Microfluidic Field-Deployable Systems for Colorimetric-Based Monitoring of Nitrogen Species in Environmental Waterbodies: Past, Present, and Future
Abstract
1. Introduction
2. Fundamentals of Microfluidic Platforms for Chemical Sensing
2.1. Non-Droplet Microfluidic Systems
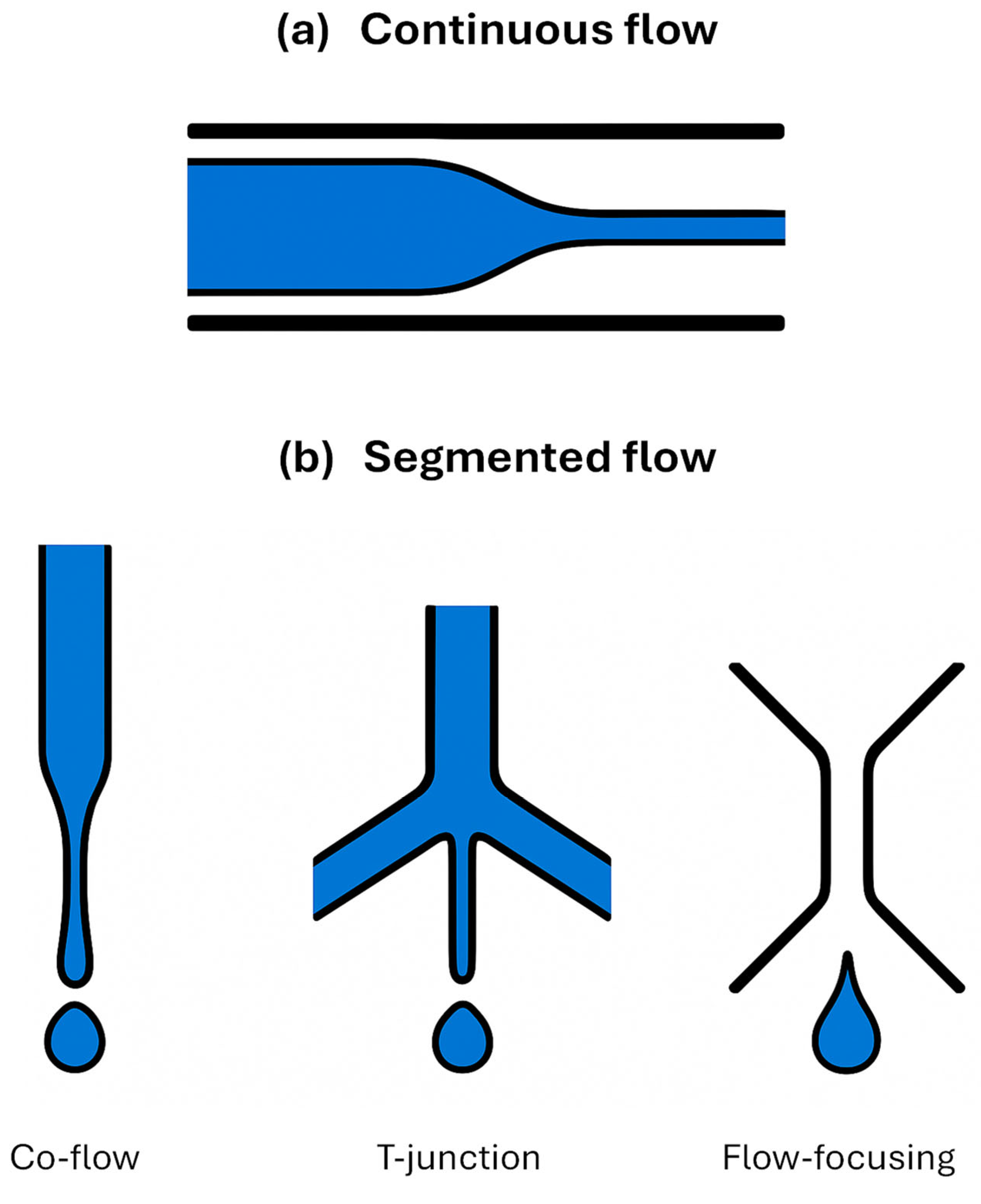
2.2. Droplet-Based Microfluidic Systems
2.3. On-Chip Droplet Operations
2.4. Intrinsic Advantages of Microfluidic Droplet-Based Systems
3. Colorimetric Detection in Microfluidic Systems
3.1. Principles of Colorimetric Measurement
3.2. Optical Detection Setups for Microfluidic Analysis
4. Application of Microfluidics to Nitrogen Species Analysis in Environmental Waterbodies
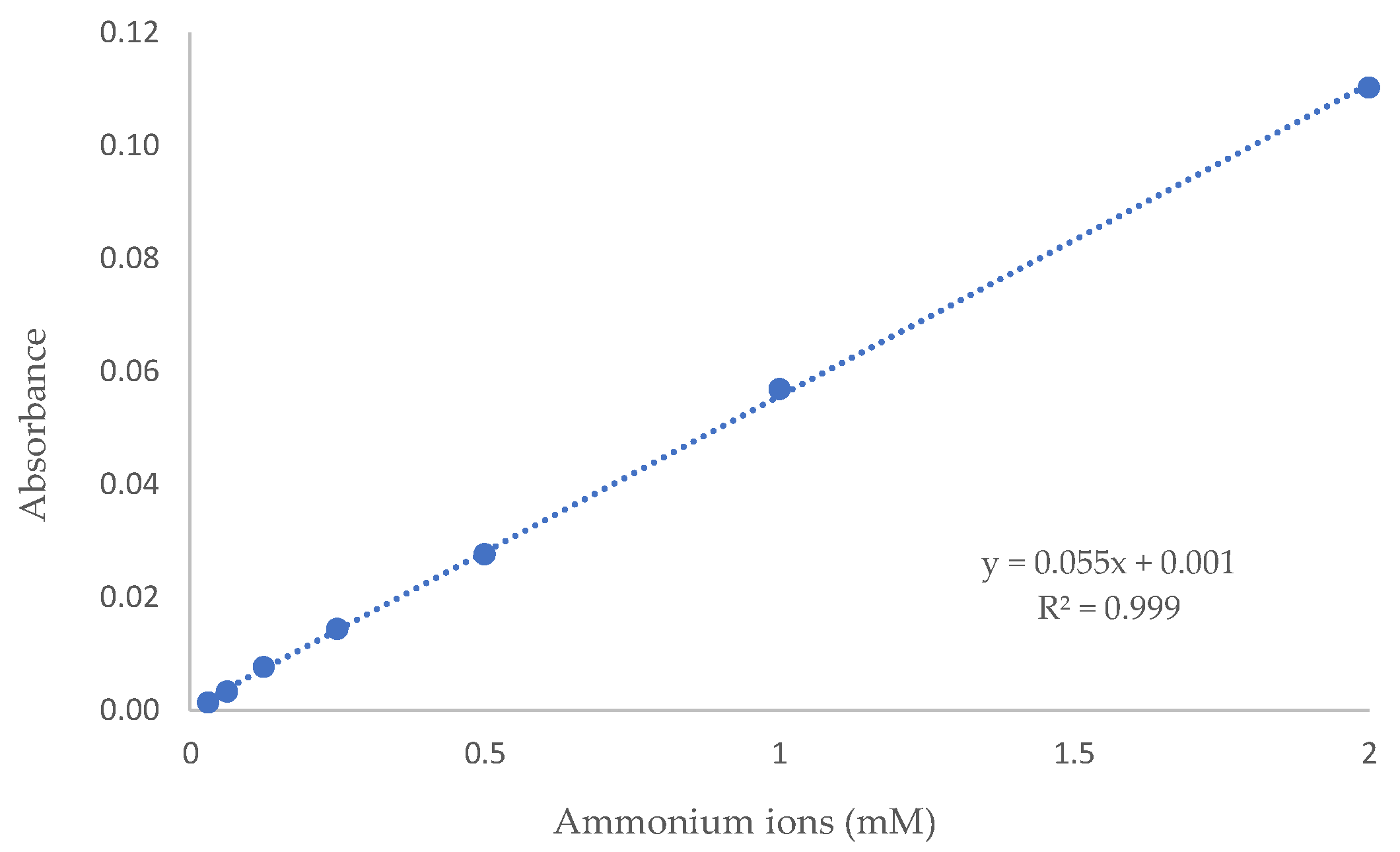
4.1. Ammonium (NH4+) Species
4.2. Nitrite (NO2−) Species
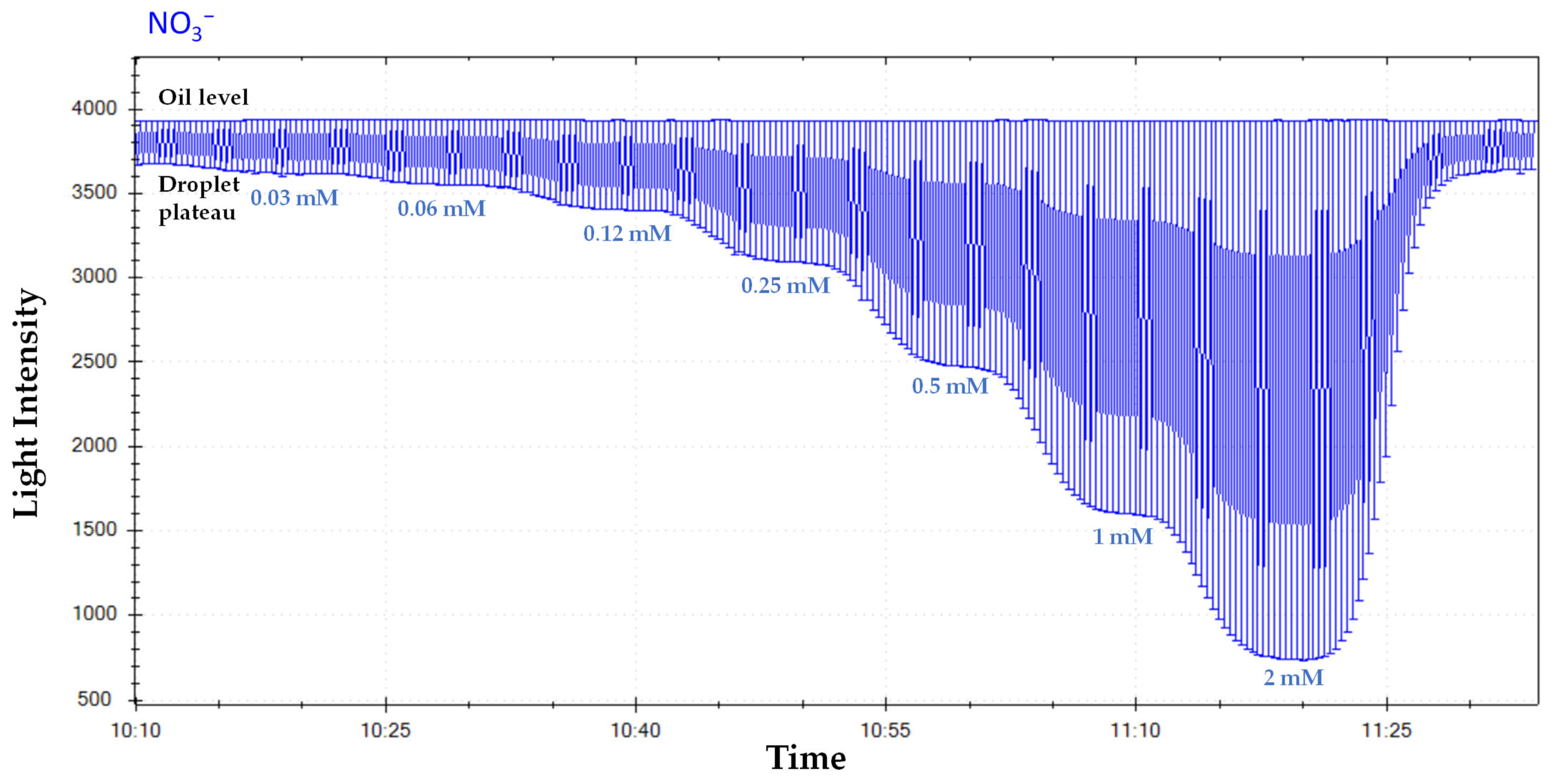
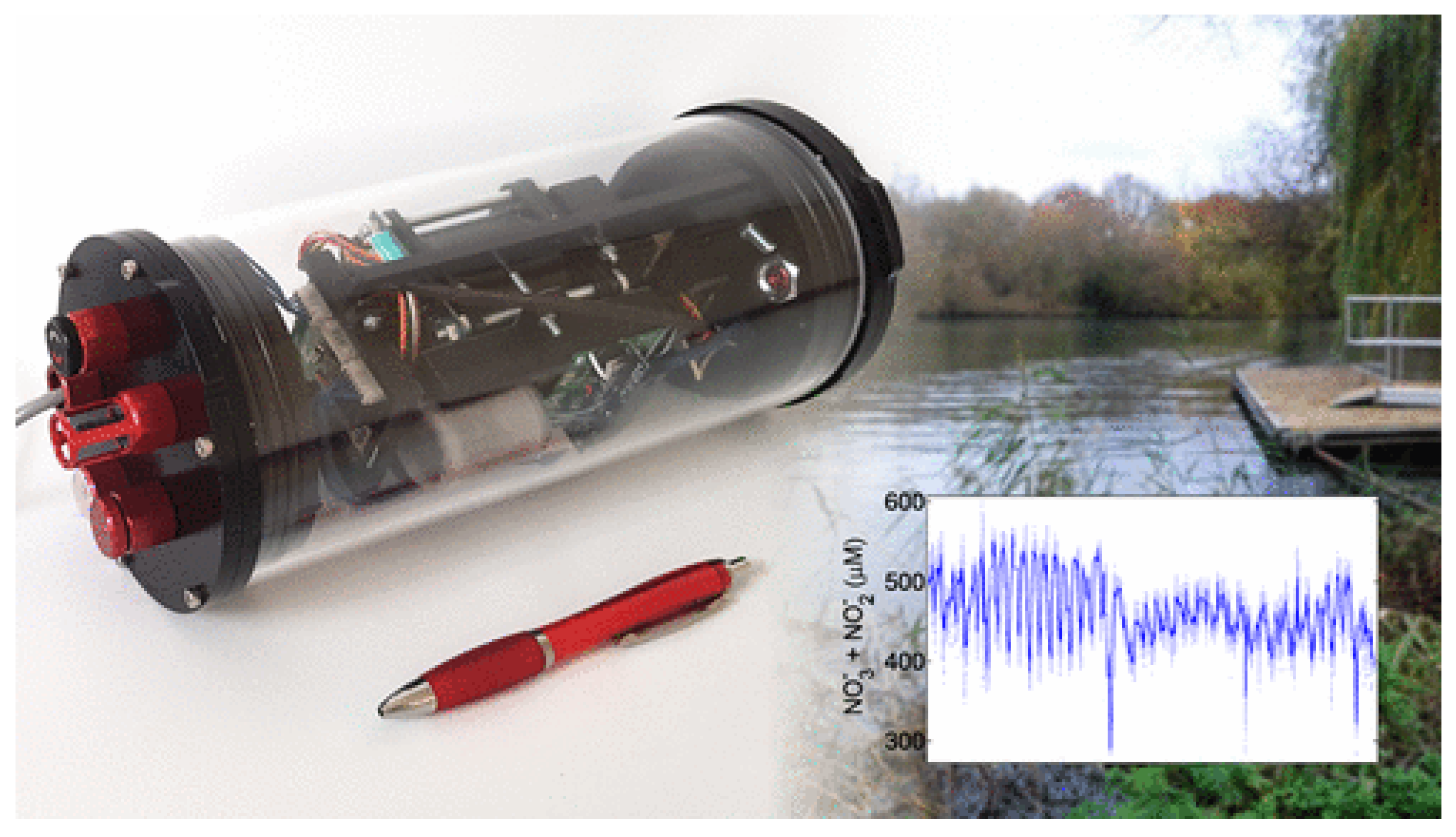
4.3. Nitrate (NO3−) Species
4.4. Total Dissolved Nitrogen (TDN) and Total Nitrogen (TN)
4.5. Organic Nitrogen
4.6. Multiplexed Detection of Nitrogen Species
5. Advantages and Overcoming Limitations in Environmental Water Analysis
5.1. Enhanced Analytical Capabilities for Environmental Science
5.2. Addressing Complex Environmental Matrix Effects
5.3. Robustness and Field Deployment Considerations
6. Future Perspectives and Emerging Trends
6.1. Advanced Materials and Manufacturing
6.2. Enhanced On-Chip Integration and Autonomy
6.3. Data Science and Sensor Networks
6.4. Novel Chemistries and Detection Schemes
6.5. Standardisation and Commercialisation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile butadiene styrene |
| AUV | Autonomous underwater vehicle |
| BTB | Bromothymol blue |
| COC | Cyclic olefine copolymer |
| DEP | Dielectrophoresis |
| DO | Dissolved oxygen |
| DOM | Dissolved organic matter |
| DON | Dissolved organic nitrogen |
| HAB | Harmful algal bloom |
| LED | Light-emitting diode |
| LOC | Lab-on-chip |
| ML | Machine learning |
| N | Nitrogen |
| NEDA·2HCl | N-(1-naphthyl) ethylenediamine dihydrochloride |
| NH4+ | Ammonium ion |
| NO2− | Nitrite ion |
| NO3− | Nitrate ion |
| NY | Nitrazine yellow |
| µPAD | Microfluidic paper-based device |
| PCB | Printed circuit board |
| PEEK | Polyetheretherketone |
| PD | Photodiode |
| PDMS | Polydimethylsiloxane |
| PLA | Polylactic acid |
| PMMA | Polymethyl methacrylate |
| PMT | Photomultiplier tube |
| PO43− | Phosphate ion |
| PON | Particulate organic nitrogen |
| PTFE | Polytetrafluoroethylene |
| SAW | Surface acoustic wave |
| TDN | Total dissolved nitrogen |
| TN | Total nitrogen |
| UV | Ultraviolet |
| VCl3 | Vanadium-(III) chloride |
References
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Sipler, R.A.; Bronk, D.A. Dynamics of dissolved organic nitrogen. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 127–232. [Google Scholar]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar]
- Chesapeake Bay Foundation. Available online: www.cbf.org (accessed on 23 June 2025).
