Abstract
Urban community gardens are valued for promoting sustainable food production, yet the accumulation of toxic heavy metals in city soils can present both ecological and public health risks. Therefore, this study was aimed at assessing the environmental and health risks of toxic heavy metals in community gardens soil contaminated by an industrial fire hazard in New Brunswick, Canada. Both top and subsoil soil samples were collected at Carleton community garden. The collected samples were examined for toxic heavy metals using inductively coupled plasma optical emission spectrometry and inductively coupled plasma mass spectrometry. Ecological risks were evaluated through the ecological risk factor and the potential ecological risk index, while human health risks were determined using a standard human health risk assessment approach. The mean concentration of Pb, Zn, Cu, and Sn exceeded permissible limits when compared to the Canadian soil quality guidelines and upper continental crust values. Findings from the ecological risk assessment showed that all metals were associated with low risk, except for nickel, which posed a high ecological risk across both soil layers. PERI results revealed a low overall ecological threat. The human health risk analysis indicated that children could face non-carcinogenic and carcinogenic risks from As exposure, while adults were not at risk from any of the studied metals. These findings identify arsenic as the primary contaminant of concern, with children representing the most vulnerable population, emphasizing the necessity for targeted mitigation strategies and protective measures to reduce their exposure. The results of this study can inform interventions aimed at safeguarding both environmental and public health, while also raising awareness about the presence and risks of toxic heavy metals, ultimately contributing to the protection of human health and the broader ecosystem.
1. Introduction
Soil is an essential resource for human survival and development, serving a key role in maintaining ecological functions [1]. As a fundamental component of ecosystems, it supports agricultural production and various human activities. The sustainable and rational use of soil contributes to the healthy and stable development of modern society [2,3]. It is a critical natural resource that underpins the safety, availability, and quality of human food, while also being central to ecological and environmental protection [4]. The quality of the soil environment is closely linked to human health and overall social development [5]. However, activities associated with urbanization, industrialization, agriculture, vehicle emissions, mining, construction, heavy road traffic, road wear, combustion processes, power generation, inadequate waste management, wastewater discharge, and the corrosion of building materials have significantly degraded fertile soils and increased heavy metal contamination [6,7,8]. In addition, unpredictable events such as forest fires and industrial explosions can further disrupt ecosystems [9,10]. These events may alter the geochemical properties of soils not only within the affected areas but also in surrounding regions. They often leave behind ash, debris, and contaminated soil containing toxic environmental pollutants, including heavy metals [11].
Various heavy metals and metalloids can be released into the environment as a result of human activities, including As (arsenic), Cd (cadmium), Cr (chromium), Pb (lead), and Hg (mercury), Fe (iron) among others. Of these elements, As, Cd, Cr, Pb, and Hg are recognized as toxic metals posing significant public health concerns [12,13]. Recent estimates suggest that over 20 million hectares of land, including urban community gardens, are affected by these toxic elements [3]. Urban community gardens play a critical role in providing local populations with access to nutritious food, offering affordable alternatives to the predominantly unhealthy fast-food options common in city environments. Urban community gardens serve as an important survival strategy for low-income populations worldwide [14,15]. However, the presence of environmental toxicants in these areas poses serious threats to ecosystem integrity, compromising both its structure and function. Heavy metals are especially concerning as they are persistent contaminants that resist natural degradation, leading to their gradual build-up in soils and surrounding ecosystems. Such accumulation can disrupt biological communities by modifying microbial diversity, accumulating in plants and soil biota, and ultimately posing risks to animals, vegetation, and human health [16,17]. In addition, these elements can be taken up by plants and transferred through the food web, thereby creating serious threats to food safety and public health [5].
Potential toxic heavy metals negatively impact plants by suppressing seed germination, impairing growth and photosynthetic activity, and disturbing redox homeostasis, which induces oxidative stress and ultimately lowers crop yields [18]. On a global scale, an estimated 1.3 billion people are exposed to elevated concentrations of these metals, with five to ten million deaths annually primarily among children attributed to severe metal-related diseases [19]. Exposure to highly contaminated soils through inhalation, ingestion, or dermal absorption can result in serious health consequences for urban residents [13,20,21,22,23]. Such exposure has been linked to a wide range of medical conditions, including cancer, miscarriages, asthma, renal failure, hypertension, headaches, reproductive disorders, cardiovascular disease, neurological effects, lung granulomas, as well as damage to endocrine and immune systems [13,24,25,26,27].
While many studies globally have documented toxic heavy metals in community garden soils and their related health risks [15,17,28,29,30,31,32,33], comparatively limited research has been carried out in Canada [30,34,35]. Notably, no studies have been conducted on the environmental and health risks of toxic metals in soils of the Carleton community garden located near an iron and metal facility in Port Saint John, Canada, which experienced a fire outbreak on 4 September 2023 are scarce. Factors such as historical emissions, tailings or slag disposal, ore composition, and particle resuspension and redeposition may contribute to local soil contamination. During operations including receiving, shredding, sorting, cleaning, storage, and loading, metallic fragments and other metal residues can accumulate in the soil layer and, through corrosion, become mobilized in the environment [36]. Furthermore, recycling processes can lead to the resuspension and redeposition of particles enriched with toxic heavy metals, further exacerbating soil contamination [37]. Additionally, historical applications of lead-based paints, residual deposition from leaded gasoline emissions, tire wear, galvanized metal structures, urban dust, fertilizers, preservatives, treated timber, plumbing materials, and fungicides may contribute to soil contamination [38]. The presence of oversized scrap metal piles at the facility may further intensify heavy metal pollution in the surrounding area. Past incidents of explosions and fires have also released airborne particulates, affecting nearby communities. As reported by Adnan et al. [39], atmospheric deposition is frequently the primary pathway through which soil contamination occurs, supporting the observed patterns of pollution in the region.
Neglecting soil quality can result in community members being exposed to hazardous levels of toxic heavy metals. Consequently, it is essential to monitor heavy metal concentrations in community gardens and evaluate their potential environmental and health risks. Conducting environmental and human health risk assessments is crucial for understanding and managing stressors that may affect both ecosystems and public health especially after such as incident. Therefore, this study seeks to address this knowledge gap by evaluating the environmental and human health risks linked to toxic heavy metals in the soils of Carleton community garden, which was affected by an industrial fire hazard in New Brunswick, Canada. The specific objectives of the study are: (i) to quantify the levels of toxic heavy metals, (ii) to assess potential ecological risks, (iii) to evaluate both carcinogenic and non-carcinogenic health risks, and (iv) to examine the implications of these findings for environmental and public health in the Carleton community garden area. The findings of this study are anticipated to raise public awareness of toxic heavy metal contamination and its potential risks, thereby supporting the safeguarding of both human health and the environment.
