Abstract
Biochar is increasingly recognized as a multifunctional soil amendment that improves soil fertility, nutrient cycling, and crop productivity. Studies across field, greenhouse, and incubation settings show that biochar enhances nutrient retention, reduces leaching, and regulates carbon, nitrogen, and phosphorus cycling. Its effects are shaped by intrinsic physicochemical properties and interactions with soil minerals, microbial communities, and enzymatic processes. Short-term benefits of biochar applications often include improved nutrient adsorption and water regulation, while long-term applications support stable soil organic matter formation, root development, and fertilizer use efficiency. Biochar also reshapes soil microbial diversity and activity. Beneficial bacterial groups such as Proteobacteria and Actinobacteria, along with fungi such as Mortierella, respond positively, enhancing nitrogen fixation, phosphorus solubilization, and organic matter decomposition. Meanwhile, biochar applications could suppress pathogens. Enzyme activities, including urease and phosphatase, are typically stimulated, driving nutrient mobilization. Yet outcomes remain context-dependent, with biochar feedstock, application rate, soil conditions, and crop type influencing results; excessive use may suppress enzymatic activity, reduce nutrient availability, or shift microbial communities unfavorably. Practically, biochar can improve fertilizer efficiency, restore degraded soils, and reduce greenhouse gas emissions, contributing to climate-smart agriculture. Future work should prioritize long-term, multi-site trials and advanced analytical tools to refine sustainable application strategies.
1. Introduction
The excessive application of chemical fertilizers has triggered multiple environmental challenges, including soil degradation caused by acidification, nutrient leaching, deterioration of soil structure, decline in beneficial microbial populations, and an increase in greenhouse gas emissions [1]. Biochar, a pyrolyzed carbon-rich solid produced under oxygen-limited conditions from diverse biomass sources, has emerged as a sustainable amendment due to its capacity to improve soil properties and foster beneficial microbial activity [2,3]. Because of its high carbon content, porous structure, negatively charged surface, and alkaline properties, biochar can greatly change soil physicochemical properties, such as lowering bulk density, increasing pH, and improving nutrient retention and availability, water holding capacity, cation exchange capacity, and aggregate stability [4,5]. Nonetheless, these effects are influenced by multiple factors, including feedstock type, pyrolysis conditions, biochar application rate, soil type, and climate [6,7].
A growing body of evidence highlights its effectiveness in lowering greenhouse gas emissions [8,9,10]. A critical review by Lyu et al. [8] demonstrated a general inhibitory effect of biochar across various environments, with the impact on greenhouse gases decreasing in the order: N2O > CH4 > CO2. This suggests that biochar modifies the physicochemical characteristics of soil and compost under diverse conditions, thereby influencing gas emissions. While relatively few, studies have also shown that biochar has no significant effect on or may even increase greenhouse gas emissions. A smaller review by Atilano-Camino et al. [11] on total greenhouse gas emissions from soils with and without biochar amendment found that the average CO2 (7.7 t/ha) and CH4 (4.9 t/ha) outputs were higher in soils amended with biochar than those from soils without biochar, whereas N2O emissions (1.6 t/ha) were lower than those in the untreated control. Additionally, biochar has been reported to promote long-term crop productivity by fostering synergistic interactions between biochar, soil, and microbial communities [12,13]. Soil is a highly intricate ecosystem that sustains diverse microorganisms essential to ecosystem functioning. The incorporation of biochar has been shown to influence these functions by enhancing enzyme activity and restructuring microbial community composition [14]. Soil enzymes are critical for mobilizing recalcitrant nutrients, thereby ensuring a continuous energy supply for soil microbiota [15]. Moreover, biochar-induced increases in the pH of acidic soil promote crop growth by releasing phosphorus (P) bound to iron (Fe) and alleviating aluminum (Al) toxicity, ultimately regulating key aspects of soil nutrient dynamics [16].
The nutrient composition of biochar is largely influenced by the type of feedstock and the pyrolysis conditions. For instance, biochar produced from manure and biosolid feedstocks generally contains higher levels of nitrogen (N) and P compared to that generated from straw or wood materials [17,18]. During pyrolysis, an increase in ash content (inorganic residues) elevates the concentrations of P, calcium (Ca), magnesium (Mg), silicon (Si), and potassium (K), whereas the N content declines at higher pyrolysis temperatures due to gaseous losses [19,20].
Biochar also functions as a nutrient reservoir, effectively retaining macronutrients such as N, P, and K, as it can store and hold these nutrients within its structure and on its surface and slowly release them, keeping them available in soil longer [21,22]. However, excessive application of biochar may raise soil pH above the optimal level for crop growth, which can alter nutrient availability and ultimately diminish soil fertility and crop productivity [23]. A study reported that potato yields dropped by over 6% with mineral and 10% with organic fertilization, likely due to biochar-induced alkalinity that raised soil pH and reduced nutrient availability [24]. Although less reported, nutrient losses, particularly N, may occur through leaching and nitrous oxide emissions. For instance, at a high application rate of 30 t/ha, biochar increased nitrate and dissolved organic nitrogen leaching by 38% and 170%, respectively, relative to the control. This treatment resulted in the highest N losses through both nitrous oxide emissions and leaching, indicating that application rate plays a crucial role in N loss [25]. Nonetheless, a meta-analysis has shown that, at application rates of up to 120 t/ha, biochar generally reduces nitrous oxide emissions, as larger quantities can limit N availability for denitrification through the adsorption of nitrate. This could raise N use efficiency [26]. Yu et al. [27] noted that the nutrient retention capacity of biochar is primarily governed by its porosity and surface charge, which determine its ability to adsorb both cations and anions.
In recent years, biochar has attracted attention as a strategy for mitigating soil pollution and enhancing soil restoration [28,29,30]. Reviews of current studies indicate that biochar contributes to the immobilization of heavy metals and persistent organic pollutants in soils, thereby lowering their bioavailability. This remediation effect is largely attributed to processes such as precipitation, electrostatic attraction, surface adsorption, structural entrapment, and promoted degradation. However, the efficiency of contaminant removal is influenced by factors including the type of biochar, the application rate, the characteristics of the soil, and the nature of the pollutants [31,32,33]. By acting as a nutrient reservoir and altering soil microbial composition, biochar contributes to both contaminant removal from soil and alterations in soil nutrient content. However, few reviews focus specifically on the latter.
Dai et al. [34] reviewed how biochar properties influence N and P cycles in soil and the abundance of soil microbial communities, but provided limited explanation on how biochar applications affect the levels and transformation of various nutrients in soil and the microbial communities for cycling of nutrients beyond N and P. Tsolis and Barouchas [35] reviewed the influence of adding biochar on soil properties and its biological interactions in soil, but did not discuss the influence of biochar on the retention, alteration, and leaching of various soil nutrients in detail. Similarly to the review of Dai et al. [34], the review of Anyebe et al. [36] highlights how biochar properties, shaped by feedstock and pyrolysis temperature, affect soil ecosystem services, nutrient cycling, and crop productivity, with the emphasis placed on biochar synthesis and properties. Singh et al. [37] reviewed biochar production, key properties, and its role in restoring degraded soils, with emphasis on its effectiveness in remediating saline and acidic soils, its interactions with soil properties, and its ecological functions influencing enzymes and crop performance. Their review did not focus on the underlying molecular and microbial mechanisms that alter soil nutrient levels.
It is important to review the effects of biochar on nutrient dynamics and microbial activity in soil because both are central to soil fertility, ecosystem functioning, and sustainable crop production. Biochar can alter nutrient availability by influencing soil pH, cation exchange capacity, and sorption processes, which in turn affect the efficiency with which plants and microbes access essential elements. The two are closely connected: microbial processes (e.g., mineralization, nitrogen fixation, and decomposition) drive nutrient cycling, while nutrient availability regulates microbial growth and metabolism. Thus, understanding how biochar simultaneously influences nutrient dynamics and microbial activity is critical for predicting its long-term impacts on soil health and agricultural productivity. Therefore, this review aims to examine how biochar influences soil nutrient levels, particularly nitrogen, phosphorus, carbon, and cations. Specifically, it aims to elucidate the physicochemical mechanisms by which biochar alters nutrient retention, transformation, and leaching. Additionally, it presents recent progress in understanding how biochar influences soil microbial communities (bacteria and fungi). It seeks to elucidate the mechanisms by which biochar–microbe interactions enhance nutrient availability. In doing so, it provides deeper mechanistic insights into how it regulates soil health.
2. Review Methodology
This narrative review was conducted to ensure a comprehensive and balanced synthesis of current knowledge on biochar amendments in soil, with a particular focus on nutrient dynamics and microbial activity. A broad search was undertaken in major scientific databases, including Web of Science, Scopus, and Google Scholar, to identify peer-reviewed journal articles, reviews, and relevant book chapters published between 2015 and 2025. Search terms were combined using Boolean operators and included variations of the following keywords: (1) “biochar” AND “soil restoration”, (2) “biochar” AND “nutrient dynamics” OR “nutrient cycling” OR “fertility”, (3) “biochar” AND “microbial activity” OR “microbial community” OR “soil microbiota”, and (4) “biochar” AND “soil health” OR “ecosystem functioning”.
Studies were included if they: (1) reported experimental, field, or modeling data on the impacts of biochar on nutrient availability, nutrient cycling, or microbial processes in soil; (2) provided mechanistic insights into biochar–soil interactions relevant to soil fertility, ecosystem functioning, or crop productivity focusing on the nutrient and microbial aspects; and (3) were published in English and in peer-reviewed outlets.
Studies were excluded if they: (1) focused solely on biochar production techniques without discussing impacts on nutrient dynamics or microbial activity; (2) addressed only physical soil properties (e.g., bulk density, porosity) without linking them to nutrient or microbial processes; (3) mixed biochar with other soil amendments or treatments except fertilization as the simplest form of soil improvement; (4) involved functionalized biochar due to the myriad of functionalizing materials and techniques; and (5) were non-peer-reviewed sources such as conference abstracts, opinion articles, or policy briefs.
Key information from each study was extracted, including biochar characteristics, soil type and experimental setting (laboratory, greenhouse, or field), reported impacts on nutrient dynamics (e.g., N, P, K availability, pH changes, cation exchange capacity), as well as reported impacts on microbial activity (e.g., microbial biomass, community composition, enzymatic activity, nutrient mineralization).
Extracted data were synthesized thematically to highlight the mechanisms by which biochar influences nutrient dynamics, the variations in microbial activity and community structure due to biochar addition, and the interlinkages between nutrient dynamics and microbial processes under biochar amendment. Thematic synthesis combined both a priori and emergent coding. Priori themes, namely nutrient dynamics and microbial community composition, were identified based on the review’s objectives. Additional subthemes, including variations by soil type, biochar feedstock, and environmental conditions, emerged inductively during literature analysis. No formal effect size calculations were performed, as this review aimed for qualitative integration rather than meta-analysis; however, directional trends were noted (e.g., the proportion of studies reporting increased nutrient availability or microbial biomass). Contradictory results were reconciled by considering contextual factors, including biochar properties, soil characteristics, and experimental duration. Study robustness was evaluated qualitatively, with greater weight given to peer-reviewed experimental data with clear methodological detail.
3. Impacts of Biochar on Nutrient Dynamics
Biochar amendments are known to significantly influence soil nutrient dynamics by altering chemical properties, nutrient availability, and nutrient cycling processes, thereby shaping soil fertility and plant productivity.
