Comparison of Uranium Leachability Between Three Groundwater Aquifers in Relation to the Degree of Bedrock Weathering: A Petro-Mineralogical and Experimental Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Geology of the Study Area and Sampling Methods
2.2. Analytical Methods
2.2.1. Sample Preparation
2.2.2. Mineralogical Analyses
2.2.3. Geochemical Analyses
2.2.4. Groundwater Analysis
2.3. Weathering Experiments
2.4. Quality Assurance and Quality Control
3. Results and Discussion
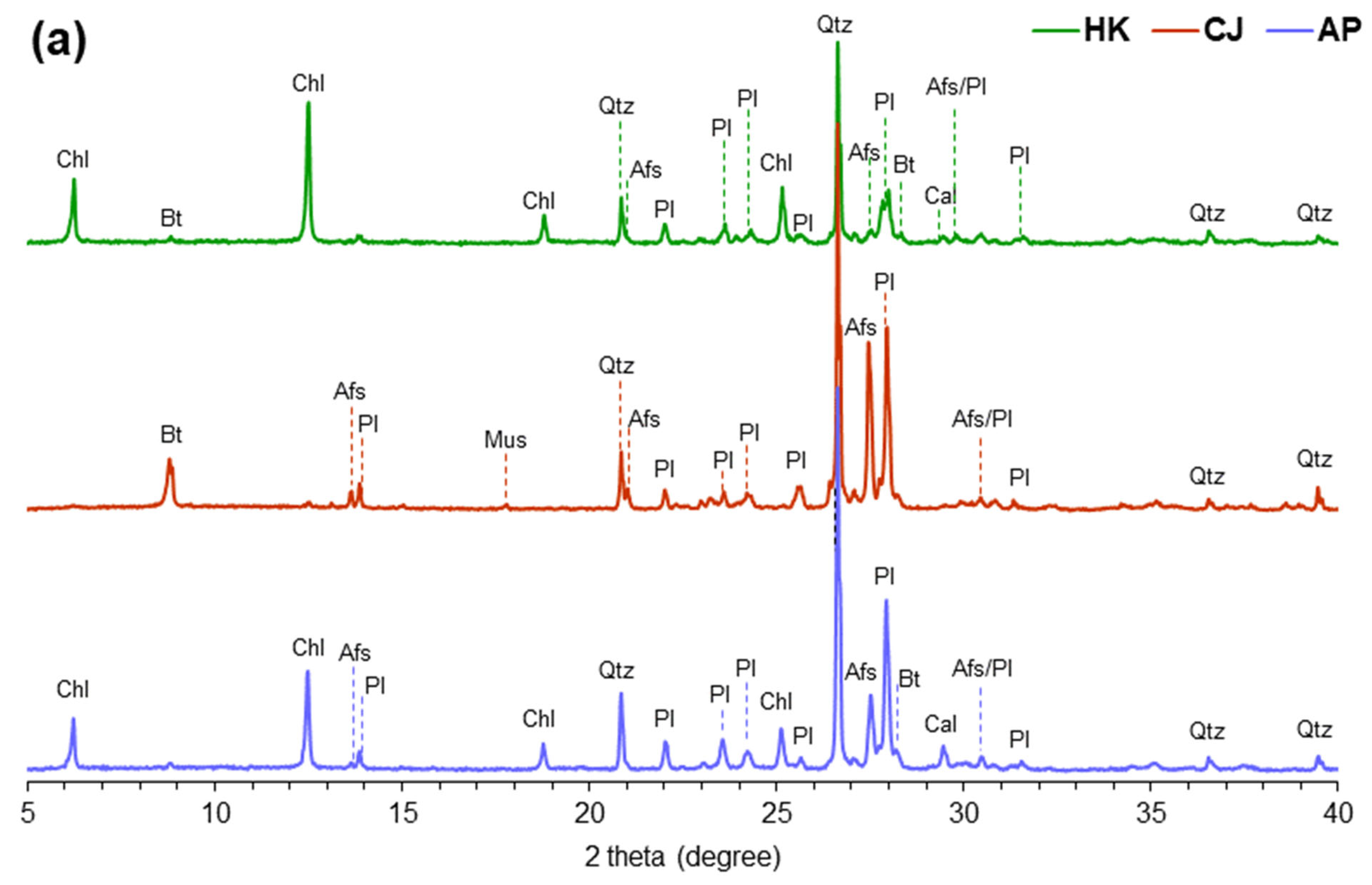
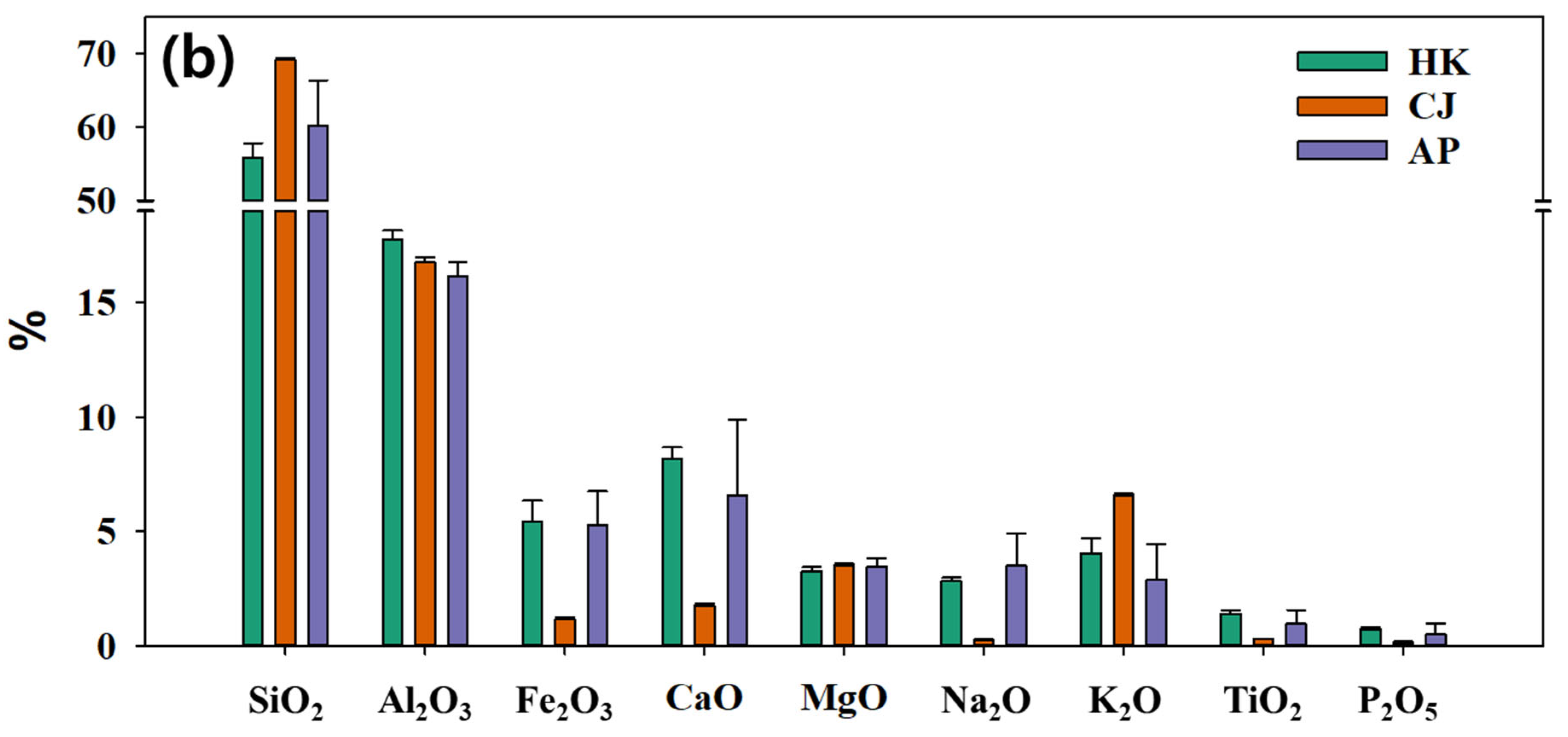
3.1. Petro-Mineralogical Features
3.2. Characteristics of Radioactive Minerals
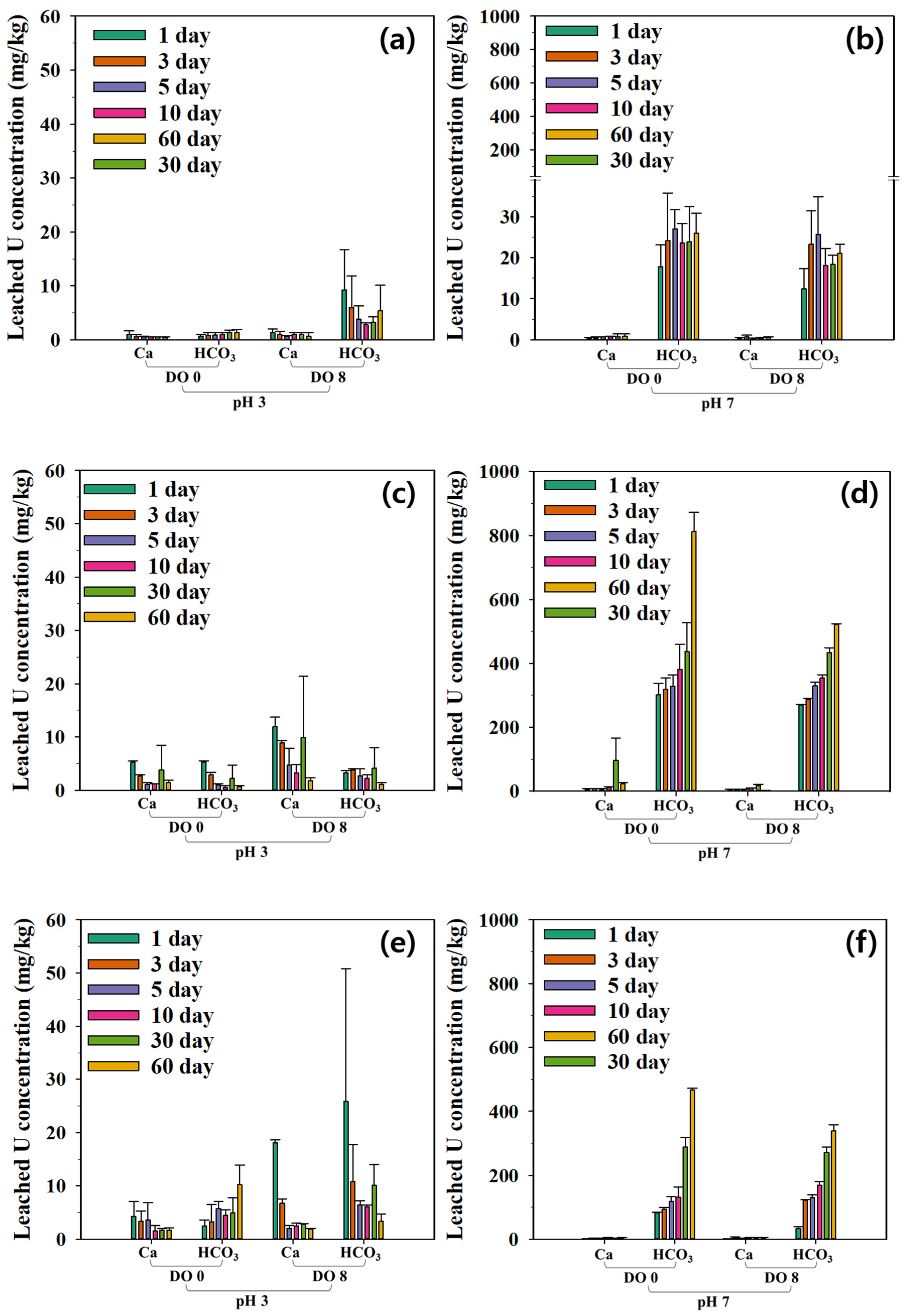
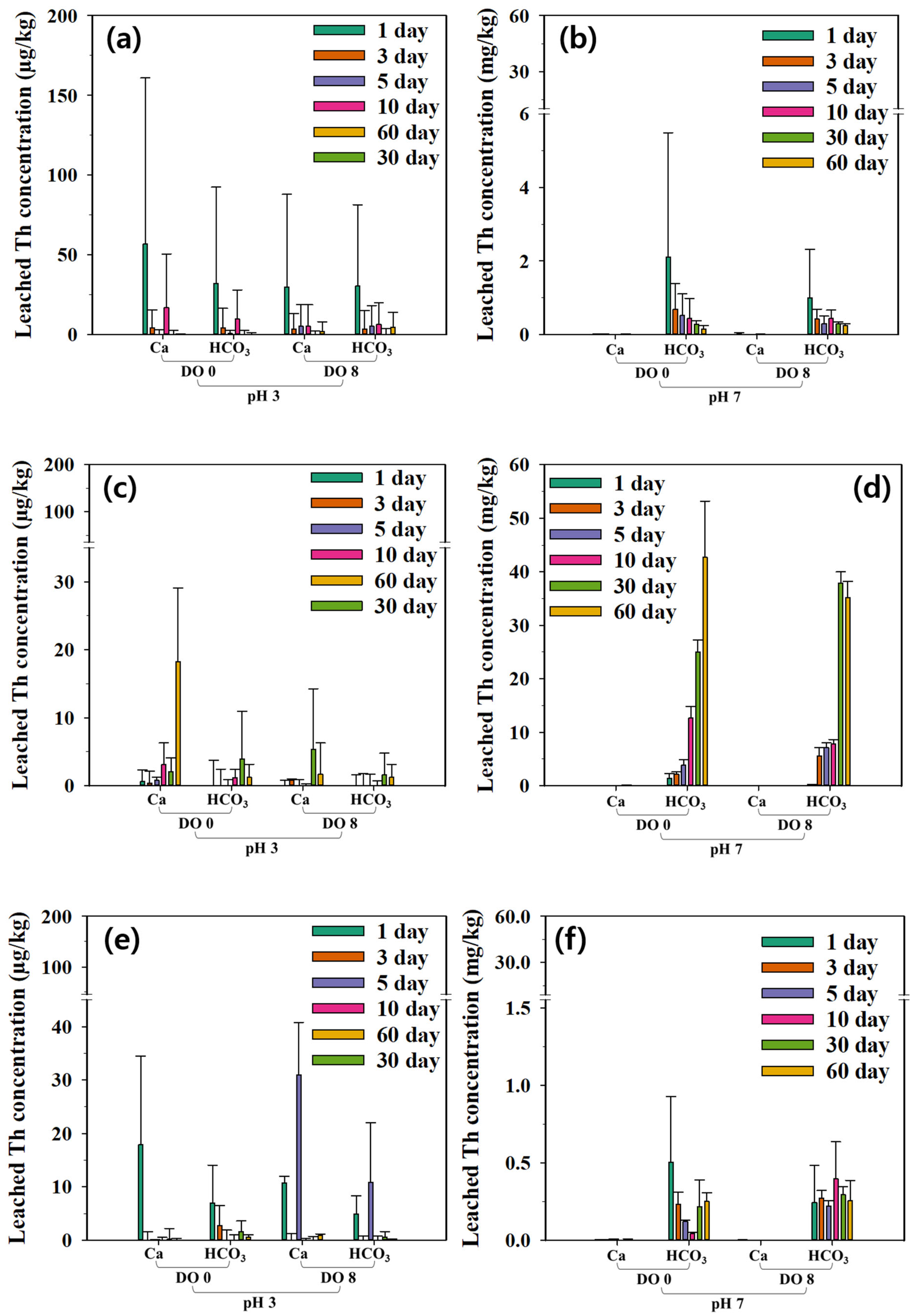
3.3. Leachability of Radioactive Minerals
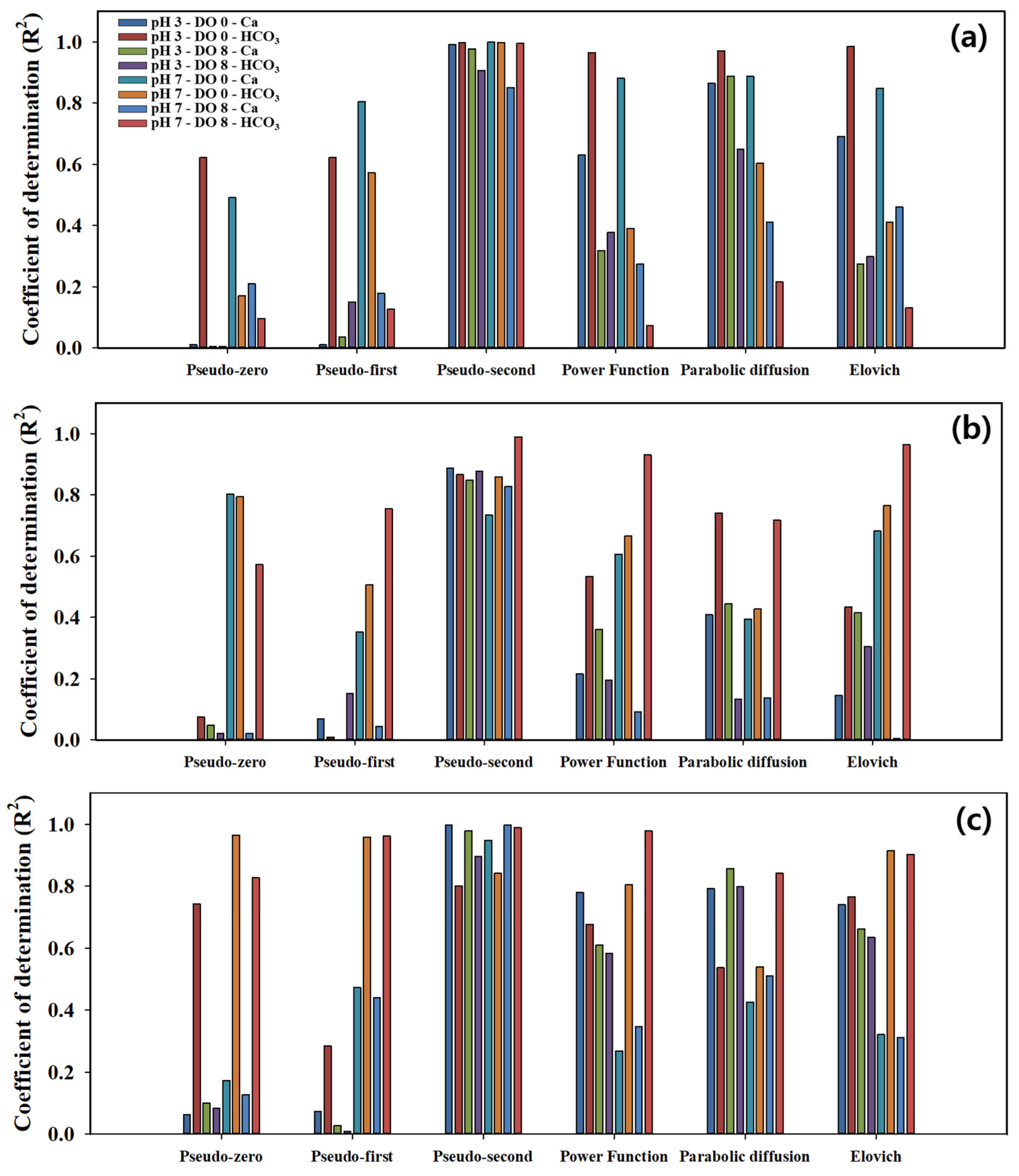
3.4. Regression Analysis of Kinetic Model Results
3.5. Implications for Groundwater Management
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.; Moon, S.-H. Geochemistry of U and Th of Mesozoic granites in South Korea: Implications of occurrences of different U-host minerals and dissolved U and Rn between Jurassic and Cretaceous granite aquifers. Geosci. J. 2021, 25, 183–195. [Google Scholar] [CrossRef]
- Lee, W.-C.; Lee, S.-W.; Jeon, J.H.; Lee, J.H.; Jeong, D.-H.; Kim, M.-S.; Kim, H.-K.; Kim, S.-O. Uranium Concentrations in Private Wells of Potable Groundwater, Korea. Toxics 2022, 10, 543. [Google Scholar] [CrossRef]
