; Dvylyuk, I.; Medici de Mattos, I.; Moshynskyy, I.; Honaramooz, A.; Simko, E. Testicular changes of honey bee drones, Apis mellifera (Hymenoptera: Apidae), during sexual maturation. J. Insect Sci. 2021, 21, 3. [Google Scholar] [CrossRef]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A.; et al. The Honey bee Apis mellifera: An insect at the interface between human and ecosystem health. Biology 2022, 11, 233. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, D.; Zou, N.; Yu, X.; Zhang, Z.; Liu, F.; Mu, W. Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honey bees (Apis mellifera L.). Chemosphere 2018, 201, 159–167. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Labella, G.F.; Giorgi, A.; Panseri, S.; Pavlovic, R.; Bonacci, S.; Arioli, F. The occurrence of pesticides and persistent organic pollutants in Italian organic honeys from different productive areas in relation to potential environmental pollution. Chemosphere 2016, 154, 482–490. [Google Scholar] [CrossRef]
- Eissa, F.; Taha, E.K.A. Contaminants in honey: An analysis of EU RASFF notifications from 2002 to 2022. J. Consum. Prot. Food Saf. 2023, 18, 393–402. [Google Scholar] [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef]
- Margaoan, R.; Papa, G.; Nicolescu, A.; Cornea-Cipcigan, M.; Kösoğlu, M.; Topal, E.; Negri, I. Environmental pollution effect on honey bees and their derived products: A comprehensive analysis. Environ. Sci. Pollut. Res. 2024, 32, 10370–10391. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Łabuć, E.; Ptaszyńska, A.A. Seasonal detection of pathogens in honey bees kept in natural and laboratory conditions. Parasitol. Int. 2025, 104, 102978. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anagnostopoulos, C.; Anastasiadou, P.; Machera, K. Pesticide residues in honey bees, honey and bee pollen by LC–MS/MS screening: Reported death incidents in honey bees. Sci. Total Environ. 2014, 485, 633–642. [Google Scholar] [CrossRef]
- Malhat, F.M.; Haggag, M.N.; Loutfy, N.M.; Osman, M.A.M. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemophere 2015, 120, 457–461. [Google Scholar] [CrossRef]
- Catalano, P.; Della Sala, F.; Cavaliere, M.; Caputo, C.; Pecoraro, D.; Crispino, G.; Lettera, S.; Caioni, G.; Esposito, M.; Verre, A.; et al. Use of honey bees and hive products as bioindicators to assess environmental contamination in targeted areas of the Campania region (Italy). Animals 2024, 14, 1446. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef]
- van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- De Jong, D.; Lester, P.J. The global challenge of improving bee protection and health. Front. Bee Sci. 2023, 1, 1118292. [Google Scholar] [CrossRef]
- Cresswell, J.E.; Desneux, N.; van Engelsdorp, D. Dietary traces of neonicotinoid pesticides as a cause of population declines in honey bees: An evaluation by Hill’s epidemiological criteria. Pest. Manag. Sci. 2012, 68, 819–827. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Gusso-Choueri, P.K.; Choueri, R.B.; de Araújo, G.S.; Cruz, A.C.F.; de Oliveira Stremel, T.R.; de Campos, S.X.; de Souza Abessa, D.M.; de Oliveira Ribeiro, C.A. Univariate or multivariate approaches for histopathological biomarkers in the context of environmental quality assessments? Mar. Pollut. Bull. 2022, 181, 113828. [Google Scholar] [CrossRef]
- Tanabe, P.; Schlenk, D.; Forsgren, K.L.; Pampanin, D.M. Using digital pathology to standardize and automate histological evaluations of environmental samples. Environ. Toxicol. Chem. 2025, 44, 306–317. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Bugeja Douglas, A.; Cadahía, L.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; et al. Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: The combined effects of operation size, migration and queen replacement. J. Apic. Res. 2022, 62, 204–210. [Google Scholar] [CrossRef]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017.