3.1. Phosphorus and Nitrogen Dynamics
Biochar plays a pivotal role in regulating soil P and N dynamics. A field study in western Montana examined the effects of wood biochar (0, 20, and 40 tons per ha), with or without short-term cattle trampling, on soil properties, nutrient dynamics, and grass nutrient uptake [38]. Trampling alone compacted surface soils and reduced nitrogen availability, whereas biochar application improved infiltration, enhanced net nitrification, and promoted biochar incorporation into soil. Biochar also increased organic P while lowering inorganic forms, with stronger effects under trampling, and these changes were mirrored in grass P concentrations (Figure 1). Cattle trampling drove biochar deeper into the soil, increasing its contact with soil matrices and enhancing P sorption, which lowered CaCl2-P and citrate-P levels. These P shifts may also reflect higher phosphatase activity from plants or microbes responding to greater P demand under adequate N and better moisture conditions [38].
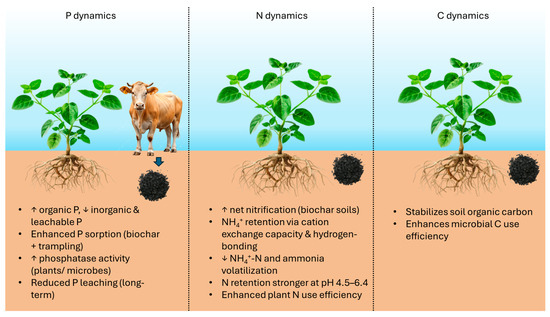
Figure 1.
Impacts of biochar on P, N, and C dynamics in soil. (Note: ↑ represents increase and ↓ represents decrease).
Long-term trials (7 years) confirm biochar’s ability to limit nutrient losses. On vegetated roofs in Finland, biochar consistently reduced P leaching in both seeded plantings and pre-grown mats, while N leaching decreased only in plantings, coinciding with improved plant cover [39]. Nutrient losses declined over time as roofs aged; however, fertilization six years post-establishment caused elevated P leaching that biochar did not mitigate. Runoff from plantings contained more total P than mats, despite mats having 1.4 times higher P in the substrate. P dynamics were governed mainly by substrate texture, chemistry, and pH, whereas N dynamics were driven by biological processes. Substrate pH declined from 7.2–7.9 (2016) to 6.3–7.0 (2020) over the study duration, a range that keeps P plant-available rather than particle-bound.
Dubrovina [40] examined how wood biochar affects phosphate forms in two Karelian soils with different phosphorus availability, namely a sandy Umbric Podzol and a heavy loamy Umbric Retisol. Biochar fractions (≤2 mm and 3–5 mm) were applied at 2% and 5% rates. Biochar, especially the ≤2 mm fraction at 5%, raised soil pH, available phosphorus (by 20–40%), and the proportions of Ca-, Al-, and loosely bound P, while enhancing phosphatase activity in the Umbric Podzol. In pot tests with spring barley, biochar further increased P2O5 levels. In the Umbric Retisol, biochar had a smaller effect, slightly increasing available and Ca-bound P but not phosphatase activity; significant P2O5 gains occurred only with the combined biochar–fertilizer treatment. Overall, fine biochar (≤2 mm) at 5% showed the strongest positive impact across parameters.
Soil incubation experiments provide further insight into N cycling. Gupta et al. [41] tested rice straw (RSB) (pH = 9.1) and acacia wood biochar (ACB) (pH = 7.3) (at 0–1% in clay loam (pH = 7.6) and loamy sand soils (pH = 7.0). Ammonium-N declined faster in biochar-treated soils, while RSB consistently raised nitrate-N more than ACB. In clay loam, nitrate levels increased with higher biochar application rates up to 15 days. However, in loamy sand, the 1% amendment reduced nitrate levels after 30–60 days compared to the 0.5% amendment. With ACB, nitrate remained higher at 1% than 0.5% for longer periods in both soils. Phosphorus stayed elevated across all biochar treatments throughout the experiment. Biochar application markedly reduced NH4+-N in both soils compared to controls throughout the 60-day incubation. This effect is linked to carboxyl and ketone groups in biochar, which enhance NH4+ retention via hydrogen bonding and electrostatic forces (Figure 1). By improving soil cation exchange capacity, biochar also suppressed ammonia volatilization, thereby lowering cumulative NH4+-N losses in a dose-dependent manner [42].
In an acidic citrus orchard soil, pH was adjusted to five levels (4.0–7.2) with CaO. The soil was subsequently amended with 0 or 1% biochar [43]. Using 15N-tracing, the study showed that biochar generally increased gross mineralization (TM) and NH4+ immobilization (TI), except for TI at pH 4.0. It also promoted heterotrophic nitrification across a pH range of 4.0–7.4, while autotrophic nitrification occurred above a pH of 6.4. Overall, biochar improved nitrogen retention within pH 4.5–6.4, with stronger effects on TI than TM, and reduced autotrophic nitrification, suggesting enhanced N use efficiency and lower NO3−-N losses in subtropical pomelo orchards (Figure 1). Soil pH strongly influenced how biochar affected nitrogen cycling in subtropical citrus soils. Biochar enhanced N retention mainly at pH 4.5–6.4, with greater effects on NH4+ immobilization than on mineralization, while suppressing autotrophic nitrification as a key mechanism for improved retention. This aligns with the findings of [41].
Ammonia nitrogen in farmland soils is easily lost due to environmental and human factors. Lu et al. [44] assessed how Gracilaria lemaneiformis biochar influences ammonium behavior in soils. Results showed that adding 10% biochar increased ammonium in the 0–10 cm layer by 1.57–2.36 times while reducing leaching losses by 44.83–72.27%. Adsorption and retention were mainly governed by electrostatic attraction and ion exchange, with additional effects from molecular interactions and system energy. Low-energy sites near C6H12O6 favored ammonium binding, while structures like C5H10O5, C-S-H, C-SO3, and C4H7NO4 contributed via physical or chemical adsorption mechanisms. The negative adsorption free energy (−394,590.84 kcal/mol) confirmed stable binding of ammonium to biochar surfaces.
Additionally, a study using pot experiments (farmland soil) reported that biochar improved soil N availability when applying corn straw biochar at rates of 0%, 0.5%, 1.0%, 2.0%, and 4.0%. It also evaluated soil nutrients and bacterial diversity [45]. Total and ammonium nitrogen rose by 4.7–32.3% and 8.3–101.5%, respectively, while microbial biomass nitrogen increased with higher biochar levels. This is primarily attributed to biochar’s highly porous structure and large surface area, which enable it to adsorb and retain nitrogen, minimize leaching losses, and enhance soil nitrogen levels [46].
Overall, biochar significantly regulates soil P and N dynamics across diverse systems by enhancing nutrient retention, limiting leaching, and improving plant uptake. Biochar increases organic P while reducing inorganic and leachable forms. It also promotes nitrification and enhances ammonium retention through electrostatic and chemical interactions. Soil type, pH, and biochar feedstock strongly mediate these effects, with optimal N retention often occurring under moderately acidic conditions. Trampling and substrate properties can further influence nutrient shifts, while crop- and system-specific responses highlight both benefits and potential constraints at high application rates.
3.2. Carbon Dynamics
Biochar influences soil carbon dynamics by stabilizing organic matter, enhancing carbon sequestration, and moderating microbial activity. Biochar stabilizes organic matter through several mechanisms. Its highly aromatic and condensed carbon structure resists microbial decomposition, thereby slowing down organic carbon mineralization. Additionally, biochar provides microsites that physically protect labile organic matter within its porous matrix and through organo-mineral associations. The surface functional groups (e.g., carboxyl, hydroxyl) can also adsorb soluble organic compounds, reducing their accessibility to decomposers. These physicochemical interactions contribute to long-term carbon stabilization and enhanced soil organic carbon persistence. The details are presented in the following reviews.
Wu et al. [47] conducted an experiment that included a no-amendment control and a treatment with peanut shell biochar. After 70 days of incubation, peanut shell biochar application increased soil organic carbon by 44.8% and particulate organic carbon by 27.4% compared to the control. Mantel analysis and structural equation modeling revealed strong links among cbbL, cbbM, Bacteroidota, total organic carbon, and particulate organic carbon. The biochar treatment enriched soil carbon fractions, enhanced Bacteroidetes abundance, and reduced Acidobacteria richness, influencing key carbon cycle genes (cbbL and cbbM) (Figure 1). Overall, peanut shell biochar markedly improved soil carbon content and fertility.
Furthermore, Abdul-Aziz et al. [48] assessed how biochar type and application rate affect soil fertility and maize yield over two field seasons (2022–2023). Biochar materials from rice husk, groundnut husk, and sawdust were applied at 0–8 t ha−1. Groundnut husk biochar proved to be the most effective, with 8 t ha−1 increasing grain yield by up to 218% in 2022 and 106% in 2023. Soil organic matter rose by 90–343%, and nitrogen availability by up to 220% (2022) and 70% (2023). Biochar’s large surface area and reactive groups (carboxyl, hydroxyl) enable it to hold NH4+ and limit NO3− leaching. Maize yield strongly correlated with soil fertility indicators, except harvest index. Regarding the increase in soil organic matter, two main mechanisms may be involved: biochar directly adds stable carbon to the soil, and it suppresses the mineralization of soil organic matter by immobilizing microbes and adsorbing easily degradable organic substances.
Liu et al. [49] compared how biochar (1.0% w/w) with and without N addition influences the fate of photosynthetically fixed carbon in rice plant–soil systems using 13CO2 pulse labeling at key growth stages. Biochar did not alter the distribution of 13C among plant–soil components but increased its accumulation in shoots, roots, and soil, particularly when combined with N addition. During the growth period, biochar raised total 13C in shoots, roots, and soil by 23%, 14%, and 20%, respectively, and improved nitrogen use efficiency by about 23% at the heading and ripening stages. This was likely due to two factors, namely its porous structure adsorbs labile compounds and enhances microbial carbon use efficiency, thereby stabilizing more photo-assimilated C [50], and greater root biomass under biochar plus N addition contributed additional 13C inputs to the soil, even though allocation patterns of 13C within the plant–soil system remained unchanged [51]. Overall, biochar promotes higher plant productivity and soil carbon sequestration through enhanced N use efficiency.
A field trial applied biochar at 0% (control), 1% (LB), 2% (MB), and 4% (HB) of topsoil dry weight before planting winter wheat [52]. Soil organic carbon and particulate organic carbon rose with higher biochar rates. After 8 months, readily oxidizable carbon was reduced but later returned to control levels. Biochar increased dissolved organic carbon without affecting water-soluble organic carbon, though both dissolved organic carbon and water-soluble organic carbon declined over time. Soil organic carbon rose with higher biochar rates due to its high stable carbon content (60–80%) [53]. While biochar did not affect water-soluble organic carbon, it was positively linked to dissolved organic carbon, likely from labile carbon release, leaching of dissolved organic matter, and selective adsorption of small dissolved organic molecules. Both water-soluble organic carbon and dissolved organic carbon declined over time, reflecting microbial carbon utilization [53].
Mesocosm experiments with Festuca arundinacea were conducted to investigate the impact of cornstalk biochar (100 g/kg soil) on soil organic carbon under simulated grazing conditions in two different soil types [54]. Biochar boosted soil organic carbon by 595% and mineral-associated organic carbon by 39%, mainly by stimulating microbes that promoted stable organo-metal complexes with Fe/Al in red soils and Ca in calcareous soils. Effects were stronger in red soils due to pH and mineral differences, while in calcareous soils, biochar also offered long-term protection against acidification. Biochar enriches soil carbon pools by directly adding carbon and stabilizing mineral-associated organic carbon through its high carbon content, aromatic structure, and resistance to decomposition [55]. It also raises soil pH, causing Fe and Al to precipitate, which allows high-molecular-weight organics to bind to these oxides and form stable Fe- or Ca-associated complexes. This process enhances the incorporation of plant inputs into stable soil organic carbon, particularly in acidic to neutral soils like red soils [56].
