Effect of Plant Growth-Promoting Bacteria on Photosynthetic Parameters of One-Year-Old Sessile Oak Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Sessile Oak Seedling Production
2.2. Bacterial Treatment Preparation
2.3. Sessile Oak Seedling Inoculation
2.4. Measurements of Photosynthetic Parameters
2.5. Statistical Analyses
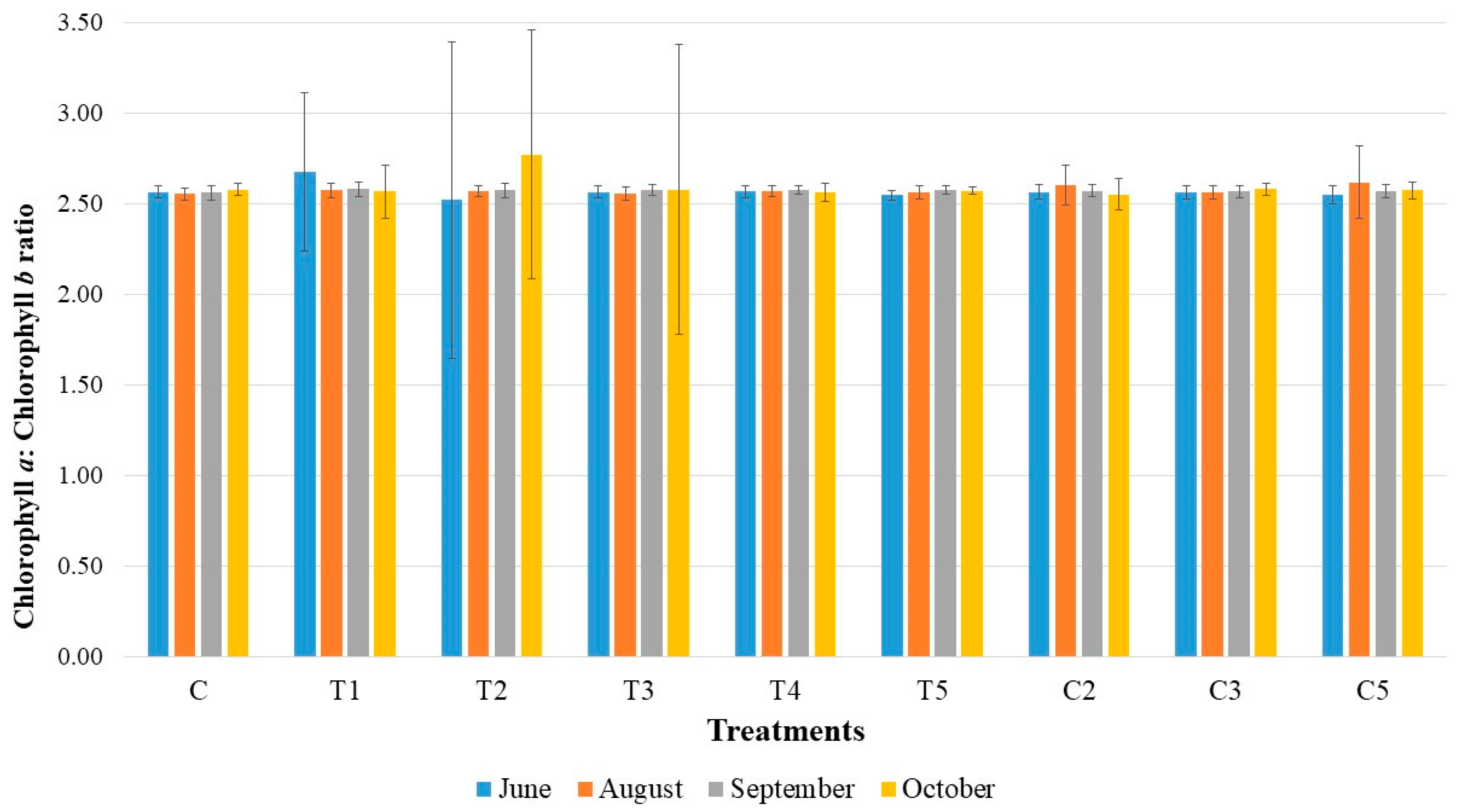
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dror, I.; Yaron, B.; Berkowitz, B. Microchemical contaminants as forming agents of anthropogenic soils. Ambio 2017, 46, 109–120. [Google Scholar] [CrossRef]
- Frazer-Williams, R.; Sankoh, A. Soil contamination resulting from inefficient solid waste management. In Environmental Pollution and Public Health; Frazer-Williams, R., Ogundiran, M.B., Unuabonah, E.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 251–264. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hjyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive Analysis of Heavy Metal Soil Contamination in Mining Environments: Impacts, Monitoring Techniques, and Remediation Strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Melnychenko, V. Phytoremediation of Soils Contaminated as a Result of Military and Anthropogenic Impact. Nauk. Dopovìdì Nacìonalʹnogo Unìversitetu Bìoresursiv ì Prir. Ukraïni 2024, 20, 72–84. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse Soil Pollution from Agriculture: Impacts and Remediation. Sci. Total Environ. 2024, 962, 178398. [Google Scholar] [CrossRef] [PubMed]
- Breś, W.; Politycka, B. Contamination of Soils and Substrates in Horticulture. In Soil Contamination—Current Consequences and Further Solutions; Larramendy, M.L., Soloneski, S., Eds.; InTechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Pahalvi, H.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A. Chemical Fertilizers and Their Impact on Soil Health. In Soil Health; Springer: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, Y.; Yu, T.; Yang, J.; Fan, S.; Feng, C.; Liu, Z.; Huang, C. A Scientometric Analysis and Visualization of Forest Soil Contamination Research from Global Perspectives. Forests 2024, 15, 1068. [Google Scholar] [CrossRef]