- Alturkistani, H.A.; Tashkandi, F.M.; Mohammedsaleh, Z.M. Histological stains: A literature review and case study. Glob. J. Health Sci. 2015, 8, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Power, K.; Martano, M.; Altamura, G.; Maiolino, P. Histopathological findings in testes from apparently healthy drones of Apis mellifera ligustica. Vet. Sci. 2020, 7, 124. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Saraiva, A.; Costa, J.; Serrão, J.; Cruz, C.; Eiras, J.C. A histology-based fish health assessment of farmed seabass (Dicentrarchus labrax L.). Aquaculture 2015, 448, 375–381. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with Python. SciPy 2010, 7, 92–96. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Services to Support EFSA Systematic Reviews: Lot 5 Systematic Literature Review on the Neonicotinoids (Namely Active Substances Clothianidin, Thiamethoxam and Imidacloprid) and the Risks to Bees. EFSA Supporting Publication 2015: EN-756, 2013. Available online: http://www.efsa.europa.eu/en/supporting/doc/756e.pdf (accessed on 15 February 2025).
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J.; van Engelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Pettis, J.S.; Tarpy, D.R.; Mullin, C.A.; Frazier, J.L.; Frazier, M. In-hive pesticide exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the eastern United States. Sci. Rep. 2016, 6, 33207. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.A.; Frazier, M.; Frazier, J.L.; Ashcroft, S.; Simonds, R.; van Engelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef]
- Rinderer, T.E.; De Guzman, L.I.; Lancaster, V.A.; Delatte, G.T.; Seltzer, J.A. Varroa in the mating yard: I. The effects of Varroa jacobsoni and Apistan® on drone honey bees. Am. Bee J. 1999, 139, 134–139. [Google Scholar]
- Benito-Murcia, M.; Botías, C.; Martín-Hernández, R.; Higes, M.; Soler, F.; Pérez-López, M.; Míguez-Santiyán, M.P.; Martínez-Morcillo, S. 2024. Biomarker responses and lethal dietary doses of tau-fluvalinate and coumaphos in honey bees: Implications for chronic acaricide toxicity. Environ. Toxicol. Pharmacol. 2024, 105, 104330. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Lim, S.; Lee, L.M.L.; Kwon, H.W.; Nikolenko, A.G. Effect of miticides amitraz and fluvalinate on reproduction and productivity of honey bee Apis mellifera. Uludağ Arıcılık Derg. 2021, 21, 21–30. [Google Scholar] [CrossRef]
- Bishop, G.H. Fertilization in the honey-bee. I. The male sexual organs: Their histological structure and physiological functioning. J. Exp. Zool. 1920, 31, 224–265. [Google Scholar] [CrossRef]