In Russia, an incubation study was conducted on three Podzol Antric soils differing in initial total organic carbon content for 90 days, using birch- and aspen-derived biochar (produced by fast pyrolysis at 550 °C) at application rates of 0.1% and 1.0%. The 0.1% dose showed no effect on soil organic matter mineralization, while 1.0% biochar increased it by 15–18%, accompanied by shifts in humic substance composition [57]. The higher proportion of aromatic humic acid fragments suggests greater stability. In humus-rich soils with abundant insoluble material, mineralization and humic acid formation occurred concurrently, likely due to enhanced transformation of resistant organic fractions and partial humification of the biochar itself.
Collectively, biochar enhances soil carbon dynamics by stabilizing organic matter, improving carbon sequestration, and boosting nutrient use efficiency. It increases soil organic and particulate carbon, stimulates carbon-fixing microbes, and reduces nitrogen losses. Higher application rates promote stable mineral-associated carbon and long-term C retention, especially in acidic and red soils. Even at moderate levels, biochar improves humic composition and aromatic carbon stability. Empirical evidence indicates that biochar carbon may persist for 100–1000 years depending on feedstock, production temperature, and environmental conditions [58], underscoring its potential as a long-term carbon sink and soil fertility enhancer. Overall, biochar enhances soil fertility and carbon storage through the activation of microbes and the chemical stabilization of soil.
3.3. Nutrient Dynamics in General
Numerous studies have examined the effects of biochar on various soil nutrients, including C, N, P, and minerals. For instance, peanut shell biochar (0–10%) was tested in acidified soils using experiments and simulations. The highest adsorption was for K+ (820.38 mg/g), followed by PO43− (270.51 mg/g), NO3− (235.65 mg/g), and NH4+ (130.93 mg/g) [59]. At 10% biochar, outlet volume decreased by 65.32%, nutrient leaching dropped by 48.40–68.28%, and surface soil nutrient retention increased by 437.80–913.87%. Nutrient concentrations declined with depth, confirming biochar’s potential for improving nutrient efficiency in acidic soils.
Similarly, Lewoyehu et al. [60] conducted an incubation study in Ethiopia, testing water hyacinth biochar (WHB) against lime and fertilizer in acidic Nitisol. WHB slowed nitrification, lowered H+ release, and improved N and P availability. Soil pH rose by 0.30–0.35 (1% WHB) and 0.72–0.86 (2% WHB), while exchangeable acidity dropped by 26.5–28.8% and 58.4–63%, respectively. The 2% WHB + fertilizer treatment further raised the pH by 0.71–0.90 and cut acidity by 49.8–64.7% compared to lime + fertilizer or fertilizer alone, showing WHB outperformed lime in resisting acidification. Biochar reduced soil acidity by retaining NH4+ and neutralizing Al3+, H+, and Fe3+ via its surface functional groups. The more porous, phenolic- and carboxyl-rich pyrolyzed WHB raised the soil pH more than the ground WHB [60]. Overall, WHB mitigated acidification by slowing nitrification and enhancing soil pH buffering capacity [61]. The improvement in soil P availability was attributed to higher soil pH dissolving Al3+/Fe3+-bound phosphates, functional groups on biochar releasing P bound to metal cations, and the direct release of soluble P from biochar.
Long-term effects were explored by Burgeon et al. [62], who studied century-old kiln charcoal (~200 years) as a proxy for historical biochar in three land covers. They compared soils with old biochar (CoBC), fresh oak biochar (YBC, 80 t ha−1), and no biochar. Results showed that the age of biochar strongly influenced nutrient dynamics. YBC reduced NO3− and K+ leaching but increased PO43− in topsoil pore water, while CoBC raised subsoil K+ and Mg2+, enhanced total N, K+, and Ca2+, but decreased available P. YBC reduced NO3− leaching via ion diffusion into its porous structure and temporarily raised topsoil PO43−, though alkaline pH limited its availability. In contrast, CoBC immobilized P through cationic bridging on oxidized surfaces but improved Ca2+ availability through functional groups. While yields were unaffected, biochar reduced N, K, and Ca uptake but increased Mg uptake, showing that YBC affects short-term pore water dynamics, whereas CoBC drives long-term nutrient cycling [62].
Long-term field experiments have further highlighted biochar’s role in crop systems. In a seven-year rice study in northwest China, annual biochar application (9 t ha−1 yr−1) improved shoot and root growth, enhanced root traits, and delayed root senescence during grain filling [63]. Biochar alone increased root biomass and length, while its combination with N fertilizer altered root morphology and growth patterns. Beyond its effects on plants, biochar reduced soil bulk density by 10–12%, increased total organic carbon, and raised available N, P, and K, underscoring its contributions to soil quality and fertility.
Additionally, dos Santos et al. [64] examined how biochar materials from sugarcane bagasse (SB), orange bagasse (OB), and corncobs (CB) affect ion leaching in clay soil, with untreated soil (CT) and gypsum-amended soil (CTG) as controls. Biochar raised water infiltration and flow by 80% and 71%, respectively. SB and CB enhanced ion removal by 48%, while OB achieved 19%, likely due to its lower surface area and water retention. Biochar reduced exchangeable Ca2+ and Mg2+ by 44% and 76% but increased K+ by 233%. Notably, SB and CB cut exchangeable Na+ by 90% and OB by 50%, indicating strong potential for improving soil quality and reclaiming salt-affected soils [64]. Biochar improved anion removal from soil, but the effect differed by type, likely due to variations in elemental composition, high electronegativity that repels similar ions, and enhanced soil water distribution and retention.
Nutrient release from animal waste biochar has also been studied. Hadroug et al. [65] tested poultry manure biochar (RPM-B, 600 °C) under static and dynamic conditions. In a 10-day leaching test, P and K were gradually but substantially released (93.6 and 17.1 mg g−1, about 95% and 43% of initial contents), outperforming many other biochar materials. Column leaching showed RPM-B (5–8% w/w) retained NO3−-N and NH4+-N in alkaline sandy soil but promoted leaching of PO43−-P, K+, Mg2+, and Ca2+ at low, sustained rates (<10 mg kg−1 d−1 over 40 days) [65]. This indicates that RPM-B could be a sustainable amendment for nutrient-poor semi-arid soils compared with synthetic fertilizers.
A six-week pot experiment assessed how wood biochar influences boreal soil chemistry and spring barley growth in sandy loam Umbric Podzol and clay loam Umbric Retisol [66]. Using two biochar fractions (3–5 mm and ≤2 mm) at 5% (w/w), soil pH, available P and K, and mineral N were measured, along with barley growth and protein content. In the Umbric Podzol, biochar raised pH by 0.5 units and increased K (17 mg/kg), P (7 mg/kg), and nitrate (4 mg/kg) levels, promoting plant growth, biomass yield, and protein accumulation, especially with fine biochar (3–5 mm) plus NPK fertilizer. In contrast, the same treatments in the Umbric Retisol slowed barley growth and lowered nitrate content by 4–7 mg/kg, likely due to enhanced denitrification.
In summary, biochar significantly improves soil nutrient dynamics by enhancing nutrient retention, reducing leaching, and alleviating acidity. Its porous structure and functional groups adsorb essential ions, buffer soil pH, and increase the availability of N, P, and K across diverse soil types. Long-term applications further stabilize nutrient cycling and improve root growth, soil structure, and fertility. Performance varies with feedstock and soil conditions, with materials like sugarcane bagasse and poultry manure biochar showing strong potential for nutrient recovery and soil reclamation. Overall, biochar enhances soil productivity by improving nutrient efficiency and providing long-term chemical stabilization. A summary of the effects of biochar on P, N, C, and nutrient dynamics, in general, is presented in Table 1.

Table 1.
Impacts of biochar on P, N, C, and general nutrient dynamics.
3.4. Nutrient Dynamics in Fertilized Soils
A two-year field experiment evaluated how rice-straw biochar influences the nutrient supply of organic fertilizer (OF) under rice–rapeseed rotation [67]. Results showed that using OF alone reduced rice yields by 2% and rapeseed yields by 6–10% compared with chemical fertilizer (CF). However, applying biochar at 15 t ha−1 with OF (OF + B15) boosted rice yields by 10–17% and rapeseed yields by 20–25% relative to OF, and still outperformed CF with yield increases of 7–14% for rice and 12–13% for rapeseed. The OF + B15 treatment also improved soil quality: during rice and rapeseed seasons, it raised soil organic carbon by 57–90% and enhanced enzyme activities, i.e., catalase (14–19%), invertase (14–20%), urease (17–24%), and phosphatase (13–17%) [67]. Biochar improved the nutrient-supplying ability of organic manure by raising soil pH and organic carbon. This pH increase was linked to the high ash content (19.43%) and alkalinity (pH 9.42) of the biochar, which released cations that neutralized soil acidity [68]. Overall, biochar addition enhanced soil properties, enzymatic activity, and nutrient availability from OF.
Lu et al. [69] tested how biochar type (fine pine wood powder, 0.1 mm, vs. coarse fescue grass, 0.5–2 mm) and application method (surface vs. incorporated at 1% w/w) influenced N and P leaching from soils with inorganic fertilizer or biosolids. Soil columns were leached for six months, yielding ~16 pore volumes of drainage. Biochar showed limited retention of ammonium (NH4+) and no sorption of nitrate (NO3−). However, incorporating powder biochar reduced cumulative leaching of NH4-N, NO3-N, and total N by about 25% compared with controls. Although biochar’s negatively charged surface was expected to strongly adsorb NH4+-N [70], powder biochar reduced NH4+–N in leachate during only 6 of 16 events, mainly in biosolids and control soils, with effects declining after day 50. Still, incorporating powder biochar lowered cumulative NH4+-N, NO3−-N, and total N losses, especially in the early leaching phase (Days 1–36) when N release was greatest. Incorporation proved more effective than surface application, likely due to greater contact between biochar and soluble N [69].
In a greenhouse pot trial, Llovet et al. [71] compared three commercial organo-mineral fertilizers with their biochar-amended versions (NPK + B, NP + B, K + B) using barley as the test crop. Results showed that NPK + B slowed nutrient leaching, particularly nitrate (−28%) and potassium (−22%) in the first two weeks, though this effect reversed after week three, likely due to microbial nutrient immobilization supported by biochar. NPK + B also boosted barley straw biomass by 23.4% compared to NPK, while all biochar-based fertilizers improved plant nutrient content and export (K, S, Ca, Mn), suggesting biochar contributed as an additional nutrient source.
Furthermore, a lab incubation study tested biochar from spent mushroom substrate (2% w/w) and inorganic P addition (62 kg ha−1) on soil P availability, pH, acid phosphatase activity, and bacterial abundance in acidic paddy and red soils from southern China at 15 °C and 25 °C [72]. Both amendments significantly raised soil pH by up to ~39–41% in paddy soil and ~43–45% in red soil. Inorganic P addition produced the highest available P, reaching 111.47 mg/kg at 15 °C and 100.17 mg/kg at 25 °C in paddy soil. The biochar contained labile P that directly released phosphate into the soil. Along with inorganic P, it reduced adsorption and complex formation, mobilizing native soil P beyond critical levels. Changes in soil pH, porosity, and microbial activity further enhanced available P.
