Linking Antibiotic Residues and Antibiotic Resistance Genes to Water Quality Parameters in Urban Reservoirs: A Seasonal Perspective
Abstract
1. Introduction
2. Materials and Methods
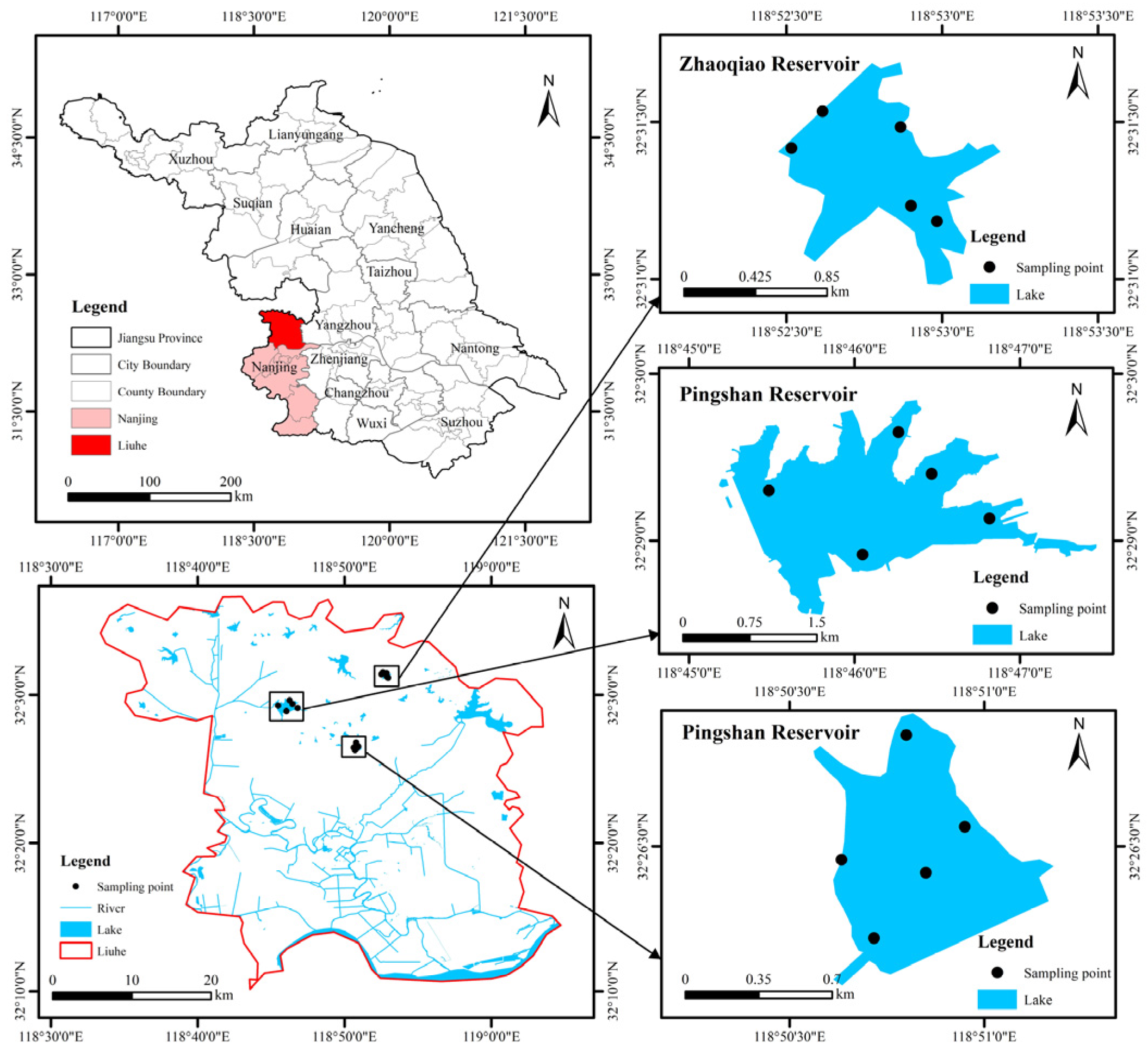
2.1. Study Area
2.2. Water Sampling
2.3. Water Quality Parameters
2.4. Heavy Metal Analysis
2.5. Antibiotic Residues
2.6. ARG Detection
2.7. Risk Quotient Calculation
2.8. Data Analysis
3. Results and Discussion
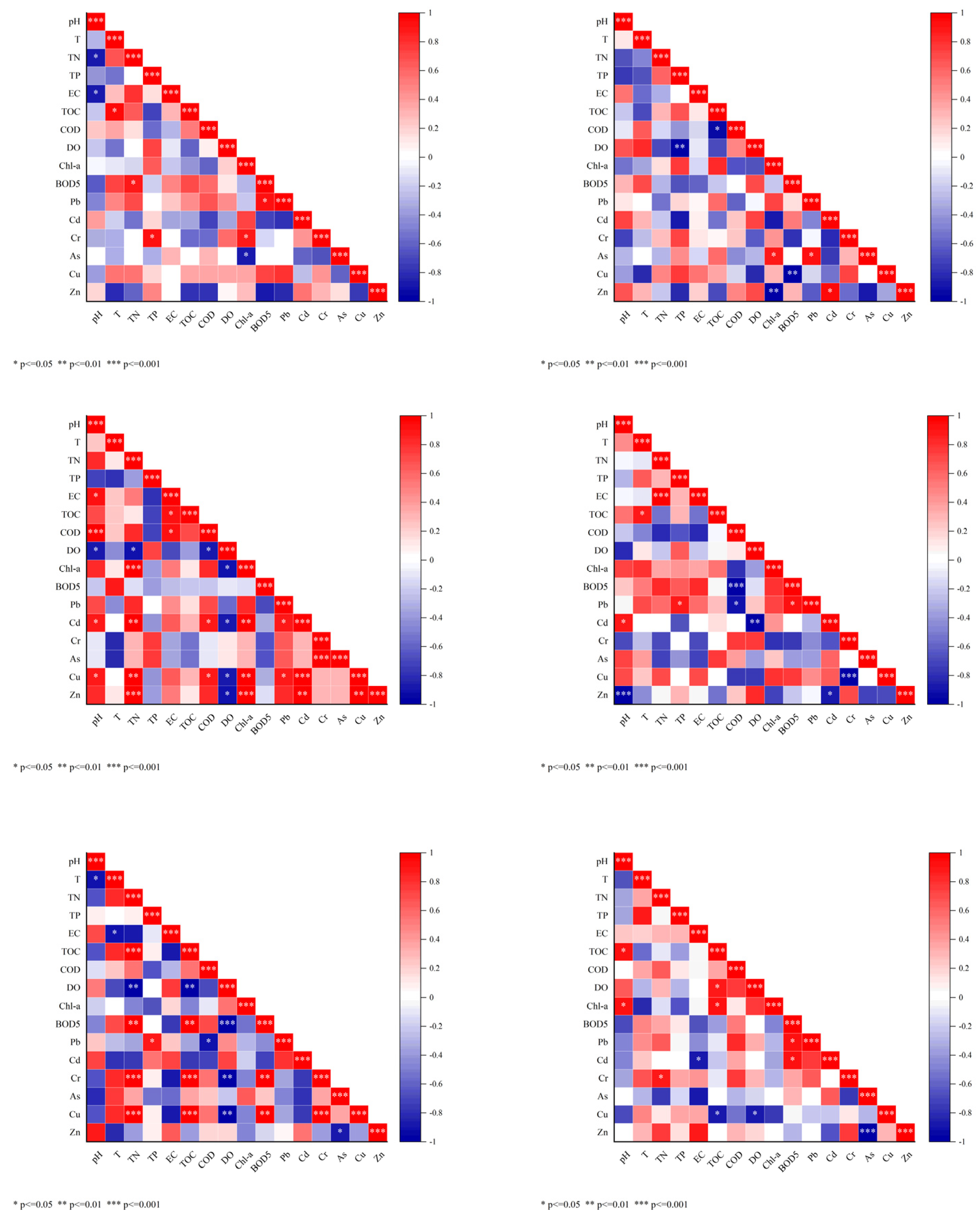
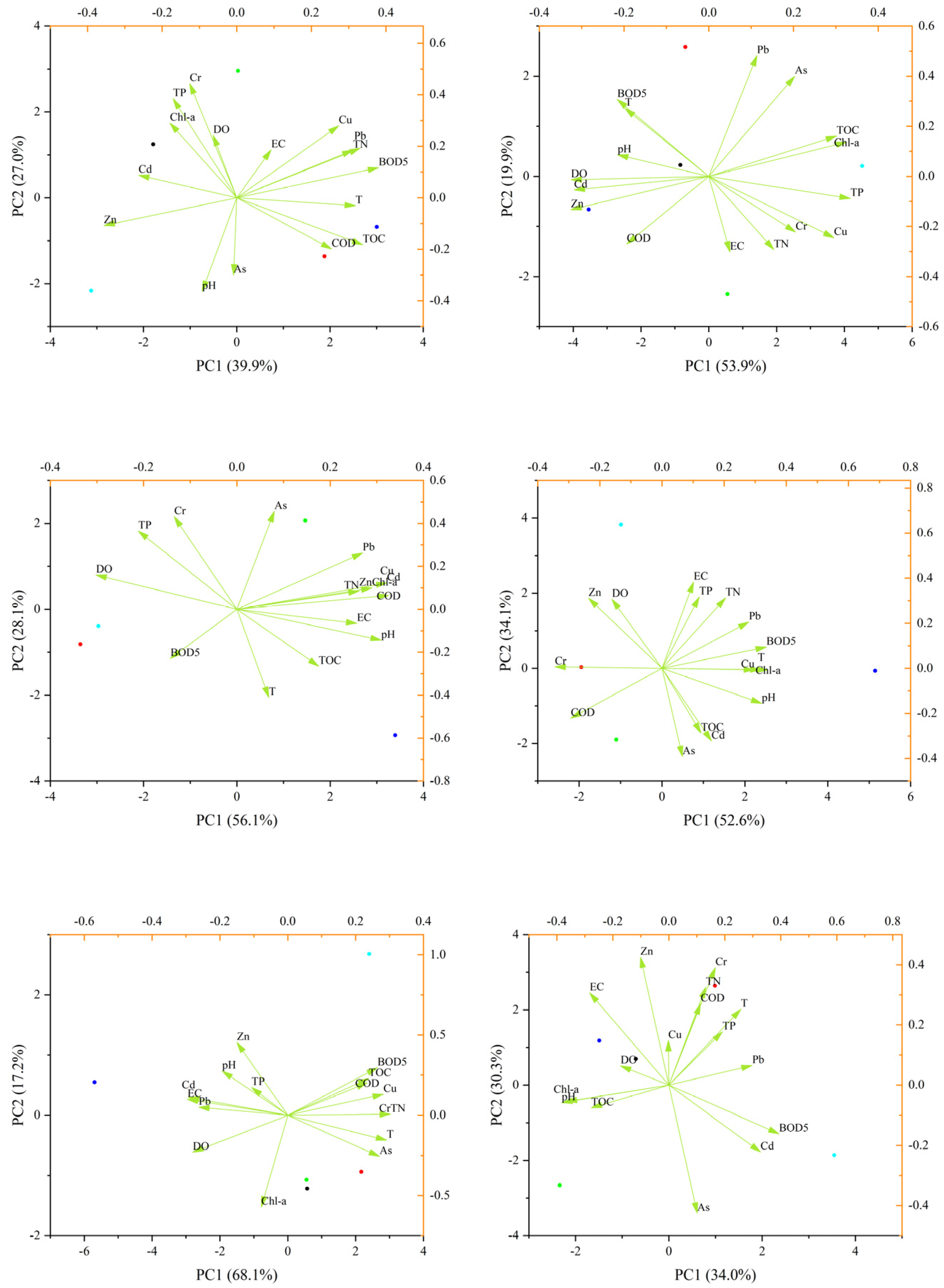
3.1. Seasonal Variations and Relationships in Regard to Water Quality Parameters and Heavy Metals Among Three Reservoirs
3.2. Role of Water Quality Parameters and Heavy Metals in ARG Proliferation
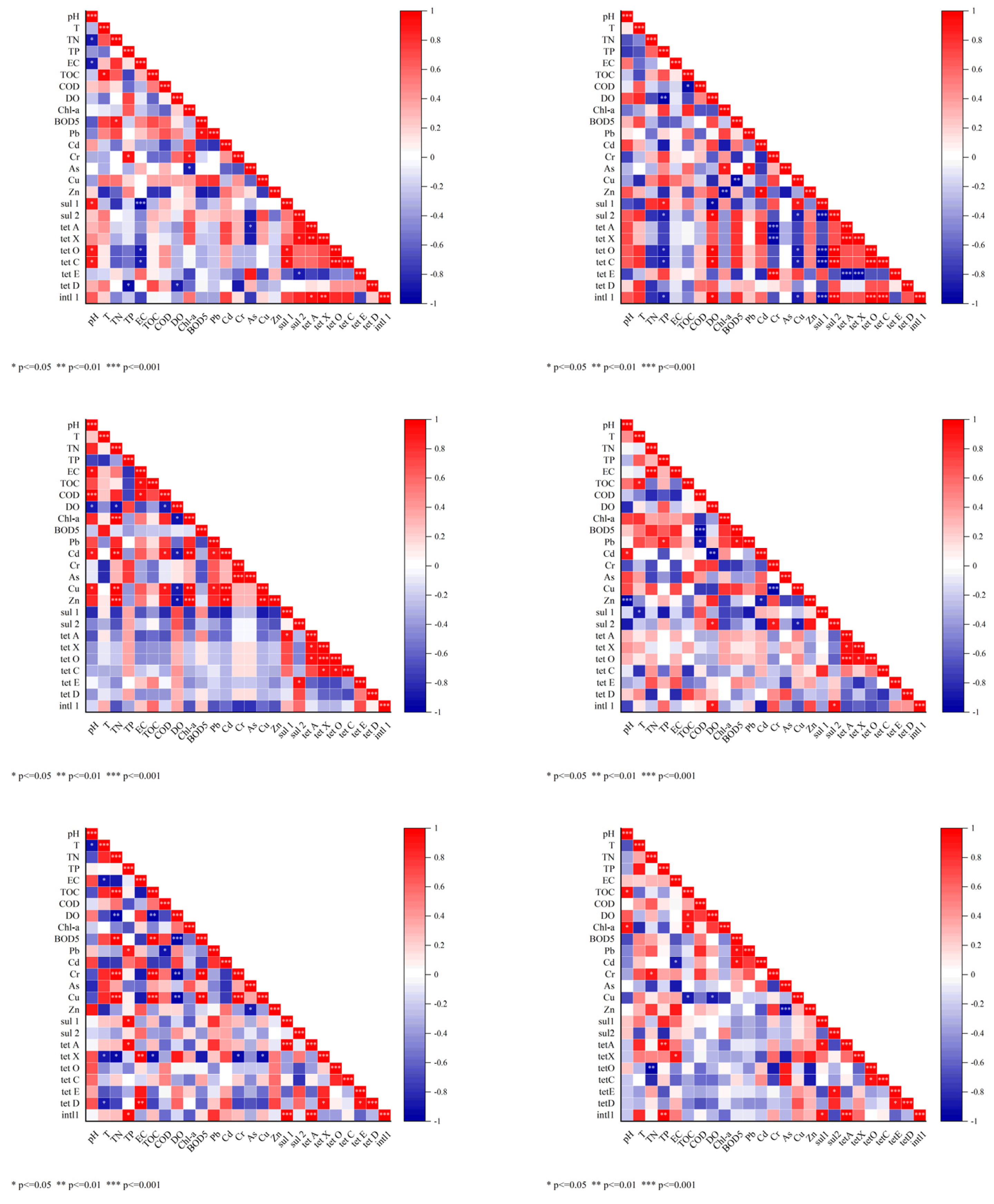
3.3. Antibiotic Resistance Gene (ARG) Dynamics Across Seasons and Their Relationship with Antibiotics
3.4. Seasonal Variation in the Antibiotics Among the Reservoirs
3.5. Correlation Between Antibiotics, Heavy Metals, and Water Quality Parameters
4. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okoye, C.O.; Nyaruaba, R.; Ita, R.E.; Okon, S.U.; Addey, C.I.; Ebido, C.C.; Opabunmi, A.O.; Okeke, E.S.; Chukwudozie, K.I. Antibiotic resistance in the aquatic environment: Analytical techniques and interactive impact of emerging contaminants. Environ. Toxicol. Pharmacol. 2022, 96, 103995. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, W.; Gu, Q.; Wang, X.; Yang, D.; Li, H.; Wang, P.; Liao, W.; Huang, J.; Yuan, Q.; et al. Spread of antibiotic resistance genes in drinking water reservoirs: Insights from a deep metagenomic study using a curated database. Water Res. 2024, 256, 121572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Liu, Y.; Chen, L.; Wang, J.; Li, Y.; Wang, K.; Wang, W. Deciphering the natural and anthropogenic drivers on the fate and risk of antibiotics and antibiotic resistance genes (ARGs) in a typical river-estuary system, China. J. Hazard. Mater. 2024, 480, 136006. [Google Scholar] [CrossRef] [PubMed]
- Cherian, T.; Ragavendran, C.; Vijayan, S.; Kurien, S.; Peijnenburg, W.J.G.M. A review on the fate, human health and environmental impacts, as well as regulation of antibiotics used in aquaculture. Environ. Adv. 2023, 13, 100411. [Google Scholar] [CrossRef]
- Dong, J.; Yan, D.; Mo, K.; Chen, Q.; Zhang, J.; Chen, Y.; Wang, Z. Antibiotics along an alpine river and in the receiving lake with a catchment dominated by grazing husbandry. J. Environ. Sci. 2022, 115, 374–382. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, B.; Zhu, D.; Cheng, X.; Zhou, Z.; Xie, J.; Chen, Z.; Sun, L.; Zhang, Y.; Xie, Y.; et al. Cross-contamination and ecological risk assessment of antibiotics between rivers and surrounding open aquaculture ponds. Environ. Pollut. 2024, 344, 123404. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Huang, H.; Zeng, S.; Dong, X.; Li, D.; Zhang, Y.; He, M.; Du, P. Diverse and abundant antibiotics and antibiotic resistance genes in an urban water system. J. Environ. Manag. 2019, 231, 494–503. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Liu, Y.; Liu, K.; Zhang, L.; Huang, F.; Wang, L.; Rashid, A.; Hu, A.; Yu, C. Horizontal and vertical gene transfer drive sediment antibiotic resistome in an urban lagoon system. J. Environ. Sci. 2021, 102, 11–23. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Sun, X.; Lu, W. Occurrence and distribution of antibiotic resistance genes in urban rivers with black-odor water of Harbin, China. Environ. Res. 2024, 259, 119497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xin, R.; Zhao, Z.; Ma, Y.; Zhang, Y.; Niu, Z. Antibiotic Resistance Genes in drinking water of China: Occurrence, distribution and influencing factors. Ecotoxicol. Environ. Saf. 2020, 188, 109837. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Lin, C.; Lian, M.; He, M.; Liu, X.; Ouyang, W. Occurrence, removal efficiency, and emission of antibiotics in the sewage treatment plants of a low-urbanized basin in China and their impact on the receiving water. Sci. Total Environ. 2024, 921, 171134. [Google Scholar] [CrossRef]
- Li, S.; Ondon, B.S.; Ho, S.-H.; Zhou, Q.; Li, F. Drinking water sources as hotspots of antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARGs): Occurrence, spread, and mitigation strategies. J. Water Process Eng. 2023, 53, 103907. [Google Scholar] [CrossRef]
- Motsinger-Reif, A.A.; Reif, D.M.; Akhtari, F.S.; House, J.S.; Campbell, C.R.; Messier, K.P.; Fargo, D.C.; Bowen, T.A.; Nadadur, S.S.; Schmitt, C.P.; et al. Gene-environment interactions within a precision environmental health framework. Cell Genom. 2024, 4, 100591. [Google Scholar] [CrossRef]
- Li, T.; Tao, S.; Ma, M.; Liu, S.; Shen, M.; Zhang, H. Is the application of organic fertilizers becoming an undeniable source of microplastics and resistance genes in agricultural systems? Sci. Total Environ. 2024, 912, 169571. [Google Scholar] [CrossRef]
- Ji, W.; Ma, J.; Zheng, Z.; Al-Herrawy, A.Z.; Xie, B.; Wu, D. Algae blooms with resistance in fresh water: Potential interplay between Microcystis and antibiotic resistance genes. Sci. Total Environ. 2024, 940, 173528. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W.; Lin, H.; Wang, W.; Du, L.; Xing, W. Antibiotics and antibiotic resistance genes in global lakes: A review and meta-analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 2021, 777, 146009. [Google Scholar] [CrossRef]
- Ohore, O.E.; Addo, F.G.; Han, N.; Li, X.; Zhang, S. Profiles of ARGs and their relationships with antibiotics, metals and environmental parameters in vertical sediment layers of three lakes in China. J. Environ. Manag. 2020, 225, 109583. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Liu, Y.; Hua, Z.; Lu, Y.; Ye, F. Spatio-temporal distribution and dynamics of antibiotic resistance genes in a water-diversion lake, China. J. Environ. Manag. 2023, 348, 119232. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, C.; Wang, Y.; Yang, Y.; Mishra, S. Occurrence, distribution and their correlation with different parameters of antibiotics and antibiotic resistance genes in lakes of China: A review. Mar. Pollut. Bull. 2023, 193, 115189. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Lu, T.; Peijnenburg, W.; Gillings, M.; Yang, X.; Chen, J.; Penuelas, J.; Zhu, Y.G.; Zhou, N.Y.; et al. Cyanobacterial blooms contribute to the diversity of antibiotic-resistance genes in aquatic ecosystems. Commun. Biol. 2020, 3, 737. [Google Scholar] [CrossRef]