- Elhamalawy, O.; Bakr, A.; Eissa, F. Impact of pesticides on non-target invertebrates in agricultural ecosystems. Pest. Biochem. Physiol. 2024, 202, 105974. [Google Scholar] [CrossRef] [PubMed]
- Mitkovska, V.; Chassovnikarova, T.; Vasileva, P.; Stoyanov, I.; Petrov, P.; Petkov, N.; Ivanova, E.N. Sperm comet assay as a novel tool in assessing genotoxicity in high-mortality honey bee (Apis mellifera) populations. Apidologie 2025, 56, 13. [Google Scholar] [CrossRef]
- Mitkovska, V.; Chassovnikarova, T.; Vasileva, P.; Stoyanov, I.; Petrov, P.; Ivanova, E.N. Pesticide stress induces spermatozoa DNA damage and morphological abnormalities in Apis mellifera populations. Environ. Toxicol. Pharmacol. 2025, 116, 104710. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Taherianfard, M. The effects of heavy metals exposure on reproductive system of cyprinid fish from Kor River. Iran. J. Fish. Sci. 2011, 10, 13–26. [Google Scholar]
- Babazadeh, M.; Najafi, G. Effect of chlorpyrifos on sperm characteristics and testicular tissue changes in adult male rats. Vet. Res. Forum. 2017, 8, 319–326. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Sandroc, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Entomol. 2014, 16, 119–128. [Google Scholar] [CrossRef]
- Baines, D.; Wilton, E.; Pawluk, A.; de Gorter, M.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 2017, 7, 10979. [Google Scholar] [CrossRef]
- Basak, M.; Choudhury, R.A.; Goswami, P.; Dey, B.K.; Laskar, M.A. A review on non-target toxicity of deltamethrin and piperonyl butoxide. J. Pharm. Res. Int. 2021, 33, 85–89. [Google Scholar] [CrossRef]
- Abderkader, F.B.; Kairo, G.; Tchamitchian, S.; Bonnet, M.; Cousin, M.; Barbouche, N.; Belzunces, L.P.; Brunet, J.L. Effects of Clothianidin exposure on semen parameters of honey bee drones. J. New Sci. 2018, 59, 3791–3798. [Google Scholar]
- Ko, C.-Y.; Nai, Y.-S.; Lo, W.; Chen, C.-T.; Chen, Y.-W. Low-level fluvalinate treatment in the larval stage induces impaired olfactory associative behavior of honey bee workers in the field. Insects 2022, 13, 273. [Google Scholar] [CrossRef]
- Arslan, O.C.; Erdem, B.; Somel, M.; Giray, T.; Kence, M. Effects of coumaphos on locomotor activities of different honey bee (Apis mellifera L.) subspecies and ecotypes. Apidologie 2023, 54, 39. [Google Scholar] [CrossRef]
