Fishponds Are Hotspots of Algal Biodiversity—Organic Carp Farming Reveals Unexpected High Taxa Richness
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site Description
2.2. Field Sampling and In Situ Measurements
2.3. Dry Mass, Ash Mass, and Pigments
2.4. Water Chemistry
2.5. Algae Identification, Diversity, and Resource Use Efficiency
2.6. Zooplankton
2.7. Statistics
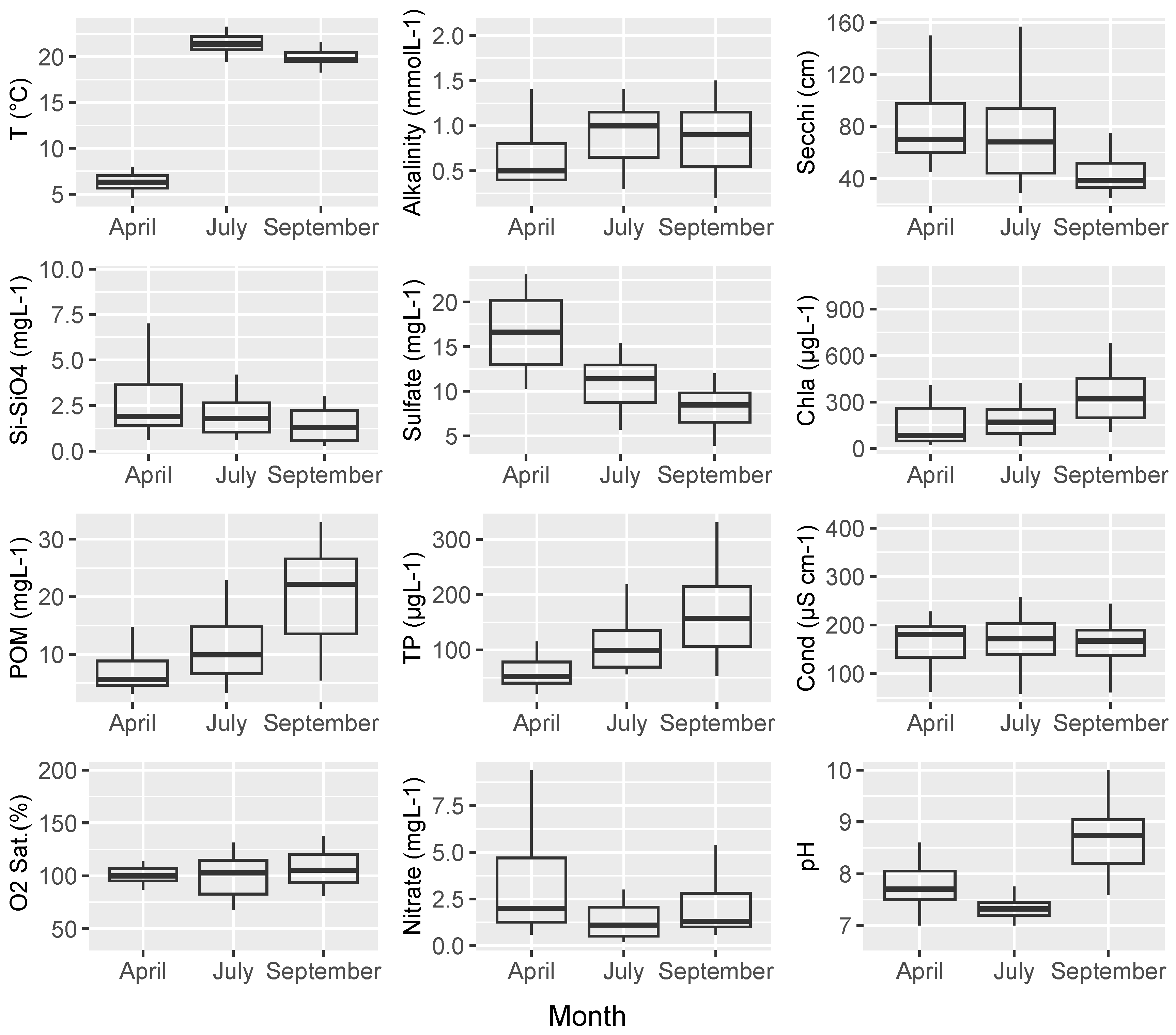
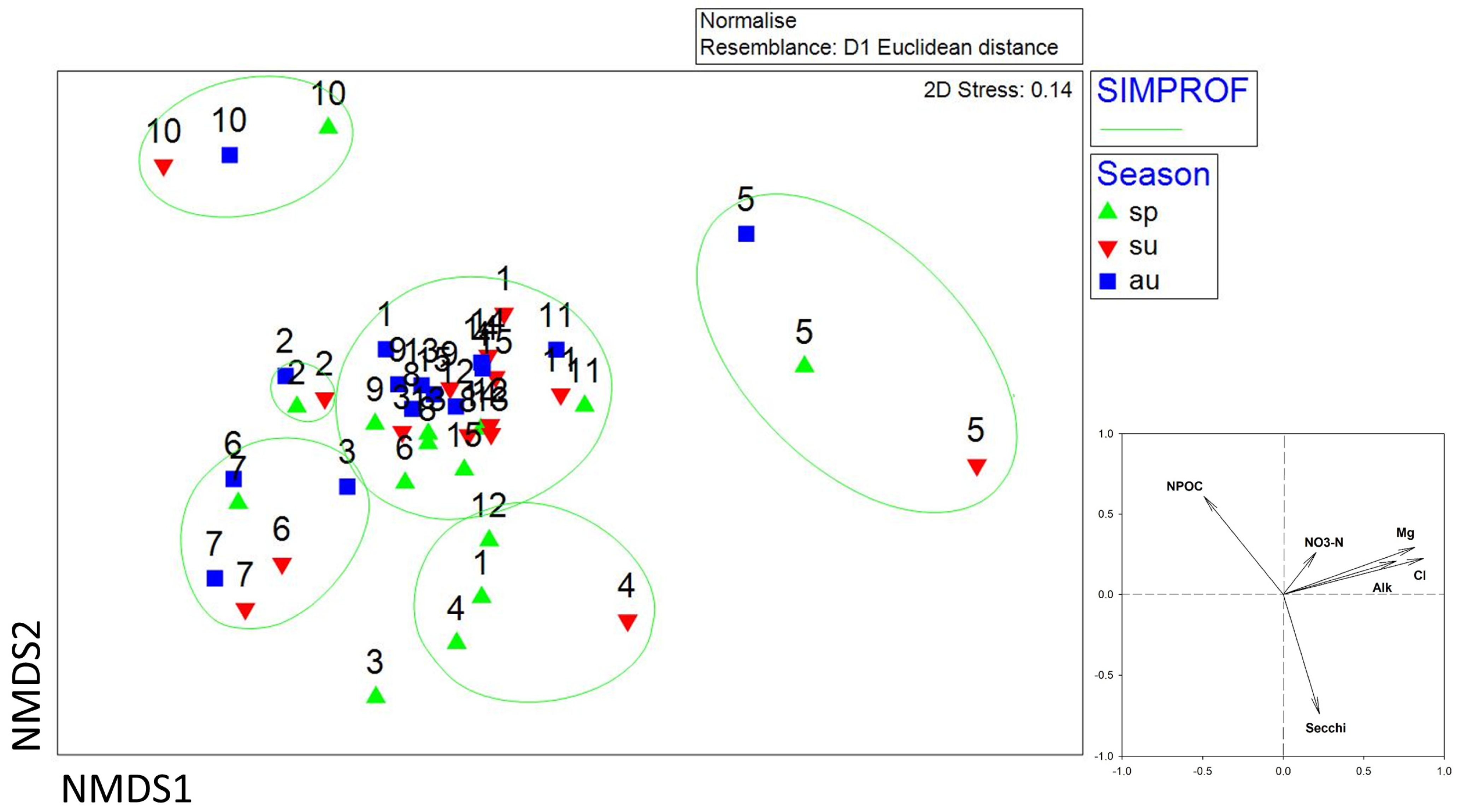
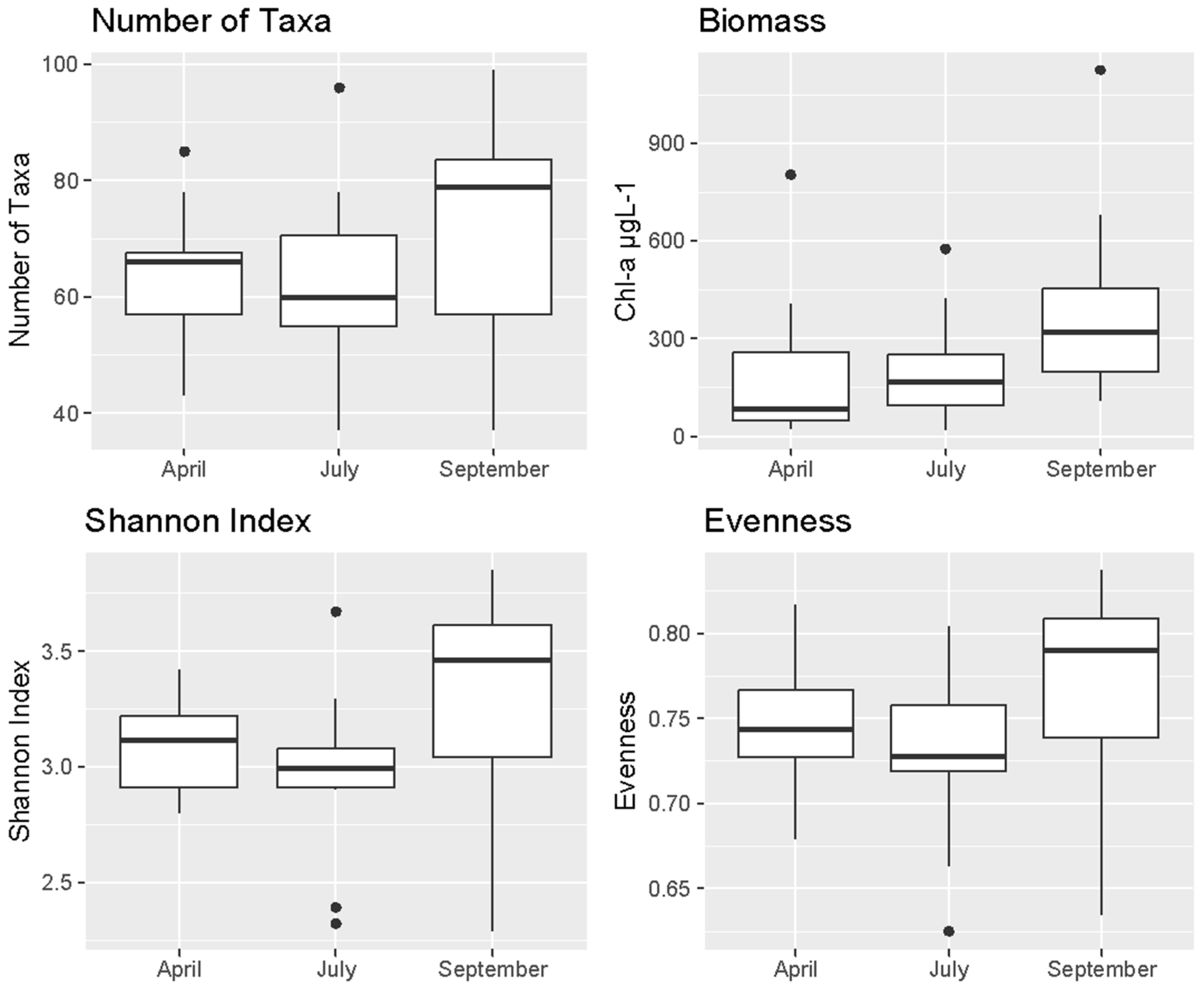
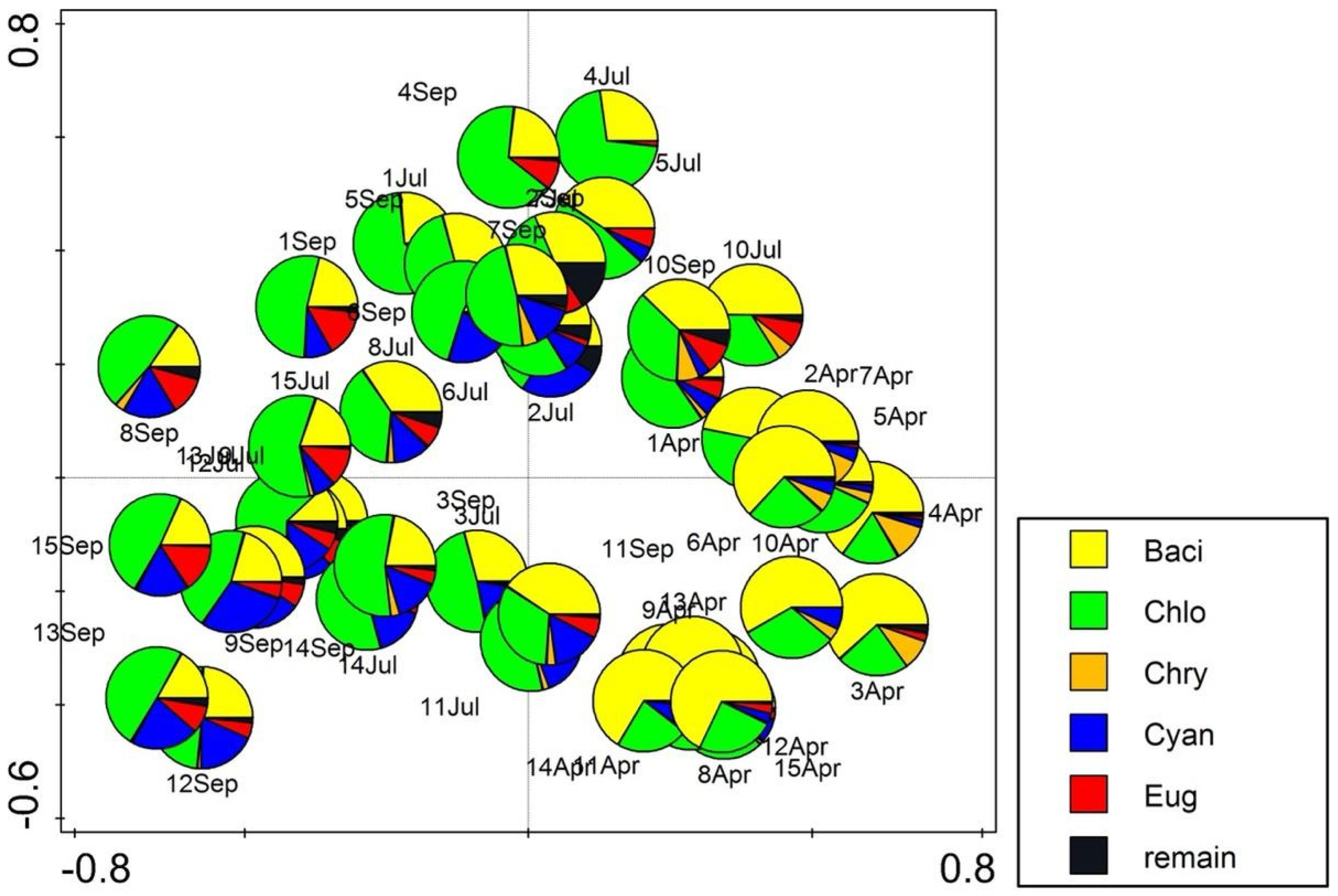
3. Results
3.1. Environment and Overall Biomass



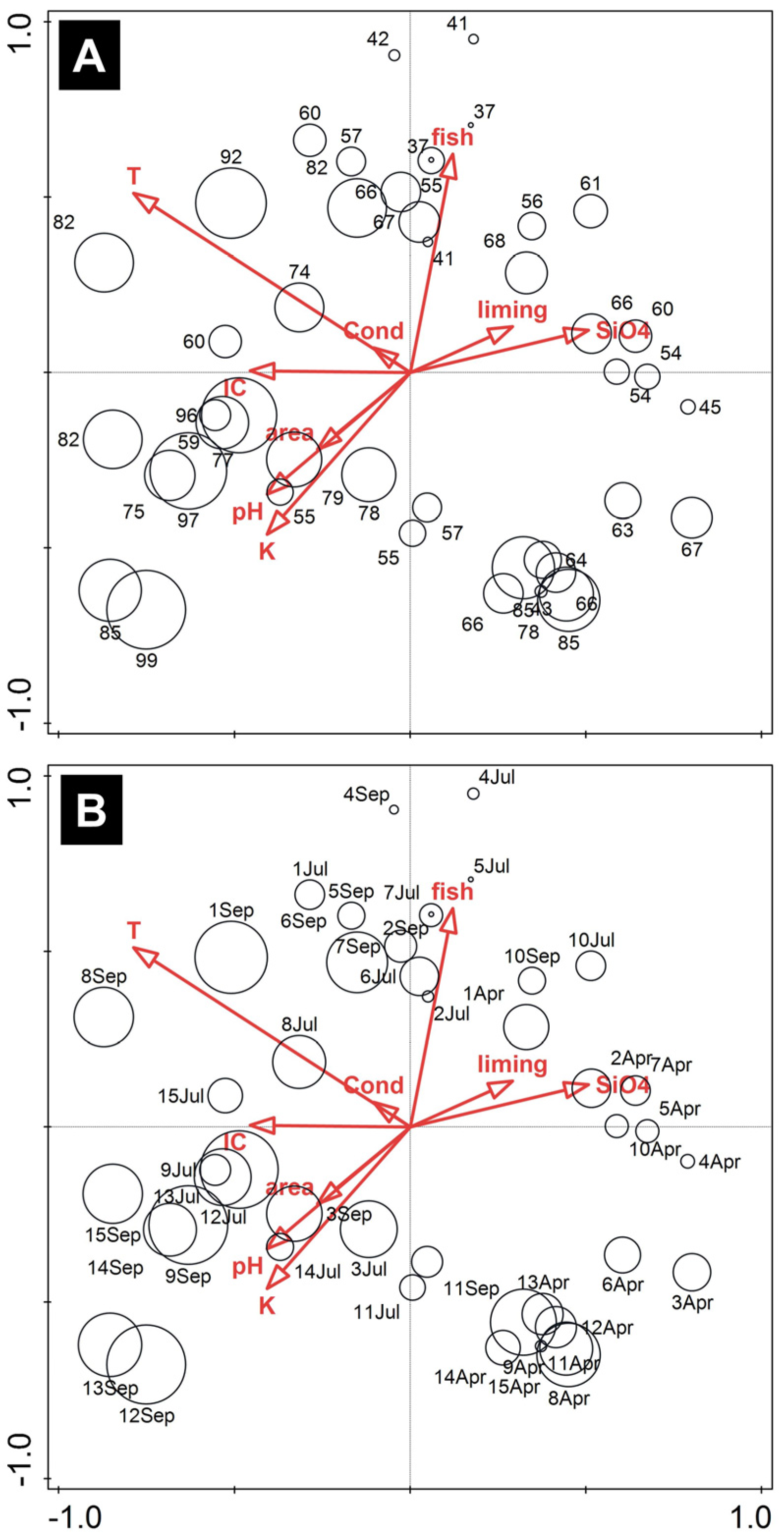
3.2. Algal Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adámek, Z.; Linhart, O.; Kratochvíl, M.; Flajšhans, M.; Randak, T.; Policar, T.; Masojídek, J.; Kozák, P. Aquaculture the Czech Republic in 2012: Modern European prosperous sector based on thousand-year history of pond culture. Aquac. Eur. 2012, 37, 5–14. [Google Scholar]
- Bauer, C. Waldviertler Teiche. Denisia 2014, 33, 157–166. [Google Scholar]
- Bezzel, E. Vögel in der Kulturlandschaft; Verlag Eugen Ulmer: Stuttgart, Germany, 1982. [Google Scholar]
- Steiner, E. Die Brutzeit bei Wasservögeln am Beispiel der Fischteiche des Waldviertels; Kataloge des OÖ. Landesmuseums: Linz, Austria, 1987; pp. 55–63. [Google Scholar]
- Fehlinger, L.; Misteli, B.; Morant, D.; Juvigny-Khenafou, N.; Cunillera-Montcusí, D.; Chaguaceda, F.; Stamenković, O.; Fahy, J.; Kolář, V.; Halabowski, D.; et al. The ecological role of permanent ponds in Europe: A review of dietary linkages to terrestrial ecosystems via emerging insects. Inland Waters 2023, 13, 30–46. [Google Scholar] [CrossRef]
- Fehlinger, L.; Mathieu-Resuge, M.; Pilecky, M.; Parmar, T.P.; Twining, C.W.; Martin-Creuzburg, D.; Kainz, M.J. Export of dietary lipids via emergent insects from eutrophic fishponds. Hydrobiologia 2023, 850, 3241–3256. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, T.M.E. Teiche in der Landschaft: Bedeutung, Funktionen und Gefährdung; Bundesamt für Wasserwirtschaft, Ökologische Station Waldviertel: Wien, Austria, 2014. [Google Scholar]
- Poschlod, P.; Rosbakh, S. Mudflat species: Threatened or hidden? An extensive seed bank survey of 108 fish ponds in Southern Germany. Biol. Conserv. 2018, 225, 154–163. [Google Scholar] [CrossRef]
- Knittler, H. Teiche als Konjunkturbarometer? Das Beispiel Niederösterreich. Water Manag. Mediev. Rural Econ. RURALIA 2005, 5, 208–221. [Google Scholar]
- Wawrik, F. Seltene Diatomeen aus Teichen des N.Ö. Waldviertels. Nova Hedwig. Beih. 1970, 31, 443–448. [Google Scholar] [CrossRef]
- Wawrik, F. Seltene “-Algen”︁ und Nannoplankter aus Teichen des Waldviertels (Nieder-Österreich). Int. Rev. Gesamten Hydrobiol. Hydrogr. 1974, 59, 247–254. [Google Scholar] [CrossRef]
- Wawrik, F. Vergleichende Braunwasser-Teichstudien im niederösterreichischen Waldviertel. Hydrobiologia 1972, 39, 17–82. [Google Scholar] [CrossRef]
- Radojičić, M.; Šorf, M.; Müllerová, B.; Kopp, R. Phytoplankton-zooplankton coupling in a cascade of hypertrophic fishponds. J. Limnol. 2023, 82, 2145. [Google Scholar] [CrossRef]
- Ivanova, A.P.; Vrba, J.; Potužák, J.; Regenda, J.; Strunecký, O. Seasonal Development of Phytoplankton in South Bohemian Fishponds (Czechia). Water 2022, 14, 1979. [Google Scholar] [CrossRef]
- Radojicic, M.; Kopp, R. Dynamic of the phytoplankton community in eutrophic fishponds. MendelNet 2016, 2016, 352–357. [Google Scholar]