- Li, X.D.; Chen, Y.H.; Liu, C.; Hong, J.; Deng, H.; Yu, D.J. Eutrophication and Related Antibiotic Resistance of Enterococci in the Minjiang River, China. Microb. Ecol. 2020, 80, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhou, X.; Cheng, W.; Ren, J.; Wan, T.; Liu, X. Transmission mechanism of antibiotic resistance genes and their differences between water and sediment in the Weihe River Basin. Environ. Res. 2024, 252, 119057. [Google Scholar] [CrossRef]
- Flach, K.A.; Bones, U.A.; Wolff, D.B.; de Oliveira Silveira, A.; da Rosa, G.M.; Carissimi, E.; Silvestri, S. Antibiotic resistant bacteria and genes (ARB and ARG) in water and sewage treatment units: A review. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100941. [Google Scholar] [CrossRef]
- Wang, M.; Masoudi, A.; Wang, C.; Wu, C.; Zhang, Z.; Zhao, X.; Liu, Y.; Yu, Z.; Liu, J. Impacts of net cages on pollutant accumulation and its consequence on antibiotic resistance genes (ARGs) dissemination in freshwater ecosystems: Insights for sustainable urban water management. Environ. Int. 2024, 183, 108357. [Google Scholar] [CrossRef]
- Du, C.; Li, G.; Xia, R.; Li, C.; Zhu, Q.; Li, X.; Li, J.; Zhao, C.; Tian, Z.; Zhang, L. New insights into cyanobacterial blooms and the response of associated microbial communities in freshwater ecosystems. Environ. Pollut. 2022, 309, 119781. [Google Scholar] [CrossRef]
- Zhao, K.; Li, C.; Wang, Q.; Lu, H. Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter. Sustainability 2022, 14, 7301. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fruci, M.; Verellen, L.A.; Skarina, T.; Mesa, N.; Flick, R.; Pham, C.; Mahadevan, R.; Stogios, P.J.; Savchenko, A. Molecular mechanism of plasmid-borne resistance to sulfonamide antibiotics. Nat. Commun. 2023, 14, 4031. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Song, R.; Lu, S.; Liu, X.; Chen, J.; Wan, Z.; Bi, B. Multi-Media Occurrence of Antibiotics and Antibiotic Resistance Genes in East Dongting Lake. Front. Environ. Sci. 2022, 10, 866332. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Hu, M.; Cai, R.; Miao, Y.; Zhu, X. Heavy metals potentially drive co-selection of antibiotic resistance genes by shifting soil bacterial communities in paddy soils along the middle and lower Yangtze River. Pedosphere 2024, 34, 606–619. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Wang, B.; Chen, C.; Cheng, Z.; Li, Y.; Wang, B.; Li, J. Antibiotic resistance genes in Chishui River, a tributary of the Yangtze River, China: Occurrence, seasonal variation and its relationships with antibiotics, heavy metals and microbial communities. Sci. Total Environ. 2022, 846, 157472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Suyamud, B.; Lohwacharin, J.; Yang, Y. Large-scale pattern of resistance genes and bacterial community in the tap water along the middle and low reaches of the Yangtze River. Ecotoxicol. Environ. Saf. 2021, 208, 111517. [Google Scholar] [CrossRef]