- Hashemi, S.; Shin, I.; Kim, S.-O.; Lee, W.-C.; Lee, S.-W.; Jeong, D.H.; Kim, M.; Kim, H.-K.; Yang, J. Health risk assessment of uranium intake from private residential drinking groundwater facilities based on geological characteristics across the Republic of Korea. Sci. Total Environ. 2024, 913, 169252. [Google Scholar] [CrossRef]
- Research, N.I.o.E. Naturally Occurring Radioactive Materials (N.O.R.M.) in Private Groundwater Wells; National Institute of Envrionmental Research: Incheon. Republic of Korea, 2024; 270p. [Google Scholar]
- Hwang, J. Geological review on the distribution and source of uraniferous groundwater in South Korea. J. Eng. Geol. 2018, 28, 593–603. [Google Scholar] [CrossRef]
- Hwang, J. Occurrence of U-minerals and Source of U in Groundwater in Daebo Granite, Daejeon Area. J. Eng. Geol. 2013, 23, 399–407. [Google Scholar] [CrossRef]
- Jeong, C.H.; Yang, J.H.; Lee, Y.J.; Lee, Y.C.; Choi, H.Y.; Kim, M.S.; Kim, H.K.; Kim, T.S.; Jo, B.U. Occurrences of Uranium and Radon-222 from Groundwaters in Various Geological Environment in the Hoengseong Area. J. Eng. Geol. 2015, 25, 557–576. [Google Scholar] [CrossRef]
- Papageorgiou, F.; McDermott, F.; Van Acken, D. Uranium in groundwaters: Insights from the Leinster granite, SE Ireland. Appl. Geochem. 2022, 139, 105236. [Google Scholar] [CrossRef]
- Kim, M.S.; Yang, J.H.; Jeong, C.H.; Kim, H.K.; Kim, D.W.; Jo, B.U. Geochemical Origins and Occurrences of Natural Radioactive Materials in Borehole Groundwater in the Goesan Area. J. Eng. Geol. 2014, 24, 535–550. [Google Scholar] [CrossRef]
- Moon, S.H.; Hwang, J.; Lee, J.Y.; Hyun, S.P.; Bae, B.K.; Park, Y. Establishing the Origin of Elevated Uranium Concentrations in Groundwater near the Central Ogcheon Metamorphic Belt, Korea. J. Environ. Qual. 2013, 42, 118–128. [Google Scholar] [CrossRef]