- Baláš, M.; Kuneš, I.; Podrázský, V.; Gallo, J.; Lopot, F. Chemical Forest Amelioration: Experience from the Czech Republic and Other Selected Countries—A Review. J. For. Sci. 2024, 70, 103–121. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Seedling Production and the Field Performance of Seedlings. Forests 2018, 9, 740. Available online: https://www.mdpi.com/1999-4907/9/12/740 (accessed on 8 September 2025). [CrossRef]
- Barros, N.F.; Dedecek, R.A.; Schumacher, M.V. The Production Chain of Tree Seedlings in the Context of Climate Change. Forests 2022, 14, 1693. Available online: https://www.mdpi.com/1999-4907/14/9/1693 (accessed on 8 September 2025).
- Yousaf, A.; Hussain, M.; Ahmad, S.; Riaz, A.; Shaukat, S.; Shaukat, S.W.A.; Mishr, R.S.; Akram, S.; Majeed, M.; Tabassum, A.; et al. Environmental Sustainability Assessment of Softwood and Hardwood Seedlings Production in Forest Nurseries: A Case Study from Pakistan. Braz. J. Biol. 2022, 84, e260615. [Google Scholar] [CrossRef]
- Rügner, H.; Forster, B. A Global Review on Innovative, Sustainable, and Effective Materials Composing Growing Media for Forest Seedling Production. Curr. For. Rep. 2023, 9, 148–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wang, L.; Li, Y. Environmental Risk Substances in Soil on Seed Germination: A Review. J. Hazard. Mater. 2024, 460, 132805. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0304389424010975 (accessed on 9 September 2025).
- Pyoabalo, A.; Tchabi, A.; Abotsi, K.; Adjonou, K.; Segla, K.; Kokutse, A.; Kokou, K. Effect of Nursery Substrate on the Growth of Pterocarpus erinaceus Poir. Seedlings. Int. J. Curr. Res. Biosci. Plant Biol. 2021, 8, 17–27. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Xu, S. Selective Biotic Stressors’ Action on Seed Germination: A Review. Plant Sci. 2024, 341, 111601. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0168945224001833 (accessed on 10 September 2025).