- Radojičić, M.; Kopp, R.; Müllerová, B.; Šorf, M. The Effect of Fish Production and Environmental Factors on Phytoplankton in Hypertrophic Fishponds. Acta Univ. Agric. Silvic. Mendel. Brun. 2022, 70, 397–406. [Google Scholar] [CrossRef]
- Rahman, M.M. Role of common carp (Cyprinus carpio) in aquaculture production systems. Front. Life Sci. 2015, 8, 399–410. [Google Scholar] [CrossRef]
- Liu, Q.-G.; Chen, Y.; Li, J.-L.; Chen, L.-Q. The food web structure and ecosystem properties of a filter-feeding carps dominated deep reservoir ecosystem. Ecol. Model. 2007, 203, 279–289. [Google Scholar] [CrossRef]
- Baxa, M.; Musil, M.; Kummel, M.; Hanzlík, P.; Tesařová, B.; Pechar, L. Dissolved oxygen deficits in a shallow eutrophic aquatic ecosystem (fishpond)—Sediment oxygen demand and water column respiration alternately drive the oxygen regime. Sci. Total Environ. 2021, 766, 142647. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef]
- Brett, M.; Müller-Navarra, D. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw. Biol. 1997, 38, 483–499. [Google Scholar] [CrossRef]
- Patil, V.; Reitan, K.I.; Knutsen, G.; Mortensen, L.M.; Källqvist, T.; Olsen, E.F.; Vogt, G.; Gislerød, H.R. Microalgae as source of polyunsaturated fatty acids for aquaculture. Curr. Top. Plant Biol. 2005, 6, 57–65. [Google Scholar]
- Pilecky, M.; Mathieu-Resuge, M.; Závorka, L.; Fehlinger, L.; Winter, K.; Martin-Creuzburg, D.; Kainz, M.J. Common carp (Cyprinus carpio) obtain omega-3 long-chain polyunsaturated fatty acids via dietary supply and endogenous bioconversion in semi-intensive aquaculture ponds. Aquaculture 2022, 561, 738731. [Google Scholar] [CrossRef]
- Mráz, J.; Máchová, J.; Kozák, P.; Pickova, J. Lipid content and composition in common carp—Optimization of n-3 fatty acids in different pond production systems. J. Appl. Ichthyol. 2012, 28, 238–244. [Google Scholar] [CrossRef]
- Jaworski, N.A.; Howarth, R.W.; Hetling, L.J. Atmospheric deposition of nitrogen oxides onto the landscape contributes to coastal eutrophication in the northeast United States. Environ. Sci. Technol. 1997, 31, 1995–2004. [Google Scholar] [CrossRef]
- Peltzer, P.M.; Lajmanovich, R.C.; Sánchez-Hernandez, J.C.; Cabagna, M.C.; Attademo, A.M.; Bassó, A. Effects of agricultural pond eutrophication on survival and health status of Scinax nasicus tadpoles. Ecotoxicol. Environ. Saf. 2008, 70, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Breukelaar, A.W.; Lammens, E.H.R.R.; Breteler, J.G.P.K.; Tatrai, I. Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Freshw. Biol. 1994, 32, 113–121. [Google Scholar] [CrossRef]
- Meijer, M.L.; De Haan, M.W.; Breukelaar, A.W.; Buiteveld, H. Is reduction of the benthivorous fish an important cause of high transparency following biomanipulation in shallow lakes? In Biomanipulation Tool for Water Management; Springer: Dordrecht, The Netherlands, 1990; pp. 303–315. [Google Scholar]
- Driver, P.D.; Closs, G.P.; Koen, T. The effects of size and density of carp (Cyprinus carpio L.) on water quality in an experimental pond. Arch. Hydrobiol. 2005, 163, 117–131. [Google Scholar] [CrossRef]
- Prescott, K.L.; Tsanis, I.K. Mass balance modelling and wetland restoration. Ecol. Eng. 1997, 9, 1–18. [Google Scholar] [CrossRef]
- Roy, K.; Vrba, J.; Kuebutornye, F.K.A.; Dvorak, P.; Kajgrova, L.; Mraz, J. Fish stocks as phosphorus sources or sinks: Influenced by nutritional and metabolic variations, not solely by dietary content and stoichiometry. Sci. Total Environ. 2024, 938, 173611. [Google Scholar] [CrossRef]