2. Material and Method
2.1. Study Area
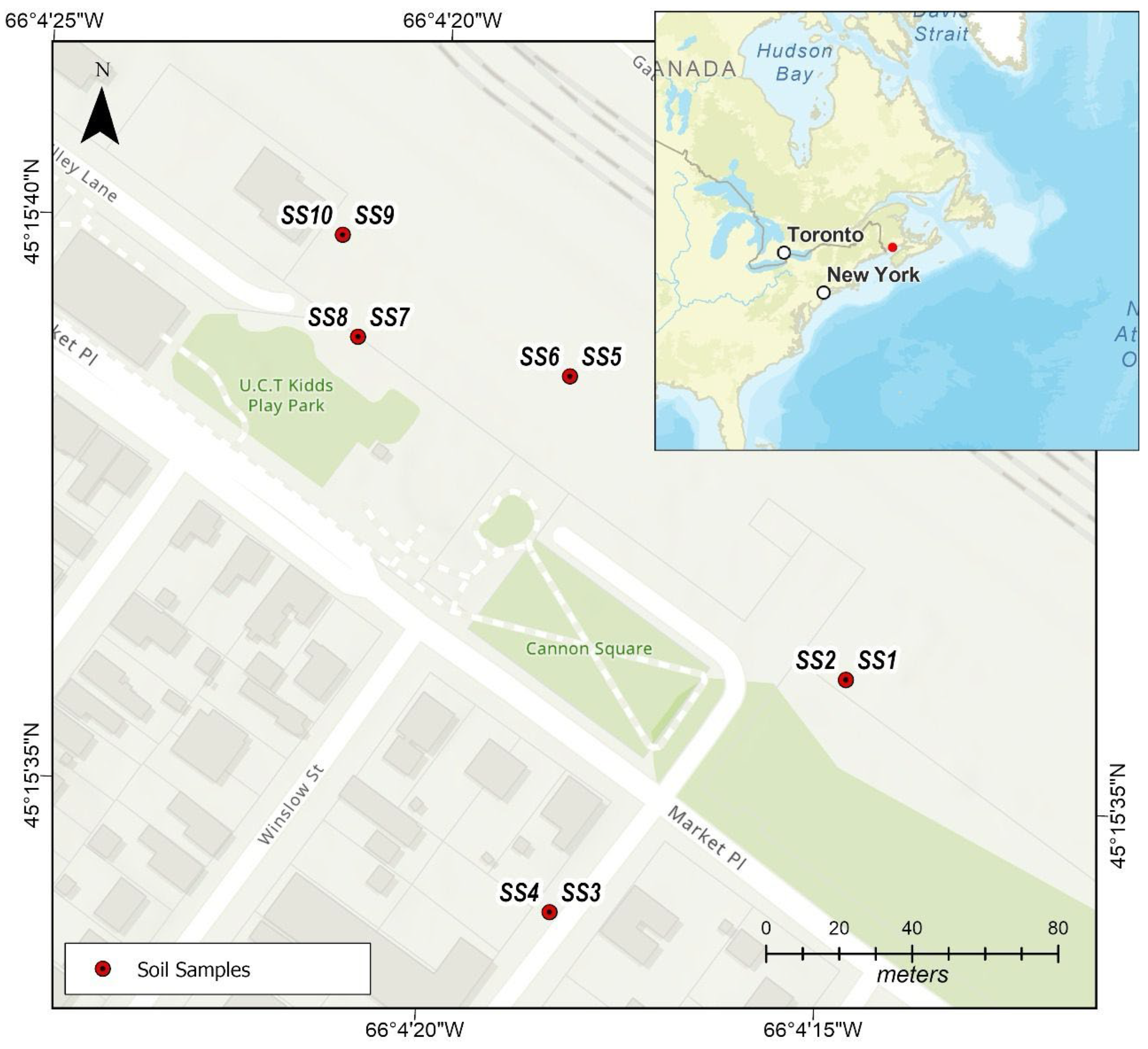
The study was conducted in Saint John, New Brunswick, Canada, with an emphasis on the Carleton Community Garden (Figure 1). The Community Garden is housed within the Carleton Community Centre on the Lower West Side of the city. The location is near the shores of the harbour and Saint John River, about at 45.2728° N, 66.0633° W. With 69,895 residents, Saint John itself is home to a number of community gardens that foster social contact and local food production, especially for lower-income households. One of the biggest scrap metal enterprises in Atlantic Canada, is located right next to the garden. This plant exports metals via the Port of Saint John and processes ferrous and non-ferrous materials, including shredding vehicles. Concerns over possible environmental effects are raised by its proximity to residential neighbourhoods, educational institutions, and green areas due to its waterfront location [40]. The area experiences a humid continental climate characterized by warm summers and cold winters, which affects the deposition and dispersion of pollutants. The local geology of Saint John, New Brunswick, is dominated by the Cambrian-aged Saint John Group, a sequence of sandstones, siltstones, and shales deposited in shallow marine settings. These sedimentary units record early Paleozoic basin development and have since been subjected to extensive tectonic deformation. Regional faulting, folding, and metamorphism have produced a structurally complex terrain, which is characteristic of the broader Appalachian orogen. The result is a diverse and deformed stratigraphic succession that forms the foundation of the Saint John area’s geological framework. The majority of the soils in the region are sandy loams and urban fill. Groundwater occurs primarily within fractured bedrock aquifers, where secondary porosity created by faults and fractures governs storage and flow, while overburden deposits provide limited shallow aquifer potential. However, the hydrogeological system is vulnerable to surface inputs, and contamination from industrial operations, harbour activities, and legacy pollutants such as leaded fuels, paints, and treated wood has historically impacted soil and groundwater quality [41].
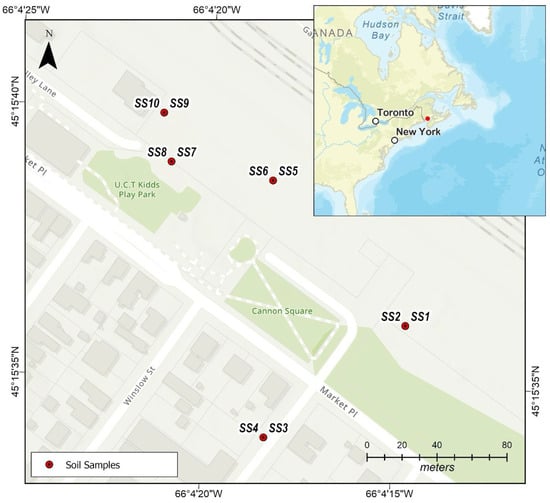
Figure 1.
Carleton community garden.
2.2. Sample Collection and Analysis
Soil samples were obtained from the Carleton community gardens, with a total of 10 samples collected (n = 10). This included five topsoil samples (0–15 cm) and five subsoil samples (15–30 cm), collected in June 2025. Sampling was performed using a random selection approach with a stainless-steel soil sampler. New nitrile gloves were worn at each sampling location to prevent contamination. Between sampling points, the equipment was thoroughly cleaned using Alconox, (White Plains, NY, USA) rinsed with deionized water, and wiped with a fresh disposable cloth. Prior to packaging, samples were field-screened to remove stones, gravel, vegetation, and other debris. Each 1 kg sample was then placed in pre-labelled (SS1, SS2, SS3, SS4, SS5, SS6, SS7, SS8, SS9 and SS10), cleaned zip-lock bags and transported to the accredited AGAT Laboratories in Dartmouth, NS, Canada, for analysis (Table S1). Soil samples were air-dried, sieved to <2 mm, and homogenized (agate mortar and pestle). Approximately 0.25 g of the homogenized <2 mm soil fraction was transferred to Teflon microwave digestion vessels; 6.0 mL concentrated HCl and 3.0 mL concentrated HNO3 (aqua regia) were added. Vessels were sealed and heated in a microwave system, ramping to 180 °C within 10 min and held for 15–20 min. After cooling, digests were quantitatively transferred to 50 mL volumetric flasks and diluted to volume with ultrapure water. Digestates were analysed by ICP-MS (Agilent 7500, Agilent Technologies, Santa Clara, CA, USA) for Sb, As, Ba, Be, B, Cd, Cr, Co, Cu, Pb, Li, Mo, Ni, Se, Ag, Sr, Tl, Sn, Ti, U, V, Zn, Hg while major elements (Al, Fe, Mn, Na) were determined using ICP-OES (Agilent Technologies, Santa Clara, CA, USA) [42,43]. Quality assurance and quality control were ensured through the use of reagent blanks, duplicate samples, and certified reference soils. The method detection limits ranged from 0.5 to 30 mg/kg, with relative percent differences below 10%. Recoveries of spiked metals ranged between 70% and 118% (Table 1). Data were processed and analysed using Microsoft Excel 2016 (Redmond, WA, USA). To facilitate clear interpretation, toxic metal concentrations were summarized using descriptive statistics, including minimum, maximum, mean, and standard deviation values.

Table 1.
Quality assurance and quality control.
