Air Pollution and Breast Cancer Risk: An Umbrella Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Umbrella Review Methods
2.2. Objective, Inclusion Criteria, and Exclusion Criteria
2.2.1. Review Question
2.2.2. Objective
2.2.3. Inclusion Criteria
2.2.4. Exclusion Criteria
- It clearly detailed the methodology used for systematic review;
- It provided details on the search strategy employed;
- It identified relevant primary studies from at least one database (e.g., PubMed OR Embase);
- It performed a quality appraisal of the include primary studies.
2.3. Search Strategy and Data Extraction
2.4. Meta-Analytical Methods Used by the Authors of the Included Studies
2.5. Quality of Assessment
2.6. Evaluation of the Strength of Evidence
3. Results
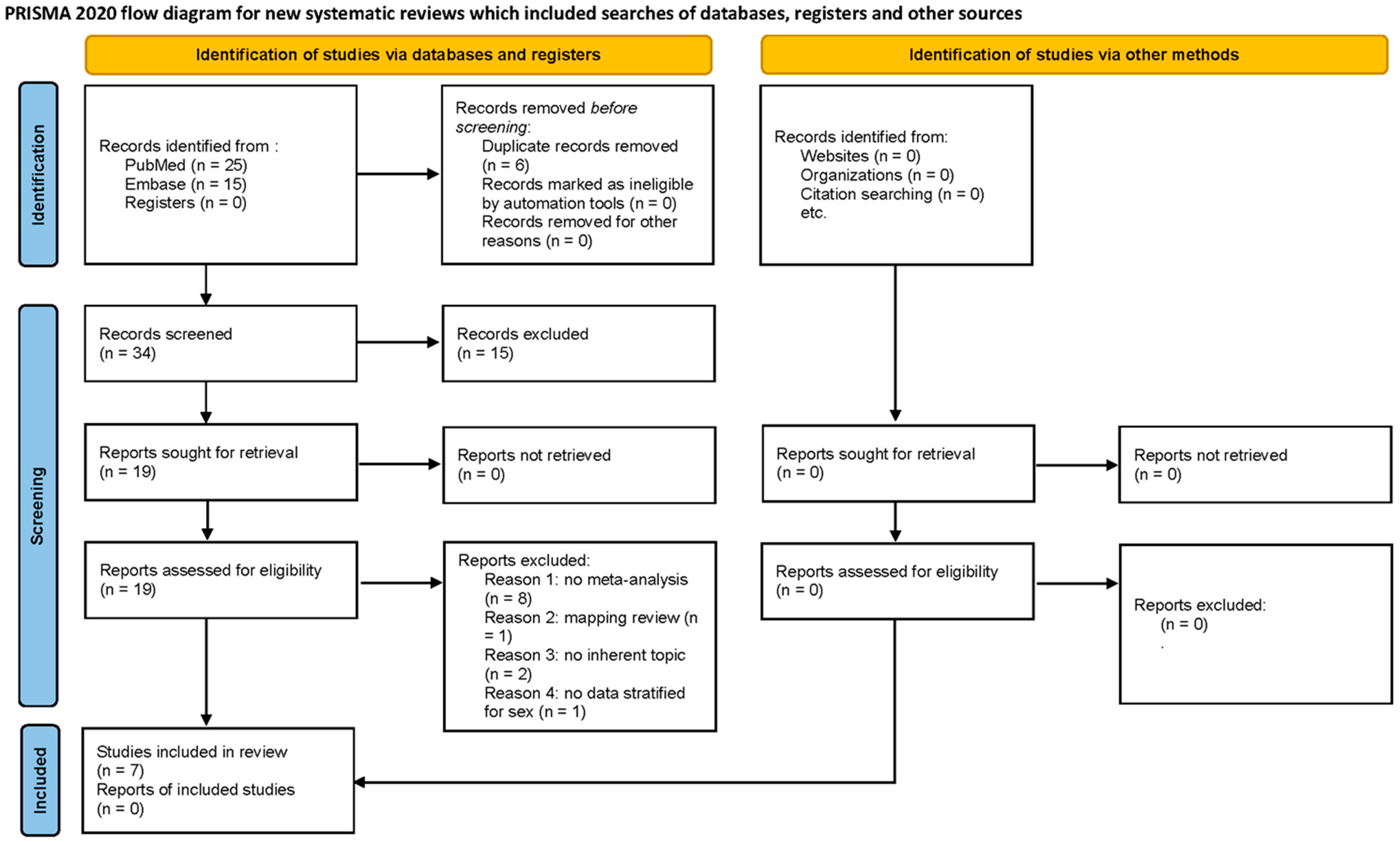
3.1. Search Strategy Outcome
3.2. Quality Assessment and Bias
3.3. Associations Between NO2, PM10, and PM2.5 Exposure and Breast Cancer
3.3.1. NO2 Exposure and BC Incidence
3.3.2. PM10 Exposure and BC Incidence
3.3.3. PM2.5 Exposure and BC Incidence
3.3.4. PM2.5 Exposure and BC Mortality
3.3.5. PM10 Exposure and BC Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, R.A.; Porco, T.C.; Liu, F.; Balke, K.; Balmain, A.; Barlow, J.; Braithwaite, D.; Diez-Roux, A.V.; Kushi, L.H.; Moasser, M.M.; et al. A multilevel model of postmenopausal breast cancer incidence. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2078–2092. [Google Scholar] [CrossRef]
- Scala, M.; Bosetti, C.; Bagnardi, V.; Possenti, I.; Specchia, C.; Gallus, S.; Lugo, A. Dose-response relationships between cigarette smoking and breast cancer risk: A systematic review and meta-analysis. J. Epidemiol. 2023, 33, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Stoll, C.R.; Anandarajah, A.; Doering, M.; Colditz, G.A. Modifiable risk factors in women at high risk of breast cancer: A systematic review. Breast Cancer Res. 2023, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Migliavacca Zucchetti, B.; Peccatori, F.A.; Codacci-Pisanelli, G. Pregnancy and Lactation: Risk or Protective Factors for Breast Cancer? Adv. Exp. Med. Biol. 2020, 1252, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Kudiarasu, C.; Lopez, P.; Galvão, D.A.; Newton, R.U.; Taaffe, D.R.; Mansell, L.; Fleay, B.; Saunders, C.; Fox-Harding, C.; Singh, F. What are the most effective exercise, physical activity and dietary interventions to improve body composition in women diagnosed with or at high-risk of breast cancer? A systematic review and network meta-analysis. Cancer 2023, 129, 3697–3712. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans: Night Shift Work 2020; IARC: Lyon, France, 2020; Volume 124.