- Roy, K.; Vrba, J.; Kajgrova, L.; Mraz, J. The concept of balanced fish nutrition in temperate European fishponds to tackle eutrophication. J. Clean. Prod. 2022, 364, 132584. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Nitrogen biogeochemistry of aquaculture ponds. Aquaculture 1998, 166, 181–212. [Google Scholar] [CrossRef]
- Durborow, R.M.; Crosby, D.M.; Brunson, M.W. Ammonia in fish ponds. J. Fish. Res. Board Can. 1997, 32, 2379–2383. [Google Scholar]
- Zhao, J.; Zhang, M.; Xiao, W.; Jia, L.; Zhang, X.; Wang, J.; Zhang, Z.; Xie, Y.; Pu, Y.; Liu, S.; et al. Large methane emission from freshwater aquaculture ponds revealed by long-term eddy covariance observation. Agric. For. Meteorol. 2021, 308–309, 108600. [Google Scholar] [CrossRef]
- Sevrin-Reyssac, J.; Pletikosic, M. Cyanobacteria in fish ponds. Aquaculture 1990, 88, 1–20. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Li, M.; Pan, B. Cyanobacterial Blooms in Environmental Water: Causes and Solutions. Curr. Pollut. Rep. 2024, 10, 606–627. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; van Donk, E.; Hansson, L.-A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Change Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Potužák, J.; Hůda, J.; Pechar, L. Changes in fish production effectivity in eutrophic fishponds—Impact of zooplankton structure. Aquac. Int. 2007, 15, 201–210. [Google Scholar] [CrossRef]
- Oliver, R.L.; Hamilton, D.P.; Brookes, J.D.; Ganf, G.G. Physiology, Blooms and Prediction of Planktonic Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 155–194. [Google Scholar]
- Pechar, L. Long-term changes in fish pond management as’ an unplanned ecosystem experiment’: Importance of zooplankton structure, nutrients and light for species composition of cyanobacterial blooms. Water Sci. Technol. 1995, 32, 187–196. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, J.; Liang, C.; Li, G.; Shen, L.; Zhang, H.; Hu, X.; Chen, X. Nitrogen limitation affects the sinking property of Microcystis by promoting carbohydrate accumulation. Chemosphere 2019, 221, 665–671. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, W.; Qin, B. An indispensable role of overlying water in nitrogen removal in shallow lakes. Sci. Total Environ. 2024, 923, 171487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, W.; Zhou, J.; Yu, J.; Kong, L.; Qin, B. Strong turbulence accelerates sediment nitrification-denitrification for nitrogen loss in shallow lakes. Sci. Total Environ. 2021, 761, 143210. [Google Scholar] [CrossRef] [PubMed]
- Brunson, M.W.; Lutz, C.G.; Durborow, R.M. Algae Blooms in Commercial Fish Production Ponds; Southern Regional Aquaculture Center: Stoneville, MS, USA, 1994. [Google Scholar]
- Landsberg, J.H.; Hendrickson, J.; Tabuchi, M.; Kiryu, Y.; Williams, B.J.; Tomlinson, M.C. A large-scale sustained fish kill in the St. Johns River, Florida: A complex consequence of cyanobacteria blooms. Harmful Algae 2020, 92, 101771. [Google Scholar] [CrossRef] [PubMed]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Ekvall, M.K.; Urrutia-Cordero, P.; Hansson, L.-A. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS ONE 2014, 9, e112956. [Google Scholar] [CrossRef]
- Wunder, W. Das Plankton als wichtiger Bestandteil der Naturnahrung des Karpfens. Methoden der Planktonvermehrung. Osterr. Fisch. 1968, 21, 97–103. [Google Scholar]