2.3. Potential Environmental and Public Health Risk Assessment of Soil Heavy Metals
2.3.1. Ecological Risk Factor
Hakanson [44] introduced the ecological risk factor (ER) index, which quantifies the ecological risk of a given chemical element in the studied medium, as expressed in Equation (1):
where Tr is the toxic-response factor of a chemical contaminant and CF is the contamination factor of a selected element. The risk level classifications based on ER index range from low to serious ecological risks as shown in Table S2 [45,46,47]. The toxicity response factors/values for selected heavy metals in this study are presented in Table S4 [42,48,49,50,51,52,53,54].
2.3.2. Potential Ecological Risk Index
The potential ecological risk index (PERI) evaluates the overall ecological risk of a study area by summing the individual risk factors of all chemical elements present, as described in Equation (2) [55]:
PERI risk classifications range from low to significantly high ecological risk as presented in Table S3 [45,56].
2.3.3. Public Health Risk Assessment
Assessing potential human health risks from both non-carcinogenic and carcinogenic agents is essential for protecting public health. The human health risk assessment (HHRA) index is a widely applied tool for evaluating individual exposure to toxic heavy metals by estimating their potential adverse effects and comparing them with established safety thresholds [57]. Ingestion is typically the dominant exposure route and was therefore considered in this study for both non-carcinogenic and carcinogenic risk evaluations [13,20,58,59,60]. To assess the population average daily dose (ADD) through ingestion exposure pathway, Equation (3) was used [46,61].
where ADD is the average daily total exposure dose (mg/kg/day) and ADDing, is the average daily dose (mg/kg/day) for ingestion pathways [46]. The parameters applied to estimate the ADDs are shown in Table S5.
To assess the non-carcinogenic risk of analysed toxic heavy metals through ingestion pathway, the hazard quotient (HQ) and hazard index (HI) are estimated using Equations (4) and (5) [13].
The reference dose (RfD) of each toxic metal (mg/kg/day) represents the daily exposure level considered safe over a lifetime without adverse effects. A hazard quotient (HQ) or hazard index (HI) greater than 1 suggests the potential for non-carcinogenic effects, whereas values below 1 indicate no significant non-carcinogenic risk [20,62].
To assess the possibility of toxic heavy metals to cause carcinogenic risk (CR) on local community, Equation (6) was used.
where CSF is the cancer slope factor (mg/kg/day) through ingestion of individual toxic heavy metal. If the CR values are greater than 1 × 10−4, the carcinogenic risk occurs; if the values are between 1 × 10−6 and 1 × 10−4, the carcinogenic risk is acceptable; if the values are below 1 × 10−6, the risk is not carcinogenic [20,63]. The toxic heavy metals oral reference doses (RfD) and cancer slope factors (CSF) used in this study are presented in Table S6 [20,61,64,65,66,67].
3. Results and Discussion
3.1. Concentration of Toxic Heavy Metals in Carleton Community Garden Soil
The mean concentrations (Table 2) of various toxic heavy metals in top soil (0–15 cm) was Aluminum (11,920.0), Antimony (0.73), Arsenic (6.6), Barium (49.5), Beryllium (0.6), Boron (3.4), Chromium (17.3), Cobalt (7.4), Copper (54.3), Iron (24,820.0), Lead (70.1), Lithium (15.5), Manganese (458.2), Molybdenum (1.0), Nickel (14.8), Sodium (119.0), Strontium (13.6), Thallium (0.1), Tin (4.2), Titanium (251.6), Uranium (0.7), Vanadium (34.7), and Zinc (116.6) mg/kg. Mercury, Cadmium, Selenium and Silver were below limit of detection (<LOD). In subsoil (15–30 cm), the mean concentration of toxic heavy metals Aluminum (12,800.0), Antimony (0.7), Arsenic (7.2), Barium (49.8), Beryllium (0.6), Boron (2.8), Chromium (19.2), Cobalt (8.3), Copper (63.5), Iron (26,680.0), Lead (71.9), Lithium (16.6), Manganese (477.0), Molybdenum (1.0), Nickel (17.1), Sodium (121.0), Strontium (14.8), Thallium (0.1), Tin (4.0), Titanium (295.2), Uranium (0.8), Vanadium (38.6), and Zinc (89.2) mg/kg. Mercury, Cadmium, Selenium and Silver were below limit of detection (Table 2).
Based on their mean concentration, their range in top soil was Fe (24,820.0) > Al (11,920.0) > Mn (458.2) > Ti (251.6) > Na (119.0) > Zn (116.6) > Pb (70.1) > Cu (53.3) > Ba (49.5) > V(34.7) > Cr (17.3) > Li (15.5) > Ni (14.8) > Sr (13.6) > Co (7.4) > As (6.6) > Sn (4.2) > B (3.4) > Mo (1.0) > U (0.7) = Sb (0.7) > Be (0.6) > Tl (0.1). In subsoil it was Fe (26,680.0) > Al (12,800.0) > Mn (477.0) > Ti (295.2) > Na (121.0) > Zn (89.2) > Pb (71.9) > Cu (63.5) > Ba (49.8) > V (38.6) > Cr (19.2) > Ni (17.1) > Li (16.6) > Sr (14.8) > Co (8.3) > As (7.2) > Sn (4.0) > B (2.8) > Mo (1.0) > U (0.8) > Sb (0.7) > Be (0.6) > Tl (0.1). The accumulation of toxic heavy metals in community garden soils has been documented in several regions, including Pittsburgh, USA [68], Wroclaw, Poland [17], Philadelphia and Pittsburgh, USA [69], Brescia, Italy [37], and the Eastern Cape, South Africa [70]. In this study, the concentrations of toxic heavy metals were compared with the Canadian soil quality guidelines (CCME) [71] and upper continental crust values (UCCV) [72]. Most elements (approximately 85%) were within acceptable limits, suggesting generally low levels of contamination. Concentrations within this range are often consistent with natural background conditions, although guideline thresholds may not always capture subtle deviations from baseline levels. Overall, the findings indicate that the study area is largely non-contaminated, with only a minority of elements requiring closer attention for potential environmental or health concerns. When compared with CCME standards, only lead (Pb) exceeded the limits in deep soil. Relative to UCCV, copper (Cu), lead (Pb), tin (Sn), and zinc (Zn) exceeded acceptable limits in topsoil, while Cu, Pb, and Sn exceeded limits in subsoil. Elevated levels of Pb, Zn, Cu, and Ni in community garden soils have also been reported in other studies [17,69,73]. These findings indicate possible contamination of the Carleton community garden soils. Elevated concentrations of toxic metals may be linked to the garden’s proximity to iron and metal recycling facilities. Previous studies have also shown that gardens near industrial operations are more likely to accumulate heavy metals [74]. Additional contributing factors may include historical emissions, tailings or slag disposal, ore composition, and particle resuspension and redeposition. During activities such as receiving, shredding, sorting, cleaning, storing, and loading, metallic fragments and other metals can accumulate in the soil layer and become mobilized through corrosion. Iron and metal recycling facilities inherently contain heavy metals, which may be released into the surrounding environment during processing [36]. Moreover, resuspension and redeposition of metal-enriched particles during recycling processes can further exacerbate soil contamination. Similarly, elevated heavy metal levels near ferroalloy plants have been attributed to historical emissions and the redistribution of metal-rich particles [37]. Improperly managed waste products, including slag and other residues containing high metal concentrations, can also lead to leaching or dispersion by wind and water, contributing to environmental contamination [75]. The elevated metal concentrations may also suggest contamination from historical sources such as lead-based paints, legacy deposition from leaded gasoline, tire wear, galvanized metal structures, urban dust, fertilizers, preservatives, treated timber, plumbing materials, and fungicides [38], as well as from fire-related emissions at the nearby iron and metal facility. The fire incident in September 2023 likely released particulate-bound metals into the surrounding environment, contributing to soil contamination. The presence of oversized scrap metal piles at the facility may further exacerbate heavy metal pollution. Previous episodes of explosions and fires in the area have resulted in airborne particulates affecting adjacent communities. According to Adnan et al. [39], atmospheric deposition is frequently the primary pathway through which soil contamination occurs, supporting these observations. Additionally, the concentrations of Al, As, Ba, Cr, Co, Cu, Fe, Pb, Li, Mn, Ni, Na, Sr, Ti, U, V, and Zn were found to increase in subsoil layers. This trend can be attributed to processes such as metal migration, groundwater movement, dilution, chemical transformations, and adsorption–desorption dynamics. Supporting this observation, Cappuyns [76] noted that elevated heavy metal concentrations at greater depths typically result from long-term surface infiltration, chemical alterations in deeper soil layers that enhance metal solubility, limited dilution, and cumulative historical deposition. Moreover, their occurrence can also be linked to the geology of the area which is the Cambrian-aged Saint John Group, a geological formation composed of sandstones, siltstones, and shales deposited under shallow marine conditions [41]. Therefore, this can lead to introduction of some trace metals such as Cu, Pb, Zn and Sn. Later intrusion of the Saint John Group by granitic bodies and hydrothermal veins, which may introduce Pb, Zn, Cu, and Sn mineralization may also be attributed to the high concentrations of these metals. This study has also shown that metal recycling and fire hazards can also contribute to heavy metals in community gardens apart from the geology of the area, traffic, industrial activities, and urbanisation [30,35]. The build-up of toxic metals in community garden soils is a major environmental and public health issue, as these contaminants can bio-accumulate and bio-magnify within the food chain, ultimately threatening human health.