- Swiatly-Blaszkiewicz, A.; Klupczynska-Gabryszak, A.; Matuszewska-Mach, E.; Matysiak, J.; Attard, E.; Kowalczyk, D.; Adamkiewicz, A.; Kupcewicz, B.; Matysiak, J. Pesticides in honey bee products—Determination of pesticides in bee pollen, propolis, and royal jelly from Polish apiary. Molecules 2025, 30, 275. [Google Scholar] [CrossRef]
- Gregorc, A.; Alburaki, M.; Rinderer, N.; Sampson, B.; Knight, P.R.; Karim, S.; Adamczyk, J. Effects of coumaphos and imidacloprid on honey bee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Sci. Rep. 2018, 8, 15003. [Google Scholar] [CrossRef] [PubMed]
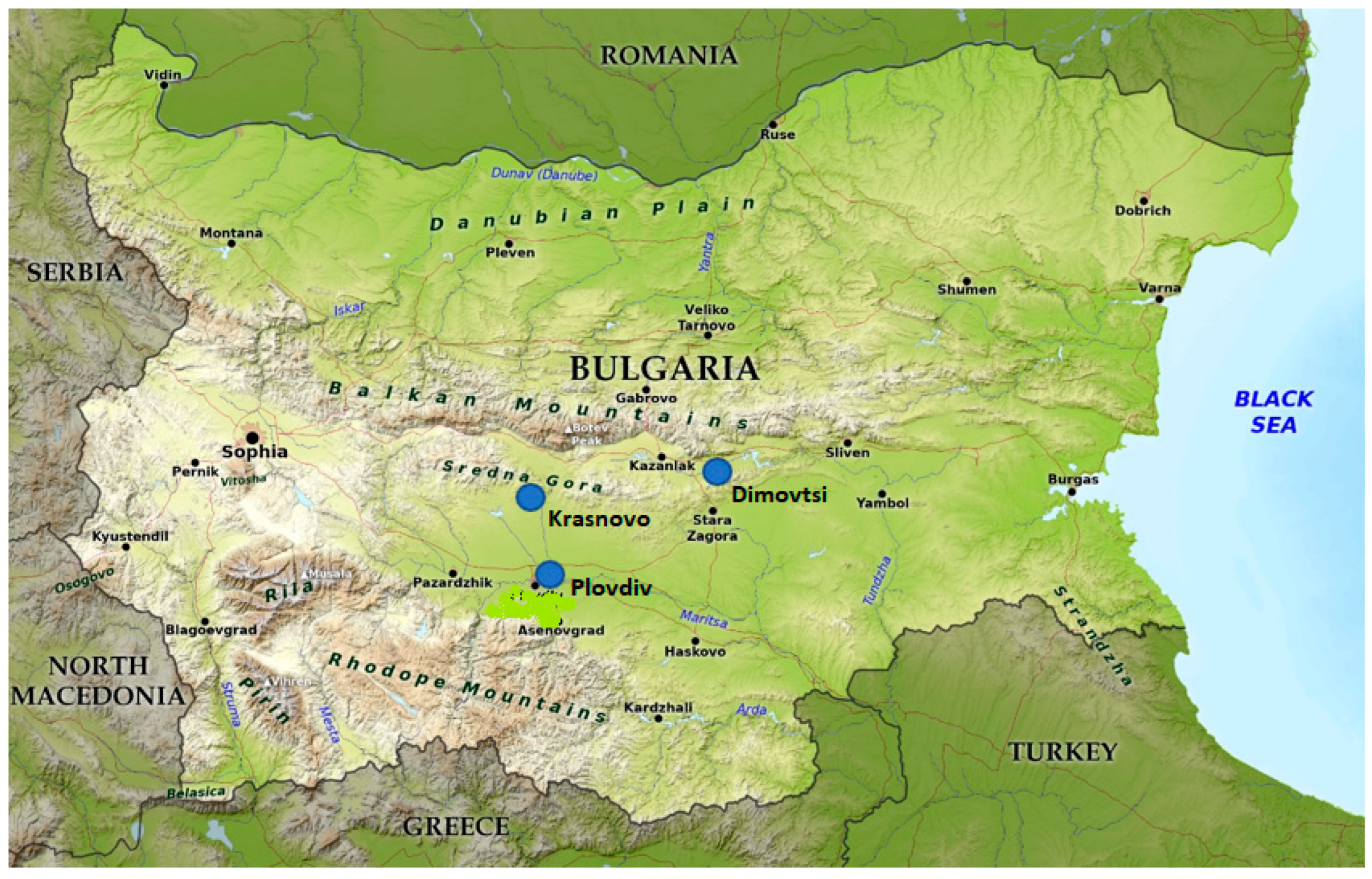

| Parameter | Krasnovo | Dimovtsi | Plovdiv |
|---|---|---|---|
| Queen problem losses % | 0 | 0 | 8.43 |
| Losses due to bee mortality % | 0 | 19.74 | 32.53 |
| Total loss % | 0 | 19.74 | 40.96 |
| Reported treatment | Oxalic acid | Oxalic acid Amitraz | No treatment |
| Risky vegetation | No | Orchards | Orchards, rapeseed, sunflower, maize |
| Chemical | Dimovtsi | Krasnovo | Plovdiv | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dead Honey Bees | Alive Honey Bees | Food Stocks | Dead Honey Bees | Alive Honey Bees | Food Stocks | Dead Honey Bees | Alive Honey Bees | Food Stocks | |
| Amitraz | BDL | BDL | BDL | BDL | BDL | BDL | 0.0039 ± 0.0003 | BDL | BDL |
| Carbendazim | BDL | BDL | BDL | BDL | BDL | BDL | 0.0011 ± 0.0001 | BDL | BDL |
| Clothianidin | 0.0203 ± 0.0015 A | BDL | BDL | BDL | BDL | BDL | 0.0030 ± 0.0002 B | BDL | BDL |
| Coumaphos | BDL | 0.0433 ± 0.0012 A | 0.3133 ± 0.0208 B | BDL | BDL | BDL | BDL | 0.0617 ± 0.0021 A | 0.0603 ± 0.0015 A |
| Cypermethrin | 0.0103 ± 0.0006 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| DEET (N,N-diethyl-M-toluamide) | BDL | BDL | BDL | BDL | BDL | BDL | BDL | 0.0387 ± 0.0015 | BDL |
| Dimethoate | BDL | BDL | BDL | BDL | BDL | BDL | 0.0011 ± 0.0002 | BDL | BDL |
| Fenamidone | BDL | BDL | BDL | BDL | BDL | BDL | 0.0013 ± 0.0002 | BDL | BDL |
| Fenbutatin oxide | BDL | BDL | BDL | BDL | BDL | BDL | 0.0032 ± 0.0002 | BDL | BDL |
| Fenoxycarb | BDL | BDL | BDL | BDL | BDL | BDL | 0.0018 ± 0.0001 | BDL | BDL |
| Flonicamid | BDL | BDL | BDL | BDL | BDL | BDL | 0.0052 ± 0.0002 | BDL | BDL |
| Fluvalinate | BDL | BDL | BDL | BDL | BDL | 0.0057 ± 0.0015 A | BDL | BDL | 0.0237 ± 0.0015 B |
| Fluxapyroxad | 0.0620 ± 0.0020 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Hexythiazox | BDL | BDL | BDL | BDL | BDL | BDL | 0.0015 ± 0.0002 | BDL | BDL |
| Linuron | BDL | BDL | BDL | BDL | BDL | BDL | 0.0023 ± 0.0002 | BDL | BDL |