- Hwang, J.; Moon, S.-H.; Ripley, E.M.; Kim, Y.H. Determining uraniferous host rocks and minerals as a source of dissolved uranium in granite aquifers near the central Ogcheon metamorphic belt, Korea. Environ. Earth Sci. 2014, 72, 4035–4046. [Google Scholar] [CrossRef]
- Kim, H.-G.; Lee, S.-W.; Kim, S.-O.; Jeong, D.-H.; Kim, M.-S.; Kim, H.-K.; Jeong, J.O. The Origin of Radioactive Elements Found in Groundwater Within the Chiaksan Gneiss Complex: Focusing on the Relationship with Minerals of the Surrounding Geology. Korean J. Mineral. Petrol. 2022, 35, 153–168. [Google Scholar] [CrossRef]
- Vesković, J.; Onjia, A. Influencing factors of groundwater 238U, 232Th, 40K, and rare earth element contamination: Insights from the two-dimensional Monte Carlo simulation of radiological risks. Mar. Pollut. Bull. 2025, 213, 117682. [Google Scholar] [CrossRef]
- Jwa, Y.-J. Possible source rocks of Mesozoic granites in South Korea: Implications for crustal evolution in NE Asia. Earth Environ. Sci. Trans. R. Soc. Edinb. 2004, 95, 181–198. [Google Scholar] [CrossRef]
- Lee, S.R.; Cho, K. Precambrian Crustal Evolution of the Korean Peninsula. J. Petrol. Soc. Korea 2012, 21, 89–112. [Google Scholar] [CrossRef]
- Kim, S.W.; Koh, H.J.; Kim, J. Geochronological study for the gneiss complex in the Wonju-Anheung-Pyeongchang area, central part of the Korean Peninsula. J. Geol. Soc. Korea 2014, 50, 327–342. [Google Scholar] [CrossRef]
- Balaram, V.; Subramanyam, K.S.V. Sample preparation for geochemical analysis: Strategies and significance. Adv. Sample Prep. 2022, 1, 100010. [Google Scholar] [CrossRef]