Table 2.
Statistical analysis of toxic heavy metals in community garden soil with upper continental crust values [72] and Canadian soil quality guidelines [71].
Table 2.
Statistical analysis of toxic heavy metals in community garden soil with upper continental crust values [72] and Canadian soil quality guidelines [71].
| Parameter | Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Topsoil (0–15 cm) | Subsoil (15–30 cm) | Background Values | ||||
| Min–Max | Mean ± SD | Min–Max | Mean ± SD | UCCV | CCME | |
| Al | 11,200–13,000 | 11,920 ± 87,579 | 11,700–14,100 | 12,800 ± 956.6 | - | - |
| Sb | 0.6–0.8 | 0.7 ± 0.1 | 0.6–0.9 | 0.7 ± 0.2 | 2.5 | - |
| As | 4.0–10.0 | 6.6 ± 2.30 | 4.0–10.0 | 7.2 ± 2.2 | 12.4 | 12 |
| Ba | 35.2–63.8 | 49.5 ± 13.3 | 41.6–65.7 | 49.8 ± 10.2 | 570 | 750 |
| Be | 0.5–0.6 | 0.6 ± 0.1 | 0.5–0.7 | 0.6 ± 0.1 | 1.84 | 4 |
| B | 2.6–4.4 | 3.4 ± 0.7 | 2.2–3.5 | 2.8 ± 0.5 | 72 | - |
| Cr | 15.4–19.8 | 17.3 ± 1.6 | 15.5–22.1 | 19.2 ± 2.8 | 68 | 64 |
| Co | 6.4–8.2 | 7.4 ± 0.8 | 6.7–10.3 | 8.3 ± 1.4 | 10.5 | 40 |
| Cu | 24.5–81.9 | 54.3 ± 25.4 | 31.9–108 | 63.5 ± 28.1 | 28.7 | 63 |
| Fe | 20,600–27,100 | 24,820 ± 2695.7 | 21,000–28,800 | 26,680 ± 3204.2 | - | |
| Pb | 15.2–171 | 70.1 ± 64.9 | 16.5–187 | 71.9 ± 70.0 | 31 | 31 |
| Li | 14.7–16.3 | 15.5 ± 0.8 | 15.2–17.3 | 16.6 ± 0.9 | - | - |
| Mn | 348–566 | 458.2 ± 84.9 | 325–693 | 477.8 ± 136.4 | - | - |
| Mo | 0.5–1.2 | 1.0 ± 0.34 | 0.9–1.1 | 1.0 ± 0.1 | 27 | 45 |
| Ni | 12–17.6 | 14.8 ± 2.2 | 13.1–21.2 | 17.1 ± 3.16 | - | 1 |
| Na | 96–155 | 119.0 ± 24.7 | 103–139 | 121.0 ± 16.85 | - | - |
| Sr | 12.5–16.5 | 13.6 ± 1.7 | 13–15.4 | 14.8 ± 0.9 | 174 | - |
| Tl | 0.1–0.1 | 0.1 ± 1.7 | 0.1–0.1 | 0.1 ± 0.0 | 0.7 | 1 |
| Sn | 1.6–6.4 | 4.2 ± 2.3 | 3–5.8 | 4.0 ± 1.1 | 3.7 | - |
| Ti | 163–441 | 251.6 ± 111.8 | 134–519 | 295.2 ± 161.0 | - | - |
| U | 0.5–0.8 | 0.7 ± 0.2 | 0.6–0.9 | 0.8 ± 0.1 | 3 | 23 |
| V | 30.5–36.6 | 34.7 ± 2.50 | 35.8–39.4 | 38.0 ± 1.5 | 131 | 130 |
| Zn | 80–205 | 116.6 ± 53.97 | 60–130 | 89.2 ± 30.8 | 103 | 250 |
Notation: mg/kg: milligram per kilogram, ±: standard deviation, min: minimum concentration, max: maximum concentration, cm: centimetres.
3.2. Ecological Risks Assessment of Toxic Heavy Metals in Carleton Community Garden Soil
The ecological risk factor (ER) and potential ecological risk index (PERI) were applied to evaluate the sensitivity of biological communities to toxic heavy metals. It was assessed based on Canadian soil quality guidelines and upper continental crust values. In this study, risk assessment was limited to metals with established toxicity response factors, and both ER and PERI were calculated using the mean concentrations of the selected metals (Table 3). Toxic heavy metals below limit of detection, with no reference values (either upper continental crust value or Canadian soil quality guidelines) were also not assessed. Based on upper continental crust values, the ER values of all selected toxic heavy metals in top soil were below 40 indicating low ecological risk. Their ER values showed the following descending order Pb (11.6) > Cu (11.1) > Be (9.9) > As (5.8) > Co (4.0) > Tl (1.4) > Zn (0.9) > V (0.6) = Cr (0.6) >= Mo (0.6) > Ba (0.09). The PERI value was 46.6 indicating low ecological risks. With reference to Canadian soil quality guidelines, the ER values for the majority of toxic metals were below 40 indicating low ecological risks except the value of Ni which was 85.6 indicating high ecological risk. Their ER values showed the following descending order Ni (85.5) > Pb (11.6) > As (6.0) > Cu (5.1) > Be (4.5) > Co (1.1) > Tl (1.0) > Zn (0.9) > V (0.6) = Cr (0.6) > Mo (0.3) > Ba (0.07). The PERI value was 117.3 which is an indication of low ecological risks. In this study Ni and Pb were the main contributing elements to the ecological risk. In subsoil, the ER values based on UCCV were also below 40 indicating low ecological risk. Their values were descending in order of Pb (11.6) > Cu (11.1) > Be (9.9) > As (5.8) > Co (4.0) > Zn (0.9) > Cr (0.6) = V (0.6) = Mo (0.6) > Tl (0.1) > Ba (0.09). The PERI value was 45.9 indicating low ecological risks. In line with CCME, the ER values were also below 40 indicating low ecological risks except the value of Ni which was 85.5 indicating high ecological risk. Their values as Ni (85.5) > Pb (11.6) > As (6.0) > Cu (5.1) > Be (4.5) > Co (1.1) > V (0.6) = Cr (0.6) > Zn (0.4) > Mo (0.3) > Tl (0.1) > Ba (0.07). The PERI values for these toxic elements were 115.7 showing low ecological risk. Majority of toxic heavy metals in this study showed low ecological risks which is similar to the study conducted in community gardens of Winnipeg, Manitoba, Canada [77]. In contrast to the findings of this study, a study conducted in Grand Forks County, North Dakota, reported low ecological risk (ER) for all investigated heavy metals in agricultural soils. Nevertheless, the same study observed low potential ecological risk index (PERI) values, which aligns with the results obtained in the present study [42]. Overall, the potential ecological risk assessment suggests that widespread ecosystem impairment is unlikely under the current conditions. However, the ecological risk is element-specific and non-uniform. Based on CCME guidelines, nickel (Ni) presents a high ecological risk and is the primary contributor, while other metals remain at low risk. The differences in ER rankings when using UCCV versus CCME reference values highlight that risk classification is highly dependent on the chosen benchmark; metals appearing acceptable against natural crustal backgrounds may still exceed regulatory thresholds. Additionally, the ecological risk patterns observed in both top and deep soils indicate that contamination is not limited to surface layers, likely resulting from historical inputs, post-fire deposition penetrating deeper soil horizons, or limited vertical attenuation. Accordingly, the community should prioritize Ni by mapping spatial hotspots, evaluating bioavailability and speciation, and assessing potential uptake by plants. Ni often exhibits high ecological risk due to its mobility and bioavailability in aquatic and soil environments. Once released, particularly from industrial sources such as electroplating, alloys, and battery production, Ni can easily migrate into the environment, i.e., soil, groundwater and surface water. Its soluble forms are readily taken up by plants and aquatic organisms, leading to bioaccumulation and trophic transfer, which threaten ecosystem integrity. Although the low total PERI suggests that broad-scale remediation may not be required, targeted interventions and monitoring are essential to mitigate Ni-driven ecological risks and limit potential transfer into the food chain. High Ni ecological risk can reduce crop yields, inhibit plant growth, and disrupt nutrient cycling by adversely affecting soil microbial activity. Furthermore, elevated Ni levels may favour metal-tolerant plant species, reducing biodiversity. Over time, Ni can accumulate in crops and enter the food chain, posing significant threats to ecosystem stability and human health [78].