- Schlott, K.; Schlott, G.; Gratzl, G.; Fichtenbauer, M.; Bauer, C. Demand-oriented feeding in carp pond Farming. The settling volume of zooplankton. Zenodo 2023, 35. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Lujić, J.; Marinović, Z.; Subakov-Simić, G.; Dulić, T.; Važić, T.; Nybom, S.; Meriluoto, J.; Codd, G.A. Cyanobacteria and cyanotoxins in fishponds and their effects on fish tissue. Harmful Algae 2016, 55, 66–76. [Google Scholar] [CrossRef]
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. Int. J. 2006, 25, 72–86. [Google Scholar] [CrossRef]
- Stepanova, N.; Nikitin, O.; Latypova, V.; Kondratyeva, T. Cyanotoxins as a possible cause of fish and waterfowl death in the Kazanka River (Russia). In Proceedings of the International Multidisciplinary Scientific GeoConference-SGEM, Albena, Bulgaria, 2–8 July 2018; pp. 2–8. [Google Scholar]
- Greer, B.; Maul, R.; Campbell, K.; Elliott, C.T. Detection of freshwater cyanotoxins and measurement of masked microcystins in tilapia from Southeast Asian aquaculture farms. Anal. Bioanal. Chem. 2017, 409, 4057–4069. [Google Scholar] [CrossRef] [PubMed]
- Varga, D.; Sándor, Z.; Hancz, C.; Csengeri, I.; Jeney, Z.; Papp, Z. Off-flavour compounds in common carp (Cyprinus carpio L.) flesh in context of type of fish pond. Acta Aliment. 2015, 44, 311–315. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Ellakany, H.F.; Abaza, S.S.; Geneedy, A.M.; Salem, H.M.; Taha, A.E.; Swelum, A.A.; Omer, F.A. Undesirable odour substances (geosmin and 2-methylisoborneol) in water environment: Sources, impacts and removal strategies. Mar. Pollut. Bull. 2022, 178, 113579. [Google Scholar] [CrossRef]
- Van der Ploeg, M.; Tucker, C.S.; Boyd, C.E. Geosmin and 2-methylisoborneol production by cyanobacteria in fish ponds in the southeastern United States. Water Sci. Technol. 1992, 25, 283–290. [Google Scholar] [CrossRef]
- van der Ploeg, M.; Boyd, C.E. Geosmin production by cyanobacteria (blue-green algae) in fish ponds at Auburn, Alabama. J. World Aquac. Soc. 1991, 22, 207–216. [Google Scholar] [CrossRef]
- Pechar, L. Impacts of long-term changes in fishery management on the trophic level water quality in Czech fish ponds. Fish. Manag. Ecol. 2000, 7, 23–31. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Orihel, D.M.; Baulch, H.M.; Casson, N.J.; North, R.L.; Parsons, C.T.; Seckar, D.C.M.; Venkiteswaran, J.J. Internal phosphorus loading in Canadian fresh waters: A critical review and data analysis. Can. J. Fish. Aquat. Sci. 2017, 74, 2005–2029. [Google Scholar] [CrossRef]
- Boyd, C.E. Practical Aspects of Chemistry in Pond Aquaculture. Progress. Fish-Cult. 1997, 59, 85–93. [Google Scholar] [CrossRef]
- Vrba, J.; Šorf, M.; Nedoma, J.; Benedová, Z.; Kröpfelová, L.; Šulcová, J.; Tesařová, B.; Musil, M.; Pechar, L.; Potužák, J.; et al. Top-down and bottom-up control of plankton structure and dynamics in hypertrophic fishponds. Hydrobiologia 2024, 851, 1095–1111. [Google Scholar] [CrossRef]
- Schlott, K. Die Planktische Naturnahrung und Ihre Bedeutung für Die Fischproduktion in Karpfenteichen; Schriftenreihe des Bundesamtes; Bundesamt für Wasserwirtschaft, Ökologische Station Waldviertel: Schrems, Austria, 2007; Volume 27. [Google Scholar]
- Gambelli, D.; Vairo, D.; Solfanelli, F.; Zanoli, R. Economic performance of organic aquaculture: A systematic review. Mar. Policy 2019, 108, 103542. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef]
- Hodapp, D.; Hillebrand, H.; Striebel, M. “Unifying” the Concept of Resource Use Efficiency in Ecology. Front. Ecol. Evol. 2019, 6, 233. [Google Scholar] [CrossRef]