- Rutkowska, A.Z.; Szybiak, A.; Serkies, K.; Rachoń, D. Endocrine disrupting chemicals as potential risk factor for estrogen-dependent cancers. Pol. Arch. Med. Wewn. 2016, 126, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.M.; Udesky, J.O.; Rudel, R.A.; Brody, J.G. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ. Res. 2018, 160, 152–182. [Google Scholar] [CrossRef] [PubMed]
- Ahern Thomas, P.; Timothy, A.B.; Lash, L.; Cronin-Fenton, D.P.; Ulrichsen, S.P.; Christiansen, P.M.; Cole, B.F.; Tamimi, R.M.; Sørensen, H.T.; Damkier, P. Phthalate exposure and breast cancer incidence: A Danish nationwide cohort study. J. Clin. Oncol. 2019, 37, 1800–1809. [Google Scholar] [CrossRef]
- Kay, J.E.; Cardona, B.; Rudel, R.A.; Vandenberg, L.N.; Soto, A.M.; Christiansen, S.; Birnbaum, L.S.; Fenton, S.E. Chemical Effects on Breast Development, Function, and Cancer Risk: Existing Knowledge and New Opportunities. Curr. Environ. Health Rpt. 2022, 9, 535–562. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, M.; Fourie, C.; Stone, W.; Engelbrecht, A.M. The impact of endocrine disrupting compounds and carcinogens in wastewater: Implications for breast cancer. Biochimie 2023, 209, 103–115. [Google Scholar] [CrossRef]
- White, A.; Teitelbaum, S.; Stellman, S.; Beyea, J.; Steck, S.; Mordukhovich, I.; McCarty, K.; Ahn, J.; Rossner, P.; Santella, R.; et al. Indoor air pollution exposure from use of indoor stoves and fireplaces in association with breast cancer: A case-control study. Environ. Health 2014, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Sandler, D.P. Indoor wood-burning stove and fireplace use and breast cancer in a prospective cohort study. Environ. Health Perspect. 2017, 125, 077011. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Hurley, S.; Nelson, D.O.; Hertz, A.; Reynolds, P. Hazardous air pollutants and breast cancer risk in California teachers: A cohort study. Environ. Health 2015, 14, 14. [Google Scholar] [CrossRef]
- Cheng, I.; Yang, J.; Tseng, C.; Wu, J.; Conroy, S.M.; Shariff-Marco, S.; Gomez, S.L.; Whittemore, A.S.; Stram, D.O.; Le Marchand, L.; et al. Outdoor ambient air pollution and breast cancer survival among California participants of the Multiethnic Cohort Study. Environ. Int. 2022, 161, 107088. [Google Scholar] [CrossRef]
- White, A.J. Invited Perspective: Air Pollution and Breast Cancer Risk: Current State of the Evidence and Next Steps. Environ. Health Perspect. 2021, 129, 51302. [Google Scholar] [CrossRef]
- Zeinomar, N.; Oskar, S.; Kehm, R.D.; Sahebzeda, S.; Terry, M.B. Environmental exposures and breast cancer risk in the context of underlying susceptibility: A systematic review of the epidemiological literature. Environ. Res. 2020, 187, 109346. [Google Scholar] [CrossRef]
- Terre-Torras, I.; Recalde, M.; Díaz, Y.; de Bont, J.; Bennett, M.; Aragón, M.; Cirach, M.; O’Callaghan-Gordo, C.; Nieuwenhuijsen, M.J.; Duarte-Salles, T. Air pollution and green spaces in relation to breast cancer risk among pre and postmenopausal women: A mega cohort from Catalonia. Environ. Res. 2022, 214, 113838. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, R.; Schiller, J. Air pollution and its impact on cancer incidence, cancer care and cancer outcomes. BMJ Oncol. 2025, 4, e000535. [Google Scholar] [CrossRef]
- Tippila, J.; Wah, N.L.S.; Akbar, K.A.; Bhummaphan, N.; Wongsasuluk, P.; Kallawicha, K. Ambient Air Pollution Exposure and Breast Cancer Risk Worldwide: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2024, 21, 1713. [Google Scholar] [CrossRef]
- Hart, J.E.; Bertrand, K.A.; DuPre, N.; James, P.; Vieira, V.M.; Tamimi, R.M.; Laden, F. Long-term particulate matter exposures during adulthood and risk of breast cancer incidence in the Nurses’ Health Study II prospective cohort. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1274–1276. [Google Scholar] [CrossRef]
- Wei, Y.; Davis, J.; Bina, W.F. Ambient air pollution is associated with the increased incidence of breast cancer in, U.S. Int. J. Environ. Health Res. 2012, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Definition of outdoor air pollution. In Outdoor Air Pollution; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. JBI Evid. Implement. 2015, 13, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. BMJ Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n 160. [Google Scholar] [CrossRef]
- Pieper, D.; Antoine, S.L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2020, 73, 1–19. [Google Scholar]
- Ioannidis, J.P. The importance of predefined rules and prespecified statistical analyses: Do not abandon significance. JAMA 2019, 321, 2067–2068. [Google Scholar] [CrossRef] [PubMed]