Table 3.
Ecological risk of toxic heavy metals in top and deep soil from Carleton community garden based on ecological risk factor and potential ecological risk index.
3.3. Non-Carcinogenic and Carcinogenic Risks of Toxic Heavy Metals in Carleton Community Garden Soil
The non-carcinogenic and carcinogenic risks of toxic heavy metals in Carleton community garden soil were assessed through ingestion which is the most critical pathway for both children and adults (Table 4 and Table 5). In this study, only toxic metals with available reference dose, and slope factors were assessed. They were assessed using their average concentrations. In addition, toxic heavy metals below limit of detection were not assessed. For non-carcinogenic risks, the hazard quotient values for children revealed all elements to have no potential for non-carcinogenic risks except As which showed potential for non-carcinogenic risks in both top (1.4 × 100) and subsoil (1.5 × 100). In addition, the total hazard quotient of toxic heavy metals showed potential for non-carcinogenic risks in both top (2.5 × 100) and subsoil (3.0 × 100). For adult group, the hazard quotients of all toxic elements in top soil and subsoil were less than 1 which is a symbol of no possibility of non-carcinogenic risks. Their total hazard quotients were also less than 1 indicating no possibility of non-carcinogenic risks. The carcinogenic risks of toxic elements such as As, Cr, Pb, and Ni were assessed (Table 4). For children group, the carcinogenic risk of these toxic elements ranged from no carcinogenic risks to carcinogenic risks. As was the only element that showed carcinogenic risks in both top soil (2.0 × 10−3) and subsoil (3.0 × 10−4). Cr and Ni carcinogenic values showed an acceptable carcinogenic risk. Their total CR values showed carcinogenic risks in both top soil (2.0 × 10−3) and subsoil (3.6 × 10−4). In addition, their CR values showed the same descending trend As > Ni > Cr > Pb in both top soil and subsoil. For adult group, none of the toxic heavy metals showed a sign of carcinogenic risk in both top soil and subsoil. It is only Pb that showed no carcinogenic risk while other toxic heavy elements showed acceptable risks in both top soil and subsoil. Their total carcinogenic values also showed an acceptable risk. In addition, the CR values showed same descending trend As > Ni > Cr > Pb (Table 5). Several studies conducted in Winnipeg, Canada [79], Guelph, Canada [80], and Puerto Rico [81], corroborate the findings of this study, highlighting As as a major contaminant in urban garden soils and emphasizing the ingestion pathway as particularly critical for children due to their increased susceptibility. Similarly, Gao et al. [82] reported that As posed the highest non-carcinogenic risk in China, consistent with the results of this study. Conversely, a study in four potato-producing regions of China found that children were primarily exposed to significant non-carcinogenic risk from Cr via ingestion, which contrasts with the findings of the present study [4]. Additionally, Alsafran et al. [83] reported carcinogenic risks from As through ingestion in agricultural soils in Qatar, which was also reported in this study. The observed non-carcinogenic and carcinogenic risks of As in Carleton community garden soils can be attributed to both natural and anthropogenic sources. Geological parent materials as well as historical and ongoing industrial activities likely contributed to elevated As concentrations, resulting in heightened human health risks. The fire incident in September 2023 at the nearby iron and metal recycling facility may have released particulate-bound metals, including As, into surrounding soils. Previous episodes of explosions and fires in the area may also have similarly contributed to airborne particulate contamination in adjacent communities. Additionally, other factors such as historical use of arsenical pesticides and herbicides, industrial emissions, irrigation with As-contaminated water, and atmospheric deposition from nearby traffic or industrial sources may further elevate soil As levels [81]. These sources underscore the persistence of arsenic in community garden soils and the associated human health risks, particularly for vulnerable populations such as children. Arsenic, on the other hand, is especially dangerous for children’s health as ingestion is often enhanced by their hand-to-mouth behaviours [84]. Prolonged exposure to arsenic presents significant public health risks, including cancers of the skin, lung, and bladder, as well as non-cancer effects such as skin lesions, cardiovascular disease, diabetes, developmental toxicity, and neurological impairments, with children and pregnant women being especially susceptible [85]. The public health risk assessment from this study indicates that children are particularly at risk from both non-carcinogenic and carcinogenic effects of soil arsenic, with hazard quotient values exceeding safe limits and carcinogenic risk values within concerning ranges. In contrast, adults exhibited no notable non-carcinogenic or carcinogenic risks from the evaluated toxic metals in either top or deep soil layers. These findings emphasize arsenic as the primary contaminant of concern and children as the most vulnerable group, highlighting the necessity for targeted mitigation strategies and protective measures to reduce child exposure.

Table 4.
Non-carcinogenic risks of toxic heavy metals in Carleton community garden soil.

Table 5.
Carcinogenic risks of toxic heavy metals in Carleton community garden soil.