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J., Jr. Gulf of Mexico hypoxia, aka “The Dead Zone”. Annu. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environ. Health Perspect. 2000, 108, 675–678. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October Establishing a Framework for Community Action in the Field of Water Policy. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0060:en:NOT (accessed on 24 June 2025).
- U.S. Environmental Protection Agency (EPA). Clean Water Act Section 303(d): Impaired Waters and Total Maximum Daily Loads (TMDLs). Available online: https://www.epa.gov/tmdl (accessed on 24 June 2025).
- APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater. Available online: https://www.standardmethods.org/doi/book/10.2105/SMWW.2882 (accessed on 24 June 2025).
- Strobl, R.O.; Robillard, P.D. Network design for water quality monitoring of surface freshwaters: A review. J. Environ. Manag. 2008, 87, 639–648. [Google Scholar] [CrossRef]
- Johnson, K.S.; Needoba, J.A.; Riser, S.C.; Showers, W.J. Chemical sensor networks for the aquatic environment. Chem. Rev. 2007, 107, 623–640. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Beaton, A.D.; Mowlem, M.C. Trends in microfluidic systems for in situ chemical analysis of natural waters. Sens. Actuators B Chem. 2015, 221, 1398–1405. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. Portable smartphone-based colorimetric system for simultaneous on-site microfluidic paper-based determination and mapping of phosphate, nitrite and silicate in coastal waters. Environ. Monit. Assess. 2022, 194, 190. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Garstecki, P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem. Soc. Rev. 2017, 46, 6210–6226. [Google Scholar] [CrossRef]
- Fornells, E.; Murray, E.; Waheed, S.; Morrin, A.; Diamond, D.; Paull, B.; Breadmore, M. Integrated 3D printed heaters for microfluidic applications: Ammonium analysis within environmental water. Anal. Chim. Acta 2020, 1098, 94–101. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.; Chun, H.; Kim, Y.S. Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens. Actuators B Chem. 2018, 256, 167–175. [Google Scholar] [CrossRef]
- Nxumalo, N.L.; Madikizela, L.M.; Kruger, H.G.; Onwubu, S.C.; Mdluli, P.S. Development of a paper-based microfluidic device for the quantification of ammonia in industrial wastewater. Water SA 2020, 46, 506–513. [Google Scholar] [CrossRef]
- Peters, J.J.; Almeida, M.I.G.S.; Šraj, L.O.; McKelvie, I.D.; Kolev, S.D. Development of a micro-distillation microfluidic paper-based analytical device as a screening tool for total ammonia monitoring in freshwaters. Anal. Chim. Acta 2019, 1079, 120–128. [Google Scholar] [CrossRef]
- Patey, M.D.; Rijkenberg, M.J.A.; Statham, P.J.; Stinchcombe, M.C.; Achterberg, E.P.; Mowlem, M.C. Determination of nitrate and phosphate in seawater at nanomolar concentrations. Trends Anal. Chem. 2010, 27, 169–182. [Google Scholar] [CrossRef]
- Šraj, L.O.C.; Almeida, M.I.G.S.; Swearer, S.E.; Kolev, S.D.; McKelvie, I.D. Analytical challenges and advantages of using flow-based methodologies for ammonia determination in estuarine and marine waters. Trends Anal. Chem. 2014, 59, 83–92. [Google Scholar] [CrossRef]