- APHA. Standard Methods For The Examination Of Water And Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2012. [Google Scholar]
- Mühr-Ebert, E.L.; Wagner, F.; Walther, C. Speciation of uranium: Compilation of a thermodynamic database and its experimental evaluation using different analytical techniques. Appl. Geochem. 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Leal Filho, W.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; del Carmen Ángeles González Chávez, M.; Carrillo-González, R.; Solís-Domínguez, F.A.; del Carmen Cuevas-Díaz, M.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 950. [Google Scholar] [CrossRef]
- Neupane, A.; Herndon, E.M.; Whitman, T.; Faiia, A.M.; Jagadamma, S. Manganese effects on plant residue decomposition and carbon distribution in soil fractions depend on soil nitrogen availability. Soil Biol. Biochem. 2023, 178, 108964. [Google Scholar] [CrossRef]
- Sparks, D.L. 7—Kinetics of Soil Chemical Processes. In Environmental Soil Chemistry, 2nd ed.; Sparks, D.L., Ed.; Academic Press: Burlington, ON, Canada, 2003; pp. 207–244. [Google Scholar]
- Almaroai, Y.A.; Usman, A.R.A.; Mahtab, A.; Kwon-Rae, K.; Meththika, V.; Sik Ok, Y. Role of chelating agents on release kinetics of metals and their uptake by maize from chromated copper arsenate-contaminated soil. Environ. Technol. 2013, 34, 747–755. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Mahdy, A.M.; ElManeah, M.M. Effects of drinking water treatment residuals on nickel retention in soils: A macroscopic and thermodynamic study. J. Soils Sediments 2013, 13, 94–105. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kihm, Y.H. Geological Report of the Jipori Sheet (Scale: 1:50,000); Korea Institute of Geoscience and Mineral Resources: Daejeon, Republic of Korea, 2007; pp. 1–67. [Google Scholar]
- Koh, H.J.; Kim, S.W.; Lee, S.R. Geological Report of the Anheungri Sheet Scale 1:50,000; Korea Institute of Geoscience and Mineral Resources: Daejeon, Republic of Korea, 2011. [Google Scholar]
- Finch, R.; Murakami, T. Systematics and Paragenesis of Uranium Minerals. In Uranium; Peter, C.B., Robert, J.F., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 1999; pp. 91–180. [Google Scholar]
- Burns, P.C.; Ewing, R.C.; Hawthorne, F.C. The crystal chemistry of hexavalent uranium; polyhedron geometries, bond-valence parameters, and polymerization of polyhedra. Can. Mineral. 1997, 35, 1551–1570. [Google Scholar]
- Piilonen, P.C.; Rowe, R.; Poirier, G.; Grice, J.D.; McDonald, A.M. Discreditation of thorogummite. Can. Mineral. 2014, 52, 769–774. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Kowacz, M.; Putnis, C.V.; Putnis, A. The role of background electrolytes on the kinetics and mechanism of calcite dissolution. Geochim. Cosmochim. Acta 2010, 74, 1256–1267. [Google Scholar] [CrossRef]
- Kopylova, Y.; Guseva, N.; Shestakova, A.; Khvaschevskaya, A.; Arakchaa, K. Uranium and thorium behavior in groundwater of the natural spa area “Choygan mineral water” (East Tuva). IOP Conf. Ser. Earth Environ. Sci. 2015, 27, 012034. [Google Scholar] [CrossRef]