3.4. Implications for the Protection of Environmental and Public Health
The presence of Pb, Zn, Cu, and Sn at quantities above both Canadian soil quality norms and upper continental crust levels indicates localized pollution in Carleton community garden soils, which may have implications for environmental and ecosystem health. While the overall ecological risk index shows a low site-wide ecological hazard, the persistently high ecological risk linked with Ni in both top and deep soils indicate a specific worry. The significant ecological risk posed by Ni in our study is consistent with CCME guidelines, which lists nickel as a priority pollutant of concern in Canadian soils due to its potential toxicity to soil biota and accumulation in food crops [86]. Nickel’s mobility and potential for bioaccumulation can harm soil microbial populations, diminish plant yield, and transfer pollutants into the food chain [87]. Elevated levels of Pb, Zn, Cu, and Sn increase the danger of chronic exposure for gardeners, local people, and urban wildlife via soil ingestion, dust inhalation, or crop uptake. It may also cause water contamination through leaching and surface discharge. While Pb and Cu bind strongly to soil particles, acidic or low-organic-matter soils can increase their solubility, allowing them to migrate into groundwater. Zn and Sn are often more mobile, especially in acidic or sandy soils, making them more susceptible to surface runoff during rainstorm events. This transit has the potential to harm aquatic species through bioaccumulation and upset ecological balance in neighbouring streams, ponds, and stormwater systems [88]. In terms of health protection, this study’s finding of arsenic at levels high enough to pose both non-carcinogenic and carcinogenic risks to children is consistent with WHO’s concerns about arsenic (classified as a Group 1 human carcinogen) exposure through soil, water, and food [89]. The study found As as the most significant pollutant of concern in Carleton community garden soils, with children being the most exposed population. Children are especially vulnerable to arsenic exposure due to hand-to-mouth behaviour, and accidental dirt ingestion during recreational activities, with higher exposure per body weight than adults. Long-term arsenic exposure has been associated with a variety of negative health effects, including poorer neurodevelopment, lower cognitive function, and an increased risk of cancer [90,91,92,93,94]. These exceedances of international and national criteria in this study, underscore the importance of conducting soil remediation, regular monitoring, and protective community measures at Port Saint John. Interventions such as regular soil and garden produce monitoring, the use of raised beds with uncontaminated soil, and community education programs to reduce children’s direct exposure should be considered. Such precautionary actions are required to reduce long-term, irreversible developmental consequences and protect public health in vulnerable communities. Sustainable remediation strategies such as phytoremediation with hyperaccumulator plant species, soil amendments (e.g., biochar and compost), and raised-bed systems may reduce heavy metal bioavailability while improving soil fertility [15,95]. Integrating these ideas into community garden management plans can help to reduce environmental concerns, improve food safety, and assure the long-term viability of urban agriculture practices.
4. Conclusions
The study sought to assess the environmental and human health risks posed by toxic heavy metals in Carleton community garden soils affected by an industrial fire hazard in New Brunswick, Canada, and to determine their implications for environmental and public health protection. Based on the study’s findings, it is possible to conclude that, while most toxic heavy metals in Carleton community garden soils pose low ecological and health risks, arsenic is a significant contaminant to be concerned about, particularly for children who face both non-carcinogenic and carcinogenic risks. Nickel also posed an increased ecological risk, indicating potential long-term environmental consequences. These findings highlight the need of implementing targeted risk management techniques, such as regular soil monitoring, phytoremediation or soil replacement in contaminated plots, and limiting children’s direct contact to garden soils. Public awareness campaigns and policy initiatives should focus on lowering arsenic exposure, assuring food safety in community gardens, and promoting safe gardening techniques. Such actions are critical for protecting vulnerable people and preserving environmental and public health. Although this study has shed light on the occurrence, ecological and public health risks associated with heavy metals in community garden soil, it has some limitations. Lack of RfD and SF for some metals may make it impossible to quantify non-carcinogenic or carcinogenic risks for specific contaminants, leading to greater uncertainty and the potential underestimation of actual health risks. Physicochemical parameters, pollution level, and source apportionment were not assessed in this study. Therefore, to provide a more realistic estimate of exposure and risk, future research should focus on bio-accessibility tests such as simulated gastric fluid extractions. Inclusion of physicochemical parameters, pollution level, and source apportionment is crucial for a comprehensive understanding of soil quality and contamination. Special emphasis should be paid to the bioavailability and bioaccumulation of metals like arsenic and nickel in food plants, as these are the principal sources of human exposure. In addition, future research should include biomonitoring studies in local people, particularly children, to measure actual exposure levels using blood, urine, or hair analysis. It is recommended that the local population employ raised beds with clean soil for food production while also applying phytoremediation or soil remediation measures to minimize arsenic and nickel concentrations over time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12100362/s1, Table S1. Sample collection points; Table S2. Interpretation of ecological risks; Table S3. potential ecological risk index; Table S4. The toxicity response factors/values for selected heavy metals; Table S5. Input parameters for average daily dose estimation via ingestion pathway; Table S6. Toxic heavy metals oral reference doses (RfD) and cancer slope factors (CSF).
Author Contributions
Data collection: H.I. and S.A.O., Data analysis: I.M., Laboratory work: H.I. and S.A.O., Writing—original draft: I.M., Writing—review and editing: H.I., I.M. and S.A.O., Supervision: S.A.O., Administration: H.I. and I.M., Sourcing Fund: S.A.O. and H.I. All authors have read and agreed to the published version of the manuscript.
Funding
The Article Processing Charge (APC) was paid by the University of New Brunswick Saint John, Canada.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used in this study are included in the article and Supplementary Materials. However, further inquiries can be directed to the corresponding author.
Acknowledgments
We appreciate the support from the Central University of Technology, Free State’s University Research Grants and Scholarships Committee (RGSC). The authors also thank the University of New Brunswick Saint John, Canada for their unceasingly support and hosting author number 3. We also acknowledge Ramukumba Tshinanne for sharing her knowledge and skills in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Wang, Q.; Zhuang, W.; Yuan, Y.; Yuan, Y.; Jiao, K.; Wang, M.; Chen, Q. Calculation of Thallium’s toxicity coefficient in the evaluation of potential ecological risk index: A case study. Chemosphere 2018, 194, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Dong, T.; He, Y.; Qu, F. Detection of soil nitrogen using near infrared sensors based on soil pretreatment and algorithms. Sensors 2017, 17, 1102. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, S.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; He, X.; Li, W.; Zhang, M.; Cai, Q. Evaluation of heavy metal contamination of soil and the health risks in four potato-producing areas. Front. Environ. Sci. 2023, 11, 1071353. [Google Scholar] [CrossRef]