- Vidal, E.; Lorenzetti, A.S.; Lista, A.G.; Domini, C.E. Micropaper-based analytical device (µPAD) for the simultaneous determination of nitrite and fluoride using a smartphone. Microchem. J. 2018, 143, 467–473. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Colorimetric determination of nitrate after reduction to nitrite in a paper-based dip strip. Chem. Proc. 2021, 5, 9. [Google Scholar] [CrossRef]
- Beaton, A.D.; Schaap, A.M.; Pascal, R.; Hanz, R.; Martincic, U.; Cardwell, C.L.; Morris, A.; Clinton-Bailey, G.; Saw, K.; Hartman, S.E.; et al. Lab-on-Chip for in situ analysis of nutrients in the deep sea. ACS Sens. 2022, 7, 89–98. [Google Scholar] [CrossRef]
- Uhlikova, N.; Almeida, M.I.G.S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the speciation of inorganic nitrogen species. Talanta 2024, 271, 125671. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Warren, B.M.; Makris, K.; Evans, G.W.H.; Papadopoulou, E.; Coleman, S.; Niu, X. A droplet microfluidic-based sensor for simultaneous in situ monitoring of nitrate and nitrite in natural waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef]
- Chiang, C.-K.; Kurniawan, A.; Kao, C.-Y.; Wang, M.-J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Gomez, I.; Ortega-Muñoz, M.; Salinas-Castillo, A.; Álvarez-Bermejo, J.A.; Ariza-Avidad, M.; de Orbe-Payá, I.; Santoyo-Gonzalez, F.; Capitan-Vallvey, L.F. Tetrazine-based chemistry for nitrite determination in a paper microfluidic device. Talanta 2016, 160, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Dudala, S.; Dubey, S.K.; Goel, S. Fully integrated, automated, and smartphone enabled point-of-source portable platform with microfluidic device for nitrite detection. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1518–1524. [Google Scholar] [CrossRef]
- Solvas, X.C.; deMello, A. Droplet microfluidics: Recent developments and future applications. Chem. Commun. 2011, 47, 1936–1942. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Utada, A.S.; Fernandez-Nieves, A.; Stone, H.A.; Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef]
- Cramer, C.; Fischer, P.; Windhab, E.J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 2004, 59, 3045–3058. [Google Scholar] [CrossRef]
- Baret, J.C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Evans, G.W.H.; Xu, P.; Kim, B.J.; Hassan, S.; Niu, X. Phased peristaltic micropumping for continuous sampling and hardcoded droplet generation. Lab Chip 2017, 17, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lunn, J.; Yeung, K.; Dhandapani, S.; Carter, L.; Roose, T.; Shaw, L.; Nightingale, A.; Niu, X. Droplet microfluidic-based in situ analyzer for monitoring free nitrate in soil. Environ. Sci. Technol. 2024, 58, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Link, D.R.; Grasland-Mongrain, E.; Duri, A.; Sarrazin, F.; Cheng, Z.D.; Cristobal, G.; Marquez, M.; Weitz, D.A. Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed. Engl. 2006, 45, 2556–2560. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Y. Bubble formation and breakup dynamics in microfluidic devices: A review. Chem. Eng. Sci. 2015, 135, 343–372. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. Engl. 2003, 42, 768–772. [Google Scholar] [CrossRef]