- Onyije, F.M.; Olsson, A.; Baaken, D.; Erdmann, F.; Stanulla, M.; Wollschlaeger, D.; Schuez, J. Environmental risk factors for childhood acute lymphoblastic leukemia: An umbrella review. Cancers 2022, 14, 382. [Google Scholar] [CrossRef]
- Gabet, S.; Lemarchand, C.; Guénel, P.; Slama, R. Breast Cancer Risk in Association with Atmospheric Pollution Exposure: A Meta-Analysis of Effect Estimates Followed by a Health Impact Assessment. Environ. Health Perspect. 2021, 129, 57012. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, X.; Gao, Y.; Zhou, J.; Huang, C.; Zhang, Z.; Chu, H. Relationship between particulate matter exposure and female breast cancer incidence and mortality: A systematic review and meta-analysis. Int. Arch. Occupupational Environ. Health 2021, 94, 191–201. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, W.; Chen, Q.; Zhou, N.; Xu, Y. The relationship between exposure to particulate matter and breast cancer incidence and mortality: A meta-analysis. Medicine 2019, 98, e18349. [Google Scholar] [CrossRef]
- Yu, P.; Guo, S.; Xu, R.; Ye, T.; Li, S.; Sim, M.R.; Abramson, M.J.; Guo, Y. Cohort studies of long-term exposure to outdoor particulate matter and risks of cancer: A systematic review and meta-analysis. Innovation 2021, 2, 100143. [Google Scholar] [CrossRef]
- Wei, W.; Wu, B.J.; Wu, Y.; Tong, Z.T.; Zhong, F.; Hu, C.Y. Association between long-term ambient air pollution exposure and the risk of breast cancer: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 63278–63296. [Google Scholar] [CrossRef]
- Praud, D.; Deygas, F.; Amadou, A.; Bouilly, M.; Turati, F.; Bravi, F.; Xu, T.; Grassot, L.; Coudon, T.; Fervers, B. Traffic-Related Air Pollution and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Cancers 2023, 15, 927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arif, I.; Adams, M.D.; Johnson, M.T.J. A meta-analysis of the carcinogenic effects of particulate matter and polycyclic aromatic hydrocarbons. Environ. Pollut. 2024, 351, 123941. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Ravnskjær, L.; Andersen, K.K.; Loft, S.; Brandt, J.; Becker, T.; Ketzel, M.; Hertel, O.; Lynge, E.; Bräuner, E.V. Long-Term Exposure to Fine Particulate Matter and Breast Cancer Incidence in the Danish Nurse Cohort Study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017, 26, 428–430. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Stafoggia, M.; Weinmayr, G.; Pedersen, M.; Galassi, C.; Jørgensen, J.T.; Oudin, A.; Forsberg, B.; Olsson, D.; Oftedal, B.; et al. Long-Term Exposure to Ambient Air Pollution and Incidence of Postmenopausal Breast Cancer in 15 European Cohorts within the ESCAPE Project. Environ. Health Perspect. 2017, 125, 107005. [Google Scholar] [CrossRef]
- Bai, L.; Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Goldberg, M.S.; Lavigne, E.; Weichenthal, S.; Martin, R.V.; et al. Exposure to Ambient Air Pollution and the Incidence of Lung Cancer and Breast Cancer in the Ontario Population Health and Environment Cohort. Int. J. Cancer 2019, 146, 2450–2459. [Google Scholar] [CrossRef]
- Cheng, I.; Tseng, C.; Wu, J.; Yang, J.; Conroy, S.M.; Shariff-Marco, S.; Li, L.; Hertz, A.; Gomez, S.L.; Le Marchand, L.; et al. Association between Ambient Air Pollution and Breast Cancer Risk: The Multiethnic Cohort Study. Int. J. Cancer 2020, 146, 699–711. [Google Scholar] [CrossRef]
- Cohen, G.; Levy, I.; Yuval; Kark, J.D.; Levin, N.; Witberg, G.; Iakobishvili, Z.; Bental, T.; Broday, D.M.; Steinberg, D.M.; et al. Chronic Exposure to Traffic-Related Air Pollution and Cancer Incidence among 10,000 Patients Undergoing Percutaneous Coronary Interventions: A Historical Prospective Study. Eur. J. Prev. Cardiol. 2018, 25, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Crouse, D.L.; Goldberg, M.S.; Ross, N.A.; Chen, H.; Labrèche, F. Postmenopausal Breast Cancer Is Associated with Exposure to Traffic-Related Air Pollution in Montreal, Canada: A Case-Control Study. Environ. Health Perspect. 2010, 118, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Datzmann, T.; Markevych, I.; Trautmann, F.; Heinrich, J.; Schmitt, J.; Tesch, F. Outdoor Air Pollution, Green Space, and Cancer Incidence in Saxony: A Semi-Individual Cohort Study. BMC Public. Health 2018, 18, 715. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Labrèche, F.; Weichenthal, S.; Lavigne, E.; Valois, M.-F.; Hatzopoulou, M.; Van Ryswyk, K.; Shekarrizfard, M.; Villeneuve, P.J.; Crouse, D.; et al. The Association between the Incidence of Postmenopausal Breast Cancer and Concentrations at Street-Level of Nitrogen Dioxide and Ultrafine Particles. Environ. Res. 2017, 158, 7–15. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Villeneuve, P.J.; Crouse, D.; To, T.; Weichenthal, S.A.; Wall, C.; Miller, A.B. Associations between Incident Breast Cancer and Ambient Concentrations of Nitrogen Dioxide from a National Land Use Regression Model in the Canadian National Breast Screening Study. Environ. Int. 2019, 133, 105182. [Google Scholar] [CrossRef] [PubMed]