Together, these studies demonstrate that biochar can significantly enhance fertilizer efficiency by improving soil fertility, reducing nutrient losses, and increasing crop yields. Its effects vary with feedstock, application method, and soil type, but overall, biochar strengthens nutrient retention, mobilizes otherwise unavailable pools, and supports long-term soil health, making it a promising amendment for sustainable crop production.
3.5. Effects of Nutrient-Enriched Biochar on Nutrient Dynamics
Sewage sludge biochar generally contains little K because much of it is lost with water during treatment. Supplementing sewage sludge biochar with mineral K can produce organo-mineral fertilizers that release K slowly, but their release patterns in different soils are not well understood. Ndoung et al. [73] examined K release from biochar-based fertilizers in pellet and granule forms in clayey soil, sandy soil, and silica over 60 days at an application rate of 4 Mg ha−1. Compared to KCl, biochar-based fertilizers released K more slowly, with pellets showing the slowest release. K availability was greatest in clay soil, followed by sandy soil and silica. K release patterns from biochar-based fertilizers and KCl were similar across soils and silica, and particle size had little effect. However, release rates differed with soil moisture at 10 kPa tension: clay soil showed the fastest K release, followed by sandy soil and silica. The higher water content in clay likely enhanced fertilizer dissolution by maintaining closer contact with soil water [73]. Overall, enriched biochar-based fertilizers act as slow-release fertilizers, suggesting potential for improved plant K uptake during growth.
Yin et al. [74] assessed the impacts of N-enriched biochar (4 and 8 t ha−1 y−1) on C emissions, storage, rice yield, and Fe dynamics in subtropical paddy fields. Both application rates raised C emissions, but 4 t ha−1 significantly improved yields. Field and culture experiments showed increased soil Fe2+ with biochar, which correlated positively with CO2, CH4, total C, and dissolved organic carbon, but negatively with Fe3+. In culture experiments without plants, biochar reduced cumulative CO2 and CH4 emissions. Both N-enriched biochar rates increased soil Fe2+, likely due to higher pH, lower redox potential, and enhanced Fe3+ reduction. Biochar’s porous structure and surface area also favor Fe-reducing microbes [75,76]. Additionally, biochar may serve as an electron donor or shuttle for Fe3+ reduction, roles considered more important than its potential function as an electron acceptor for Fe2+ oxidation [77]. Overall, moderate application (4 t ha−1) enhanced yield, biomass, and dissolved organic carbon while helping regulate C emissions through effects on Fe dynamics.
Javeed et al. [78] tested the application of date palm biochar (at 2%) enriched with buffalo slurry (SEB) on soil C–N dynamics, nutrient leaching, and wheat growth. Enrichment raised biochar C and N contents by up to 310% and 286%, respectively. Both standard and enriched biochar reduced nitrification (81–94%), ammonification (48–74%), and CO2 emissions (50–92%) compared to the control. SEB was especially effective, cutting leaching losses of C, N, P, K, Na, Ca, and Mg by 9–125%. Dissolved organic carbon supplied electrons for denitrification, releasing CO2 as oxygen was consumed. In SEB treatments, higher water-filled pore space and organic carbon created anaerobic conditions that accelerated denitrification while reducing microbial respiration [78].
Additionally, a study developed two enriched biochar (EB) fertilizers with a 6–6–4 N-P2O5-K2O grade: EB-1 (NPK intercalated into biochar) and EB-2 (EB-1 supplemented with humic acid and seaweed extract) [79]. Nutrient release tests showed EB fertilizers slowed the release of NH4+, P, and K+ compared to conventional fertilizers, while NO3− release remained similar. Although total N release was comparable, EB fertilizers released considerably less P and K within 36 h. In field trials with direct-seeded rice, EB fertilizers improved apparent nutrient use efficiency, achieving 11.7–29.5% higher N, 32.9–64.0% higher P, and 31.4–38.0% higher K efficiency than conventional fertilizers at full application rates. These results highlight the potential of EB fertilizers for sustainable rice production by reducing nutrient losses and enhancing nutrient use efficiency.
Enriched biochar materials demonstrate strong potential as sustainable slow-release fertilizers that enhance nutrient efficiency and soil health. Sewage sludge- and organo-mineral-based biochar materials released K gradually, particularly in clay soils, improving nutrient availability over time. N-enriched biochar optimized rice yield and carbon regulation by promoting Fe reduction and moderating greenhouse gas emissions. Similarly, slurry- and nutrient-enriched biochar materials improved C–N cycling, reduced nutrient leaching, and increased fertilizer use efficiency in cereals. Overall, moderate application of enriched biochar supports higher productivity while minimizing nutrient losses and environmental impacts.
3.6. Implications
Biochar plays a multifaceted role in soil nutrient and carbon dynamics by combining physical, chemical, and biological mechanisms. Its porous structure and stable carbon backbone enhance soil organic carbon storage while also influencing CO2 and CH4 fluxes through changes in redox potential and microbial respiration [54,78,80]. Acting as an electron shuttle or donor, biochar supports Fe-reducing microbes, which further regulate carbon turnover and greenhouse gas emissions [74,75].
Nutrient cycling is similarly affected, as biochar improves N and P retention while reducing losses through leaching, runoff, and volatilization [38,39,41]. Functional groups such as carboxyl and ketone bond with NH4+, while its high cation exchange capacity stabilizes mineral N [42]. Phosphorus is retained in more stable pools but is also made more plant-available through enhanced phosphatase activity and P-solubilizing microbes [38,72]. These processes increase nutrient-use efficiency and promote sustained plant uptake.
The magnitude and direction of these effects depend on soil type, feedstock, and environmental context. Acidic soils show greater ammonium immobilization and suppress nitrification, while clay soils enhance K and P availability through better water–fertilizer interactions [41,73]. Feedstocks such as rice straw enriched with Mg increase P solubility, whereas sewage sludge biochar requires mineral supplementation to supply K [73,81]. Moisture, pH buffering, and redox conditions further shape how biochar interacts with soil nutrients and microbial communities.
Mechanistically, biochar mediates nutrient dynamics not only through sorption and ion exchange but also by reshaping microbial networks. Its porous surface provides habitats for microbes, promoting groups such as Proteobacteria and Actinobacteria that drive organic matter decomposition, nitrification, and P solubilization [81]. In this way, biochar integrates physical stabilization with microbial mediation, supporting both soil fertility and environmental sustainability. Overall, its potential as a tool for nutrient management and climate mitigation is clear, though context-specific application strategies remain essential.
A zonal comparison of soils reveals that biochar’s influence on nutrient dynamics varies strongly with soil type, texture, and pH. In sandy Umbric Podzols, biochar (especially fine fractions ≤ 2 mm at 5%) significantly elevated soil pH, available P, and phosphatase activity, enhancing plant P uptake and growth [40]. In contrast, in heavy loamy Umbric Retisols, biochar produced smaller improvements in P availability and negligible effects on enzyme activity, with major benefits only when combined with fertilizer [40]. Similarly, studies on clay loam and loamy sand soils showed that biochar improved N retention and reduced NH4+ losses more effectively in fine-textured soils, while in coarse soils, the benefits declined over time due to lower cation exchange capacity [66]. In acidic soils, biochar buffered pH and enhanced N and P availability, whereas in neutral to calcareous soils, its main role was improving C stabilization and reducing nutrient leaching [43,54,72,82]. Collectively, these zonal variations demonstrate that biochar’s efficiency depends on the inherent physicochemical properties of soils, where fine-textured and moderately acidic soils show the most consistent gains in nutrient retention, biological activity, and fertility improvement.
4. Impacts of Biochar on Microbial Activity
Biochar has gained significant attention as a soil amendment due to its potential to enhance soil fertility, sequester carbon, and mitigate environmental pollution. Beyond its physical and chemical contributions to soil systems, biochar exerts profound effects on soil microbial communities, which are central to nutrient cycling, organic matter decomposition, and overall ecosystem functioning. By modifying soil properties such as pH, porosity, moisture retention, and nutrient availability, biochar can create favorable or, in some cases, inhibitory conditions for microbial growth and activity. These shifts in microbial dynamics not only influence soil health and productivity but also determine the efficacy of biochar in delivering long-term agronomic and environmental benefits.
4.1. Recent Findings on the Influence of Biochar on Microbial Activity
Zhang et al. [83] examined the impacts of straw-based carbon substrate (100 kg), a new form of biochar, on soil fertility, microbial communities, and maize yield compared with traditional straw amendments. Both crop stage and treatment significantly shaped bacterial and fungal diversity. Key taxa, including Rhizobiales, Saccharimonadales, and Eurotiales, responded positively to the biochar substrate by enhancing nutrient cycling. Rhizobiales abundance was linked to higher soil nitrogen during early maize growth, while Saccharimonadales and Eurotiales correlated with elevated carbon and phosphorus at later stages (Figure 2). These findings indicate that straw-based carbon substrate fosters stage-specific recruitment of beneficial microbes, thereby improving nutrient supply and boosting crop productivity.
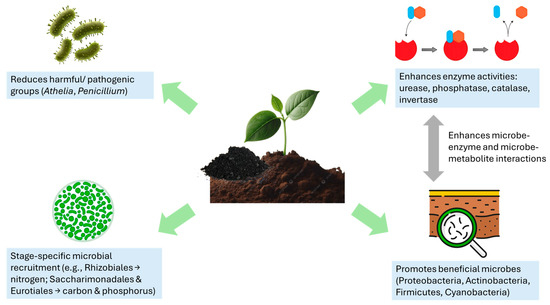
Figure 2.
Impacts of biochar on soil microbial dynamics.
A two-year pot experiment (2020–2021) investigated how biochar (0–60 t ha−1) and nitrogen fertilizer (0–450 kg ha−1) influence soil properties, enzyme activity, nitrogen use efficiency, and rapeseed yield [84]. Compared with the control, biochar with high nitrogen markedly enhanced soil enzymes: B30 + N450 increased urease by 73–75%, B60 + N450 raised catalase by 16–17%, and B60 + N225 improved alkaline phosphatase by 17–19% (Figure 2). Although excessive nitrogen alone reduced plant nitrogen metabolism enzymes, adding 30 t ha−1 biochar alleviated this stress and stimulated nitrate reductase, nitrite reductase, glutamate synthase, and glutamine synthetase at both seedling and flowering stages. The B30 + N450 treatment also increased plant height (11–18%), pods per plant (32–39%), and grain yield (54–64%), while B15 + N225 achieved the highest nitrogen use efficiency (29%) [84]. These results demonstrate that biochar enhances enzyme activity, offsets nitrogen stress, and improves nutrient efficiency and rapeseed productivity. Biochar addition enhanced key soil enzymes by improving nutrient cycling and microbial activity. Urease activity rose due to greater nitrogen mineralization and retention, while higher phosphatase activity was linked to biochar’s porous structure, ash content, and organic carbon, which increased soil pH and moisture [85]. Catalase activity improved as biochar boosted microbial oxidative metabolism through better soil aeration and moisture, and invertase activity increased with higher soil organic carbon and nutrient availability. However, excessive biochar (60 t ha−1) reduced urease and invertase, likely due to elevated pH and adsorption effects [86].