- Gilbert, B.; Carrero, S.; Dong, W.; Joe-Wong, C.; Arora, B.; Fox, P.; Nico, P.; Williams, K.H. River thorium concentrations can record bedrock fracture processes including some triggered by distant seismic events. Nat. Commun. 2023, 14, 2395. [Google Scholar] [CrossRef]
- Abrão, A.; de Freitas, A.A.; de Carvalho, F.M.S. Preparation of highly pure thorium nitrate via thorium sulfate and thorium peroxide. J. Alloys Compd. 2001, 323–324, 53–56. [Google Scholar] [CrossRef]
- Krupka, K.; Serne, R. Geochemical Factors Affecting the Behavior of Antimony, Cobalt, Europium, Technetium, and Uranium in Vadose Sediments; Pacific Northwest National Laboratory: Richland, WA, USA, 2002. [Google Scholar] [CrossRef]
- Lee, W.-C.; Kim, S.-H.; Lee, B.-T.; Lee, S.-H.; Kim, K.-W.; Shim, Y.-S.; Park, H.-S.; Kim, S.-O. The Hydrogeochemical Study on the Passive Treatment System of the Dalseong Mine. J. Korean Soc. Miner. Energy Resour. Eng. 2013, 50, 56–69. [Google Scholar] [CrossRef]
- Aharoni, C.; Ungarish, M. Kinetics of activated chemisorption. Part 2.—Theoretical models. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1977, 73, 456–464. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford Science Publications: Oxford, UK, 1975. [Google Scholar]
- García, A.C.; Latifi, M.; Amini, A.; Chaouki, J. Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals. Metals 2020, 10, 1524. [Google Scholar] [CrossRef]
- Biedunkova, O.; Kuznietsov, P. Integration of water management in the assessment of the impact of heavy metals discharge from the power plant with mitigation strategies. Ecol. Indic. 2025, 175, 113618. [Google Scholar] [CrossRef]






| Parameters | Aquifer | ||
|---|---|---|---|
