Influence of Vermicompost Tea on Metabolic Profile of Diplotaxis muralis: An NMR Spectroscopic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Preparation for NMR Metabolomics Analysis
2.3. NMR Measurements and Metabolite Identification
2.4. 1H NMR Data Processing and Multivariate Statistical Analysis
2.5. Chemicals
3. Results
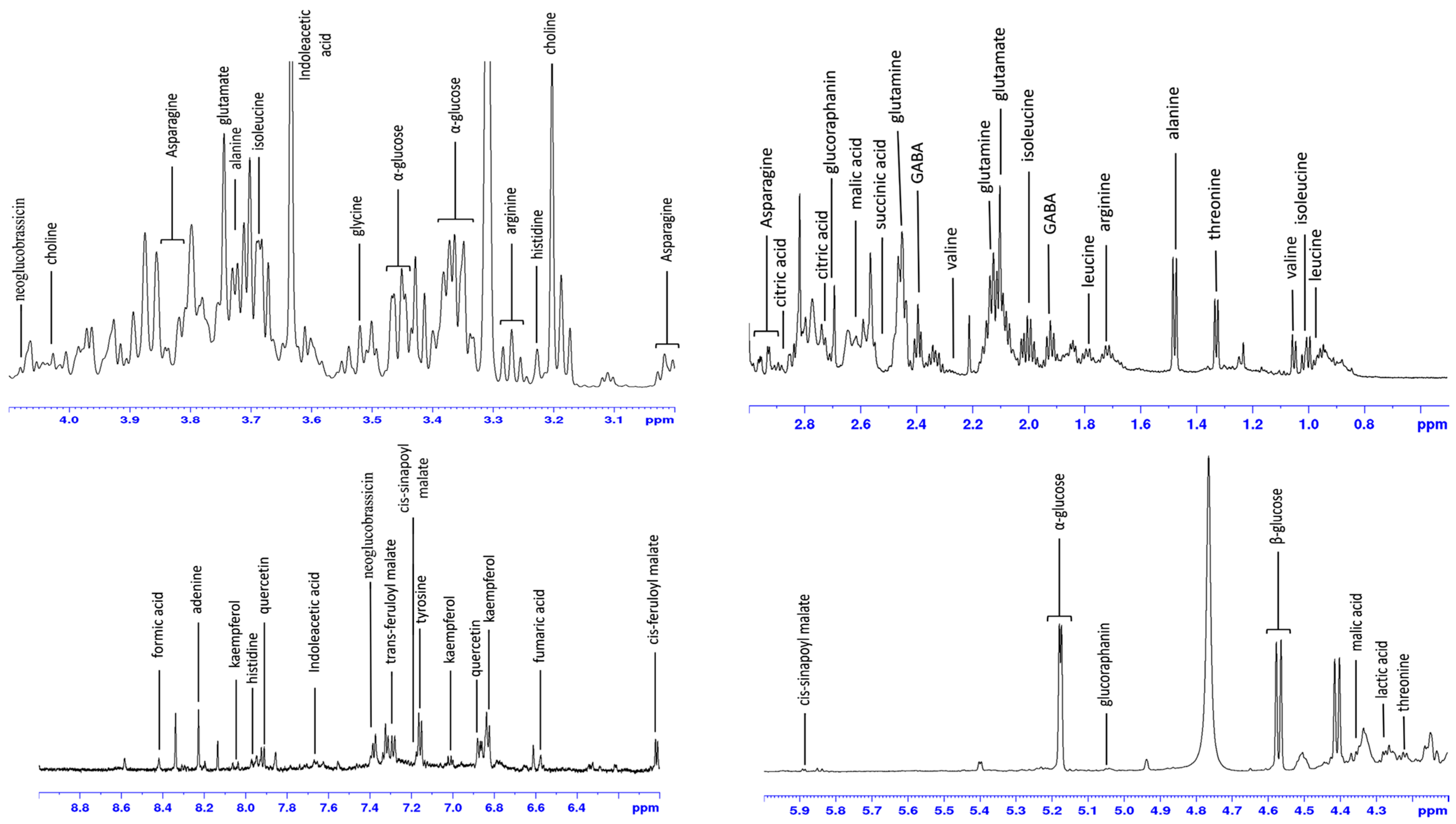
3.1. 1H-NMR Characterization of D. muralis Metabolic Extract
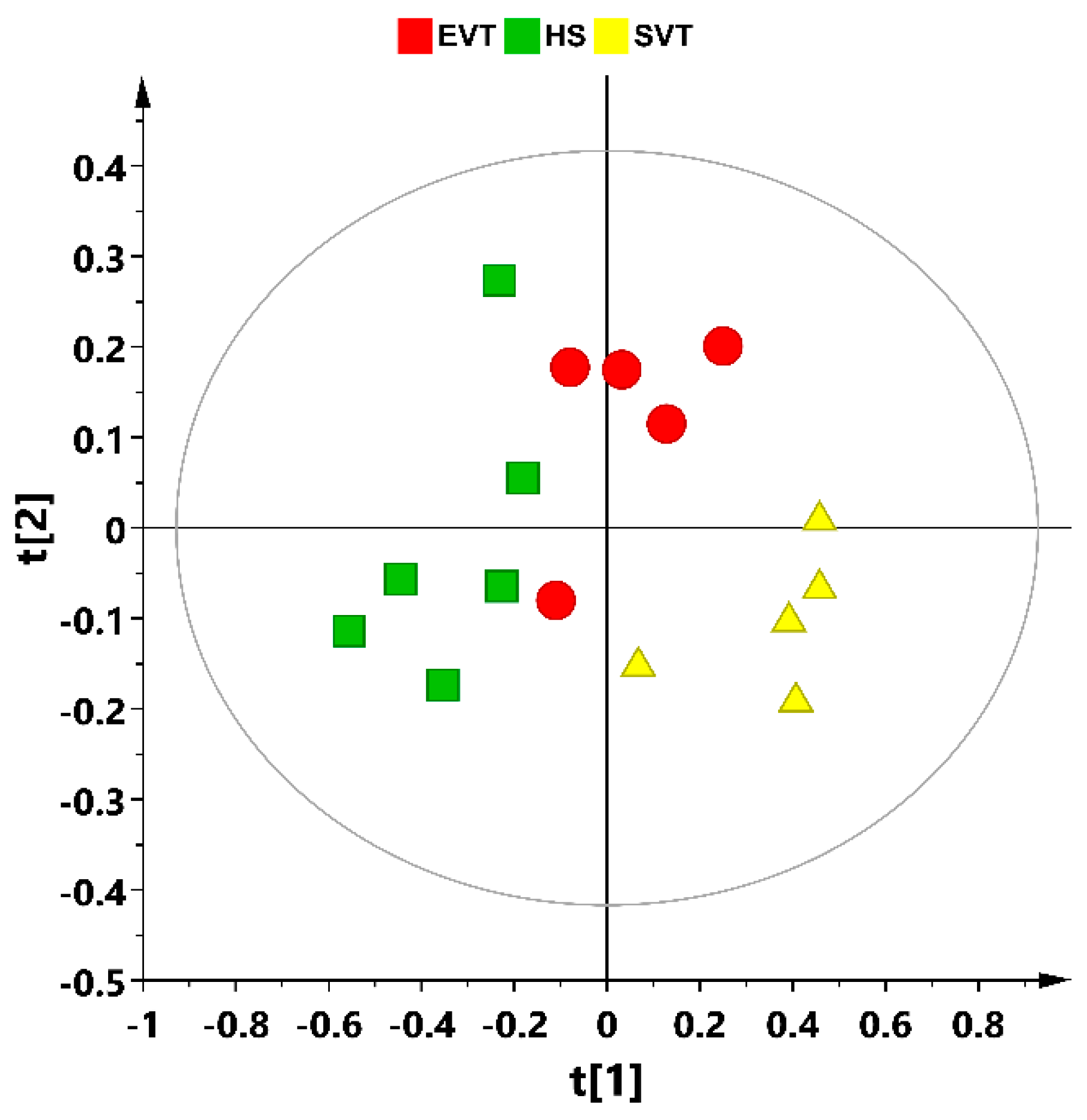
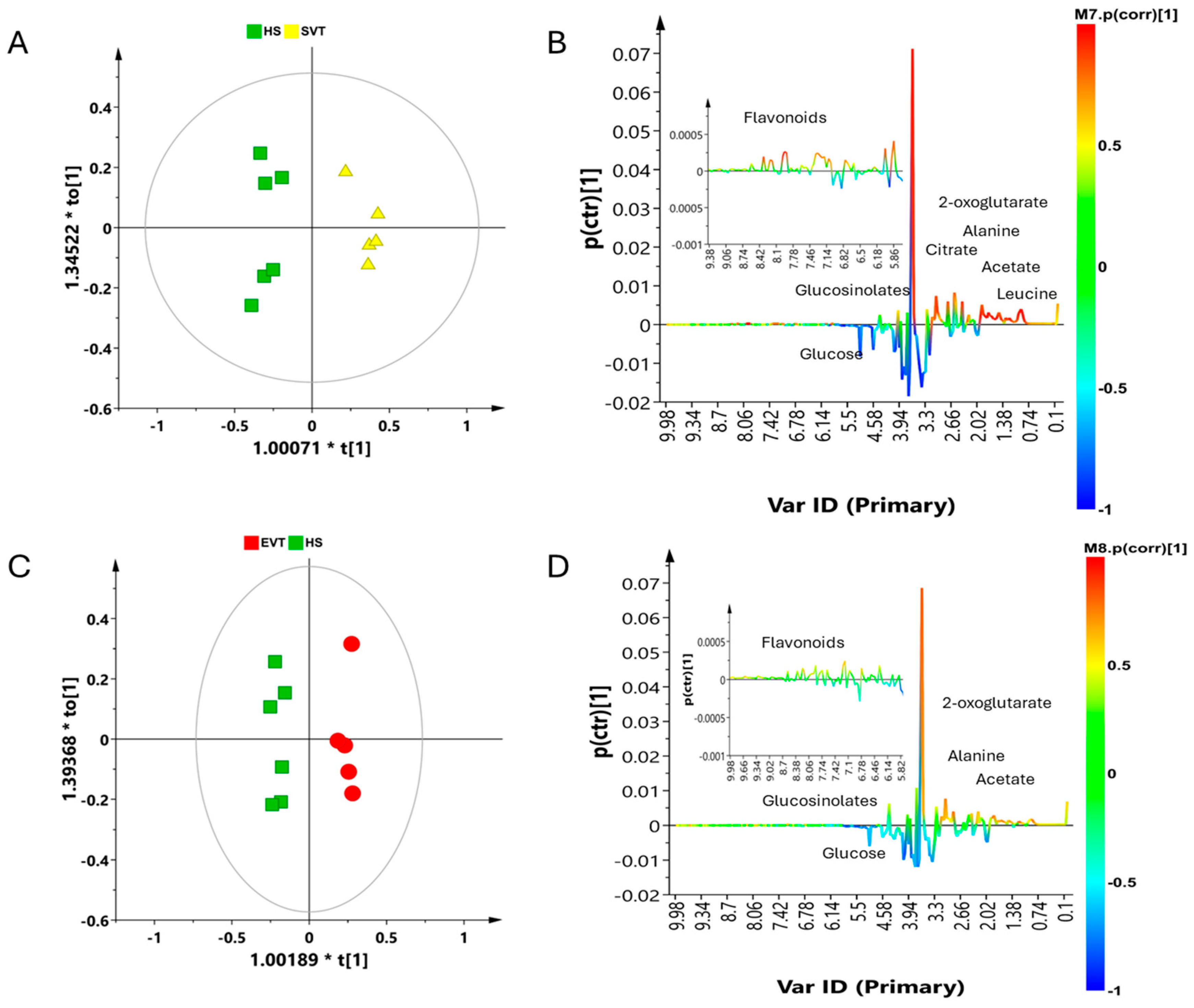
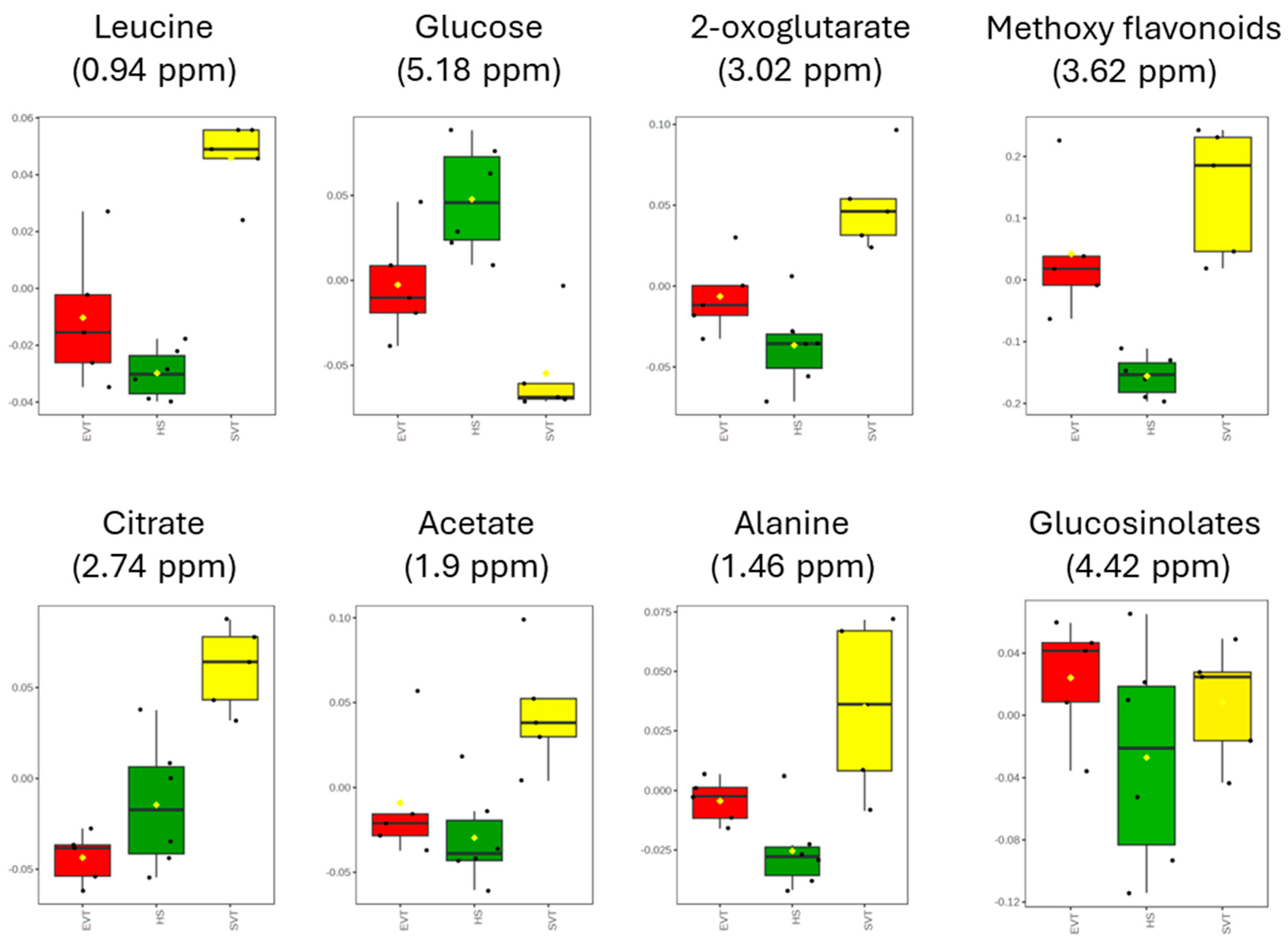
3.2. Multivariate Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMR | Nuclear magnetic resonance |
| EVT | Enhanced vermitea |
| SVT | Standard vermitea |
| HS | Hoagland solution |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PLS-DA | Partial least squares discriminant analysis |
References
- Tripodi, P.; Francese, G.; Mennella, G. Rocket salad: Crop description, bioactive compounds and breeding perspectives. Adv. Hortic. Sci. 2017, 31, 107–114. [Google Scholar]
- Pasini, F.; Verardo, V.; Cerretani, L.; Caboni, M.F.; D’Antuono, L.F. Rocket salad (Diplotaxis and Eruca spp.) sensory analysis and relation with glucosinolate and phenolic content. J. Sci. Food Agric. 2011, 91, 2858–2864. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Zhang, Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2004, 555, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bueno, R.; Rincón-Cervera, M.; González-Fernández, M.; Guil-Guerrero, J. Phytochemical composition and antitumor activities of new salad greens: Rucola (Diplotaxis tenuifolia) and corn salad (Valerianella locusta). Plant Foods Hum. Nutr. 2016, 71, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lu, C.; Hu, R.; Shi, W.; Zhou, L.; Wen, P.; Jiang, Y.; Lo, Y.M. Nutritional and microbiological effects of vermicompost tea in hydroponic cultivation of maple peas (Pisum sativum var. arvense L.). Food Sci. Nutr. 2023, 11, 3184–3202. [Google Scholar] [CrossRef]