- Migoń, P.; Michniewicz, A.; Różycka, M. Granite tors of the Waldviertel region in Lower Austria. In Landscapes and Landforms of Austria; Springer Nature: Cham, Switzerland, 2022; pp. 137–145. [Google Scholar]
- Boehm, M.; Bauer, C. Changes in water parameters in carp ponds of northern Austrian (Waldviertel) over the last 30 years. J. Appl. Ichthyol. 2015, 31, 15–17. [Google Scholar] [CrossRef]
- BML Bundesministerium für Land- und Forstwirtschaft, Regionen und Wasserwirtschaft. Waldviertler Karpfen. Available online: https://info.bml.gv.at/themen/lebensmittel/trad-lebensmittel/fisch/waldviertler_karpfen.html (accessed on 6 March 2025).
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Konopáčová, E.; Schagerl, M.; Bešta, T.; Čapková, K.; Pouzar, M.; Štenclová, L.; Řeháková, K. An assessment of periphyton mats using CHEMTAX and traditional methods to evaluate the seasonal dynamic in post-mining lakes. Hydrobiologia 2023, 850, 3143–3160. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef]
- Paulic, M.; Hand, J.; Lord, L. 1996 Water-Qualitiy Assessment for the State of Florida Section 305(B) Main Report; Bureau of Water Resources Protection: Tallahassee, FL, USA, 1996; p. 317. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 19/2, Oscillatoriales. In Süßwasserflora von Mitteleuropa, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Elsevier Spektrum Akademischer Verlag: Heidelberg, Germany, 2005; p. 759. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 19/1, Chroococcales. In Süßwasserflora von Mitteleuropa, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Jena, Germany, 1999; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 19/3, Heterocytous Genera. In Süßwasserflora von Mitteleuropa, 1st ed.; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; p. 1130. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae (Grünalgen), Ordnung Chlorococcales. In Das Phytoplankton des Süßwassers, 1st ed.; l 7/1; Huber-Pestalozzi, G., Ed.; E. Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1983; p. 1104. [Google Scholar]
- Rieth, A. Xanthophyceae. 4/2. In Süßwasserflora von Mitteleuropa, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Jena, Germany, 1980; p. 147. [Google Scholar]
- Ettl, H. Xanthophyceae. 3/1. In Süßwasserflora von Mitteleuropa, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Eds.; G. Fischer: Jena, Germany, 1978; p. 530. [Google Scholar]
- Ettl, H.; Gärtner, G. Chlorophyta. 10/2, Tetrasporales, Chlorococcales, Gloeodendrales. In Süßwasserflora von Mitteleuropa, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Stuttgart, Germany; New York, NY, USA, 1988; p. 436. [Google Scholar]
- Kristiansen, J.; Preisig, H.R. Chrysophyte and Haptophyte Algae. 1/2, Synurophyceae. In Süßwasserflora von Mitteleuropa, 2nd ed.; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Spektrum, Akad. Verl.: Heidelberg, Germany, 2007; p. 252. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die ökologische Praxis: Über 700 der häufigsten Arten und ihrer Ökologie; Koeltz Scientific Books; Gantner: Rugell, Liechtenstein, 2011; p. 908. [Google Scholar]
- Starmach, K. Chrysophyceae und Haptophyceae, 1/1. In Süßwasserflora von Mitteleuropa, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Eds.; G. Fischer: Stuttgart, Germany, 1985; p. 515. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: https://www.algaebase.org (accessed on 6 March 2025).