| Hoengseong-Kangrim (HK) | Cheorwon-Jadeung (CJ) | Asan-Punggi (AP) | |
| Uranium (µg/L) | 414.4 | 12.0 | 174.7 |
| Temperature (℃) | 13.3 | 12.0 | 16.3 |
| pH | 7.9 | 5.6 | 7.9 |
| Eh (mV) | 49 | 182 | 113 |
| EC (μS/cm) | 129 | 263 | 313 |
| DO (mg/L) | 0.7 | 0.5 | 0.4 |
| Si | 7.5 | 11.8 | 9.3 |
| Na | 8.0 | 14.3 | 16.6 |
| K | 0.50 | 1.92 | 3.31 |
| Mg | 0.27 | 5.69 | 6.65 |
| Ca | 16.9 | 41.7 | 40.8 |
| F | 0.36 | 0.37 | 0.27 |
| Cl | 1.6 | 20.8 | 8.4 |
| SO4 | 15.1 | 5.5 | 31.9 |
| NO3 | 0.0 | 2.4 | 36.2 |
| HCO3 | 63 | 165 | 118 |
| Water type | Ca-HCO3 | Ca-HCO3 | Ca-HCO3 |
| Aquifer | |||
|---|---|---|---|
| Hoengseong-Kangrim (HK) | Cheorwon-Jadeung (CJ) | Asan-Punggi (AP) | |
| Rock type | Granite gneiss | Biotite Granite | Porphyritic Granite |
| Major minerals | Plagioclase feldspar Biotite Quartz Alkali feldspar Chlorite Amphibole Calcite | Plagioclase feldspar Biotite Quartz Alkali feldspar Chlorite Muscovite | Plagioclase feldspar Biotite Quartz Alkali feldspar Chlorite Calcite |
| Accessory minerals | Sphene Allanite Apatite Zircon Thorite Ilmenite Pyrite Galena Sphalerite Monazite | Epidote Apatite Zircon Monazite Thorite Ilmenite | Sphene Monazite Apatite Zircon Thorite Ilmenite Pyrite Rutile |
| Experimental Condition | Aquifer | ||||
|---|---|---|---|---|---|
| Hoengseong Kangrim (HK) | Cheorwon Jadeung (CJ) | Asan Punggi (AP) | |||
| pH 3 | DO 0 | 0.1 M Ca | 12387 *** | 2865 | 2729 *** |
| 0.1 M HCO3 | 3164 *** | 6773 | 469 | ||
| DO 8 | 0.1 M Ca | 6475 ** | 2442 | 2370 *** | |
| 0.1 M HCO3 | 900 * | 3985 | 1337 | ||
| pH 7 | DO 0 | 0.1 M Ca | 5154 *** | 159 | 1505 * |