- Igere, B.E.; Onohuean, H.; Nwodo, U.U. Modern knowledge-scape possess petite influence on the factual persistence of resistance determinants (ARGs/MGEs): A map and assessment of discharged wastewater and water bodies. Heliyon 2022, 8, e12253. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Wang, Y.; Liu, X.; Liu, Y.; Xu, J.; Zhang, T.; Wang, Z.; Yang, Y. Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China. Environ. Pollut. 2020, 257, 113365. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wang, C.; Wang, P.; Gao, H.; Feng, B.; Fu, J. Vertical variation of antibiotic resistance genes and their interaction with environmental nutrients in sediments of Taihu lake. J. Environ. Manag. 2024, 370, 122661. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Rajasekar, A.; Murava, R.T.; Norgbey, E.; Zhu, X. Spatial Distribution, Risk Index, and Correlation of Heavy Metals in the Chuhe River (Yangtze Tributary): Preliminary Research Analysis of Surface Water and Sediment Contamination. Appl. Sci. 2024, 14, 904. [Google Scholar] [CrossRef]
- Lu, S.; Lin, C.; Lei, K.; Wang, B.; Xin, M.; Gu, X.; Cao, Y.; Liu, X.; Ouyang, W.; He, M. Occurrence, spatiotemporal variation, and ecological risk of antibiotics in the water of the semi-enclosed urbanized Jiaozhou Bay in eastern China. Water Res. 2020, 184, 116187. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I.; Garrigues-Jeanjean, N.; Mackie, R.I. Molecular Ecology of Tetracycline Resistance: Development and Validation of Primers for Detection of Tetracycline Resistance Genes Encoding Ribosomal Protection Proteins. Appl. Environ. Microbiol. 2001, 67, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical Evaluation of Two Primers Commonly Used for Amplification of Bacterial 16S rRNA Genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.; Li, E.; Liu, X.; Wei, H.; Yang, C.; Lu, S.; Ning, K. Distribution of antibiotic resistance genes in an agriculturally disturbed lake in China: Their links with microbial communities, antibiotics, and water quality. J. Hazard. Mater. 2020, 393, 122426. [Google Scholar] [CrossRef]
- European Commission. AC01769567, A. Technical Guidance Document in Support of Commission Directive 93 67 EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No. 1488 94 on Risk Assessment for Existing Substances; Office for Official Publications of the European Communities: Luxembourg, 1996.
- Jiang, Y.; Li, M.; Guo, C.; An, D.; Xu, J.; Zhang, Y.; Xi, B. Distribution and ecological risk of antibiotics in a typical effluent–receiving river (Wangyang River) in north China. Chemosphere 2014, 112, 267–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Cheng, W.; Huang, C.; Ren, J.; Wan, T.; Gao, K. Effects of water environmental factors and antibiotics on bacterial community in urban landscape lakes. Aquat. Toxicol. 2023, 265, 106740. [Google Scholar] [CrossRef]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef]