- Bertoldi, F.; Sant’Anna, E.; Barcelos-Oliveira, J. Chlorella vulgaris cultivated in hydroponic wastewater. In Proceedings of the International Symposium on Soilless Culture and Hydroponics 843, Lima, Peru, 25–28 August 2008; pp. 203–210. [Google Scholar]
- Hajam, Y.A.; Kumar, R.; Kumar, A. Environmental waste management strategies and vermi transformation for sustainable development. Environ. Chall. 2023, 13, 100747. [Google Scholar] [CrossRef]
- Abdel Salam, M.M.; Roshdy, N.M. The influence of different applications of vermicompost tea on the quality of pomegranate fruits. SVU-Int. J. Agric. Sci. 2022, 4, 107–118. [Google Scholar] [CrossRef]
- Pearson, C.; Gawel, R.; Maki, D. Effects of vermicompost and beneficial microbes on biomass and nutrient density in purple lady bok choy (Brassica rapa var. chinensis) in a vertical hydroponic grow tower system. Fine Focus 2023, 9, 46–64. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Manning, W.J.; Paoletti, E.; Sandermann, H., Jr.; Ernst, D. Ethylenediurea (EDU): A research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ. Pollut. 2011, 159, 3283–3293. [Google Scholar] [CrossRef]
- Rehman, S.u.; De Castro, F.; Marini, P.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermibiochar: A novel approach for reducing the environmental impact of heavy metals contamination in agricultural land. Sustainability 2023, 15, 9380. [Google Scholar] [CrossRef]
- Rehman, S.u.; Aprile, A.; De Castro, F.; Negro, C.; Migoni, D.; Benedetti, M.; Sabella, E.; Fanizzi, F.P. Assessing the Effectiveness of Vermi-Liquids as a Sustainable Alternative to Inorganic Nutrient Solutions in Hydroponic Agriculture: A Study on Diplotaxis muralis. Agronomy 2024, 14, 1310. [Google Scholar] [CrossRef]
- Kumar, D. Nuclear magnetic resonance (NMR) spectroscopy for metabolic profiling of medicinal plants and their products. Crit. Rev. Anal. Chem. 2016, 46, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Schiattone, M.I.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Di Venere, D.; Perniola, M.; Sergio, L.; Todorovic, M.; Candido, V. Effect of water regime, nitrogen level and biostimulants application on yield and quality traits of wild rocket [Diplotaxis tenuifolia (L.) DC.]. Agric. Water Manag. 2023, 277, 108078. [Google Scholar] [CrossRef]