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive Use of Nitrogenous Fertilizers: An Unawareness Causing Serious Threats to Environment and Human Health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef] [PubMed]
- Stekolnikov, K.E.; Gasanova, E.S.; Stekolnikova, N.V. Agrogenic Transformation (Degradation) of Chernozems of the Central Chernozem Region. BIO Web Conf. 2021, 36, 03021. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of Uncontrolled Fertilization and Heavy Metal Toxicity Associated with Arsenic (As), Lead (Pb) and Cadmium (Cd), and Possible Remediation. Toxicology 2022, 479, 153274. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, B.; Wang, L.; Xie, Z.; Wang, J.; Yang, Z. Fertilization Restructures Nematode Assemblages by Modifying Soil pH in Croplands of Northeast China. Front. Environ. Sci. 2023, 11, 1207379. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of Long-Term Fertilization on Soil Organic Carbon Mineralization and Microbial Community Structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Daws, M.; Blackburn, C.; Standish, R.; Tibbett, M. Canary in the Coal Mine: Lessons from the Jarrah Forest Suggest Long-Term Negative Effects of Phosphorus Fertilizer on Biodiverse Restoration after Surface Mining. Front. For. Glob. Change 2022, 5, 786305. [Google Scholar] [CrossRef]
- Petraityte, D.; Arlauskienė, A.; Cesevičienė, J. Use of Digestate as an Alternative to Mineral Fertilizer: Effects on Soil Mineral Nitrogen and Winter Wheat Nitrogen Accumulation in Clay Loam. Agronomy 2022, 12, 402. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Liu, B.; Du, Z.; Ma, B.; Jing, D. Effects of Plant Growth-Promoting Rhizobacteria on the Physioecological Characteristics and Growth of Walnut Seedlings under Drought Stress. Agronomy 2023, 13, 290. [Google Scholar] [CrossRef]
- Keyhani, A.; He, W.; Teng, M.; Yan, Z.; Xu, J.; Fayaz, M.; Zhou, C.; Wei, P.; Wang, P. Effect of Mineral Fertilizers on Microorganisms Community Characteristic during Leaf Litter Decomposition under Pinus massoniana in a Subtropical Forest. Appl. Soil Ecol. 2024, XX, 105421. [Google Scholar] [CrossRef]
- Çığ, F.; Sönmez, F.; Nadeem, M.A.; Sabagh, A.E. Effect of Biochar and PGPR on the Growth and Nutrients Content of Einkorn Wheat (Triticum monococcum L.) and Post-Harvest Soil Properties. Agronomy 2021, 11, 2418. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Peng, L.; Du, Z.; Ma, B.; Liu, X. Effect of the Inoculation of Plant Growth-Promoting Rhizobacteria on the Photosynthetic Characteristics of Sambucus williamsii Hance Container Seedlings under Drought Stress. AMB Express 2019, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hua, M.; Guo, D.; Xue, Y.; Chen, X.; Rui, L.; Zhou, N. Effects of Plant Growth-Promoting Rhizobacteria on Growth Indicators and Physiological Characteristics of Peucedanum praeruptorum Dunn Leaves. Plant Signal. Behav. 2023, 18, 2203571. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Święcicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Q.; Wang, X.; Haider, F.; Kučerík, J.; Mumtaz, M.; Holátko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Mishra, A.; Jan, S.; Bhat, M.; Kamal, M.; Rahman, S.; Shah, A.; Jan, A. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants 2023, 12, 629. [Google Scholar] [CrossRef]