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2/1, Naviculaceae. In Süßwasserflora von Mitteleuropa, reprint of the 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Eds.; Spektrum: Heidelberg, Germany, 2010; p. 876. [Google Scholar]
- Fleming, W.D. Naphrax: A synthetic mounting medium of high refractive Index new and improved methods of preparation. J. R. Microsc. Soc. 1954, 74, 42–44. [Google Scholar] [CrossRef]
- Alster, A.; Kaplan-Levy, R.N.; Barinova, S.S.; Zohary, T. Analyzing semiquantitative phytoplankton counts. Hydrobiologia 2024, 851, 1079–1090. [Google Scholar] [CrossRef]
- Pielou, E.C. An introduction to Mathematical Ecology; Wiley-Inter-Science: New York, NY, USA, 1969. [Google Scholar]
- Shannon, C.E.; Weaver, W. The mathematical theory of communication. University of Illinois. Urbana 1949, 117, 10. [Google Scholar]
- Zhang, Y.; Yu, Y.; Liu, J.; Guo, Y.; Yu, H.; Liu, M. The Driving Mechanism of Phytoplankton Resource Utilization Efficiency Variation on the Occurrence Risk of Cyanobacterial Blooms. Microorganisms 2024, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, P.; Padisák, J.; Koschel, R.; Krienitz, L.; Gervais, F. Chlorophyll a concentration across a trophic gradient of lakes: An estimator of phytoplankton biomass? Limnologica 2008, 38, 327–338. [Google Scholar] [CrossRef]
- Riemann, B.; Simonsen, P.; Stensgaard, L. The carbon and chlorophyll content of phytoplankton from various nutrient regimes. J. Plankton Res. 1989, 11, 1037–1045. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial. PRIMER-E; PRIMER-E Ltd.: Devon, UK, 2015; 296p. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, 7.10; Wild Blueberry Media: Corvallis, OR, USA, 2018. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.10); Biometris, Wageningen University & Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Kajgrová, L.; Kolar, V.; Roy, K.; Adámek, Z.; Blabolil, P.; Kopp, R.; Mráz, J.; Musil, M.; Pecha, O.; Pechar, L.; et al. A stoichiometric insight into the seasonal imbalance of phosphorus and nitrogen in central European fishponds. Environ. Sci. Eur. 2024, 36, 139. [Google Scholar] [CrossRef]
- Ma, M.; Li, J.; Lu, A.; Zhu, P.; Yin, X. Effects of phytoplankton diversity on resource use efficiency in a eutrophic urban river of Northern China. Front. Environ. Sci. 2024, 12, 1389220. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Li, Y.; Jeppesen, E.; Zhang, M.; Chen, F. Nitrogen Reduction Causes Shifts in Winter and Spring Phytoplankton Composition and Resource Use Efficiency in a Large Subtropical Lake in China. Ecosystems 2023, 26, 1640–1655. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Abdullah Al, M.; Ndayishimiye, J.C.; Yang, J.R.; Isabwe, A.; Luo, A.; Yang, J. Urbanization reduces resource use efficiency of phytoplankton community by altering the environment and decreasing biodiversity. J. Environ. Sci. 2022, 112, 140–151. [Google Scholar] [CrossRef]
- Oertli, B. Editorial: Freshwater biodiversity conservation: The role of artificial ponds in the 21st century. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 264–269. [Google Scholar] [CrossRef]
- Palásti, P.; Gulyás, Á.; Kiss, M. Mapping Freshwater Aquaculture’s Diverse Ecosystem Services with Participatory Techniques: A Case Study from White Lake, Hungary. Sustainability 2022, 14, 16825. [Google Scholar] [CrossRef]
- Li, Y.; Geng, M.; Yu, J.; Du, Y.; Xu, M.; Zhang, W.; Wang, J.; Su, H.; Wang, R.; Chen, F. Eutrophication decrease compositional dissimilarity in freshwater plankton communities. Sci. Total Environ. 2022, 821, 153434. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; García Molinos, J.; Heino, J.; Zhang, H.; Zhang, P.; Xu, J. Eutrophication causes invertebrate biodiversity loss and decreases cross-taxon congruence across anthropogenically-disturbed lakes. Environ. Int. 2021, 153, 106494. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Peder Jensen, J.; SØndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Zhang, M.; Straile, D.; Chen, F.; Shi, X.; Yang, Z.; Cai, Y.; Yu, J.; Kong, F. Dynamics and drivers of phytoplankton richness and composition along productivity gradient. Sci. Total Environ. 2018, 625, 275–284. [Google Scholar] [CrossRef]
- Matsumura-Tundisi, T.; Tundisi, J.G. Plankton richness in a eutrophic reservoir (Barra Bonita Reservoir, SP, Brazil). Hydrobiologia 2005, 542, 367–378. [Google Scholar]
- Wezel, A.; Oertli, B.; Rosset, V.; Arthaud, F.; Leroy, B.; Smith, R.; Angélibert, S.; Bornette, G.; Vallod, D.; Robin, J. Biodiversity patterns of nutrient-rich fish ponds and implications for conservation. Limnology 2014, 15, 213–223. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisak, J. Functional classifications and their application in phytoplankton ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, B.; Teubner, K.; Dokulil, M.T. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003, 25, 445–453. [Google Scholar] [CrossRef]
- Wu, T.; Dai, R.; Chu, Z.; Cao, J. Rapid Recovery of Buoyancy in Eutrophic Environments Indicates That Cyanobacterial Blooms Cannot Be Effectively Controlled by Simply Collapsing Gas Vesicles Alone. Water 2023, 15, 1898. [Google Scholar] [CrossRef]
- Mur, L.R.; Gons, H.J.; Van Liere, L. Some experiments on the competition between green algae and blue-green bacteria in light-limited environments. FEMS Microbiol. Lett. 1977, 1, 335–338. [Google Scholar] [CrossRef]
- Zevenboom, W.; Mur, L.R. N2-fixing cyanobacteria: Why they do not become dominant in Dutch, hypertrophic lakes. In Hypertrophic Ecosystems., 1st ed.; Barica, J., Mur, L.R., Eds.; Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 1980; Volume 2, pp. 123–130. [Google Scholar]
- Mur, L.R.; Beijdorff, R.O. A model of the succession from green to blue-green algae based on light limitation: With 7 figures and 1 table in the text. Int. Ver. Für Theor. Angew. Limnol. Verhandlungen 1978, 20, 2314–2321. [Google Scholar] [CrossRef]
- Tilman, D.; Kiesling, R. Freshwater algal ecology: Taxonomic trade-offs in the temperature dependence of nutrient competitive abilities. In Proceedings of the Third International Symposium on Microbial Ecology, East Lansing, MI, USA, 7–12 August 1983; Michigan State University: East Lansing, MI, USA, 1984; pp. 314–324. [Google Scholar]