Application of an enriched rice straw biochar at 4.24 g per treatment altered soil microbial communities by promoting beneficial groups like Proteobacteria and Actinobacteria, which support phosphorus solubilization and organic matter decomposition, while reducing taxa such as Methylomirabilota and Desulfobacterota (Figure 2) [81]. Biochar treatments increased the number of unique microbial operational taxonomic units, indicating community shifts that support nutrient cycling, plant–microbe interactions, and overall soil health. Biochar-based phosphate fertilizers further enriched rhizosphere diversity, enhancing nutrient solubilization, organic matter breakdown, and pathogen suppression [17].
In a seven-year maize-field trial testing biochar applications (0–126 Mg ha−1) and their effects on root traits and soil microbes, biochar was found to significantly enhance root length, surface area, volume, and biomass. It also increased bacterial diversity and altered community structure, though fungal communities showed little change [87]. Root development was strongly associated with bacterial, rather than fungal, communities, with microbial network analysis linking key bacterial taxa to root growth parameters. Generally, fungi can utilize the stable carbon in biochar more efficiently than bacteria, colonizing its pores and exploiting available resources [88]. However, in this study, the limited fungal response may be due to the long interval since application (7 years), the low proportion of stable carbon, and the gradual collapse of biochar pores over time. Consequently, biochar had a stronger influence on bacterial communities than on fungal ones. Biochar application (15.75–126 Mg ha−1) was observed to increase Firmicutes while reducing Actinobacteria and Acidobacteria in rhizosphere soils. Biochar can reshape rhizosphere microbial communities by stimulating root exudation and supplying nutrients and energy that support microbial activity, potentially favoring Firmicutes [89].
A study separating corn-straw biochar into aqueous extractable (AE), organic extractable (OE), and residual solid fractions (EBC) and incubating it with purple soil for 30 days revealed that biochar increased available P, K, and organic C but lowered pH and available N [90]. Sequencing showed fungal richness and diversity increased, while bacterial richness remained unchanged. Community shifts differed by group: AE mainly shaped bacterial composition through nutrient inputs, whereas EBC influenced fungal communities via its structure and stable compounds. The limited impact of biochar on fungi observed by Yan et al. [87] may be due to the seven-year gap since application, the low proportion of stable carbon in the biochar, and the gradual collapse of its pore structure over time.
In apple root zone soils, apple wood biochar (1% w/w) was found to reshape bacterial, fungal, and archaeal communities, raising bacterial α-diversity in the rhizoplane but lowering fungal and archaeal diversity in the rhizosphere [91]. It boosted nitrogen-fixing microbes and N2O-reducing activity in root-associated soils, cutting N2O emissions by up to 35%, while showing little effect on nifH and amoA genes in non-rhizosphere soils. Network analysis further linked nitrogen-cycling genes to diverse microbial groups.
Shang et al. [92] examined enzyme activity, microbial growth, and bacterial community dynamics in maize rhizospheres following straw and straw-derived biochar addition. Straw returned to soil boosted microbial biomass and specific growth rates (1.2–1.6 and 1.7–2.0 times higher, respectively), favoring fast-growing Firmicutes, which carried more β-1,4-glucosidase (BG) and β-N-acetylglucosaminidase (NAG) genes. Correspondingly, BG activity and affinity rose 2.2- and 1.8-fold, while NAG rose 4.0- and 2.0-fold. By contrast, biochar favored slower-growing Actinobacteria (K-strategists), resulting in weaker enzymatic responses but enhanced root biomass (+31%) and expanded rhizosphere hotspots for BG (+26%) and NAG (+47%). Biochar also increased network stability, whereas straw mainly accelerated nutrient cycling by promoting rapid microbial proliferation and enzyme production. Biochar enhanced nutrient mobilization in the rhizosphere by enlarging hotspot areas, allowing nutrient release from a broader soil volume [92].
Akumuntu et al. [93] assessed the impact of rice husk biochar (0–1.5%) on soil properties, lettuce growth, and earthworm toxicity. Rice husk biochar did not significantly alter soil pH but enhanced enzyme activities (dehydrogenase, alkaline phosphatase, and beta-glucosidase) after 28 days and increased beneficial growth-promoting microbes, such as Massilia, Bacillus, and Trichocladium (Figure 2). Lettuce root weight and leaf number rose with higher rice husk biochar levels, with growth improvements linked to enhanced nutrient availability driven by microbial and enzymatic shifts. Liu et al. [94] reported that the porous nature of biochar supports microbial diversity and protects microbes against stresses such as freeze–thaw events. Consequently, biochar contributes to creating favorable habitats for soil microorganisms and promotes the stability of microbial networks.
A six-year field study (2013–2018) examined how different biochar application rates (0, 15.75, 31.5, and 47.25 t ha−1) with fertilizers influence soil fertility, organic N fractions, and microbial communities [95]. Biochar raised soil organic matter, C/N ratio, and pH, and significantly altered acid-hydrolyzable N and its fractions. Amino acid N was associated with enriched Verrucomicrobia, Cyanobacteria, Glucosylbacteria, and Nitrospira; ammonium N with Verrucomicrobia, Bacteroidetes, Gemmatimonadetes, Acidobacteria, and Proteobacteria; amino sugar N with Acidobacteria; and unknown N with Gemmatimonadetes (Figure 2). These shifts, along with changes in soil enzyme activities, indicate that biochar drives N cycling through specific microbial–enzyme interactions.
Moreover, Yin et al. [96] investigated the impact of rice and corn straw biochar materials on microbial communities in the rhizosphere of rice grown in albic soil. In a pot experiment, the application of biochar increased soil nutrient levels and enhanced bacterial and fungal abundance and diversity. Compared with the control, rice straw biochar increased the bacterial abundance-based coverage estimate (ACE) by 3.1%, and the fungal ACE and Chao indices by 7.9% and 14.2%, respectively. Both biochar materials increased the operational taxonomic units. Biochar also strengthened bacteria–fungi interactions, with shifts in community structure closely tied to changes in soil nutrients. Overall, rice straw biochar had the strongest positive effect on microbial diversity and soil quality. Biochar boosts microbial abundance by supplying labile C and minor nutrients, while in nutrient-poor, compact albic soil, it also alters community composition indirectly through improvements in pH, structure, and related properties. Biochar raised the relative abundance of Proteobacteria, Verrucomicrobia, and Bacteroidetes. Proteobacteria, the dominant phylum, increased by 13.95% with rice straw biochar and 9.94% with corn straw biochar compared to the control. Their enrichment reflects their eutrophic nature, as biochar improves nutrient conditions in albic soils, consistent with findings of Di et al. [95].
A three-month field trial tested peanut shell biochar (0, 5, and 10% v/v) in landfill cover soil to assess its impacts on plants and microbes [97]. Biochar raised soil pH, nutrients, and organic matter up to two times, boosting plant growth. It also increased bacterial richness, diversity, and community heterogeneity. However, plant diversity declined, and dominant species shifted. Specifically, it promoted Cyperus rotundus L. dominance, likely through its allelopathic traits in nutrient-rich conditions. Biochar significantly increased bacterial richness and diversity at all levels, while only 5% biochar boosted fungal richness and diversity. Functional genes linked to amino acid metabolism were abundant, likely from root growth, and biochar also raised genes for cofactor and vitamin metabolism. Improved soil chemistry from biochar also reinforced interactions between fungi and Cyperus rotundus L. [97].
In addition, a field experiment tested three treatments: no biochar, 10 t/m3, and 20 t/m3, with soil microbial diversity analyzed using high-throughput sequencing [98]. Biochar improved soil properties, and alpha diversity was highest at 20 t/m3. The dominant phyla included Proteobacteria, Cyanobacteria, and Actinobacteria, with Skermanella, Nostoc, Frankia, and unclassified Proteobacteria as key genera (Figure 2). Actinobacteria varied significantly across treatments. Microbial community structure was mainly shaped by soil porosity, moisture, and nitrogen and potassium fertilizers, rather than phosphate or organic matter.
Zhang et al. [99] tested four wheat straw biochar levels (0, 5, 10, 15 kg/plant) across two soil depths (0–20 cm, 20–40 cm). Biochar improved citrus fruit quality and soil properties (pH, organic matter, nutrients). It enhanced bacterial richness, evenness, and diversity but reduced fungal evenness, while enriching beneficial bacteria and saprophytic (Basidiomycota) fungi involved in nutrient cycling. Furthermore, Yang et al. [100] examined Panax quinquefolium L. under different rice straw biochar levels (0–2.4%) to assess plant performance, active compounds, soil enzymes, and microbial communities. Biochar enhanced plant growth, photosynthesis, chlorophyll b, and ginsenoside content, while also increasing sucrase, urease, and acid phosphatase activities (Figure 2). In contrast, catalase activity and microbial diversity declined. Notably, bacterial phyla (e.g., Proteobacteria) and fungal genera (e.g., Chalara, Sistotrema) varied between the control and 1.8% biochar. Linear discriminant analysis further indicated significant shifts in rhizosphere microbial taxa under 1.8% and 2.4% treatments. Biochar can enhance microbial growth by supplying organic carbon and nutrients [100]. In this study, the rhizosphere fungal community of P. quinquefolium was more affected by biochar, differing from Zhang et al. [98], who reported increased bacterial diversity but reduced fungal evenness.
In a separate study, Ren et al. [101] assessed biochar (20 kg/plant) effects on bayberry decline by examining tree growth, fruit quality, soil properties, microbial communities, and metabolites. Biochar improved tree vigor, fruit quality, and rhizosphere microbial diversity. It increased Mycobacterium, Crossiella, Geminibasidium, and Fusarium, while reducing Acidothermus, Bryobacter, Acidibacter, Cladophialophora, Mycena, and Rickenella. Redundancy analysis showed that soil pH, organic matter, nutrients, and exchangeable cations strongly shaped microbial composition, with a greater influence on fungi than bacteria at the genus level, in line with the findings of Yang et al. [100].
A pot experiment with cotton seedlings was conducted to test rice straw biochar at three levels (0, 1, and 2%, w/w) for saline–alkaline soil treatment [102]. At 2%, biochar lowered soil pH, EC, and soluble salts, while increasing organic matter, nutrients, and enzyme activity. It reduced salinity in both soil and plant tissues and altered microbial community composition, enhancing salt-tolerant phyla like Proteobacteria, Bacteroidota, Acidobacteriota, and Actinobacteriota. Biochar markedly enhanced extracellular enzyme activity, with nitrogen- and phosphorus-cycling enzymes rising sharply by 32.79% and 34.25%, respectively. Biochar also strengthened microbe–metabolite interactions, mitigating salt stress on microorganisms [102].
A field study in a tropical ecosystem tested biochar from corn cobs at 15 and 30 t/ha, with or without phosphate fertilizer, on soil microbes and enzymes [103]. Biochar boosted microbial biomass C (4.5–8.2×), N (1.4–2.7×), and mineralized C (1.2–1.7×), while 30 t/ha amendments reduced specific maintenance respiration rates by 66–73%, indicating higher efficiency. It also increased dehydrogenase and urease activity, as well as the abundance of arbuscular mycorrhiza and soil fungi. Only 30 t/ha and 30 t/ha + P raised Gram-positive (1.7–1.9×) and Gram-negative (1.5–1.6×) bacteria, and total phospholipid fatty acid was highest under 30 t/ha treatments [103]. Specific maintenance respiration reflects microbial oxygen use and stress levels. Biochar improves soil porosity and gas diffusion [104], raising O2 availability and stimulating microbial activity. This explains the sharp decline in specific maintenance respiration with biochar, while higher specific maintenance respiration in controls indicates carbon stress. The lower stress in biochar-amended soils also favors greater fungal abundance, as fungi typically thrive under stable conditions [105].