- Khoso, M.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress 2023, 11, 100341. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Jabborova, D. The effects of Pseudomonas koreensis IGPEB 17 and arbuscular mycorrhizal fungi on growth and physiological properties of ginger. Turk. J. Agric. For. 2022, 46, 488–495. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Sikder, M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, X. Pseudomonas fluorescens Promote Photosynthesis, Carbon Fixation and Cadmium Phytoremediation of Hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Benedikty, Z.; Morina, F.; Andersen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP Chlorophyll Fluorescence Kinetics and QA Reoxidation Kinetics by Direct Fast Imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papadogeorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Karličić, V.; Golubović Ćurguz, V.; Raičević, V. The Alleviation of Reforestation Challenges by Beneficial Soil Microorganisms. Reforesta 2016, 1, 238–260. [Google Scholar] [CrossRef]
- Mohan, E.; Rajendran, K. Sustainable Development of Horticulture and Forestry through Bio-Inoculants. In Sustainable Crop Production; Hasanuzzaman, M., Fujita, M., Filho, M.C.M.T., Nogueira, T.A.R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van Der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van Der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Hengeveld, G.; Reyer, C.; Hanewinkel, M.; Zimmermann, N.E.; Cullmann, D. Alternative forest management strategies to account for climate change-induced productivity and species suitability changes in Europe. Reg. Environ. Change 2015, 15, 1581–1594. [Google Scholar] [CrossRef]
- Perkins, D.; Uhl, E.; Biber, P.; Du Toit, B.; Carraro, V.; Rötzer, T.; Pretzsch, H. Impact of climate trends and drought events on the growth of oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond their natural range. Forests 2018, 9, 108. [Google Scholar] [CrossRef]
- Kunz, J.; Löffler, G.; Bauhus, J. Minor European broadleaved tree species are more drought-tolerant than Fagus sylvatica but not more tolerant than Quercus petraea. For. Ecol. Manag. 2018, 414, 15–27. [Google Scholar] [CrossRef]
- Modrow, T.; Kuehne, C.; Saha, S.; Bauhus, J.; Pyttel, P.L. Photosynthetic performance, height growth, and dominance of naturally regenerated sessile oak (Quercus petraea [Mattuschka] Liebl.) seedlings in small-scale canopy openings of varying sizes. Eur. J. For. Res. 2020, 139, 41–52. [Google Scholar] [CrossRef]
- Ministère de l’Agriculture. Résultats de l’enquête statistique annuelle MAAF/IRSTEA sur les ventes en France de plants forestiers pour la campagne de plantation 2015–2016. In 2017-229 NdSD.; Ministère de l’Agriculture: Paris, France, 2017; p. 32. Available online: https://info.agriculture.gouv.fr/gedei/site/bo-agri/instruction-2017-229/telechargement (accessed on 14 September 2025).
- Popović, V.; Lučić, A.; Rakonjac, L.; Kerkez-Janković, I. Analysis of morphological quality parameters of one-year old bare root sessile oak (Quercus petraea (Matt.) Liebl) seedlings. Sustain. For. 2019, 79–80, 59–64. [Google Scholar]
- Girard, Q.; Ducousso, A.; De Gramont, C.; Louvet, J.; Reynet, P.; Musch, B.; Kremer, A. Provenance variation and seed sourcing for sessile oak (Quercus petraea (Matt.) Liebl.) in France. Ann. For. Sci. 2022, 79, 27. [Google Scholar] [CrossRef]
- Jovanović, S.; Popović, V.; Lučić, A.; Rakonjac, L. Bacterial Treatment Impact on Morphological Traits of One-Year Old Sessile Oak Seedlings of Two Serbian Provenances. Sustain. For. 2023, 87–88, 79–87. [Google Scholar] [CrossRef]
- Jovanović, S.; Lučić, A.; Rakonjac Lj Popović, V. Uticaj bakterijskih tretmana na dvogodišnje sadnice hrasta kitnjaka (Quercus petraea (Matt.)Liebl). Šumarstvo 2023, 3–4, 127–137. [Google Scholar]