- Hystad, P.; Villeneuve, P.J.; Goldberg, M.S.; Crouse, D.L.; Johnson, K. Canadian Cancer Registries Epidemiology Research Group Exposure to Traffic-Related Air Pollution and the Risk of Developing Breast Cancer among Women in Eight Canadian Provinces: A Case-Control Study. Environ. Int. 2015, 74, 240–248. [Google Scholar] [CrossRef]
- Lemarchand, C.; Gabet, S.; Cénée, S.; Tvardik, N.; Slama, R.; Guénel, P. Breast Cancer Risk in Relation to Ambient Concentrations of Nitrogen Dioxide and Particulate Matter: Results of a Population-Based Case-Control Study Corrected for Potential Selection Bias (the CECILE Study). Environ. Int. 2021, 155, 106604. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Sørensen, M.; Hansen, J.; Loft, S.; Overvad, K.; Tjønneland, A. Air Pollution from Traffic and Cancer Incidence: A Danish Cohort Study. Environ. Health Glob. Access Sci. Source 2011, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Reding, K.W.; Young, M.T.; Szpiro, A.A.; Han, C.J.; DeRoo, L.A.; Weinberg, C.; Kaufman, J.D.; Sandler, D.P. Breast cancer risk in relation to ambient air pollution exposure at residences in the Sister Study cohort. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Keller, J.P.; Zhao, S.; Carroll, R.; Kaufman, J.D.; Sandler, D.P. Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A U.S.-Wide Cohort. Environ. Health Perspect. 2019, 127, 107002. [Google Scholar] [CrossRef]
- White, A.J.; Gregoire, A.M.; Niehoff, N.M.; Bertrand, K.A.; Palmer, J.R.; Coogan, P.F.; Bethea, T.N. Air Pollution and Breast Cancer Risk in the Black Women’s Health Study. Environ. Res. 2021, 194, 110651. [Google Scholar] [CrossRef]
- Amadou, A.; Praud, D.; Coudon, T.; Deygas, F.; Grassot, L.; Dubuis, M.; Faure, E.; Couvidat, F.; Caudeville, J.; Bessagnet, B.; et al. Long-Term Exposure to Nitrogen Dioxide Air Pollution and Breast Cancer Risk: A Nested Case-Control within the French E3N Cohort Study. Environ. Pollut. 2022, 317, 120719. [Google Scholar] [CrossRef]
- Shin, M.; Kim, O.J.; Yang, S.; Choe, S.A.; Kim, S.Y. Different mortality risks of long-term exposure to particulate matter across different cancer sites. Int. J. Environ. Res. Public. Health 2022, 19, 3180. [Google Scholar] [CrossRef]
- Coleman, N.C.; Burnett, R.T.; Ezzati, M.; Marshall, J.D.; Robinson, A.L.; Pope, C.A., III. Fine particulate matter exposure and cancer incidence: Analysis of SEER cancer registry data from 1992–2016. Environ. Health Perspect. 2020, 128, 107004. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Goldberg, M.S.; Crouse, D.L.; To, T.; Weichenthal, S.A.; Wall, C.; Miller, A.B. Residential exposure to fine particulate matter air pollution and incident breast cancer in a cohort of Canadian women. Environ. Epidemiol. 2018, 2, e021. [Google Scholar] [CrossRef]
- Poulsen, A.H.; Hvidtfeldt, U.A.; Sørensen, M.; Pedersen, J.E.; Ketzel, M.; Brandt, J.; Geels, C.; Christensen, J.H.; Raaschou-Nielsen, O. Air pollution with NO2, PM2.5, and elemental carbon in relation to risk of breast cancer– a nationwide case-control study from Denmark. Environ. Res. 2023, 216, 11474. [Google Scholar] [CrossRef]
- Prada, D.; Baccarelli, A.A.; Terry, M.B.; Valdéz, L.; Cabrera, P.; Just, A.; Kloog, I.; Caro, H.; García-Cuellar, C.; Sánchez-Pérez, Y.; et al. Long-term PM2.5 exposure before diagnosis is associated with worse outcome in breast cancer. Breast Cancer Res. Treat. 2021, 188, 525–533. [Google Scholar] [CrossRef]
- To, T.; Zhu, J.; Villeneuve, P.J.; Simatovic, J.; Feldman, L.; Gao, C.; Williams, D.; Chen, H.; Weichenthal, S.; Wall, C.; et al. Chronic disease prevalence in women and air pollution—A 30-year longitudinal cohort study. Environ. Int. 2015, 80, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lee, P.H.; Chen, L.C.; Lin, B.C.; Lin, C.; Chan, T.C. Relationships among green space, ambient fine particulate matter, and cancer incidence in Taiwan: A 16-year retrospective cohort study. Environ. Res. 2022, 212, 113416. [Google Scholar] [CrossRef] [PubMed]