- Paradiso, R.; Di Mola, I.; Cozzolino, E.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Nutrient and nutraceutical quality of rocket as a function of greenhouse cover film, nitrogen dose and biostimulant application. Agronomy 2023, 13, 638. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Influence of the Culture System and Harvest Time on the Specialized Metabolite Composition of Rocket Salad (Eruca sativa) Leaves. Horticulturae 2023, 9, 235. [Google Scholar] [CrossRef]
- Burdziej, A.; Da Costa, G.; Gougeon, L.; Le Mao, I.; Bellée, A.; Corio-Costet, M.-F.; Mérillon, J.-M.; Richard, T.; Szakiel, A.; Cluzet, S. Impact of different elicitors on grapevine leaf metabolism monitored by 1H NMR spectroscopy. Metabolomics 2019, 15, 67. [Google Scholar] [CrossRef]
- Colì, C.S.; Girelli, C.R.; Cesari, G.; Hussain, M.; Fanizzi, F.P. Biodynamic, organic and integrated agriculture effects on cv. Italia table grapes juice, over a 3-year period experiment: An 1H NMR spectroscopy-based metabolomics study. Chem. Biol. Technol. Agric. 2024, 11, 35. [Google Scholar] [CrossRef]
- Lanzotti, V.; Anzano, A.; Grauso, L.; Zotti, M.; Sacco, A.; Senatore, M.; Moreno, M.; Diano, M.; Parente, M.; Esposito, S. NMR metabolomics and chemometrics of lettuce, Lactuca sativa L., under different foliar organic fertilization treatments. Plants 2022, 11, 2164. [Google Scholar] [CrossRef]
- De Castro, F.; Benedetti, M.; Del Coco, L.; Fanizzi, F.P. NMR-based metabolomics in metal-based drug research. Molecules 2019, 24, 2240. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- De Castro, F.; Stefano, E.; De Luca, E.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. A NMR-Based Metabolomic Approach to Investigate the Antitumor Effects of the Novel [Pt (η1-C2H4OMe)(DMSO)(phen)]+ (phen = 1,10-Phenanthroline) Compound on Neuroblastoma Cancer Cells. Bioinorg. Chem. Appl. 2022, 2022, 8932137. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Metabolic characterization of Brassica rapa leaves by NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 7936–7943. [Google Scholar] [CrossRef]