- Song, H.; Ismagilov, R.F. Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J. Am. Chem. Soc. 2003, 125, 14613–14619. [Google Scholar] [CrossRef]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.M.; Link, D.R.; Weitz, D.A. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 024104. [Google Scholar] [CrossRef]
- Franke, T.; Abate, A.R.; Weitz, D.; Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip 2009, 9, 2625–2627. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Johnson, K.S.; Coletti, L.J.; Jannasch, H.W.; Sakamoto, C.M.; Swift, D.D.; Riser, S.C. Long-term nitrate measurements in the ocean using the in situ ultraviolet spectrophotometer: Sensor integration into the APEX profiling float. J. Atmos. Ocean. Technol. 2007, 30, 1854–1866. [Google Scholar] [CrossRef]
- Bhuiyan, W.T.; Milinovic, J.; Warren, B.; Liu, Y.; Nightingale, A.M.; Niu, X. Automated droplet-based microfluidic analyser for in-situ monitoring of ammonium ions in river water. ACS ES T Water 2025, 5, 4387–4394. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Makris, K.; Bhuiyan, W.T.; Harvey, T.J.; Niu, X. Easily fabricated monolithic fluoropolymer chips for sensitive long-term absorbance measurement in droplet microfluidics. RSC Adv. 2020, 10, 30975–30981. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Cheng, J.; Zou, Y.; Guo, J.; Guo, J. Detection and analysis of droplets in microfluidic devices: A review. IEEE Sens. J. 2024, 24, 33881–33902. [Google Scholar] [CrossRef]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef]
- Geng, Z.; Miao, Y.; Zhang, G.; Liang, X. Colorimetric biosensor based on smartphone: State-of-art. Sens. Actuators A Phys. 2023, 349, 114056. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y. Development and application of analytical detection techniques for droplet-based microfluidics. Anal. Chim. Acta 2020, 1113, 66–84. [Google Scholar] [CrossRef]
- Lu, B.; Lunn, J.; Nightingale, A.M.; Niu, X. Highly sensitive absorbance measurement using droplet microfluidics integrated with an oil extraction and long pathlength detection flow cell. Front. Chem. 2024, 12, 1394388. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Oveys, H.; Fan, X. Liquid-core optical ring-resonator sensors. Opt. Lett. 2006, 31, 1319–1321. [Google Scholar] [CrossRef]
- Khongpet, W.; Pencharee, S.; Puangpila, C.; Hartwell, S.K.; Lapanantnoppakhun, S.; Jakmunee, J. A compact hydrodynamic sequential injection system for consecutive on-line determination of phosphate and ammonium. Microchem. J. 2019, 147, 403–410. [Google Scholar] [CrossRef]
- Man, Y.; Yu, K.; Tan, H.; Jin, X.; Tao, J.; Pan, L. A microfluidic colorimetric system for rapid detection of nitrite in surface water. J. Hazard. Mater. 2024, 465, 133133. [Google Scholar] [CrossRef]
- Beaton, A.D.; Sieben, V.J.; Floquet, C.F.A.; Waugh, E.M.; Bey, S.A.K.; Ogilvie, I.R.G.; Mowlem, M.C.; Morgan, H. An automated microfluidic colourimetric sensor applied in situ to determine nitrite concentration. Sens. Actuators B Chem. 2011, 156, 1009–1014. [Google Scholar] [CrossRef]