- Relic, D.; Sakan, S.; Anđelkovic, I.; Popovic, A.; Đorđevic, D. Pollution and Health Risk Assessments of Potentially Toxic Elements in Soil and Sediment Samples in a Petrochemical Industry and Surrounding Area. Molecules 2019, 24, 2139. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Subramanian, K.S.; Jessiman, B.J. A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci. Total Environ. 2001, 267, 125–140. [Google Scholar] [CrossRef]
- Manta, D.S.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy metals in urban soils: A case study from the city of Palermo (Sicily), Italy. Sci. Total Environ. 2002, 300, 229–243. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wang, L.; Cai, D.; Zhang, C.S.; Zhao, S.C. Risk assessment on dietary exposure to aflatoxin B1, heavy metals and phthalates in peanuts, a case study of Shandong province, China. J. Food Compos. Anal. 2023, 120, 105359. [Google Scholar] [CrossRef]
- Kizer, K.W. Extreme wildfires—A growing population health and planetary problem. JAMA 2020, 324, 1605–1606. [Google Scholar] [CrossRef]
- Singh, S.; Suresh, B.K.V. Forest fire susceptibility mapping for Uttarakhand state by using geospatial techniques. In Recent Technologies for Disaster Management and Risk Reduction; Springer International Publishing: Cham, Switzerland, 2021; pp. 173–188. [Google Scholar]
- Ozgeldinova, Z.; Mukayev, Z.; Zhanguzhina, A.; Ulykpanova, M.; Aidarkhanova, G. Impact of forest fire on the heavy metal content in the soil cover of the Amankaragay pine forest, Kostanay Region. J. Ecol. Eng. 2025, 26, 350–364. [Google Scholar] [CrossRef]
- Mouri, H. Medical Geology and its relevance in Africa. S. Afr. J. Sci. 2020, 116, 7699. [Google Scholar] [CrossRef]
- Mugudamani, I.; Oke, S.A.; Gumede, T.P. Influence of Urban Informal Settlements on Trace Element Accumulation in Road Dust and Their Possible Health Implications in Ekurhuleni Metropolitan Municipality, South Africa. Toxics 2022, 10, 253. [Google Scholar] [CrossRef]
- Hunter, C.M.; Williamson, D.H.Z.; Gribble, M.O.; Bradshaw, H.; Pearson, M.; Saikawa, E.; Ryan, P.B.; Kegler, M. Perspectives on Heavy Metal Soil Testing Among Community Gardeners in the United States: A Mixed Methods Approach. Int. J. Environ. Res. Public Health 2019, 16, 2350. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Shakya, K.M. Trace Metal Contamination in Community Garden Soils across the United States. Sustainability 2024, 16, 1831. [Google Scholar] [CrossRef]
- Nie, X.; Huang, X.; Li, M.; Lu, Z.; Ling, X. Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects. Toxics 2024, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D.; Gruss, I.; Szopka, K. Assessing Environmental Risks of Local Contamination of Garden Urban Soils with Heavy Metals Using Ecotoxicological Tests. Toxics 2024, 12, 873. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Gao, S.; Shan, C.J. Effects of sodium selenite on the antioxidant capacity and the fruit yield and quality of strawberry under cadmium stress. Sci. Hortic. 2020, 260, 108876. [Google Scholar] [CrossRef]
- Adewumi, A.J.; Ogundele, O.D. Hidden hazards in urban soils: A meta-analysis review of global heavy metal contamination (2010-2022), sources and its Ecological and health consequences. Sustain. Environ. 2024, 10, 2293239. [Google Scholar] [CrossRef]
- Mugudamani, I.; Oke, S.A.; Gumede, T.P.; Senbore, S. Herbicides in Water Sources: Communicating Potential Risks to the Population of Mangaung Metropolitan Municipality, South Africa. Toxics 2023, 11, 538. [Google Scholar] [CrossRef]
- Karthikayini, S.; Chandrasekaran, A.; Manjunatha Bennal, A.S. Ecological and human health risk assessment of heavy metal contamination in soils of major industrial estates of Tamil Nadu, India. Int. J. Environ. Anal. Chem. 2024, 1–20. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Izah, S.C.; Sawyer, W.E.; Amabie, T. Environmental Risk Assessment of Trace Metal Pollution: A Statistical Perspective. Environ. Geochem. Health 2025, 47, 94. [Google Scholar] [CrossRef]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, K.; Wang, M.; Wan, X.; Chen, W. Estimation of the accumulation rates and health risks of heavy metals in residential soils of three metropolitan cities in China. J. Environ. Sci. 2022, 115, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Pascaud, G.; Leveque, T.; Soubrand, M.; Boussen, S.; Joussein, E.; Dumat, C. Environmental and health risk assessment of Pb, Zn, As and Sb in soccer field soils and sediments from mine tailings: Solid speciation and bioaccessibility. Environ. Sci. Pollut. Res. Int. 2014, 21, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Praveena, S.M.; Abidin, E.Z.; Cheema, M.S. A review of heavy metals in indoor dust and its human health-risk implications. Rev. Environ. Health. 2016, 31, 447–456. [Google Scholar] [CrossRef]
- Bidar, G.; Pelfrêne, A.; Schwartz, C.; Waterlot, C.; Sahmer, K.; Marot, F.; Douay, F. Urban Kitchen Gardens: Effect of the Soil Contamination and Parameters on the Trace Element Accumulation in Vegetables–A Review. Sci. Total Environ. 2020, 738, 139569. [Google Scholar] [CrossRef]
- Bosiacki, M.; Bednorz, L.; Spiżewski, T. Concentration of Heavy Metals in Urban Allotment Soils and their Uptake by Selected Vegetable Crop Species-A Case Study from Gorzów Wielkopolski, Poland. J. Elem. 2022, 27, 405–421. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Zereini, F.; Püttmann, W. Metal and metalloid accumulation in cultivated urban soils: A medium-term study of trends in Toronto, Canada. Sci. Total Environ. 2015, 538, 564–572. [Google Scholar] [CrossRef]
- Cooper, A.M.; Felix, D.; Alcantara, F.; Zaslavsky, I.; Work, A.; Watson, P.L.; Pezzoli, K.; Yu, Q.; Zhu, D.; Scavo, A.J.; et al. Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: A case study of an urban community garden. Plant Direct 2020, 4, e00198. [Google Scholar] [CrossRef]
- Goswami, O.; Rouff, A.A. Soil Lead Concentration and Speciation in Community Farms of Newark. New Jersey, USA. Soil Syst. 2020, 5, 2. [Google Scholar] [CrossRef]
- Spliethoff, H.M.; Mitchell, R.G.; Shayler, H.; Marquez-Bravo, L.G.; Russell-Anelli, J.; Ferenz, G.; McBride, M. Estimated lead (Pb) exposures for a population of urban community gardeners. Environ. Geochem. Health 2016, 38, 955–971. [Google Scholar] [PubMed]
- Monfared, H.S. Community Garden Heavy Metal Contamination Study. Environ. Can. Ecol. Action Cent. 2011, 4–20. [Google Scholar]
- Montaño-López, F.; Biswas, A. Are heavy metals in urban garden soils linked to vulnerable populations? A case study from Guelph, Canada. Sci. Rep. 2021, 11, 11286. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, J.; Wang, Y.; Du, X.; Li, G.; Li, B. Identifying the Source of Heavy Metal Pollution and Apportionment in Agricultural Soils Impacted by Different Smelters in China by the Positive Matrix Factorization Model and the Pb Isotope Ratio Method. Sustainability 2021, 13, 6526. [Google Scholar] [CrossRef]
- Ferri, R.; Hashim, D.; Smith, D.R.; Guazzetti, S.; Donna, F.; Ferretti, E.; Curatolo, M.; Moneta, C.; Beone, G.M.; Lucchini, R.G. Metal contamination of home garden soils and cultivated vegetables in the province of Brescia, Italy: Implications for human exposure. Sci. Total Environ. 2015, 518–519, 507–517. [Google Scholar] [CrossRef]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Carleton Community Centre. About Us. 2025. Available online: https://www.carletoncommunitycentre.ca/about-us-1 (accessed on 26 August 2025).