- Konopka, A.; Brock, T.D. Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [CrossRef]
- Robarts, R.D.; Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N. Z. J. Mar. Freshw. Res. 1987, 21, 391–399. [Google Scholar] [CrossRef]
- Korinek, V.; Fott, J.; Fuksa, J.; Lellák, J.; Prazakova, M. Carp Ponds of Central Europe. In Managed Aquatic Ecosystems. Ecosystems of the World, 29; Elsevier Science Publishing Co.: New York, NY, USA, 1987. [Google Scholar]
- Pražáková, M. Impact of Fishery Management on Cladoceran Populations. Hydrobiologia, 1991; 225, 209–216. [Google Scholar]
- Schlott-idl, K. Development of zooplankton in fishponds of the Waldviertel (Lower Austria). J. Appl. Ichthyol. 1991, 7, 223–229. [Google Scholar] [CrossRef]
- Kopp, R.; Ziková, A.; Mareš, J.; Navrátil, S.; Adamovský, O.; Palíková, M. Diversity and toxin content of cyanobacteria in fish ponds (South Moravia, Czech Republic) related to fishery management intensity. Acta Univ. Agric. Silvic. Mendel. Brun. 2008, 5, 111–118. [Google Scholar]
- Nõges, P.; Ott, I. Occurrence, coexistence and competition of Limnothrix redekei and Planktothrix agardhii: Analysis of Danish-Estonian lake database. Arch. Hydrobiol. Suppl. Algol. Stud. 2003, 148, 429–441. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Walsby, A.E. Water-blooms. Biol. Rev. 1975, 50, 437–481. [Google Scholar] [CrossRef]
- Rojo, C.; Cobales, M.A. Taxonomy and ecology of phytoplankton in a hypertrophic gravel-pit lake. I. Blue-green algae. Arch. Protistenkd. 1992, 142, 77–90. [Google Scholar] [CrossRef]
- Rücker, J.; Wiedner, C.; Zippel, P. Factors controlling the dominance of Planktothrix agardhii and Limnothrix redekei in eutrophic shallow lakes. Hydrobiologia 1997, 342/343, 107–115. [Google Scholar] [CrossRef]
- Romo, S.; Miracle, M.R. Diversity of the phytoplankton assemblages of a polymictic hypertrophic lake. Arch. Hydrobiol. 1995, 132, 363–384. [Google Scholar] [CrossRef]
- Donabaum, K.; Schagerl, M.; Dokulil, M.T. Integrated Management to Restore Macrophyte Domination. Hydobiologia 1999, 395, 87–97. [Google Scholar] [CrossRef]
- Fott, J.; Kořínek, V.; Pražáková, M.; Vondruš, B.; Forejt, K. Seasonal development of phytoplankton in fish ponds. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1974, 59, 629–641. [Google Scholar] [CrossRef]
- Borics, G.; Grigorszky, I.; Szabó, S.; Padisák, J. Phytoplankton associations in a small hypertrophic fishpond in East Hungary during a change from bottom-up to top-down control. In The Trophic Spectrum Revisited: The Influence of Trophic State on the Assembly of Phytoplankton Communities, Proceedings of the 11th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP), Shrewsbury, UK, 15–23 August 1998; Springer: Dordrecht, The Netherlands, 2000; pp. 79–90. [Google Scholar]
- Wawrik, F. Waldviertler Fischteiche II—Die Jaidhof-Teiche. Sitzungsberichte Akad. Wiss. Math. -Naturwissenschaftliche Kl. 1960, 169, 341–381. [Google Scholar]
- Mandal, B.K. Limnological studies of a freshwater fish pond at Burdwan, West Bengal, India. Jpn. J. Limnol. 1980, 41, 10–18. [Google Scholar] [CrossRef]
- Osti, J.A.S.; Tucci, A.; Camargo, A.F.M. Changes in the structure of the phytoplankton community in a Nile tilapia fishpond. Acta Limnol. Bras. 2018, 30, e213. [Google Scholar] [CrossRef]
- Fastner, J.; Erhard, M.; Carmichael, W.W.; Sun, F.; Rinehart, K.L.; Rönicke, H.; Chorus, I. Characterization and diversity of microcystins in natural blooms and strains of the genera Microcystis and Planktothrix from German freshwaters. Arch. Hydrobiol. 1999, 145, 147–164. [Google Scholar] [CrossRef]
- Keil, C.; Forchert, A.; Fastner, J.; Szewzyk, U.; Rotard, W.; Chorus, I.; Krätke, R. Toxicity and microcystin content of extracts from a Planktothrix bloom and two laboratory strains. Water Res. 2002, 36, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Mareš, J.; Palíková, M.; Navrátil, S.; Kubíček, Z.; Ziková, A.; Hlávková, J.; Bláha, L. Biochemical parameters of blood plasma and content of microcystins in tissues of common carp (Cyprinus carpio L.) from a hypertrophic pond with cyanobacterial water bloom. Aquac. Res. 2009, 40, 1683–1693. [Google Scholar] [CrossRef]
- Radojicic, M.; Hetesa, J.; Musilova, B.; Kopp, R. Quantitative Analyses of Phytoplankton in Zámecký Pond–Three Years Research. 2018. Available online: http://www.rybarstvi.eu/pub%20rybari/2018%20Marija%20mendelnet.pdf (accessed on 6 March 2025).
- Boyd, C.E. Summer algal communities and primary productivity in fish ponds. Hydrobiologia 1973, 41, 357–390. [Google Scholar] [CrossRef]
- Wawrik, F. Phytoplankton aus neu angelegten Streckteichen (Waldviertel, Niederösterreich). Int. Rev. Gesamten Hydrobiol. Hydrogr. 1977, 62, 295–313. [Google Scholar] [CrossRef]
- Kopp, R.; Řezníčková, P.; Hadašová, L.; Petrek, R.; Brabec, T. Water quality and phytoplankton communities in newly created fishponds. Acta Univ. Agric. Silvic. Mendel. Brun. 2016, 64, 71–80. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Hutorowicz, A.; Tucholski, S. Dynamics and structure of phytoplankton in fishponds fed with treated wastewater. Pol. J. Environ. Stud. 2011, 20, 151. [Google Scholar]
- Adámek, Z.; Mössmer, M.; Hauber, M. Current principles and issues affecting organic carp (Cyprinus carpio) pond farming. Aquaculture 2019, 512, 734261. [Google Scholar] [CrossRef]
- Haney, J.F. Field studies on zooplankton-cyanobacteria interactions. N. Z. J. Mar. Freshw. Res. 1987, 21, 467–475. [Google Scholar] [CrossRef]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) Model: Mechanisms Driving Plankton Succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Grover, J.P.; Chrzanowski, T.H. Seasonal dynamics of phytoplankton in two warm temperate reservoirs: Association of taxonomic composition with temperature. J. Plankton Res. 2006, 28, 1–17. [Google Scholar] [CrossRef]
- Komárková, J.; Komárek, O.; Hejzlar, J. Evaluation of the long term monitoring of phytoplankton assemblages in a canyon-shape reservoir using multivariate statistical methods. Hydrobiologia 2003, 504, 143–157. [Google Scholar] [CrossRef]