- Okeke, E.S.; Nwankwo, C.E.I.; Ezeorba, T.P.C.; Ogugofor, M.O.; Nwuche, C.O. Antibiotic residues and antibiotic resistance genes in African water systems: Implications for safe drinking water, aquatic ecosystems, and Sustainable Development Goals. J. Water Process Eng. 2025, 69, 106636. [Google Scholar] [CrossRef]
- Ripanda, A.; Rwiza, M.J.; Nyanza, E.C.; Hossein, M.; Alfred, M.S.; Mahmoud, A.E.D.; Murthy, H.C.A.; Bakari, R.; Vuai, S.A.H.; Machunda, R.L. Ecological Consequences of Antibiotics Pollution in Sub-Saharan Africa: Understanding Sources, Pathways, and Potential Implications. Emerg. Contam. 2025, 11, 100475. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, J.; Xu, X.; Wang, X.; Yang, C.; Song, C.; Wang, S.; Zhao, S. Bioaccumulation and trophic transfer of antibiotics in the aquatic and terrestrial food webs of the Yellow River Delta. Chemosphere 2023, 323, 138211. [Google Scholar] [CrossRef] [PubMed]
- SEPA. Environmental Quality Standard for Surface Water; Ministry of Environmental Protection: Beijing, China, 2002.
- Wu, R.; Liu, Y.; Zhang, S.; Shi, X.; Zhao, S.; Lu, J.; Kang, X.; Wang, S.; Wu, Y.; Arvola, L. Characterization of nitrogen and phosphorus at the ice-water-sediment interface and the effect of their migration on overlying water quality in Daihai Lake (China) during the freezing period. Sci. Total Environ. 2023, 893, 164863. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hou, S.; Zhang, H.; Sun, S.; Guo, C.; Zhang, X.; Song, G.; Xu, J. Spatiotemporal variations and priority ranking of emerging contaminants in nanwan reservoir: A case study from the agricultural region in huaihe river basin in China. J. Environ. Manag. 2024, 368, 122195. [Google Scholar] [CrossRef]
- Lin, S.-S.; Shen, S.-L.; Zhou, A.; Lyu, H.-M. Assessment and management of lake eutrophication: A case study in Lake Erhai, China. Sci. Total Environ. 2021, 751, 141618. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Wang, S.; Sun, M.; Wang, M.; Ren, X.; Gao, Z.; Zhou, Y.; Zhang, J.; Zhuang, W.; et al. Temperature and phosphorus: The main environmental factors affecting the seasonal variation of soil bacterial diversity in Nansi Lake Wetland. Front. Microbiol. 2023, 14, 1169444. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Zhang, M.; Lin, K.; Wang, Z.; Liu, L. Seasonal responses of microbial communities to water quality variations and interaction of eutrophication risk in Gehu Lake. Sci. Total Environ. 2024, 955, 177199. [Google Scholar] [CrossRef]
- Xia, K.; Wu, T.; Li, X.; Wang, S.; Tang, H.; Zu, Y.; Yang, Y. A novel method for assessing water quality status using MODIS images: A case study of large lakes and reservoirs in China. J. Hydrol. 2024, 638, 131545. [Google Scholar] [CrossRef]
- Jiang, Y.; He, K.; Li, Y.; Qin, M.; Cui, Z.; Zhang, Y.; Yao, Y.; Chen, X.; Deng, M.; Gray, A.; et al. Driving Factors of Total Organic Carbon in Danjiangkou Reservoir Using Generalized Additive Model. Water 2022, 14, 891. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kam, J. Spatiotemporal patterns of water volume and total organic carbon concentration of agricultural reservoirs over South Korea. Water Res. 2024, 256, 121610. [Google Scholar] [CrossRef]