- Martinez-Cisneros, C.; da Rocha, Z.; Seabra, A.; Valdés, F.; Alonso-Chamarro, J. Highly integrated autonomous lab-on-a-chip device for on-line and in situ determination of environmental chemical parameters. Lab Chip 2018, 18, 1884–1890. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, H.L.; Zhu, X.Q.; Zhu, J.M.; Zuo, Y.F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Han, X.T. Optofluidic differential colorimetry for rapid nitrite determination. Lab Chip 2018, 18, 2994. [Google Scholar] [CrossRef]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low cost lab on chip for the colorimetric detection of nitrate in mineral water products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef]
- Murphy, B.J.; Luy, E.A.; Panzica, K.L.; Johnson, G.; Sieben, V.J. An energy efficient thermally regulated optical spectroscopy cell for lab-on-chip devices: Applied to nitrate detection. Micromachines 2021, 12, 861. [Google Scholar] [CrossRef]
- Vincent, A.G.; Pascal, R.W.; Beaton, A.D.; Walk, J.; Hopkins, J.E.; Woodward, E.M.S.; Mowlem, M.; Lohan, M.C. Nitrate drawdown during a shelf sea spring bloom revealed using a novel microfluidic in situ chemical sensor deployed within an autonomous underwater glider. Mar. Chem. 2018, 205, 29–36. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Hu, X.; Chen, L.; Zuo, Y.; Yang, Y.; Jiang, F.; Sun, C.; Zhao, W.; Han, X. Rapid nitrate determination with a portable lab-on-chip device based on double microstructured assisted reactors. Lab Chip 2021, 21, 1109. [Google Scholar] [CrossRef]
- Cogan, D.; Cleary, J.; Fay, C.; Rickard, A.; Jankowski, K.; Phelan, T.; Bowkett, M.; Diamond, D. The development of an autonomous sensing platform for the monitoring of ammonia in water using a simplified Berthelot method. Anal. Methods 2014, 6, 7606–7614. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. Trends Environ. Anal. Chem. 2019, 24, e00073. [Google Scholar] [CrossRef]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Yuan, M.; Trinh, M.V.; Chen, Y.; Lu, Y.; Wang, L.; Cheng, S.; Li, Z.; Santikunaporn, M.; Asavatesanupap, C. Steam stripping for recovery of ammonia from wastewater using a high-gravity rotating packed bed. Environments 2024, 11, 206. [Google Scholar] [CrossRef]
- Salee, P.; Nitiyanontakit, S.; Tungkijanansin, N.; Varanusupakul, P.; Unob, F. A new gel-based platform for an LED-based colorimetric flow-analysis detection of ammonia in water. Microchem. J. 2025, 215, 114352. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, X.; Niu, W.; Zhang, J.; Shen, Y.; Li, H.; Chen, J. Evaluation of matrix effects on automated nutrient determination in seawater using segmented flow analysis. Microchem. J. 2025, 212, 113459. [Google Scholar] [CrossRef]
- Beaton, A.D.; Cardwell, C.L.; Thomas, R.S.; Sieben, V.J.; Legiret, F.E.; Waugh, E.M.; Statham, P.J.; Mowlem, M.C.; Morgan, H. Lab-on-chip measurement of nitrate and nitrite for in situ analysis of natural waters. Environ. Sci. Technol. 2012, 46, 9548–9556. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, L.; Liu, Y.; Lin, L.; Lu, R.; Zhu, J.; He, L.; Lu, Z. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.B. The Griess reaction. A survey of the chemistry. Anal. Chem. 1979, 51, 1493–1502. [Google Scholar] [CrossRef]