- Sipaúba-Tavares, L.H.; Millan, R.N.; Capitano, É.C.O.; Scardoelli-Truzzi, B. Abiotic parameters and planktonic community of an earthen fish pond with continuous water flow. Acta Limnol. Bras. 2019, 31, e13. [Google Scholar] [CrossRef]
- Sipaúba-Tavares, L.H.; Seto, L.M.; Millan, R.N. Seasonal variation of biotic and abiotic parameters in parallel neotropical fishponds. Braz. J. Biol. 2014, 74, 166–174. [Google Scholar] [CrossRef]
- Baker, K.G.; Geider, R.J. Phytoplankton mortality in a changing thermal seascape. Glob. Change Biol. 2021, 27, 5253–5261. [Google Scholar] [CrossRef] [PubMed]
- Striebel, M.; Schabhüttl, S.; Hodapp, D.; Hingsamer, P.; Hillebrand, H. Phytoplankton responses to temperature increases are constrained by abiotic conditions and community composition. Oecologia 2016, 182, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Temponeras, M.; Kristiansen, J.; Moustaka-Gouni, M. Seasonal variation in phytoplankton composition and physical-chemical features of the shallow Lake Doïrani, Macedonia, Greece. In The Trophic Spectrum Revisited: The Influence of Trophic State on the Assembly of Phytoplankton Communities, Proceedings of the 11th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP), Shrewsbury, UK, 15–23 August 1998; Springer: Dordrecht, The Netherlands, 2000; pp. 109–122. [Google Scholar]
- Verbeek, L.; Gall, A.; Hillebrand, H.; Striebel, M. Warming and oligotrophication cause shifts in freshwater phytoplankton communities. Glob. Change Biol. 2018, 24, 4532–4543. [Google Scholar] [CrossRef] [PubMed]

| Labelling | District | Pond | Area (ha) | Age (y) | Sea Level (m) | Type | Stocking Amount (Indivudual ha−1) | Usage | Liming | GPS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HD | Steinbruckteich | 8.73 | 250 | 605 | H | 3000 | C | No | 48.8591, 15.1464 |
| 2 | HD | Winkelauerteich | 49.27 | 250 | 597 | H/S | 650 | C | Yes | 48.8455, 15.1457 |
| 3 | HD | Edelwehrteich | 3.13 | 210 | 561 | S | F | No | 48.8728, 15.1333 | |
| 4 | HD | Streitteich | 2.76 | 250 | 574 | H | 3000 | C | No | 48.8792, 15.1270 |
| 5 | HD | Neuteich | 4.36 | 250 | 564 | H | 3000 | C | No | 48.8713, 15.1235 |
| 6 | HD | Gr. Brünaiteich | 30.00 | 250 | 550 | H | 550 | C | Yes | 48.8720, 15.0647 |
| 7 | HD | Brandteich | 15.22 | 250 | 524 | H | 3000 | C | Yes | 48.8592, 15.0308 |
| 8 | SCH | Haslauerteich | 50.00 | 50 | 560 | S | 650 | C | No | 48.8230, 15.1331 |
| 9 | SCH | Gebhartsteich | 57.00 | 250 | 547 | S | 650 | C | Yes | 48.80214, 15.1385 |
| 10 | SCH | Moorbad | 3.00 | 250 | 532 | H | F/B | Yes | 48.8000, 15.0793 | |
| 11 | SCH | Höfentöckteich | 15.00 | 250 | 517 | H | F | Yes | 48.7893, 15.0380 | |
| 12 | PÜR | Pürbacher Teich | 21.20 | 250 | 526 | H/S | 650 | C | No | 48.7704, 15.0752 |
| 13 | PÜR | Althöllteich | 19.00 | 250 | 572 | H/S | 650 | C | No | 48.7660, 15.0724 |
| 14 | PÜR | Frauenteich | 26.58 | 250 | 536 | H/S | 650 | C | No | 48.7540, 15.0875 |
| 15 | PÜR | Edlauteich | 20.96 | 250 | 534 | H/S | 650 | C | No | 48.7542, 15.0691 |
| Source | df | SS | MS | Pseudo-F | p (perm) | Unique perms |
| season | 2 | 10,140 | 5070.2 | 2.5428 | 0.0001 | 9870 |
| stock | 1 | 6505.5 | 6505.5 | 3.2626 | 0.0001 | 9862 |
| season x stock | 2 | 2457.7 | 1228.8 | 0.61629 | 0.9954 | 9836 |
| Res | 39 | 77,763 | 1993.9 | |||
| Total | 44 | 1.04 × 105 | ||||
| Pairwise Tests for | Season | t | p(perm) | Unique perms | ||
| spring–summer | 1.6764 | 0.0001 | 9897 | |||
| spring–autumn | 1.9395 | 0.0001 | 9892 | |||
| summer–autumn | 1.0859 | 0.2233 | 9900 | |||
| Taxon | Group | IV | Mean | St.Dev |
|---|---|---|---|---|
| Pseudopediastrum boryanum | aquaculture | 79 | 56.4 | 5.63 |
| Craticula cuspidata | aquaculture | 63 | 37.5 | 9.15 |
| Eudorina elegans | aquaculture | 61 | 38.1 | 9.09 |
| Navicula cryptocephala | aquaculture | 61 | 44.4 | 8.04 |
| Desmodesmus armatus var. longispina | recreation | 65 | 45.1 | 9.31 |
| Aulacoseira muzzanensis | recreation | 63 | 45.1 | 9.51 |
| Ankistrodesmus arcuatus | spring | 82 | 22.7 | 6.68 |
| Pinnilaria viridis | spring | 78 | 22.1 | 7.46 |
| Cymatopleura solea | spring | 67 | 26.6 | 8.89 |
| Ulnaria ulna | spring | 65 | 19.5 | 7.32 |
| Asterionella formosa | spring | 65 | 37.1 | 6.24 |
| Dinobryon cylindricum | spring | 60 | 15.4 | 6.14 |
| Trachelomonas volvocina | autumn | 84 | 20.2 | 6.67 |
| Scenedesmus obtusus f. disciformis | autumn | 61 | 25.3 | 6.86 |
| Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|
| Eigenvalues | 0.12 | 0.08 | 0.06 | 0.04 |
| Explained variation (cumulative) | 11.69 | 19.45 | 25.17 | 29.44 |
| Pseudo-canonical correlation | 0.94 | 0.95 | 0.94 | 0.91 |
| Explained fitted variation (cumulative) | 29.12 | 48.42 | 62.67 | 73.31 |
| p value | 0.0001 | 0.0001 | 0.0001 | 0.0003 |
| Name | Simple | Conditional | Pseudo-F | p | p(adj) |
|---|---|---|---|---|---|
| Temp | 9.8 | 9.8 | 4.7 | 0.0001 | 0.0009 |
| K+ | 6.4 | 6.3 | 3 | 0.0001 | 0.0009 |
| DIC | 6.2 | 3.1 | 2.9 | 0.0002 | 0.0012 |
| SiO4 | 5.9 | 4.4 | 2.7 | 0.0001 | 0.0009 |
| fish | 5.5 | 5.3 | 2.5 | 0.0003 | 0.0015 |
| liming | 5.2 | 2.9 | 2.4 | 0.0004 | 0.0016 |
| pH | 5 | 2.9 | 2.2 | 0.0009 | 0.0027 |
| area | 4.7 | 3.4 | 2.1 | 0.0013 | 0.0027 |
| Cond | 4.3 | 2 | 1.9 | 0.0035 | 0.0035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schagerl, M.; Yen, C.-C.; Bauer, C.; Gaspar, L.; Waringer, J. Fishponds Are Hotspots of Algal Biodiversity—Organic Carp Farming Reveals Unexpected High Taxa Richness. Environments 2025, 12, 92. https://doi.org/10.3390/environments12030092
Schagerl M, Yen C-C, Bauer C, Gaspar L, Waringer J. Fishponds Are Hotspots of Algal Biodiversity—Organic Carp Farming Reveals Unexpected High Taxa Richness. Environments. 2025; 12(3):92. https://doi.org/10.3390/environments12030092
Chicago/Turabian StyleSchagerl, Michael, Chun-Chieh Yen, Christian Bauer, Luka Gaspar, and Johann Waringer. 2025. "Fishponds Are Hotspots of Algal Biodiversity—Organic Carp Farming Reveals Unexpected High Taxa Richness" Environments 12, no. 3: 92. https://doi.org/10.3390/environments12030092
APA StyleSchagerl, M., Yen, C.-C., Bauer, C., Gaspar, L., & Waringer, J. (2025). Fishponds Are Hotspots of Algal Biodiversity—Organic Carp Farming Reveals Unexpected High Taxa Richness. Environments, 12(3), 92. https://doi.org/10.3390/environments12030092