- Abdel-Farid, I.; Jahangir, M.; Van Den Hondel, C.; Kim, H.; Choi, Y.; Verpoorte, R. Fungal infection-induced metabolites in Brassica rapa. Plant Sci. 2009, 176, 608–615. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Metabolomic response of Brassica rapa submitted to pre-harvest bacterial contamination. Food Chem. 2008, 107, 362–368. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Choi, Y.H.; Kim, H.K.; Linthorst, H.J.; Verpoorte, R. Metabolomic analysis of methyl jasmonate treated Brassica rapa leaves by 2-dimensional NMR spectroscopy. Phytochemistry 2006, 67, 2503–2511. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Veskovski, L.; Andersson, P.-O.; Turesson, I.; Malmodin, D.; Pedersen, A.; Mellqvist, U.-H. Serum Metabolomic Profiling Correlated with ISS and Clinical Outcome for Multiple Myeloma Patients Treated with High Dose Melphalan and Autologous Stem Cell Transplantation. Blood 2019, 134, 1771. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Llorach, R.; Gil, M.I.; Ferreres, F. Identification of new flavonoid glycosides and flavonoid profiles to characterize rocket leafy salads (Eruca vesicaria and Diplotaxis tenuifolia). J. Agric. Food Chem. 2007, 55, 1356–1363. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef]
- Lim, J.-E.; Cho, M.-J.; Yun, H.-J.; Ha, S.-K.; Lee, D.-B.; Sung, J.-K. The relation between fertilization practices and functional metabolites of crops: A review. Korean J. Soil Sci. Fertil. 2016, 49, 168–180. [Google Scholar] [CrossRef][Green Version]
- Radić, S.; Vujčić, V.; Glogoški, M.; Radić-Stojković, M. Influence of pH and plant growth regulators on secondary metabolite production and antioxidant activity of Stevia rebaudiana (Bert). Period. Biol. 2016, 118, 9–19. [Google Scholar] [CrossRef]