| 0.1 M HCO3 | 181 *** | 5.8 | 8.4 | ||
| DO 8 | 0.1 M Ca | 8.4 | 1987 | 1577 *** | |
| 0.1 M HCO3 | 226 *** | 8.7 *** | 12 ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-G.; Lee, W.-C.; Lee, S.-W.; Lee, J.-H.; Jeong, D.-H.; Jeong, Y.-Y.; Kim, M.-S.; Kim, S.-O. Comparison of Uranium Leachability Between Three Groundwater Aquifers in Relation to the Degree of Bedrock Weathering: A Petro-Mineralogical and Experimental Investigation. Environments 2025, 12, 415. https://doi.org/10.3390/environments12110415
Kim H-G, Lee W-C, Lee S-W, Lee J-H, Jeong D-H, Jeong Y-Y, Kim M-S, Kim S-O. Comparison of Uranium Leachability Between Three Groundwater Aquifers in Relation to the Degree of Bedrock Weathering: A Petro-Mineralogical and Experimental Investigation. Environments. 2025; 12(11):415. https://doi.org/10.3390/environments12110415
Chicago/Turabian StyleKim, Hyeong-Gyu, Woo-Chun Lee, Sang-Woo Lee, Jong-Hwan Lee, Do-Hwan Jeong, Yu-Yeon Jeong, Moon-Su Kim, and Soon-Oh Kim. 2025. "Comparison of Uranium Leachability Between Three Groundwater Aquifers in Relation to the Degree of Bedrock Weathering: A Petro-Mineralogical and Experimental Investigation" Environments 12, no. 11: 415. https://doi.org/10.3390/environments12110415
APA StyleKim, H.-G., Lee, W.-C., Lee, S.-W., Lee, J.-H., Jeong, D.-H., Jeong, Y.-Y., Kim, M.-S., & Kim, S.-O. (2025). Comparison of Uranium Leachability Between Three Groundwater Aquifers in Relation to the Degree of Bedrock Weathering: A Petro-Mineralogical and Experimental Investigation. Environments, 12(11), 415. https://doi.org/10.3390/environments12110415