- DuPré, N.C.; Hart, J.E.; Holmes, M.D.; Poole, E.M.; James, P.; Kraft, P.; Laden, F.; Tamimi, R.M. Particulate matter and traffic-related exposures in relation to breast cancer survival. Cancer Epidemiol. Biomarkers Prev. 2019, 2, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dailey, A.B.; Kan, H.; Xu, X. The effect of atmospheric particulate matter on survival of breast cancer among US females. Breast Cancer Res. Treat. 2013, 139, 217–226. [Google Scholar] [CrossRef]
- Hung, L.J.; Chan, T.F.; Wu, C.H.; Chiu, H.F.; Yang, C.Y. Traffic air pollution and risk of death from ovarian cancer in Taiwan: Fine particulate matter (PM2.5) as a proxy marker. J. Toxicol. Environ. Health A 2012, 75, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Mizuno, S.; Miyasaka, Y.; Mori, T. Correlation between suspended particles in the environmental air and causes of disease among inhabitants: Cross-sectional studies using the vital statistics and air pollution data in Japan. Environ. Res. 2005, 99, 106–117. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope CA3rd Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient air pollution and cancer mortality in the Cancer Prevention Study II. Environ. Health Perspect. 2017, 125, 087013. [Google Scholar] [CrossRef]
- Wong, C.M.; Tsang, H.; Lai, H.K.; Thomas, G.N.; Lam, K.B.; Chan, K.P.; Zheng, Q.; Ayres, J.G.; Lee, S.Y.; Lam, T.H.; et al. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. Biomark. Prev. 2016, 25, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Yang, J.; Tseng, C.; Wu, J.; Shariff-Marco, S.; Park, S.L.; Conroy, S.M.; Inamdar, P.P.; Fruin, S.; Larson, T.; et al. Traffic-related air pollution and lung cancer incidence: The California Multiethnic Cohort Study. Am. J. Respir. Crit. Care Med. 2022, 206, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xu, R.; Li, S.; Coelho, M.S.Z.S.; Saldiva, P.H.N.; Sim, M.R.; Abramson, M.J.; Guo, Y. Associations between long-term exposure to PM2.5 and site-specific cancer mortality: A nationwide study in Brazil between 2010 and 2018. Environ. Pollut. 2022, 302, 119070. [Google Scholar] [CrossRef]
- White, A.J.; Fisher, J.A.; Sweeney, M.R.; Freedman, N.D.; Kaufman, J.D.; Silverman, D.T.; Jones, R.R. Ambient fine particulate matter and breast cancer incidence in a large prospective US cohort. JNCI J. Natl. Cancer Inst. 2024, 116, 53–60. [Google Scholar] [CrossRef]
- Plusquin, M.; Guida, F.; Polidoro, S.; Vermeulen, R.; Raaschou-Nielsen, O.; Campanella, G.; Hoek, G.; Kyrtopoulos, S.A.; Georgiadis, P.; Naccarati, A.; et al. DNA methylation and exposure to ambient air pollution in two prospective cohorts. Environ. Int. 2017, 108, 127–136. [Google Scholar] [CrossRef] [PubMed]
- van Veldhoven, K.; Polidoro, S.; Baglietto, L.; Severi, G.; Sacerdote, C.; Panico, S.; Mattiello, A.; Palli, D.; Masala, G.; Krogh, V.; et al. Epigenome-wide association study reveals decreased average methylation levels years before breast cancer diagnosis. Clin. Epigenetics 2015, 7, 67. [Google Scholar] [CrossRef]
- Li, S.; Liao, X.; Ma, R.; Deng, N.; Wu, H.; Zhang, Z.; Chen, L.; Wang, Q.; Liao, Q.; Li, Q.; et al. Effects of Co-Exposure to Benzene, Toluene, and Xylene, Polymorphisms of microRNA Genes, and Their Interactions on Genetic Damage in Chinese Petrochemical Workers. Toxics 2024, 12, 821. [Google Scholar] [CrossRef]
- Mordukhovich, I.; Beyea, J.; Herring, A.H.; Hatch, M.; Stellman, S.D.; Teitelbaum, S.L.; Richardson, D.B.; Millikan, R.C.; Engel, L.S.; Shantakumar, S.; et al. Vehicular traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence: The Long Island Breast Cancer Study Project (LIBCSP). Environ. Health Perspect. 2016, 124, 30–38. [Google Scholar] [CrossRef]
- Agudo, A.; Peluso, M.; Munnia, A.; Lujan-Barroso, L.; Barricarte, A.; Amiano, P.; Navarro, C.; Sanchez, M.J.; Quiros, J.R.; Ardanaz, E.; et al. Aromatic DNA adducts and breast cancer risk: A case-cohort study within the EPIC-Spain. Carcinogenesis 2017, 38, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Shu, X.O.; Gao, Y.T.; Ji, B.T.; Yang, G.; Blair, A.; Rothman, N.; Zheng, W.; Chow, W.H.; Kang, D. Breast cancer and urinary biomarkers of polycyclic aromatic hydrocarbon and oxidative stress in the Shanghai Women’s Health Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 877–883. [Google Scholar] [CrossRef]
- Plísková, M.; Vondrácek, J.; Vojtesek, B.; Kozubík, A.; Machala, M. Deregulation of cell proliferation by polycyclic aromatic hydrocarbons in human breast carcinoma MCF-7 cells reflects both genotoxic and nongenotoxic events. Toxicol. Sci. 2004, 83, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Gammon, M.D.; Santella, R.M.; Neugut, A.I.; Eng, S.M.; Teitelbaum, S.L.; Paykin, A.; Levin, B.; Terry, M.B.; Young, T.L.; Wang, L.W.; et al. Environmental toxins and breast cancer on Long Island. I. Polycycl. Aromat. Hydrocarb. DNA Adducts Cancer Epidemiol. Biomark. Prev. 2002, 11, 677–685. [Google Scholar]