- Ahmad, N.B.; Jaafaru, M.S.; Isa, Z.; Abdulhamid, Y.; Kakudi, R.A.; Ugya, A.Y.; Meguellati, K. High pollution loads engineer oxygen dynamics, ecological niches, and pathogenicity shifts in freshwater environments. J. Hazard. Mater. Adv. 2024, 14, 100425. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Sun, Y.; Zhang, Y.; Chen, W.; Yuan, Y.; Hu, S.; Li, S. Machine learning-based analysis of heavy metal contamination in Chinese lake basin sediments: Assessing influencing factors and policy implications. Ecotoxicol. Environ. Saf. 2024, 283, 116815. [Google Scholar] [CrossRef] [PubMed]
- Tshisikhawe, C.S.; Ngole-Jeme, V.M. Temperature induced changes on heavy metal geochemical partitioning and mobility in contaminated and uncontaminated soils. Environ. Pollut. Bioavailab. 2024, 36, 2317747. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, W.; Wang, Y.; Tysklind, M.; Hao, f.; Liu, H.; Hao, X.; Xu, Y.; Lin, C.; Su, L. Heavy metal accumulation, geochemical fractions, and loadings in two agricultural watersheds with distinct climate conditions. J. Hazard. Mater. 2020, 389, 122125. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Chen, X.; Sha, A.; Xiong, Z.; Luo, Y.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. The Easily Overlooked Effect of Global Warming: Diffusion of Heavy Metals. Toxics 2024, 12, 400. [Google Scholar] [CrossRef]
- Rajasekar, A.; Qiu, M.; Wang, B.; Murava, R.T.; Norgbey, E. Relationship between water quality, heavy metals and antibiotic resistance genes among three freshwater lakes. Environ. Monit. Assess. 2022, 194, 64. [Google Scholar] [CrossRef]
- Shi, X.; Xia, Y.; Wei, W.; Ni, B.-J. Accelerated spread of antibiotic resistance genes (ARGs) induced by non-antibiotic conditions: Roles and mechanisms. Water Res. 2022, 224, 119060. [Google Scholar] [CrossRef]
- Alves, J.; Dias, L.; Mateus, J.; Marques, J.; Graças, D.; Ramos, R.; Seldin, L.; Henriques, I.; Silva, A.; Folador, A. Resistome in Lake Bolonha, Brazilian Amazon: Identification of Genes Related to Resistance to Broad-Spectrum Antibiotics. Front. Microbiol. 2020, 11, 67. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Hu, C.; Marion, J.W. Beyond cyanotoxins: Increased Legionella, antibiotic resistance genes in western Lake Erie water and disinfection-byproducts in their finished water. Front. Microbiol. 2023, 14, 1233327. [Google Scholar] [CrossRef]
- Huang, Y.; Fu, M.; Chen, G.; Zhang, J.; Xu, P.; Pan, L.; Zhang, X.; Chen, X. Reducing the water residence time is inadequate to limit the algal proliferation in eutrophic lakes. J. Environ. Manag. 2023, 330, 117177. [Google Scholar] [CrossRef]