- Manzano-Gómez, L.A.; Guzmán-Albores, J.M.; Rincón-Rosales, R.; Winkler, R.; Rincón-Molina, C.I.; Castañón-González, J.H.; Ruiz-Lau, N.; Gutiérrez-Miceli, F.A.; Rincón-Molina, F.A.; Ruíz-Valdiviezo, V.M. Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types. Agronomy 2021, 11, 2061. [Google Scholar] [CrossRef]
- Kosem, H.; Kocak, M.Z.; Kaysim, M.G.; Celikcan, F.; Kulak, M. Liquid leachate produced from vermicompost effects on some agronomic attributes and secondary metabolites of sweet basil (Ocimum basilicum L.) exposed to severe water stress conditions. Horticulturae 2022, 8, 1190. [Google Scholar] [CrossRef]
- Wong, W.S.; Zhong, H.T.; Cross, A.T.; Yong, J.W.H. Plant biostimulants in vermicomposts: Characteristics and plausible mechanisms. In The Chemical Biology of Plant Biostimulants; Wiley: Hoboken, NJ, USA, 2020; pp. 155–180. [Google Scholar]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef]
- Türkay, I.; Öztürk, L. The form, dose, and method of application of vermicompost differentiate the phenylpropene biosynthesis in the peltate glandular trichomes of methylchavicol chemotype of Ocimum basilicum L. Ind. Crops Prod. 2023, 198, 116688. [Google Scholar] [CrossRef]
- Latifi, M.; Kheiry, A.; Razavi, F.; Sanikhani, M. Influence of vermicompost and nitrogen treatments on the enhancement of morphophysiological traits and essential oil composition of spearmint (Mentha spicata L.) for double harvesting seasons. J. Med. Plants By-Prod. 2024, 13, 1016–1025. [Google Scholar]
- Souffront, D.K.S.; Salazar-Amoretti, D.; Jayachandran, K. Influence of vermicompost tea on secondary metabolite production in tomato crop. Sci. Hortic. 2022, 301, 111135. [Google Scholar] [CrossRef]
- Moffett, J.R.; Puthillathu, N.; Vengilote, R.; Jaworski, D.M.; Namboodiri, A.M. Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—Part 1: Acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases. Front. Physiol. 2020, 11, 580167. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Lin, J.; Feng, B.; Zhu, A.; Zhao, X.; Wang, D.; Zeng, Y.; Yang, H.; Wang, S.; et al. Acetate prevents pistil dysfunction in rice under heat stress by inducing methyl jasmonate and quercetin synthesis. J. Adv. Res. 2025; in press. [Google Scholar]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Van Thang, D.; Sohng, J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzym. Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, R.; Gao, S.; Yue, M.; Zhang, X.; Zeng, W.; Tang, B.; Zhou, J.; Huang, D.; Xu, S. Identification of two new flavone 4′-O-methyltransferases and their application in de novo biosynthesis of (2S)-hesperetin in Yarrowia lipolytica. Synth. Syst. Biotechnol. 2025, 10, 728–736. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Baldelli, S.; Lombardo, M.; D’Amato, A.; Karav, S.; Tripodi, G.; Aiello, G. Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods 2025, 14, 912. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Elementi, S.; Neri, R. Glucosinolates in Diplotaxis and Eruca leaves: Diversity, taxonomic relations and applied aspects. Phytochemistry 2008, 69, 187–199. [Google Scholar] [CrossRef]
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Magan, J.; Zhang, Y.; Rowland, I.R.; Wagstaff, C. Analysis of phytochemical composition and chemoprotective capacity of rocket (Eruca sativa and Diplotaxis tenuifolia) leafy salad following cultivation in different environments. J. Agric. Food Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef]
- Ali, A.; Franzoni, G.; Petrini, A.; Santoro, P.; Mori, J.; Ferrante, A.; Cocetta, G. Investigating physiological responses of Wild Rocket subjected to artificial Ultraviolet B irradiation. Sci. Hortic. 2023, 322, 112415. [Google Scholar] [CrossRef]
- Neugart, S.; Krumbein, A.; Zrenner, R. Influence of light and temperature on gene expression leading to accumulation of specific flavonol glycosides and hydroxycinnamic acid derivatives in kale (Brassica oleracea var. sabellica). Front. Plant Sci. 2016, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Egamberdieva, D.; Bhardwaj, R.; Ashraf, M. Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J. Plant Interact. 2017, 12, 429–437. [Google Scholar]