- Zhan, Y.; Lin, H.; Xiang, J.; Zhang, B.; Zhao, Z.; Lin, J.; Li, J.; Cheng, Y. A low-cost read-out photoresponsive colorimetric platform for nitrite detection based on smartphone APP. Microchem. J. 2025, 215, 114365. [Google Scholar] [CrossRef]
- Jones, M.N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 1984, 18, 643–646. [Google Scholar] [CrossRef]
- Cogan, D.; Fay, C.; Boyle, D.; Osborne, C.; Kent, N.; Cleary, J.; Diamond, D. Development of a low cost microfluidic sensor for the direct determination of nitrate using chromotropic acid in natural waters. Anal. Methods 2015, 7, 5396. [Google Scholar] [CrossRef]
- Schnetger, B.; Lehners, C. Determination of nitrate plus nitrite in small volume marine water samples using vanadium(III)chloride as a reduction agent. Mar. Chem. 2014, 160, 91–98. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Evans, G.W.H.; Coleman, S.M.; Niu, X. Nitrate measurement in droplet flow: Gas-mediated crosstalk and correction. Lab Chip 2018, 18, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Li, H.; Bo, G.; Lin, K.; Yuan, D.; Ma, J. On-site detection of nitrate plus nitrite in natural water samples using smartphone-based detection. Microchem. J. 2021, 165, 106117. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Wang, D.; Zheng, M.; Yang, M.; Yang, Y.; Ren, T. Recent advances in microfluidic sensors for nutrients detection in water. Trends Analyt. Chem. 2023, 158, 116790. [Google Scholar] [CrossRef]
- Plant, J.N.; Sakamoto, C.M.; Jonhson, K.S.; Maurer, T.L.; Bif, M.B. Updated temperature correction for computing seawater nitrate with in situ ultraviolet spectrophotometer and submersible ultraviolet nitrate analyzer nitrate sensors. Limnol. Oceanogr. Methods 2023, 21, 581–593. [Google Scholar] [CrossRef]
- Valderrama, J.C. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 1981, 10, 109–122. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, C.; Wang, Y.; Wu, S.; Jin, Y.; Yang, M.; Liu, H. Effects of nitrate and nitrite on the UV/PDS process: Performance and byproduct formation from the perspective of substituents. RSC Adv. 2025, 15, 7480. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, L.; Zhong, G.; Wu, Q.; Liu, J.; Liu, X. A portable analytical system for rapid on-site determination of total nitrogen in water. Water Res. 2021, 202, 117410. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Kalsoom, U.; Farajikhah, S.; Innis, P.C.; Nesterenko, P.N.; Lewis, T.W.; Breadmore, M.C.; Paull, B. Enhanced physicochemical properties of polydimethylsiloxane based microfluidic devices and thin films by incorporating synthetic microdiamond. Sci. Rep. 2017, 8, 15109. [Google Scholar]
- Bittig, H.C.; Maurer, T.L.; Plant, J.N.; Schmechtig, C.; Wong, A.P.S.; Claustre, H.; Trull, T.W.; Bhaskar, T.V.S.U.; Boss, E.; Dall’Olmo, G.; et al. A BGC-Argo Guide: Planning, Deployment, Data Handling and Usage Mission Considerations or the Global. Front. Mar. Sci. 2019, 6, 502. [Google Scholar] [CrossRef]