- Rundle, A.; Tang, D.L.; Hibshoosh, H.; Estabrook, A.; Schnabel, F.; Cao, W.F.; Grumet, S.; Perera, F.P. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis 2000, 21, 1281–1289. [Google Scholar] [CrossRef]
- Korsh, J.; Shen, A.; Aliano, K.; Davenport, T. Polycyclic aromatic hydrocarbons and breast cancer: A review of the literature. Breast Care 2015, 10, 316–318. [Google Scholar] [CrossRef]
- Madrigal, J.M.; Pruitt, C.N.; Fisher, J.A.; Liao, L.M.; Graubard, B.I.; Gierach, G.L.; Silverman, D.T.; Ward, M.H.; Jones, R.R. Carcinogenic industrial air pollution and postmenopausal breast cancer risk in the National Institutes of Health AARP Diet and Health Study. Environ. Int. 2024, 191, 108985. [Google Scholar] [CrossRef] [PubMed]
- Benbrahim-Tallaa, L.; Baan, R.A.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Loomis, D.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012, 13, 663–664. [Google Scholar] [CrossRef]
- González, L.T.; Pérez-Rodríguez, M.; Rodríguez, F.L.; Mancilla, Y.; Acuña-Askar, K.; Campos, A.; Luis, A.; González, P.; Vidaurri, L.G.S.; Zapata, A.A.; et al. Insights from the combined bulk chemical and surface characterization of airborne PM10 on source contributions and health risk: The case of three Mexican cities. Air Qual. Atmos. Health 2023, 16, 1455–1477. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Chen, J.; Rodopoulou, S.; Strak, M.; de Hoogh, K.; Andersen, Z.J.; Bellander, T.; Brandt, J.; Fecht, D.; Forastiere, F.; et al. Breast cancer incidence in relation to long-term low-level exposure to air pollution in the ELAPSE pooled cohort. Cancer Epidemiol. Biomark. Prev. 2023, 32, 105–113. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; Kang, N.; Wang, N.; Zhang, X.; Liu, S.; Li, H.; Xue, T.; Ji, J. Associations of incident female breast cancer with long-term exposure to PM2.5 and its constituents: Findings from a prospective cohort study in Beijing, China. J. Hazard. Mater. 2024, 473, 134614. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Erdal, S.; Chen, H.Y.; Gann, P.H.; Argos, M.; Rauscher, G.H. Metallic air pollutants and breast cancer heterogeneity. Environ. Res. 2019, 177, 108639. [Google Scholar] [CrossRef]
- Liu, R.; Nelson, D.O.; Hurley, S.; Hertz, A.; Reynolds, P. Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: The California Teachers Study. Epidemiology 2015, 26, 365–373. [Google Scholar] [CrossRef]
- White, A.J.; O’Brien, K.M.; Niehoff, N.M.; Carroll, R.; Sandler, D.P. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology 2019, 30, 20–28. [Google Scholar] [CrossRef]
- Farahzadi, R.; Valipour, B.; Fathi, E.; Pirmoradi, S.; Molavi, O.; Montazersaheb, S.; Sanaat, Z. Oxidative stress regulation and related metabolic pathways in epithelial-mesenchymal transition of breast cancer stem cells. Stem Cell Res. Ther. 2023, 14, 342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazzuferi, G.; Bacchetti, T.; Islam, M.O.; Ferretti, G. High density lipoproteins and oxidative stress in breast cancer. Lipids Health Dis. 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahiraee, A.; Ebrahimi, R.; Halabian, R.; Aghabozorgi, A.S.; Amani, J. The role of inflammation and its related microRNAs in breast cancer: A narrative review. J. Cell Physiol. 2019, 234, 19480–19493. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.; Bandera, E.V.; Greenwood, D.C.; Norat, T. Circulating C-Reactive Protein and Breast Cancer Risk-Systematic Literature Review and Meta-analysis of Prospective Cohort Studies. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Michels, K.B.; Brody, J.G.; Byrne, C.; Chen, S.; Jerry, D.J.; Malecki, K.M.; Martin, M.B.; Miller, R.L.; Neuhausen, S.L.; et al. Environmental exposures during windows of susceptibility for breast cancer: A framework for prevention research. Breast Cancer Res. 2019, 21, 96. [Google Scholar] [CrossRef]
- Board on Health Sciences Policy, Committee on Breast Cancer, The Scientific Evidence, Research Methodology, & Future Directions. Breast Cancer and the Environment: A Life Course Approach; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Cohn, B.A.; Cirillo, P.M.; Terry, M.B. DDT and breast cancer: Prospective study of induction time and susceptibility windows. JNCI J. Natl. Cancer Inst. 2019, 111, 803–810. [Google Scholar] [CrossRef]