- Komijani, M.; Shamabadi, N.S.; Shahin, K.; Eghbalpour, F.; Tahsili, M.R.; Bahram, M. Heavy metal pollution promotes antibiotic resistance potential in the aquatic environment. Environ. Pollut. 2021, 274, 116569. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-T.; Song, X.; Yuan, S.; Zhao, H.-P. Unveiling the inhibitory mechanisms of chromium exposure on microbial reductive dechlorination: Kinetics and microbial responses. Water Res. 2024, 253, 121328. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Sun, X.; Wu, J.; Ma, L.; Zhou, Y.; Lin, K.; Luo, Y.; Cui, C. Risk assessment of antibiotic resistance genes in the drinking water system. Sci. Total Environ. 2021, 800, 149650. [Google Scholar] [CrossRef]
- Yu, W.; Xu, Y.; Wang, Y.; Sui, Q.; Xin, Y.; Wang, H.; Zhang, J.; Zhong, H.; Wei, Y. An extensive assessment of seasonal rainfall on intracellular and extracellular antibiotic resistance genes in Urban River systems. J. Hazard. Mater. 2023, 455, 131561. [Google Scholar] [CrossRef]
- Zhang, R.-D.; Gao, F.-Z.; Shi, Y.-J.; Zhao, J.-L.; Liu, Y.-S.; He, L.-Y.; Ying, G.-G. Metagenomic investigation of antibiotic resistance genes and resistant bacteria contamination in pharmaceutical plant sites in China. Environ. Pollut. 2024, 357, 124482. [Google Scholar] [CrossRef]
- Yin, F.; Hao, W.; Zhang, H.; Miao, J.; Shi, H. Pollution status and spatial distribution of antibiotic resistance genes in urban surface water and surrounding soil media. J. Environ. Chem. Eng. 2024, 12, 113856. [Google Scholar] [CrossRef]
- Szekeres, E.; Chiriac, C.M.; Baricz, A.; Szoke-Nagy, T.; Lung, I.; Soran, M.L.; Rudi, K.; Dragos, N.; Coman, C. Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas. Environ. Pollut. 2018, 236, 734–744. [Google Scholar] [CrossRef]
- Hao, H.; Shi, D.-y.; Yang, D.; Yang, Z.-w.; Qiu, Z.-g.; Liu, W.-l.; Shen, Z.-q.; Yin, J.; Wang, H.-r.; Li, J.-w.; et al. Profiling of intracellular and extracellular antibiotic resistance genes in tap water. J. Hazard. Mater. 2019, 365, 340–345. [Google Scholar] [CrossRef]
- Lu, L.; Liu, J.; Li, Z.; Zou, X.; Guo, J.; Liu, Z.; Yang, J.; Zhou, Y. Antibiotic resistance gene abundances associated with heavy metals and antibiotics in the sediments of Changshou Lake in the three Gorges Reservoir area, China. Ecol. Indic. 2020, 113, 106275. [Google Scholar] [CrossRef]
- Qi, M.; He, P.; Hu, H.; Zhang, T.; Li, T.; Zhang, X.; Qin, Y.; Zhu, Y.; Guo, Y. An Automated Solid-Phase Extraction–UPLC–MS/MS Method for Simultaneous Determination of Sulfonamide Antimicrobials in Environmental Water. Molecules 2023, 28, 4694. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, W.; Shen, X.; Zhang, F.; Zhou, X.; Shen, J.; Lu, M. Occurrence, Distribution, and Ecological Risk Assessment of Antibiotics in Different Environmental Media in Anqing, Anhui Province, China. Int. J. Environ. Res. Public Health 2021, 18, 8112. [Google Scholar] [CrossRef] [PubMed]
- Maghsodian, Z.; Sanati, A.M.; Mashifana, T.; Sillanpää, M.; Feng, S.; Nhat, T.; Ramavandi, B. Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review. Antibiotics 2022, 11, 1461. [Google Scholar] [CrossRef]