- Lin, K.; Xu, J.; Guo, H.; Huo, Y.; Zhang, Y. Flow injection analysis method for determination of total dissolved nitrogen in natural waters using on-line ultraviolet digestion and vanadium chloride reduction. Microchem. J. 2021, 164, 105993. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.U.; deMello, A.J. Multiplexed microfluidic systems for high-throughput colorimetric analysis. Lab Chip 2015, 15, 1995–2002. [Google Scholar]
- Johnson, K.S.; Coletti, L.J. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean. Deep Sea Res. 2002, 49, 1291–1305. [Google Scholar] [CrossRef]
- Altahan, M.F.; Esposito, M.; Achterberg, E.P. Improvement of on-site sensor for simultaneous determination of phosphate, silicic acid, nitrate plus nitrite in seawater. Sensors 2022, 22, 3479. [Google Scholar] [CrossRef]
- Neumann, K.C.; La, D.; Yoo, H.; Burkepile, D.E. Programmable autonomous water samplers (PAWS): An inexpensive, adaptable and robust submersible system for time-integrated water sampling in freshwater and marine ecosystems. HardwareX 2023, 13, e00392. [Google Scholar] [CrossRef]
- Grand, M.M.; Clinton-Bailey, G.S.; Beaton, A.D.; Schaap, A.M.; Johengen, T.H.; Tamburri, M.N.; Connelly, D.P.; Mowlem, M.C.; Achterberg, E.P. A lab-on-chip phosphate analyzer for long-term in situ monitoring at fixed observatories: Optimization and performance evaluation in estuarine and oligotrophic coastal waters. Front. Mar. Sci. 2017, 4, 255. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Ward, D.; Reynolds, D.M.; Brunsdon, C.; Carliell-Marquet, C.; Browning, S. Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 2008, 391, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiang, Y.; Liang, W.; Zeng, H.; Chen, H.; Zhang, M. Affordable droplet-based flow analyzer with peristaltic micro-pumps for fluorescent ammonium sensing. Talanta Open 2024, 10, 100356. [Google Scholar] [CrossRef]
- Yang, T.; Stavrakis, S.; deMello, A. A high-sensitivity, integrated absorbance and fluorescence detection scheme for probing picoliter-volume droplets in segmented flows. Anal. Chem. 2017, 89, 12880–12887. [Google Scholar] [CrossRef]
- Delaunay, L.; Compère, C.; Lehaitre, M. Biofouling protection for marine environmental sensors. Ocean Sci. 2010, 6, 503–511. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, G.S.; Chiu, D.T. Disposable microfluidic devices: Fabrication, function, and application. Biotechniques 2005, 38, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. Engl. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Ray, P.P. A survey on Internet of Things architectures. J. King Saud Univ. Comput. Inf. Sci. 2018, 30, 291–319. [Google Scholar] [CrossRef]
- Calvo-López, A.; Alonso-Chamarro, J.; Puyol, M. Highly versatile and automated total ammonia nitrogen compact analyzer suitable for different types of water samples. Environ. Sci. Water Res. Technol. 2023, 9, 3366. [Google Scholar] [CrossRef]
- Fluidion: Water Intelligence Company. Available online: www.fluidion.com (accessed on 27 June 2025).