- Nie, J.; Beyea, J.; Bonner, M.R.; Han, D.; Vena, J.E.; Rogerson, P.; Vito, D.; Muti, P.; Trevisan, M.; Edge, S.B.; et al. Exposure to traffic emissions throughout life and risk of breast cancer: The Western New York Exposures and Breast Cancer (WEB) study. Cancer Causes Control. 2007, 18, 947–955. [Google Scholar] [CrossRef]
- Smotherman, C.; Sprague, B.; Datta, S.; Braithwaite, D.; Qin, H.; Yaghjyan, L. Association of air pollution with postmenopausal breast cancer risk in UK Biobank. Breast Cancer Res. 2023, 25, 83. [Google Scholar] [CrossRef]
- Lunny, C.; Pieper, D.; Thabet, P.; Kanji, S. Managing overlap of primary study results across systematic reviews: Practical considerations for authors of overviews of reviews. BMC Med. Res. Methodol. 2021, 21, 140. [Google Scholar] [CrossRef]
- Duboeuf, M.; Amadou, A.; Coudon, T.; Grassot, L.; Ramel-Delobel, M.; Faure, E.; Salizzoni, P.; Gulliver, J.; Severi, G.; Mancini, F.R.; et al. Long-term exposure to air pollution at residential and workplace addresses and breast cancer risk: A case-control study nested in the French E3N-Générations cohort from 1990 to 2011. Eur. J. Cancer. 2024, 210, 114293. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Michl, E.L.; Melius, J.M.; Kallenbach, L.R.; Ju, C.L.; Talbot, T.O.; Orr, M.F. Breast cancer risk and residence near industry or traffic in Nassau and Suffolk Counties, Long Island, New York. Arch. Environ. Health An. Int. J. 1996, 51, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Giampiccolo, C.; Amadou, A.; Coudon, T.; Praud, D.; Grassot, L.; Faure, E.; Couvidat, F.; Severi, G.; Mancini, F.R.; Fervers, B.; et al. Multi-pollutant exposure profiles associated with breast cancer risk: A Bayesian profile regression analysis in the French E3N cohort. Environ. Int. 2024, 190, 108943. [Google Scholar] [CrossRef]
| Rating | Description |
|---|---|
| High | No or one non-critical weakness: the meta-analysis provides an accurate and comprehensive summary of the results of the available studies that address the question of interest. |
| Moderate | More than one non-critical weakness *: the meta-analysis has more than one weakness but no critical flaws; it may provide an accurate summary of the results of the available studies that were included in the review. |
| Low | One critical flaw with or without non-critical weaknesses: the review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest. |
| Critically low | More than one critical flaw with or without non-critical weaknesses: the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies. |
| Evidence | Positive Association | Cases | Heterogeneity I2 | Publication Bias |
|---|---|---|---|---|
| Strong | ≥75% | >1000 | 0–40% | Absent: Egger’s test p > 0.10 or Negligible difference in case of trim-and-fill |
| Moderate | ≥50–75% | >1000 | 41–60% | Possible: Egger’s test p = 0.05–<0.10 |
| Modest | ≥25–<50% | <1000 | 61–80% | High: Egger’s test p ≤ 0.05 |
| Weak | 0–<25% | <1000 | 81–100% | High: Egger’s test p ≤ 0.05 |
| Gabet et al., 2021 [39] | Guo et al., 2021 [40] | Zhang et al., 2019 [41] | Yu et al., 2021 [42] | Wei et al., 2021 [43] | Praud et al., 2023 [44] | Arif et al., 2024 [45] | |
|---|---|---|---|---|---|---|---|
| Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Partial Yes | Yes | Yes | Partial Yes |
| Yes | No | Yes | Yes | Yes | Yes | Yes |
| No | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | No | No | Yes | Yes | Yes | Yes |
| No | Yes | Yes | Yes | No | No | No |
| No | No | No | No | No | No | No |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | No | No |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | No |
| Yes | Yes | Yes | Yes | Yes | Yes | No |
| Yes | Yes | Yes | No | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| No | No | No | No | No | Yes | Yes |
| Total of yes | 13/16 (81.2%) | 13/16 (81.2%) | 14/16 (87.5%) | 14/16 (87.5%) | 14/16 (87.5%) | 13/16 (81.2%) | 10.5/16 |
| Rating overall confidence | Low | Moderate | Moderate | Moderate | Moderate | Moderate | Critically low |
| Pollutant | Author, Year | Design of Included Studies | Total Population | Age (Years) | Country | Effect Size RR (95% CI) | Heterogeneity I2 * | Publication Bias ** |
|---|---|---|---|---|---|---|---|---|
| NO2 | Gabet et al., 2021 [39] | 6 cohort, 4 case-control | 3,914,690 | 25–75 | 1 Germany, 2 Sweden, 5 Canada, 2 Denmark, 2 France, 1 Netherlands, 1 UK, 1 Spain, 2 Italy, 1 Norway, 2 USA, 1 Austria | 1.03 (1.01–1.05) | 24 | 0.018 |
| Wei et al., 2021 [43] | 11 cohort, 3 case-control | 4,002,546 | 35–85 | 3 Denmark, 4 USA, 1 Europe, 1 Germany, 1 Israel, 5 Canada | 1.02 (1.01–1.04) | 46.8 | 0.024 | |