| Piperonyl-butoxide | BDL | BDL | BDL | BDL | BDL | 0.0073 ± 0.0021 | BDL | BDL | BDL |
| Prosulfocarb | BDL | BDL | BDL | BDL | BDL | BDL | 0.0035 ± 0.0002 | BDL | BDL |
| Pyraclostrobin | 0.1433 ± 0.0208 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Tebuconazole | BDL | BDL | BDL | BDL | BDL | BDL | 0.0026 ± 0.0004 | BDL | BDL |
| Thiamethoxam | 0.0071 ± 0.0052 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Thiophanate-methyl | BDL | BDL | BDL | BDL | BDL | BDL | 0.1367 ± 0.0153 | BDL | BDL |
| Histopathological Lesion | Krasnovo (Control) | Dimovtsi | Plovdiv | |||
|---|---|---|---|---|---|---|
| Mature Drones | Immature Drones | Mature Drones | Immature Drones | Mature Drones | Immature Drones | |
| Thinning of the covering epithelium of the seminiferous tubules | 0 A | 0 A | 1.90 ± 0.74 B | 1.00 ± 0.67 C | 0.80 ± 0.63 C | 0.90 ± 0.57 C |
| Detachment of the basement membrane of the seminiferous tubule | 0 A | 0 A | 2.20 ± 0.79 B | 1.30 ± 0.48 C | 1.00 ± 0.67 C | 1.00 ± 0.67 C |
| Necrotic areas | 0 A | 0 A | 0.70 ± 0.67 A | 0.60 ± 0.52 A | 2.10 ± 0.74 B | 2.00 ± 0.94 B |
| Lack of spermatozoa (only in mature drones) | 0 A | 0 A | 0.20 ± 0.42 A | - | 0 A | - |
| Absence of lumen (only in mature drones) | 0 A | 0 A | 1.00 ± 0.67 B | - | 0 A | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, S.; Georgieva, E.; Petrov, P.; Yancheva, V.; Antal, L.; Somogyi, D.; Nyeste, K.; Ivanova, E.N. Pesticide Pollution Provokes Histopathological Alterations in Apis mellifera (Linnaeus, 1758) Drone Gonads. Environments 2025, 12, 173. https://doi.org/10.3390/environments12060173
Stoyanova S, Georgieva E, Petrov P, Yancheva V, Antal L, Somogyi D, Nyeste K, Ivanova EN. Pesticide Pollution Provokes Histopathological Alterations in Apis mellifera (Linnaeus, 1758) Drone Gonads. Environments. 2025; 12(6):173. https://doi.org/10.3390/environments12060173
Chicago/Turabian StyleStoyanova, Stela, Elenka Georgieva, Plamen Petrov, Vesela Yancheva, László Antal, Dóra Somogyi, Krisztián Nyeste, and Evgeniya N. Ivanova. 2025. "Pesticide Pollution Provokes Histopathological Alterations in Apis mellifera (Linnaeus, 1758) Drone Gonads" Environments 12, no. 6: 173. https://doi.org/10.3390/environments12060173
APA StyleStoyanova, S., Georgieva, E., Petrov, P., Yancheva, V., Antal, L., Somogyi, D., Nyeste, K., & Ivanova, E. N. (2025). Pesticide Pollution Provokes Histopathological Alterations in Apis mellifera (Linnaeus, 1758) Drone Gonads. Environments, 12(6), 173. https://doi.org/10.3390/environments12060173