- Sea-Bird Scientific Company. Available online: www.seabird.com (accessed on 27 June 2025).





| Sample | Detection Method | Chip Material | Detection Time | Reagent Consumption | Detection Range | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Ammonium ions | |||||||
| Natural waters: river water samples, ammonium standard solutions | Colorimetric/FIA | D-ABS/graphene PLA | 1.6 min | 100 µL | 28–280 µM | 8.4 µM | [24] |
| Water | Colorimetric (Berthelot’s) | Paper | 15 min | 1.2 mL | 0.55–11 mM | <0.55 mM | [25] |
| Industrial wastewater | Colorimetric (Nessler’s) | Paper | 2 min | 15 µL | 0–280 µM | 187 µM | [26] |
| Freshwater: park pond, garden lake | Colorimetric | Paper BTB-based NY based | 1 min 5 min | 8 µL | 28–1680 µM 112–560 µM | 17.9 µM 26.3 µM | [27] |
| River | Colorimetric (Berthelot’s) | PLA | 10 s | 0.3 µL | 10–110 µM | 6.1 µM | [56] |
| Water | Colorimetric (Berthelot’s) | PMMA | 205 s | 100 µL | 21–286 µM | 19 µM | [64] |
| Nitrite ions | |||||||
| Groundwater, tap water | Colorimetric | Paper | 10 min | 20.5 µL | 1.09–217 µM | 651 nM | [30] |
| Aqueous samples | Colorimetric | Paper/PMMA | 15 min | 0.5 µL | 3.9–1000 µM | 14.8 µM | [36] |
| Aqueous sample | Colorimetric | Paper | 5 min | 11 µL | 5–500 µM | 1.3 µM | [37] |
| Aqueous samples | Spectrophotometric | PDMS/PMMA | N/A | <1.25 mL | 4.3–97.6 µM | 1.54 µM | [38] |
| River and lake water, tap water | Colorimetric | PMMA | 45 min | 10 µL | 0–0.28 mM | 9.35 µM | [65] |
| Seawater | Colorimetric | PMMA | 30 min | 12 µL h−1 | 0–5 µM | 15 nM | [66] |
| Aqueous samples | Spectrophotometric | PDMS | N/A | 2.5 mL | 2.17–217 µM | 1.17 µM | [67] |
| Water solutions | Differential Colorimetric | PDMS | >90 s | N/A | 0.25–4 mM | 0.33 µM | [68] |
| Nitrate ions | |||||||
| Sargasso Sea | Colorimetric | Paper | 21 min | 95 µL | 0–80 mM | 8.48 µM | [32] |
| Atlantic | Colorimetric | PMMA | 11 min 20 s | 0.66 mL | 0.25–750 µM | 0.03 ± 0.01 µM | [33] |
| Mineral water | Colorimetric | PMMA | ~14 min | N/A | 0.02–0.3 mM | 1.25 µM | [69] |
| Water solutions | Colorimetric | PMMA | <30 min | 0.4 µL min−1 | 0.25–50 µM | 20 nM | [70] |
| Seawater | Colorimetric | PMMA | 17 min | 4.71 mL | N/A | <0.1 µM | [71] |
| Water | Spectrophotometric | PMMA/PDMS | 115 s (per sample) | 26.8 µL (per sample) | 0.1–30 µM | 0.05 µM | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milinovic, J.; Lunn, J.; Attia, S.; Slavik, G. Microfluidic Field-Deployable Systems for Colorimetric-Based Monitoring of Nitrogen Species in Environmental Waterbodies: Past, Present, and Future. Environments 2025, 12, 434. https://doi.org/10.3390/environments12110434
Milinovic J, Lunn J, Attia S, Slavik G. Microfluidic Field-Deployable Systems for Colorimetric-Based Monitoring of Nitrogen Species in Environmental Waterbodies: Past, Present, and Future. Environments. 2025; 12(11):434. https://doi.org/10.3390/environments12110434
Chicago/Turabian StyleMilinovic, Jelena, James Lunn, Sherif Attia, and Gregory Slavik. 2025. "Microfluidic Field-Deployable Systems for Colorimetric-Based Monitoring of Nitrogen Species in Environmental Waterbodies: Past, Present, and Future" Environments 12, no. 11: 434. https://doi.org/10.3390/environments12110434
APA StyleMilinovic, J., Lunn, J., Attia, S., & Slavik, G. (2025). Microfluidic Field-Deployable Systems for Colorimetric-Based Monitoring of Nitrogen Species in Environmental Waterbodies: Past, Present, and Future. Environments, 12(11), 434. https://doi.org/10.3390/environments12110434