| Praud et al., 2023 [44] | 8 cohort, 5 case-control | 128,618 | Not reported | 3 USA, 5 Canada, 1 Denmark, 2 France, 1 Germany, 1 Europe | 1.02 (1.00–1.03) | 16.9 | 0.27 | |
| PM10 | Zhang et al., 2019 [41] | 8 cohort | 592,369 | 25–65+ | 5 USA, 2 Denmark, 1 Netherlands, 1 UK, 1 Italy, 1 Norway, 1 Germany | 1.05 (0.98–1.12) | 72.7 | Not Reported |
| Yu et al., 2021 [42] | 4 cohort | 2,107,018 | 0–90 | 1 Denmark, 1 USA, 1 Germany | 1.05 (0.93–1.19) | 68 | 0.030 | |
| Gabet et al., 2021 [39] | 1 case-control, 5 cohort | 1,326,524 | 0–90 | 1 France, 1 Germany, 1 Sweden, 2 Denmark, 1 Netherlands, 1 UK, 1 Italy, 1 Norway, 3 USA, 1 Austria | 1.06 (0.99–1.13) | 27.6 | 0.41 | |
| Guo et al., 2021 [40] | 1 case-control, 8 cohort | 2,552,761 | 0–90 | 5 USA, 1 Canada, 2 Denmark, 1 Italy | 1.03 (0.98–1.09) | 65.1 | 0.009 | |
| Wei et al., 2021 [43] | 7 cohort | 2,290,241 | 0–90 | 4 USA, 1 Germany, 1 Denmark, 1 Europe | 1.04 (0.98–1.10) | 70.3 | 0.06 | |
| Arif et al., 2024 [45] | 10 | Not reported | Not reported | 4 Europe, 4 Americas | 1.14 (0.97–1.30) | 84.0 | 0.00 | |
| PM2.5 | Yu et al., 2021 [42] | 6 cohort | 2,871,705 | 25–85 | 1 Denmark, 1 USA, 3 Canada | 1.03 (0.93–1.13) | 63 | 0.020 |
| Wei et al., 2021 [43] | 11 cohort | 11,755,200 | 0–90 | 2 Denmark, 6 USA, 1 Europe (Sweden, Norway, Italy, UK, Netherlands, Austria), 3 Canada | 1.03 (0.99–1.06) | 8.2 | 0.00023 | |
| Gabet et al., 2021 [39] | 1 case-control, 6 cohort | 2,848,486 | 25–85 | 1 France, 1 Sweden, 2 Canada, 2 Denmark, 1 Netherlands, 1 UK, 1 Italy, 1 Norway, 3 USA, 1 Austria | 1.01 (0.93–1.11) | 37.4 | 0.72 | |
| Guo et al., 2021 [40] | 11 cohort, 1 case-control, 1 ecological, 1 cross-sectional | 6,643,972 | 25–85 | 7 USA, 2 Canada, 2 China, 2 Denmark, 1 Italy, 1 Japan | 1.04 (0.98–1.10) | 17,4 | 0,293 | |
| Zhang et al., 2019 [41] | 11 cohort, 2 ecological | 994,551 | 25–>65 | 1 Canada, 6 USA, 1 Sweden, 2 Denmark, 1 Netherlands, 1 UK, 1 Austria, 1 France, 3 Italy, 1 Spain, 1 Germany, 1 China, 1 Puerto Rico, 1 Taiwan, 1 Japan | 1.02 (0.93–1.11) | 30.6 | 0.218 | |
| Arif et al., 2024 [45] | 14 | Not reported | Not reported | 4 Europe, 7 Americas | 1.05 (0.98–1.12) | 55.7 | 0.00 |
| Pollutant | Author, Year | Study Design | Total Population (nr) | Cases | Age (Years) | Country | Effect Size RR (95% CI) | Heterogeneity I2 * | Publication Bias ** |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | Yu et al., 2021 [42] | 4 cohort | 756,393 | 7895 | 0–90 | 3 USA, 1 Hong Kong | 1.18 (0.81–1.73) | 70 | 0.02 |
| Zhang et al., 2019 [41] | 5 cohort, 2 ecological | 913,779 | 5439 | 25–65+ | 1 Canada, 6 USA, 1 Sweden, 2 Denmark, 1 Netherlands, 1 UK, 1 Austria, 1 France, 3 Italy, 1 Spain, 1 Germany, 1 China, 1 Puerto Rico, 1 Taiwan, 1 Japan | 1.17 (1.05–1.30) | 73.1 | 0.122 | |
| Guo et al., 2021 [40] | 3 cohort, 1 case-control, 1 ecological, 1 cross-sectional | 692,257 | 51,661 | 25 to 85 | 7 USA, 2 Canada, 2 China, 2 Denmark, 1 Italy, 1 Japan | 1.20 (0.92–1.48) | 52.5 | 0.12 | |
| Arif et al., 2024 [45] | Not reported | Not reported | Not reported | Not reported | Not reported | 1.17 (1.07–1.27) | 55.2 | 0.04 | |
| PM10 | Zhang et al., 2019 [41] | cohort/case-control | 264,064 | 25–55+ | USA | 1.11 (1.02–1.21) | 0.0 | ||
| Guo et al., 2021 [40] | cohort/case-control | 264,064 | 25–55+ | USA | 1.07 (0.93–1.20) | 56.4 | Not NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Palella, M.; Ferroni, E.; Miligi, L.; Portaluri, M.; Marchese, C.A.; Mensi, C.; Civitelli, S.; Tanturri, G.; Mangia, C. Air Pollution and Breast Cancer Risk: An Umbrella Review. Environments 2025, 12, 153. https://doi.org/10.3390/environments12050153
Fiore M, Palella M, Ferroni E, Miligi L, Portaluri M, Marchese CA, Mensi C, Civitelli S, Tanturri G, Mangia C. Air Pollution and Breast Cancer Risk: An Umbrella Review. Environments. 2025; 12(5):153. https://doi.org/10.3390/environments12050153
Chicago/Turabian StyleFiore, Maria, Marco Palella, Eliana Ferroni, Lucia Miligi, Maurizio Portaluri, Cristiana Alessandra Marchese, Carolina Mensi, Serenella Civitelli, Gabriella Tanturri, and Cristina Mangia. 2025. "Air Pollution and Breast Cancer Risk: An Umbrella Review" Environments 12, no. 5: 153. https://doi.org/10.3390/environments12050153
APA StyleFiore, M., Palella, M., Ferroni, E., Miligi, L., Portaluri, M., Marchese, C. A., Mensi, C., Civitelli, S., Tanturri, G., & Mangia, C. (2025). Air Pollution and Breast Cancer Risk: An Umbrella Review. Environments, 12(5), 153. https://doi.org/10.3390/environments12050153