- Mamat, D.D.; Chong, C.-S.; Abd Samad, A.; Chai, T.-T.; Abd Manan, F. Effects of copper on total phenolics, flavonoids and mitochondrial properties of Orthosiphon stamineus callus culture. Int. J. Agric. Biol. 2015, 17, 1243–1248. [Google Scholar] [CrossRef]
- Son, Y.-J.; Park, J.-E.; Lee, N.; Ju, Y.-W.; Pyo, S.-H.; Oh, C.; Yoo, G.; Nho, C.W. Copper-or Zinc-Fortified Nutrient Solution in Vertical Farming System Enriches Copper or Zinc and Elevates Phenolic Acid and Flavonoid Contents in Artemisia annua L. Agronomy 2024, 14, 135. Agronomy 2024, 14, 135. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Jin, X.; Liu, N.; Fan, X.; Dong, C.; Shen, Q.; Xu, Y. Potassium enhances the sugar assimilation in leaves and fruit by regulating the expression of key genes involved in sugar metabolism of Asian pears. Plant Growth Regul. 2017, 83, 287–300. [Google Scholar] [CrossRef]
- Yan, J.; Ye, X.; Song, Y.; Ren, T.; Wang, C.; Li, X.; Cong, R.; Lu, Z.; Lu, J. Sufficient potassium improves inorganic phosphate-limited photosynthesis in Brassica napus by enhancing metabolic phosphorus fractions and Rubisco activity. Plant J. 2023, 113, 416–429. [Google Scholar] [CrossRef]
- Nabil-Adam, A.; Elnosary, M.E.; Ashour, M.L.; Abd El-Moneam, N.M.; Shreadah, M.A. Flavonoids biosynthesis in plants as a defense mechanism: Role and function concerning pharmacodynamics and pharmacokinetic properties. In Flavonoid Metabolism-Recent Advances and Applications in Crop Breeding; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Jogawat, A. Osmolytes and their role in abiotic stress tolerance in plants. In Molecular Plant Abiotic Stress: Biology and Biotechnology; Wiley: Hoboken, NJ, USA, 2019; pp. 91–104. [Google Scholar]