- Samaddar, S.; Chatterjee, P.; Choudhury, A.; Ahmed, S.; Sa, T. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019, 219, 66–73. [Google Scholar] [CrossRef]
- Ahsan, N.; Shimizu, M. Lysinibacillus Species: Their Potential as Effective Bioremediation, Biostimulant, and Biocontrol Agents. Rev. Agric. Sci. 2021, 9, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Dasila, H.; Rana, A.; Maithani, D.; Rana, A.; Sahgal, M.; Tewari, S. Interaction between Dalbergia sissoo Roxb. and Pseudomonas koreensis AS15 Strain is Cultivar Specific. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 297–306. [Google Scholar] [CrossRef]
- Bielinis, E.; Jóźwiak, W.; Robakowski, P. Modelling of the relationship between the SPAD values and photosynthetic pigments content in Quercus petraea and Prunus serotina leaves. Dendrobiology 2015, 73, 125–134. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Petković, B.; Merkulov, L.; Duletić-Laušević, S. Anatomija i Morfologija Biljaka sa Praktikumom; Stanković, S., Ed.; Biološki fakultet Univerziteta u Beogradu: Beograd, Srbija, 2014; ISBN 978-86-7078-084-2. [Google Scholar]
- Hall, D.O.; Rao, K.K. Photosynthesis; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Willows, R.D. Chlorophylls. In Plant Pigments and Their Manipulation; Davies, K.M., Ed.; Blackwell Publishing: Oxford, UK, 2004; pp. 23–46. [Google Scholar]
- Mattila, H.; Valev, D.; Havurinne, V.; Khorobrykh, S.; Virtanen, O.; Antinluoma, M.; Mishra, K.B.; Tyystjärvi, E. Degradation of chlorophyll and synthesis of flavonols during autumn senescence—The story told by individual leaves. AoB Plants 2018, 10, ply028. [Google Scholar] [CrossRef]
- Donnelly, A.; Yu, R.; Rehberg, C.; Meyer, G.; Young, E.B. Leaf chlorophyll estimates of temperate deciduous shrubs during autumn senescence using a SPAD-502 meter and calibration with extracted chlorophyll. Ann. For. Sci. 2020, 77, 30. [Google Scholar] [CrossRef]
- Babu, A.; Shea, P.; Sudhakar, D.; Jung, I.; Oh, B. Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J. Environ. Manag. 2015, 151, 160–166. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.; Lee, K.; Kang, S.; Dhungana, S.; Bhusal, N.; Lee, I. The Halotolerant Rhizobacterium—Pseudomonas koreensis MU2 Enhances Inorganic Silicon and Phosphorus Use Efficiency and Augments Salt Stress Tolerance in Soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, Y.; Shi, M.; Han, X.; Jing, Y.; Li, Y.; Li, H.; Lai, H. Pseudomonas koreensis promotes tomato growth and shows potential to induce stress tolerance via auxin and polyphenol-related pathways. Plant Soil 2021, 462, 141–158. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, C.; Jing, Y.; Yang, S.; Li, H.; Xue, Q.; Lai, H. Pseudomonas koreensis Culture Filtrate Alleviates Tomato Drought Stress: Modulation of Antioxidant Systems Coupled with the Porphyrin and Chlorophyll–Photosynthesis–Fructose and Mannose Axis. Plant Soil 2022, 484, 237–256. [Google Scholar] [CrossRef]
- Kalleku, J.; Ihsan, S.; Al-Azzawi, T.; Khan, M.; Hussain, A.; Chebitok, F.; Das, A.; Moon, Y.; Mun, B.; Lee, I.; et al. Halotolerant Pseudomonas koreensis S4T10 Mitigates Salt and Drought Stress in Arabidopsis thaliana. Physiol. Plant. 2024, 176, e14258. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, M.; Moon, Y. Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L. Curr. Issues Mol. Biol. 2025, 47, 194. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown Gold of Marginal Soil: Plant Growth Promoting Bacteria to Overcome Plant Abiotic Stress for Agriculture, Biofuels and Carbon Sequestration. Sci. Total Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Li, H.; Guo, D.; Sharma, A.; Verma, K.; Solanki, M.; Upadhyay, S.; Lakshmanan, P.; Yang, L.; et al. Nitrogen Fixation and Phytohormone Stimulation of Sugarcane Plant through Plant Growth Promoting Diazotrophic Pseudomonas. Biotechnol. Genet. Eng. Rev. 2023, 40, 15–35. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Natarajan, D.; Ma, Y. Plant Growth-Promoting Bacteria in Metal-Contaminated Soil: Current Perspectives on Remediation Mechanisms. Front. Microbiol. 2022, 13, 966226. [Google Scholar] [CrossRef] [PubMed]
- Suhr, M.; Lederer, F.L.; Günther, T.J.; Raff, J.; Pollmann, K. Characterization of Three Different Unusual S-Layer Proteins from Viridibacillus arvi JG-B58 That Exhibits Two Super-Imposed S-Layer Proteins. PLoS ONE 2016, 11, e0156785. [Google Scholar] [CrossRef]
- Ye, J.; Tian, W.; Jin, C. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The Utilization and Roles of Nitrogen in Plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, Z.; Glick, B.; He, S.; Huang, C.; Wu, L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Kuroda, M.; Inoue, D.; Morikawa, M.; Ike, M. Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2020, 96, fiaa101. [Google Scholar] [CrossRef]
- Barriuso, J.; Solano, B.R.; Gutiérrez Mañero, F.J. Biological Control Protection Against Pathogen and Salt Stress by Four Plant Growth-Promoting Rhizobacteria Isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 2008, 98, 666–672. [Google Scholar] [CrossRef] [PubMed]



| Bacterial Isolate | Most Similar Reference from the NCBI Database (Based on 16S rDNA) | Percentage of Identity (%) | NCBI GenBank Database Accession Number | 16S rRNA Sequence Size (bp) | IAA Production | Siderophore Production | Phosphate Solubilization | ACC Deaminase Production |
|---|---|---|---|---|---|---|---|---|
| R3.17 | Viridibacillus sp. | 100 | PQ590395 | 1144 | + | + | - | + |
| R4.2.1P | Pseudomonas sp. | 99.18 | PQ590398 | 854 | + | - | + | + |
| R4.29P | Pseudomonas sp. | 99.12 | PQ590401 | 1137 | + | + | + | + |
| R1.4 | Lysinibacillus sp. | 99.39 | PQ590391 | 814 | + | - | - | + |
| R4.45P | Pseudomonas sp. | 99.16 | PQ590403 | 1067 | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazić, S.; Berić, T.; Milanović, S.; Medić, O.; Vemić, A.; Lučić, A.; Stanković, S.; Rakonjac, L.; Popović, V. Effect of Plant Growth-Promoting Bacteria on Photosynthetic Parameters of One-Year-Old Sessile Oak Seedlings. Environments 2025, 12, 409. https://doi.org/10.3390/environments12110409
Lazić S, Berić T, Milanović S, Medić O, Vemić A, Lučić A, Stanković S, Rakonjac L, Popović V. Effect of Plant Growth-Promoting Bacteria on Photosynthetic Parameters of One-Year-Old Sessile Oak Seedlings. Environments. 2025; 12(11):409. https://doi.org/10.3390/environments12110409
Chicago/Turabian StyleLazić, Sanja, Tanja Berić, Slobodan Milanović, Olja Medić, Aleksandar Vemić, Aleksandar Lučić, Slaviša Stanković, Ljubinko Rakonjac, and Vladan Popović. 2025. "Effect of Plant Growth-Promoting Bacteria on Photosynthetic Parameters of One-Year-Old Sessile Oak Seedlings" Environments 12, no. 11: 409. https://doi.org/10.3390/environments12110409
APA StyleLazić, S., Berić, T., Milanović, S., Medić, O., Vemić, A., Lučić, A., Stanković, S., Rakonjac, L., & Popović, V. (2025). Effect of Plant Growth-Promoting Bacteria on Photosynthetic Parameters of One-Year-Old Sessile Oak Seedlings. Environments, 12(11), 409. https://doi.org/10.3390/environments12110409