In a double rice system, the effects of wheat straw biochar on microbes and soil fertility were assessed 3–4 years after a single application (0, 24, 48 t/ha) [106]. Biochar increased bacterial and fungal abundances by up to 102% and 178%, linked to higher total organic carbon, nitrogen, and rice biomass, while archaea rose only slightly. The bacteria/fungi ratio fell by up to 61.4%, as fungi dominated in decomposing recalcitrant carbon. Biochar also promoted Acidobacteria, Mortierella, and Westerdykella, which support carbon cycling and plant growth, while reducing phytopathogens like Athelia and Penicillium. A summary of these recent findings is shown in Table 2.

Table 2.
Biochar effects on soil microbial communities.
4.2. Variations in Bacterial and Fungal Communities
Biochar generally exerts positive effects on bacterial communities. It increases bacterial richness, diversity, and network stability by improving soil conditions, such as pH, porosity, nutrient availability, and aeration. Beneficial bacterial phyla, such as Proteobacteria, Actinobacteria, Rhizobiales, and Firmicutes, as well as genera like Massilia, often proliferate under biochar amendment, thereby supporting nutrient cycling, nitrogen fixation, and root development. Biochar’s porous structure provides microhabitats and labile carbon that stimulate bacterial activity, while its ability to retain nutrients enhances bacterial-mediated processes such as phosphorus solubilization, nitrogen mineralization, and organic matter decomposition [81,92,93,95]. However, at very high application rates, biochar can reduce bacterial enzyme activity or shift community composition in ways that are less favorable, often due to excessive pH increases or adsorption of available nutrients [86].
The effects of biochar on fungi are more variable and context-dependent. In many cases, biochar increases fungal abundance and richness, particularly of beneficial saprophytic fungi (e.g., Basidiomycota, Mortierella, Westerdykella) that drive carbon cycling and plant–soil symbioses [99,106]. Fungal communities often show stronger responses to biochar’s structural fractions (e.g., stable carbon compounds and pore architecture) than bacteria do [96,100]. Positive impacts include enhanced phosphorus mobilization, pathogen suppression, and increased resilience under stressors such as salinity.
However, biochar can also reduce fungal evenness or suppress certain groups, especially under high application rates, long-term use, or when biochar significantly alters soil pH [98]. Some studies reported little change in fungi compared to bacteria, while others observed declines in diversity or abundance of specific fungal taxa (e.g., Mycena, Rickenella) [100].
Biochar generally plays a suppressive role against soil-borne pathogens by modifying the soil environment in ways that reduce their competitiveness. Many studies show that biochar lowers the abundance of harmful fungi such as Athelia and Penicillium in rice systems, as well as Cladophialophora, Mycena, and Rickenella in bayberry soils [100,106]. These reductions are linked to improvements in soil fertility, organic matter, pH, and redox conditions, which create less favorable niches for pathogens to thrive. By improving overall soil quality, biochar makes conditions more conducive to beneficial microbes while simultaneously weakening pathogen survival.
An important mechanism for pathogen suppression is competitive exclusion by beneficial microbial taxa. Biochar stimulates the proliferation of groups like Proteobacteria, Actinobacteria, Mortierella, and Westerdykella, which are known for their roles in nutrient cycling, organic matter decomposition, and plant growth promotion [106]. These beneficial organisms occupy ecological niches and utilize resources that might otherwise support pathogens, thereby reducing their abundance and influence.
Enhanced soil enzyme activity also contributes to pathogen suppression. Biochar addition increases activities of urease, phosphatase, catalase, invertase, and other enzymes that strengthen nutrient cycling and microbial oxidative metabolism [67,84,103]. This enzymatic activity supports antagonistic microbial populations and biochemical processes that hinder pathogen growth. In particular, biochar’s porous structure and high surface area provide habitats for beneficial microbes that can outcompete or inhibit pathogens.
However, biochar does not always act purely suppressively. Some evidence suggests that, under certain conditions, biochar may shift microbial communities in ways that favor particular taxa with pathogenic potential. For example, increases in Fusarium in bayberry soils following biochar application suggest that some pathogenic groups may benefit from the altered nutrient and pH environment [101].
4.3. Connections Between Microbial Activity and Nutrient Dynamics
Biochar alters nutrient dynamics largely through its influence on microbial activity. By improving soil conditions, such as pH, aeration, and organic matter content, biochar creates favorable habitats that stimulate microbial growth and activity. Beneficial groups such as Proteobacteria and Actinobacteria become more abundant, alongside fungi like Mortierella and Westerdykella, which contribute to organic matter decomposition and nutrient release [92,98,106]. These shifts in community composition strengthen soil biochemical cycling.
Nitrogen cycling is particularly sensitive to microbial responses under biochar amendment. Proteobacteria, many of which include nitrogen-fixing (Rhizobium) and denitrifying (Pseudomonas) genera, are stimulated by improved soil conditions [83,107]. Increased urease activity, often linked to bacterial and fungal groups, accelerates N mineralization, raising the pool of plant-available nitrogen. Biochar also promotes Fe-reducing microbes, such as members of the Geobacter genus within Deltaproteobacteria, which drive Fe3+ reduction [74,75]. This process lowers redox potential and indirectly regulates denitrification pathways, thereby affecting nitrogen losses and greenhouse gas emissions.
Phosphorus dynamics are also strongly microbially mediated. Enrichment of Proteobacteria and Actinobacteria enhances the activity of phosphatase-producing taxa, which liberate inorganic phosphate from organic matter [81]. Some groups within Actinobacteria are known for secreting organic acids that solubilize phosphate minerals, complementing biochar’s direct effects on P sorption–desorption equilibria [108]. In this way, microbial turnover ensures greater and more sustained phosphorus availability.
For potassium, microbial activity plays a supporting role through mineral weathering and organic matter decomposition. Certain bacterial genera within Acidobacteria and Bacteroidetes contribute to the breakdown of plant residues, gradually releasing K+. This complements the cation exchange capacity of biochar, which stabilizes K in the soil and prolongs its availability [65,109].
Overall, microbial activities under biochar amendment drive nutrient dynamics by accelerating organic matter decomposition, stimulating enzyme-mediated processes, and regulating redox-sensitive pathways. The enhanced abundance of phyla such as Proteobacteria, Actinobacteria, Bacteroidetes, and Acidobacteria, along with fungal genera like Mortierella and Westerdykella, underscores the biological mechanisms linking biochar’s physicochemical properties with nutrient cycling. These microbial shifts underpin improvements in N, P, and K availability, thereby supporting soil fertility and plant nutrition.
5. Conclusions
Across a zonal series of soils, from acidic Podzols and Retisols to neutral loams and calcareous soils, biochar exerts soil-specific effects on nutrient dynamics, particularly phosphorus (P) and nitrogen (N) transformations. In coarse-textured, acidic soils such as Umbric Podzols, biochar markedly enhances nutrient availability by raising pH, stimulating phosphatase activity, and increasing exchangeable and Ca-bound P. Conversely, in fine-textured or neutral soils like Umbric Retisols and loamy sands, biochar’s influence is more moderate, often improving nutrient retention but showing limited stimulation of microbial processes. High surface area and functional groups in biochar promote cation exchange and ammonium adsorption, thereby reducing volatilization and leaching losses. However, its efficacy depends strongly on soil reaction and mineralogy: optimal N retention typically occurs under mildly acidic to neutral conditions (pH 4.5–6.4), where biochar enhances NH4+ immobilization and suppresses nitrification. These zonal contrasts demonstrate that while biochar universally contributes to nutrient conservation and soil fertility enhancement, its mechanisms and magnitudes vary with soil type, texture, and pH. Hence, region-specific optimization of biochar formulation and application rate is essential for maximizing nutrient efficiency and sustaining soil productivity across diverse agroecosystems. For instance, higher application rates may be required for acidic soil to raise pH, increase available P, and improve nutrient retention, while lower rates are beneficial for calcareous or neutral to alkaline soils to avoid further alkalinization, which can immobilize P and hinder nutrient uptake.
Biochar has demonstrated consistent potential to improve soil fertility by enriching microbial diversity, stimulating enzyme activity, and supporting nutrient cycling, thereby boosting crop productivity. Its porous structure and nutrient content provide favorable habitats for beneficial bacterial and fungal taxa, which drive nitrogen fixation, phosphorus solubilization, and organic matter decomposition, while also suppressing certain pathogens. However, outcomes remain context-dependent, influenced by soil type, biochar feedstock, application rate, and crop species, with excessive use sometimes reducing enzyme activity, shifting microbial communities unfavorably, raising pH, and reducing nutrient availability.
Future research should prioritize long-term, multi-site field trials across varying climates, soils, and cropping systems to better understand biochar’s role in microbial succession and nutrient-use efficiency. Combining molecular, isotopic, and metabolomic tools will help clarify the mechanisms underlying biochar–microbe interactions. In addition, integrating biochar with nanotechnology, organic amendments, and precision fertilization strategies may reveal synergies that optimize soil health, crop productivity, and climate resilience in sustainable agricultural systems.
Funding
This research received no external funding.
Data Availability Statement
This review paper does not report original data. All data presented or discussed are derived from previously published studies, which are cited throughout the manuscript. No new datasets were generated or analyzed for this study.