- Salma, U.; Nishimura, Y.; Tokumura, M.; Hossain, A.; Watanabe, K.; Noro, K.; Raknuzzaman, M.; Amagai, T.; Makino, M. Occurrence, seasonal variation, and environmental risk of multiclass antibiotics in the urban surface water of the Buriganga River, Bangladesh. Chemosphere 2025, 370, 143956. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Laht, M.; Karkman, A.; Voolaid, V.; Ritz, C.; Tenson, T.; Virta, M.; Kisand, V. Abundances of Tetracycline, Sulphonamide and Beta-Lactam Antibiotic Resistance Genes in Conventional Wastewater Treatment Plants (WWTPs) with Different Waste Load. PLoS ONE 2014, 9, e103705. [Google Scholar] [CrossRef]
- Tong, L.; Qin, L.; Guan, C.; Wilson, M.E.; Li, X.; Cheng, D.; Ma, J.; Liu, H.; Gong, F. Antibiotic resistance gene profiling in response to antibiotic usage and environmental factors in the surface water and groundwater of Honghu Lake, China. Environ. Sci. Pollut. Res. 2020, 27, 31995–32005. [Google Scholar] [CrossRef]
- Han, Q.F.; Zhao, S.; Zhang, X.R.; Wang, X.L.; Song, C.; Wang, S.G. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ. Int. 2020, 138, 105551. [Google Scholar] [CrossRef]
- Li, Q.; Na, G.; Zhang, L.; Lu, Z.; Gao, H.; Li, R.; Jin, S. Effects of corresponding and non-corresponding contaminants on the fate of sulfonamide and quinolone resistance genes in the Laizhou Bay, China. Mar. Pollut. Bull. 2018, 128, 475–482. [Google Scholar] [CrossRef]
- Guo, X.; Feng, C.; Gu, E.; Tian, C.; Shen, Z. Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze Estuary. Sci. Total Environ. 2019, 671, 548–557. [Google Scholar] [CrossRef]

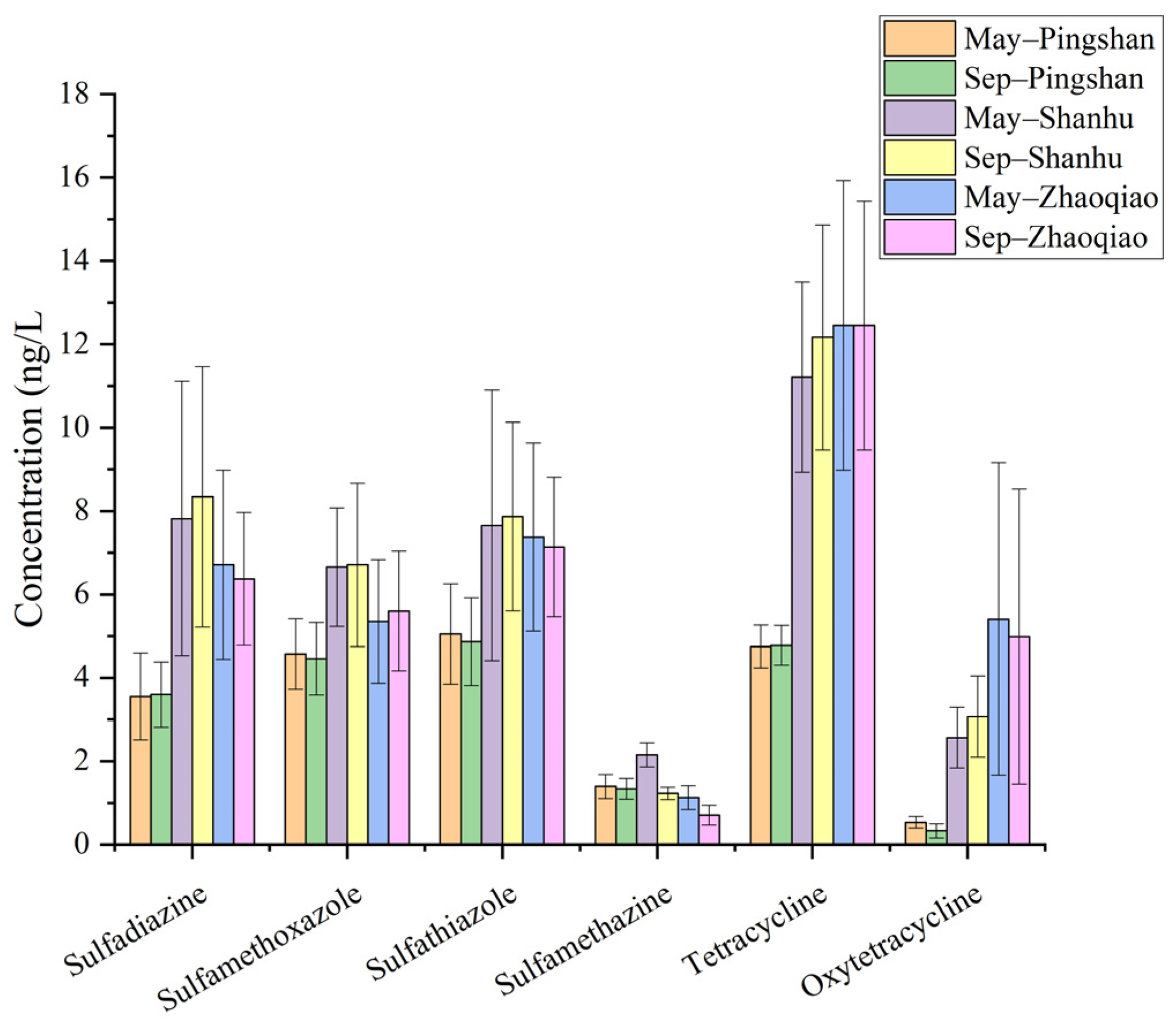
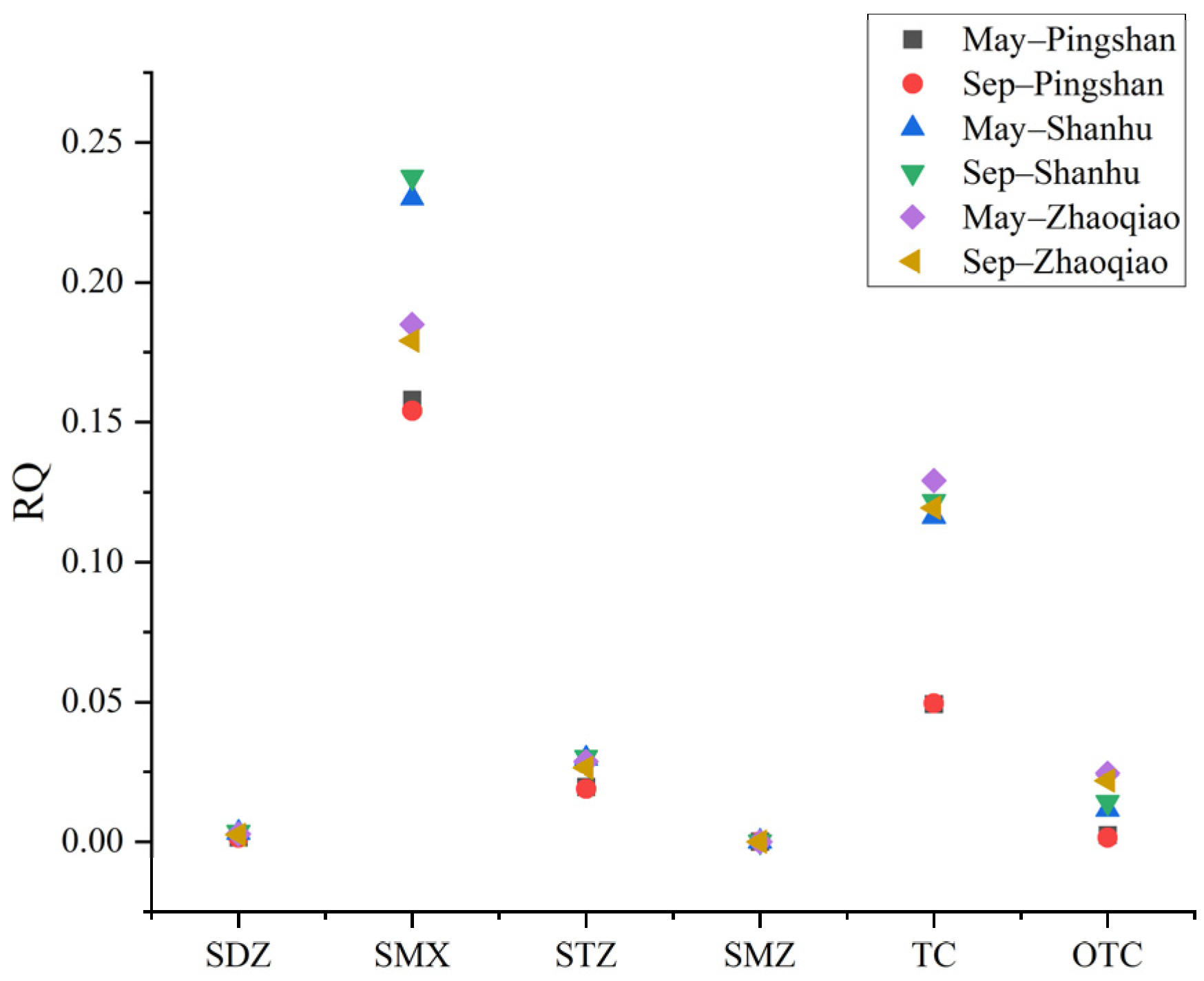



| May– Pingshan | Sep– Pingshan | May– Shanhu | Sep– Shanhu | May– Zhaoqiao | Sep– Zhaoqiao | |
|---|---|---|---|---|---|---|
| Sulfonamides | 0.16 ± 0.14 | 0.31 ± 0.08 | 0.6 ± 0.18 | 0.73 ± 0.16 | 0.13 ± 0.02 | 0.34 ± 0.08 |
| Tetracyclines | 1.91 ± 0.68 | 2.5 ± 0.92 | 0.42 ± 0.18 | 1.22 ± 0.15 | 0.52 ± 0.16 | 0.52 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Murava, R.T.; Zhang, Q.; Zhou, T.; Omoregie, A.I.; Rajasekar, A.; Ouahbi, T. Linking Antibiotic Residues and Antibiotic Resistance Genes to Water Quality Parameters in Urban Reservoirs: A Seasonal Perspective. Environments 2025, 12, 96. https://doi.org/10.3390/environments12030096
Li S, Murava RT, Zhang Q, Zhou T, Omoregie AI, Rajasekar A, Ouahbi T. Linking Antibiotic Residues and Antibiotic Resistance Genes to Water Quality Parameters in Urban Reservoirs: A Seasonal Perspective. Environments. 2025; 12(3):96. https://doi.org/10.3390/environments12030096
Chicago/Turabian StyleLi, Sihan, Raphinos Tackmore Murava, Qiyue Zhang, Tong Zhou, Armstrong Ighodalo Omoregie, Adharsh Rajasekar, and Tariq Ouahbi. 2025. "Linking Antibiotic Residues and Antibiotic Resistance Genes to Water Quality Parameters in Urban Reservoirs: A Seasonal Perspective" Environments 12, no. 3: 96. https://doi.org/10.3390/environments12030096
APA StyleLi, S., Murava, R. T., Zhang, Q., Zhou, T., Omoregie, A. I., Rajasekar, A., & Ouahbi, T. (2025). Linking Antibiotic Residues and Antibiotic Resistance Genes to Water Quality Parameters in Urban Reservoirs: A Seasonal Perspective. Environments, 12(3), 96. https://doi.org/10.3390/environments12030096