- Kocaman, A.; İnci, Y.; Kıtır, N.; Turan, M.; Argın, S.; Yıldırım, E.; Giray, G.; Ersoy, N.; Güneş, A.; Katırcıoğlu, H.; et al. The effect of novel biotechnological vermicompost on tea yield, plant nutrient content, antioxidants, amino acids, and organic acids as an alternative to chemical fertilizers for sustainability. BMC Plant Biol. 2024, 24, 868. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, Q.; Wang, X.; Wei, M.; Hu, J.; Liu, J.; Yang, F. Influence of cow manure vermicompost on the growth, metabolite contents, and antioxidant activities of Chinese cabbage (Brassica campestris ssp. chinensis). Biol. Fertil. Soils 2010, 46, 689–696. [Google Scholar] [CrossRef]
- Turan, M.; Kocaman, A.; Tüfenkçi, Ş.; Katırcıoğlu, H.; Güneş, A.; Kıtır, N.; Giray, G.; Gürkan, B.; Ersoy, N.; Yildirim, E. Development of organic phosphorus vermicompost from raw phosphate rock using microorganisms and enzymes and its effect on tomato yield. Sci. Hortic. 2023, 321, 112323. [Google Scholar] [CrossRef]
- Abbey, L.; Annan, N.; Asiedu, S.K.; Esan, E.O.; Iheshiulo, E.M.-A. Amino acids, mineral nutrients, and efficacy of vermicompost and seafood and municipal solid wastes composts. Int. J. Agron. 2018, 2018, 6419467. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.u.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Influence of Vermicompost Tea on Metabolic Profile of Diplotaxis muralis: An NMR Spectroscopic Analysis. Environments 2025, 12, 366. https://doi.org/10.3390/environments12100366
Rehman Su, De Castro F, Aprile A, Benedetti M, Fanizzi FP. Influence of Vermicompost Tea on Metabolic Profile of Diplotaxis muralis: An NMR Spectroscopic Analysis. Environments. 2025; 12(10):366. https://doi.org/10.3390/environments12100366
Chicago/Turabian StyleRehman, Sami ur, Federica De Castro, Alessio Aprile, Michele Benedetti, and Francesco Paolo Fanizzi. 2025. "Influence of Vermicompost Tea on Metabolic Profile of Diplotaxis muralis: An NMR Spectroscopic Analysis" Environments 12, no. 10: 366. https://doi.org/10.3390/environments12100366
APA StyleRehman, S. u., De Castro, F., Aprile, A., Benedetti, M., & Fanizzi, F. P. (2025). Influence of Vermicompost Tea on Metabolic Profile of Diplotaxis muralis: An NMR Spectroscopic Analysis. Environments, 12(10), 366. https://doi.org/10.3390/environments12100366