Acknowledgments
The author wishes to thank the University of Arizona for the administrative support provided.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Ren, T.; Feng, H.; Xu, C.; Xu, Q.; Fu, B.; Azwar, E.; Wei, Y.; Lam, S.S.; Liu, G. Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere’s microbial community and health. Chemosphere 2022, 294, 133710. [Google Scholar] [CrossRef]
- Qian, R.; Yu, K.; Chen, N.; Li, R.; Tang, K.H.D. Adsorptive immobilization of cadmium and lead using unmodified and modified biochar: A review of the advances, synthesis, efficiency and mechanisms. Chemosphere 2025, 370, 143988. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Kang, M.W.; Yibeltal, M.; Kim, Y.H.; Oh, S.J.; Lee, J.C.; Kwon, E.E.; Lee, S.S. Enhancement of soil physical properties and soil water retention with biochar-based soil amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef]
- Kuok Ho Daniel, T. Valorization of organic waste as biosorbents for wastewater treatment. Water Emerg. Contam. Nanoplastics 2024, 3, 25. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Wang, J.; Yang, X.; Zhang, W.; Tang, K.H.D.; Wang, H.; Zhang, X.; Gao, R.; Yang, G.; et al. Insight into the humification and carbon balance of biogas residual biochar amended co-composting of hog slurry and wheat straw. Environ. Sci. Pollut. Res. 2025, 32, 21919–21930. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, H.; Chu, M.; Zhang, C.; Tang, J.; Chang, S.X.; Mašek, O.; Ok, Y.S. Biochar affects greenhouse gas emissions in various environments: A critical review. Land Degrad. Dev. 2022, 33, 3327–3342. [Google Scholar] [CrossRef]
- Xia, F.; Zhang, Z.; Zhang, Q.; Huang, H.; Zhao, X. Life cycle assessment of greenhouse gas emissions for various feedstocks-based biochars as soil amendment. Sci. Total Environ. 2024, 911, 168734. [Google Scholar] [CrossRef]
- Tang, K.H.D. Urban Solutions to Climate Change: An Overview of Latest Progress. Acad. Environ. Sci. Sustain. 2024, 1. [Google Scholar] [CrossRef]
- Atilano-Camino, M.M.; Canizales Laborin, A.P.; Ortega Juarez, A.M.; Valenzuela Cantú, A.K.; Pat-Espadas, A.M. Impact of Soil Amendment with Biochar on Greenhouse Gases Emissions, Metals Availability and Microbial Activity: A Meta-Analysis. Sustainability 2022, 14, 5648. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, C.; Chen, Z.; Liu, Y.; Zhang, Y.; Gou, P.; Zhao, X.; Ma, D.; Kang, G.; Wang, C.; et al. Successive biochar amendment affected crop yield by regulating soil nitrogen functional microbes in wheat-maize rotation farmland. Environ. Res. 2021, 194, 110671. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Liu, X.; Huang, Y.; Cai, A.; Xu, H.; Ran, J.; Xiao, J.; Zhang, W. A global dataset of biochar application effects on crop yield, soil properties, and greenhouse gas emissions. Sci. Data 2024, 11, 57. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Mishra, V.K.; Choudhury, B.U.; Dutta, S.K.; Hazarika, S.; Kalita, H.; Roy, A.; Singh, N.U.; Gopi, R.; et al. Utilizing dissimilar feedstocks derived biochar amendments to alter soil biological indicators in acidic soil of Northeast India. Biomass Convers. Biorefinery 2023, 13, 10203–10214. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Hajiboland, R.; Panda, C.K.; Lastochkina, O.; Gavassi, M.A.; Habermann, G.; Pereira, J.F. Aluminum Toxicity in Plants: Present and Future. J. Plant Growth Regul. 2023, 42, 3967–3999. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Christel, W.; Bruun, S.; Magid, J.; Kwapinski, W.; Jensen, L.S. Pig slurry acidification, separation technology and thermal conversion affect phosphorus availability in soil amended with the derived solid fractions, chars or ashes. Plant Soil 2016, 401, 93–107. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing Cation Exchange Capacity of Weathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Salem, T.M.; Refaie, K.M.; Sherif, A.E.-H.E.-G.A.E.-L.; Eid, M.A.M. Biochar application in alkaline soil and its effect on soil and plant. Acta Agric. Slov. 2019, 114, 85–96. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, Z.; Ei-Naggar, A.; Tian, L.; Huang, C.; Zhang, Z.; Palansooriya, K.N.; Li, Y.; Yu, B.; Chang, S.X.; et al. Contrasting effects of rice husk and its biochar on N2O emissions and nitrogen leaching from Lei bamboo soils under subtropical conditions. Biol. Fertil. Soils 2023, 59, 803–817. [Google Scholar] [CrossRef]
- Kaur, N.; Kieffer, C.; Ren, W.; Hui, D. How much is soil nitrous oxide emission reduced with biochar application? An evaluation of meta-analyses. GCB Bioenergy 2023, 15, 24–37. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Effect of steam activated biochar application to industrially contaminated soils on bioavailability of polycyclic aromatic hydrocarbons and ecotoxicity of soils. Sci. Total Environ. 2016, 566–567, 1023–1031. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Berry, C.M.; Strawn, D.G.; Novak, J.M.; Levine, J.; Harley, A. Biochars Reduce Mine Land Soil Bioavailable Metals. J. Environ. Qual. 2017, 46, 411–419. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Kristanti, R.A. Bioremediation of perfluorochemicals: Current state and the way forward. Bioprocess Biosyst. Eng. 2022, 45, 1093–1109. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Reid, B.J.; Arp, H.P.H.; Sun, G.-X.; Yuan, H.-Y.; Zhu, Y.-G. Advances in research on the use of biochar in soil for remediation: A review. J. Soils Sediments 2018, 18, 2433–2450. [Google Scholar] [CrossRef]
- Kumpiene, J.; Antelo, J.; Brännvall, E.; Carabante, I.; Ek, K.; Komárek, M.; Söderberg, C.; Wårell, L. In situ chemical stabilization of trace element-contaminated soil—Field demonstrations and barriers to transition from laboratory to the field—A review. Appl. Geochem. 2019, 100, 335–351. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Tsolis, V.; Barouchas, P. Biochar as Soil Amendment: The Effect of Biochar on Soil Properties Using VIS-NIR Diffuse Reflectance Spectroscopy, Biochar Aging and Soil Microbiology—A Review. Land 2023, 12, 1580. [Google Scholar] [CrossRef]
- Anyebe, O.; Sadiq, F.K.; Manono, B.O.; Matsika, T.A. Biochar Characteristics and Application: Effects on Soil Ecosystem Services and Nutrient Dynamics for Enhanced Crop Yields. Nitrogen 2025, 6, 31. [Google Scholar] [CrossRef]
- Singh, S.; Luthra, N.; Mandal, S.; Kushwaha, D.P.; Pathak, S.O.; Datta, D.; Sharma, R.; Pramanick, B. Distinct Behavior of Biochar Modulating Biogeochemistry of Salt-Affected and Acidic Soil: A Review. J. Soil Sci. Plant Nutr. 2023, 23, 2981–2997. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Rangeland application of biochar and rotational grazing interact to influence soil and plant nutrient dynamics. Geoderma 2022, 408, 115572. [Google Scholar] [CrossRef]
- Kuoppamäki, K.; Setälä, H.; Hagner, M. Nutrient dynamics and development of soil fauna in vegetated roofs with the focus on biochar amendment. Nat.-Based Solut. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Dubrovina, I.A. Changes in the Phosphate Regime of Soils in the Middle Taiga under the Impact of Biochar. Eurasian Soil Sci. 2023, 56, 363–370. [Google Scholar] [CrossRef]
- Gupta, R.K.; Vashisht, M.; Naresh, R.K.; Dhingra, N.; Sidhu, M.S.; Singh, P.K.; Rani, N.; Al-Ansari, N.; Alataway, A.; Dewidar, A.Z.; et al. Biochar influences nitrogen and phosphorus dynamics in two texturally different soils. Sci. Rep. 2024, 14, 6533. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.E.-E.A.Z. Carbon sequestration, kinetics of ammonia volatilization and nutrient availability in alkaline sandy soil as a function on applying calotropis biochar produced at different pyrolysis temperatures. Sci. Total Environ. 2020, 726, 138489. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, Q.; Chen, H.; Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J.; Müller, C.; Yi, Z. Enhancing Soil Nitrogen Retention Capacity by Biochar Incorporation in the Acidic Soil of Pomelo Orchards: The Crucial Role of pH. Agronomy 2023, 13, 2110. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Wang, B.; Hou, B.; Du, G.; Si, H. Analysis of the adsorption and fixation process of ammonium nitrogen in arable soil by biochar based on molecular dynamics simulation. Sci. Total Environ. 2024, 930, 172815. [Google Scholar] [CrossRef]
- Hu, T.; Wei, J.; Du, L.; Chen, J.; Zhang, J. The effect of biochar on nitrogen availability and bacterial community in farmland. Ann. Microbiol. 2023, 73, 4. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, M.; Zhai, Z.; Dai, H.; Yang, M.; Zhang, Y.; Liang, T. Soil organic carbon, carbon fractions, and microbial community under various organic amendments. Sci. Rep. 2024, 14, 25431. [Google Scholar] [CrossRef]
- Abdul-Aziz, A.-L.; Abukari, I.A.; Galadima, M.M.; Haruna, A.; Abubakari, M.; Abdulai, R. Biochar effects on soil properties and yield of maize in Northern region, Ghana. Discov. Agric. 2025, 3, 103. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Li, S.; Liu, W.; Bian, R.; Zhang, X.; Zheng, J.; Drosos, M.; Li, L.; Pan, G. Quantitative assessment of the effects of biochar amendment on photosynthetic carbon assimilation and dynamics in a rice–soil system. New Phytol. 2021, 232, 1250–1258. [Google Scholar] [CrossRef]
- Ge, T.; Liu, C.; Yuan, H.; Zhao, Z.; Wu, X.; Zhu, Z.; Brookes, P.; Wu, J. Tracking the photosynthesized carbon input into soil organic carbon pools in a rice soil fertilized with nitrogen. Plant Soil 2015, 392, 17–25. [Google Scholar] [CrossRef]
- Zang, H.; Xiao, M.; Wang, Y.; Ling, N.; Wu, J.; Ge, T.; Kuzyakov, Y. Allocation of assimilated carbon in paddies depending on rice age, chase period and N fertilization: Experiment with 13CO2 labelling and literature synthesis. Plant Soil 2019, 445, 113–123. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, J.; Boorboori, M.R.; Li, D.; Chen, S.; Ma, X.; Cheng, P.; Zhang, H. Effect of biochar application rate on changes in soil labile organic carbon fractions and the association between bacterial community assembly and carbon metabolism with time. Sci. Total Environ. 2023, 855, 158876. [Google Scholar] [CrossRef]
- Chagas, J.K.M.; Figueiredo, C.C.d.; Ramos, M.L.G. Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Zhou, H.; Luo, W.; Yi, X.; Zhou, Y.; Wu, Y.; Petticord, D.F.; Song, X. Biochar efficacy in enhancing soil carbon fractions is mediated by parent soil type in grazing karst grassland. Carbon Res. 2025, 4, 52. [Google Scholar] [CrossRef]
- Patel, M.R.; Panwar, N.L. Biochar from agricultural crop residues: Environmental, production, and life cycle assessment overview. Resour. Conserv. Recycl. Adv. 2023, 19, 200173. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Orlova, N.; Abakumov, E.; Orlova, E.; Yakkonen, K.; Shahnazarova, V. Soil organic matter alteration under biochar amendment: Study in the incubation experiment on the Podzol soils of the Leningrad region (Russia). J. Soils Sediments 2019, 19, 2708–2716. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Lu, J.; Luo, Y.; Huang, J.; Hou, B.; Wang, B.; Ogino, K.; Zhao, J.; Si, H. The effect of biochar on the migration theory of nutrient ions. Sci. Total Environ. 2022, 845, 157262. [Google Scholar] [CrossRef]
- Lewoyehu, M.; Kohira, Y.; Fentie, D.; Addisu, S.; Sato, S. Water Hyacinth Biochar: A Sustainable Approach for Enhancing Soil Resistance to Acidification Stress and Nutrient Dynamics in an Acidic Nitisol of the Northwest Highlands of Ethiopia. Sustainability 2024, 16, 5537. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Ni, N.; Wang, R.-H.; Nkoh, J.N.; Pan, X.-Y.; Dong, G.; Xu, R.-K.; Cui, X.-M.; Li, J.-Y. Dissolved biochar fractions and solid biochar particles inhibit soil acidification induced by nitrification through different mechanisms. Sci. Total Environ. 2023, 874, 162464. [Google Scholar] [CrossRef]
- Burgeon, V.; Fouché, J.; Garré, S.; Dehkordi, R.H.; Colinet, G.; Cornelis, J.-T. Young and century-old biochars strongly affect nutrient cycling in a temperate agroecosystem. Agric. Ecosyst. Environ. 2022, 328, 107847. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Li, H.; Zhang, A.; Rengel, Z. Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. GCB Bioenergy 2021, 13, 257–268. [Google Scholar] [CrossRef]
- dos Santos, W.M.; Gonzaga, M.I.S.; da Silva, A.J.; de Almeida, A.Q. Improved water and ions dynamics in a clayey soil amended with different types of agro-industrial waste biochar. Soil Tillage Res. 2022, 223, 105482. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Jeguirim, M.; Kwapinska, M.; Hamdi, H.; Leahy, J.J.; Kwapinski, W. Static and Dynamic Investigations on Leaching/Retention of Nutrients from Raw Poultry Manure Biochars and Amended Agricultural Soil. Sustainability 2021, 13, 1212. [Google Scholar] [CrossRef]
- Dubrovina, I. Biochar has Different Effects on Soil Fertility and Spring Barley Productivity in Two Boreal Soils with Contrasting Textures. Agric. Res. 2025. [Google Scholar] [CrossRef]
- Zhang, K.; Khan, Z.; Khan, M.N.; Luo, T.; Luo, L.; Bi, J.; Hu, L. The application of biochar improves the nutrient supply efficiency of organic fertilizer, sustains soil quality and promotes sustainable crop production. Food Energy Secur. 2024, 13, e520. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Lu, Y.; Silveira, M.L.; O’Connor, G.A.; Vendramini, J.M.B.; Li, Y.C. Biochar type and application methods affected nitrogen and phosphorus leaching from a sandy soil amended with inorganic fertilizers and biosolids. Agrosyst. Geosci. Environ. 2022, 5, e20236. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Llovet, A.; Vidal-Durà, A.; Alcañiz, J.M.; Ribas, A.; Domene, X. Biochar addition to organo-mineral fertilisers delays nutrient leaching and enhances barley nutrient content. Arch. Agron. Soil Sci. 2023, 69, 2537–2551. [Google Scholar] [CrossRef]
- Sarfraz, R.; Nadeem, F.; Yang, W.; Tayyab, M.; Khan, M.I.; Mahmood, R.; Guo, X.; Xing, S.; Kim, G.W. Evaluation of Biochar and Inorganic Fertilizer on Soil Available Phosphorus and Bacterial Community Dynamics in Acidic Paddy Soils for Different Incubation Temperatures. Agronomy 2024, 14, 26. [Google Scholar] [CrossRef]
- Ndoung, O.C.N.; Souza, L.R.d.; Fachini, J.; Leão, T.P.; Sandri, D.; Figueiredo, C.C.d. Dynamics of potassium released from sewage sludge biochar fertilizers in soil. J. Environ. Manag. 2023, 346, 119057. [Google Scholar] [CrossRef]
- Yin, X.; Peñuelas, J.; Sardans, J.; Xu, X.; Chen, Y.; Fang, Y.; Wu, L.; Singh, B.P.; Tavakkoli, E.; Wang, W. Effects of nitrogen-enriched biochar on rice growth and yield, iron dynamics, and soil carbon storage and emissions: A tool to improve sustainable rice cultivation. Environ. Pollut. 2021, 287, 117565. [Google Scholar] [CrossRef]
- Suksabye, P.; Pimthong, A.; Dhurakit, P.; Mekvichitsaeng, P.; Thiravetyan, P. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 962–973. [Google Scholar] [CrossRef]
- Wang, M.; Lan, X.; Xu, X.; Fang, Y.; Singh, B.P.; Sardans, J.; Romero, E.; Peñuelas, J.; Wang, W. Steel slag and biochar amendments decreased CO2 emissions by altering soil chemical properties and bacterial community structure over two-year in a subtropical paddy field. Sci. Total Environ. 2020, 740, 140403. [Google Scholar] [CrossRef]
- Joseph, S.; Husson, O.; Graber, E.R.; Van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The Electrochemical Properties of Biochars and How They Affect Soil Redox Properties and Processes. Agronomy 2015, 5, 322–340. [Google Scholar] [CrossRef]
- Javeed, H.M.; Ali, M.; Ahmed, I.; Wang, X.; Al-Ashkar, I.; Qamar, R.; Ibrahim, A.; Habib-Ur-Rahman, M.; Ditta, A.; El Sabagh, A. Biochar Enriched with Buffalo Slurry Improved Soil Nitrogen and Carbon Dynamics, Nutrient Uptake and Growth Attributes of Wheat by Reducing Leaching Losses of Nutrients. Land 2021, 10, 1392. [Google Scholar] [CrossRef]
- Roy, A.; Chaturvedi, S.; Singh, S.V.; Kasivelu, G.; Dhyani, V.C.; Pyne, S. Preparation and evaluation of two enriched biochar-based fertilizers for nutrient release kinetics and agronomic effectiveness in direct-seeded rice. Biomass Convers. Biorefinery 2024, 14, 2007–2018. [Google Scholar] [CrossRef]
- Lan, Y.; Chu, Q.; Liu, X.; Xu, S.; Li, D.; Zhang, C.; He, P.; Feng, X.; Zhang, H.; Sha, Z. Oxygen-nanobubble-loaded biochar increases soil carbon sequestration in rice paddies. Soil Environ. Health 2025, 3, 100174. [Google Scholar] [CrossRef]
- Deng, L.; Tu, P.; Ahmed, N.; Zhang, G.; Cen, Y.; Huang, B.; Deng, L.; Yuan, H. Biochar-based phosphate fertilizer improve phosphorus bioavailability, microbial functioning, and citrus seedling growth. Sci. Hortic. 2024, 338, 113699. [Google Scholar] [CrossRef]
- Mandal, S.; Donner, E.; Smith, E.; Sarkar, B.; Lombi, E. Biochar with near-neutral pH reduces ammonia volatilization and improves plant growth in a soil-plant system: A closed chamber experiment. Sci. Total Environ. 2019, 697, 134114. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, L.; Chang, J.; Wang, E.; Wang, C.; Zhang, H.; Pang, Y.; Tian, C. Straw Soil Conditioner Modulates Key Soil Microbes and Nutrient Dynamics across Different Maize Developmental Stages. Microorganisms 2024, 12, 295. [Google Scholar] [CrossRef]
- Khan, Z.; Zhang, K.; Khan, M.N.; Bi, J.; Zhu, K.; Luo, L.; Hu, L. How Biochar Affects Nitrogen Assimilation and Dynamics by Interacting Soil and Plant Enzymatic Activities: Quantitative Assessment of 2 Years Potted Study in a Rapeseed-Soil System. Front. Plant Sci. 2022, 13, 853449. [Google Scholar] [CrossRef]
- Gong, X.; Cai, L.; Li, S.; Chang, S.X.; Sun, X.; An, Z. Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotoxicol. Environ. Saf. 2018, 156, 197–204. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Yan, H.; Cong, M.; Hu, Y.; Qiu, C.; Yang, Z.; Tang, G.; Xu, W.; Zhu, X.; Sun, X.; Jia, H. Biochar-mediated changes in the microbial communities of rhizosphere soil alter the architecture of maize roots. Front. Microbiol. 2022, 13, 1023444. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Xue, Y. Nickel-loaded shrimp shell biochar enhances batch anaerobic digestion of food waste. Bioresour. Technol. 2022, 352, 127092. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Shen, F.; Tian, D.; Zeng, Y.; Yang, G.; Zhang, Y.; Deng, S. Partitioning biochar properties to elucidate their contributions to bacterial and fungal community composition of purple soil. Sci. Total Environ. 2019, 648, 1333–1341. [Google Scholar] [CrossRef]
- Cao, H.; Jia, M.; Xun, M.; Wang, X.; Chen, K.; Yang, H. Nitrogen transformation and microbial community structure varied in apple rhizosphere and rhizoplane soils under biochar amendment. J. Soils Sediments 2021, 21, 853–868. [Google Scholar] [CrossRef]
- Shang, W.; Razavi, B.S.; Yao, S.; Hao, C.; Kuzyakov, Y.; Blagodatskaya, E.; Tian, J. Contrasting mechanisms of nutrient mobilization in rhizosphere hotspots driven by straw and biochar amendment. Soil Biol. Biochem. 2023, 187, 109212. [Google Scholar] [CrossRef]
- Akumuntu, A.; Hong, J.-K.; Jho, E.H.; Omidoyin, K.C.; Park, S.-J.; Zhang, Q.; Zhao, X. Biochar derived from rice husk: Impact on soil enzyme and microbial dynamics, lettuce growth, and toxicity. Chemosphere 2024, 349, 140868. [Google Scholar] [CrossRef]
- Liu, X.; Zhiming, Q.I.; Wang, Q.; Zhiwen, M.A.; Lanhai, L.I. Effects of biochar addition on CO2 and CH4 emissions from a cultivated sandy loam soil during freeze-thaw cycles. Plant Soil Environ. 2017, 63, 243–249. [Google Scholar] [CrossRef]
- Di, W.; Lan, Y.; Chen, W.; Liu, Z.; Gao, J.; Cao, D.; Wang, Q.; Mazhang, C.; An, X. Response of bacterial communities, enzyme activities and dynamic changes of soil organic nitrogen fractions to six-year different application levels of biochar retention in Northeast China. Soil Tillage Res. 2024, 240, 106097. [Google Scholar] [CrossRef]
- Yin, D.; Li, H.; Wang, H.; Guo, X.; Wang, Z.; Lv, Y.; Ding, G.; Jin, L.; Lan, Y. Impact of Different Biochars on Microbial Community Structure in the Rhizospheric Soil of Rice Grown in Albic Soil. Molecules 2021, 26, 4783. [Google Scholar] [CrossRef]
- Liao, J.; Hao, G.; Guo, H.; Chen, H. Effects of biochar on plant and microbial communities in landfill soil. Appl. Soil Ecol. 2024, 204, 105749. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.-L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann. Microbiol. 2022, 72, 35. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Yang, X.; Ran, Z.; Li, R.; Fang, L.; Zhou, J.; Guo, L. Effects of Biochar on the Growth, Ginsenoside Content, and Soil Microbial Community Composition of Panax quinquefolium L. J. Soil Sci. Plant Nutr. 2022, 22, 2670–2686. [Google Scholar] [CrossRef]
- Ren, H.; Guo, H.; Shafiqul Islam, M.; Zaki, H.E.M.; Wang, Z.; Wang, H.; Qi, X.; Guo, J.; Sun, L.; Wang, Q.; et al. Improvement effect of biochar on soil microbial community structure and metabolites of decline disease bayberry. Front. Microbiol. 2023, 14, 1154886. [Google Scholar] [CrossRef]
- Wang, X.; Riaz, M.; Babar, S.; Eldesouki, Z.; Liu, B.; Xia, H.; Li, Y.; Wang, J.; Xia, X.; Jiang, C. Alterations in the composition and metabolite profiles of the saline-alkali soil microbial community through biochar application. J. Environ. Manag. 2024, 352, 120033. [Google Scholar] [CrossRef]
- Amoakwah, E.; Arthur, E.; Frimpong, K.A.; Lorenz, N.; Rahman, M.A.; Nziguheba, G.; Islam, K.R. Biochar amendment impacts on microbial community structures and biological and enzyme activities in a weathered tropical sandy loam. Appl. Soil Ecol. 2022, 172, 104364. [Google Scholar] [CrossRef]
- Arthur, E.; Ahmed, F. Rice straw biochar affects water retention and air movement in a sand-textured tropical soil. Arch. Agron. Soil Sci. 2017, 63, 2035–2047. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, J.C.; Cale, J.A.; Cahill, J.F., Jr.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2021, 229, 1105–1117. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Shen, J.; Yuan, Q.; Fan, F.; Wei, W.; Li, Y.; Wu, J. Biochar alters soil microbial communities and potential functions 3–4 years after amendment in a double rice cropping system. Agric. Ecosyst. Environ. 2021, 311, 107291. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Yang, Z.; Xiao, L. Synergetic effects of biochars and denitrifier on nitrate removal. Bioresour. Technol. 2021, 335, 125245. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Messuti, M.I.; Reiner, G.; Boenel, M.; Vobis, G.; Wall, L.G.; Scervino, J.M. Exploring the response of Actinobacteria to the presence of phosphorus salts sources: Metabolic and co-metabolic processes. J. Basic Microbiol. 2019, 59, 487–495. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).