- Tanoli, S.; Pickerill, R.K. Cambrian shelf deposits of the King Square Formation, Saint John Group, southern New Brunswick. Atlantic Geology. Atl. Geol. 1989, 25, 129–139. [Google Scholar] [CrossRef]
- Saleem, M.; Pierce, D.; Wang, Y.; Sens, D.A.; Somji, S.; Garrett, S.H. Heavy Metal(oid)s Contamination and Potential Ecological Risk Assessment in Agricultural Soils. J. Xenobiot. 2024, 14, 634–650. [Google Scholar] [CrossRef]
- Sanad, H.; Moussadek, R.; Mouhir, L.; Lhaj, M.O.; Zahidi, K.; Dakak, H.; Manhou, K.; Zouahri, A. Ecological and Human Health Hazards Evaluation of Toxic Metal Contamination in Agricultural Lands Using Multi-Index and Geostatistical Techniques across the Mnasra Area of Morocco’s Gharb Plain Region. J. Hazard. Mater. Adv. 2025, 18, 100724. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; da Silva, J.B.J.; dos Santos, I.F.; de Oliveira, O.M.C.; Cerda, V.; Antonio, F.S.; Queiroz, A.F.C. Use of pollution indices and ecological risk in the assessment of contamination from chemical elements in soils and sediments—Practical aspects. Trends Environ. Anal. Chem. 2022, 35, e00169. [Google Scholar] [CrossRef]
- Tytła, M.; Widziewicz-Rzońca, K. Ecological and human health risk assessment of heavy metals in sewage sludge produced in Silesian Voivodeship, Poland: A case study. Environ. Monit. Assess. 2023, 195, 1373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Li, X.; Zhang, Z.; Chen, Z.; Ren, D.; Zhang, S. Ecological and health risk assessments of heavy metals and their accumulation in a peanut-soil system. Environ. Res. 2024, 252, 118946. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, C.; Sönmez, V.Z.; Sivri, N. Potential Ecological Risk Assessment of Critical Raw Materials: Gallium, Gadolinium, and Germanium. Arch. Environ. Contam. Toxicol. 2023, 84, 368–376. [Google Scholar] [CrossRef]
- Shahla, K.; Sakine, S.; Gholamreza, M. Health and ecological risk assessment and simulation of heavy metal-contaminated soil of Tehran landfill. R. Soc. Chem. 2021, 11, 8080–8095. [Google Scholar]
- Liu, X.; Liu, F.; Huang, W.; Peng, J.; Shen, T.; He, Y. Quantitative Determination of Cd in Soil Using Laser-Induced Breakdown Spectroscopy in Air and Ar Conditions. Molecules 2018, 23, 2492. [Google Scholar] [CrossRef]
- Xu, D.; Gao, B.; Peng, W.; Liu, L.; Wu, W.; Liu, X. Boron toxicity coefficient calculation and application for ecological risk assessment in reservoir sediments. Sci. Total Environ. 2020, 739, 139703. [Google Scholar] [CrossRef]
- Gu, Y.G. Calculation of beryllium toxic factor for potential ecological risk evaluation: A case study. Environ. Technol. Innov. 2021, 21, 101361. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X. Quantitative source apportionment and ecological risk assessment of heavy metals in soil of a grain base in Henan Province, China, using PCA, PMF modeling, and geostatistical techniques. Environ. Monit. Assess. 2021, 193, 655. [Google Scholar] [CrossRef]
- Ennaji, W.; Barakat, A.; El Baghdadi, M.; Rais, J. Heavy metal contamination in agricultural soil and ecological risk assessment in the northeast area of Tadla plain, Morocco. J. Sediment. Environ. 2020, 5, 307–320. [Google Scholar] [CrossRef]
- Weissmannova, H.D.; Pavlovsky, J. Indices of soil contamination by heavy metals—Methodology of calculation for pollution assessment (minireview). Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef] [PubMed]
- Klik, B.K.; Gusiatin, Z.M.; Kulikowska, D. Suitability of environmental indices in assessment of soil remediation with conventional and next generation washing agents. Sci. Rep. 2020, 10, 20586. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, H.; Nawaz, R.; Nasim, I.; Irshad, M.A.; Irfan, A.; Khurshid, I.; Okla, M.K.; Wondmie, G.F.; Ahmed, Z.; Bourhia, M. Comprehensive human health risk assessment of heavy metal contamination in urban soils: Insights from selected metropolitan zones. Front. Environ. Sci. 2023, 11, 1260317. [Google Scholar] [CrossRef]
- Anaman, R.; Peng, C.; Jiang, Z.; Liu, X.; Zhou, Z.; Guo, Z.; Xiao, X. Identifying sources and transport routes of heavy metals in soil with different land uses around a smelting site by GIS based PCA and PMF. Sci. Total Environ. 2022, 823, 153759. [Google Scholar] [CrossRef]
- Liang, Q.; Tian, K.; Li, L.; He, Y.; Zhao, T.; Liu, B.; Wu, Q.; Huang, B.; Zhao, L.; Teng, Y. Ecological and human health risk assessment of heavy metals based on their source apportionment in cropland soils around an e-waste dismantling site, Southeast China. Ecotoxicol. Environ. Saf. 2022, 242, 113929. [Google Scholar] [CrossRef]
- Saleem, M.; Sens, D.A.; Somji, S.; Pierce, D.; Wang, Y.; Leopold, A.; Haque, M.E.; Garrett, S.H. Contamination Assessment and Potential Human Health Risks of Heavy Metals in Urban Soils from Grand Forks, North Dakota, USA. Toxics 2023, 11, 132. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; Office of Emergency and Remedial Response: Washington, DC, USA, 2002. Available online: https://epa.gov/superfund/superfund-soil-screening-guidance (accessed on 18 August 2025).
- Pavlovic, D.; Pavlovic, M.; Perovic’, V.; Mataruga, Z.; Cakmak, D.; Mitrovic, M.; Pavlovic, P. Chemical Fractionation, Environmental, and Human Health Risk Assessment of Potentially Toxic Elements in Soil of Industrialised Urban Areas in Serbia. Int. J. Environ. Res. Public Health 2021, 18, 9412. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Islam, S.M.; Ghosh, G.C.; Ghosh, P.; Zaman, S.; Hossain, M.R.; Habib, A.; Nice, M.S.; Rahman, M.S.; Islam, K.R.; et al. Receptor model-based sources and risks appraisal of potentially toxic elements in the urban soils of Bangladesh. Toxicol. Rep. 2023, 10, 308–319. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency (USEPA). IRIS Toxicological Review of Inorganic Arsenic (Final Report, 2025); U.S. EPA: Washington, DC, USA, EPA/635/R-25/005; 2025. Available online: https://iris.epa.gov/document/&deid=363892 (accessed on 19 August 2025).
- United State Environmental Protection Agency (USEPA). Assessment. Risk Assessment Guidance for Superfund (Rags), Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Interim. 2009. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf (accessed on 10 August 2025).
- Tan, B.; Wang, H.; Wang, X.; Ma, C.; Zhou, J.; Dai, X. Health Risks and Source Analysis of Heavy Metal Pollution from Dust in Tianshui, China. Minerals 2021, 11, 502. [Google Scholar] [CrossRef]
- Gabarrón, M.; Faz, A.; Martínez-Martínez, S.; Zornoza, R.; Acosta, J.A. Assessment of metals behaviour in industrial soil using sequential extraction, multivariable analysis and a geostatistical approach. J. Geochem. Explor. 2017, 172, 174–183. [Google Scholar] [CrossRef]
- McDonough, R.; Shakya, K.M. Trace Metal Contamination in Community Gardens in Pittsburgh, Pennsylvania. Environments 2025, 12, 159. [Google Scholar] [CrossRef]
- Bassetti, O.G.; McDonough, R.A.; Shakya, K.M. Soil contamination in community gardens of Philadelphia and Pittsburgh, Pennsylvania. Environ. Monit. Assess. 2023, 195, 782. [Google Scholar] [CrossRef]
- Bvenura, C.; Afolayan, A.J. Heavy metal contamination of vegetables cultivated in home gardens in the Eastern Cape. S. Afr. J. Sci. 2012, 108, 696. [Google Scholar] [CrossRef]
- CCME (Canadian Council of Ministers of the Environment). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2013; Available online: https://ccme.ca/en/current-activities/canadian-environmental-quality-guidelines (accessed on 8 August 2025).
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Sage, L.; Bassetti, O.; Johnson, E.; Shakya, K.; Weston, N. Assessment of heavy metal contamination in soil and produce of Philadelphia community gardens. Environ. Pollut. Bioavailab. 2023, 35, 2209283. [Google Scholar] [CrossRef]
- Evseev, A.V.; Krasovskaya, T.M. Toxic metals in soils of the Russian North. J. Geochem. Explor. 2017, 174, 128–131. [Google Scholar] [CrossRef]
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef]
- Cappuyns, V. Downstream Distribution and Postdepositional Mobilization of Cadmium in Alluvial Soils. Appl. Environ. Soil Sci. 2023, 2023, 9915654. [Google Scholar] [CrossRef]
- Senderewich, T.; Goltz, D.; Rodriguez-Gil, J.L.; Laird, B.; Prosser, R.S.; Hanson, M.L. Human health and environmental risk assessment of metals in community gardens of Winnipeg, Manitoba, Canada. Environ. Sci. Pollut. Res. 2024, 1, 20293–20310. [Google Scholar] [CrossRef]
- Rizwan, M.; Usman, K.; Alsafran, M. Ecological impacts and potential hazards of nickel on soil microbes, plants, and human health. Chemosphere 2024, 357, 142028. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Hossain, M.S.; Singh, B.; Ahmed, T.; Anawar, H.M.; Shakil, M.A. Toxic trace elements in urban community gardens in Winnipeg, Canada: Human health risk assessment. Environ. Sci. Pollut. Res. 2024, 31, 39152–39165. [Google Scholar]
- Antwi-Agyei, P.; Yousefi, M.; Guo, D.; Lake, C.B.; Bonnycastle, M.M. Soil contamination in community gardens in the city of Guelph, Canada: Implications for gardeners’ health and wellbeing. Int. J. Environ. Res. Public Health 2021, 18, 4932. [Google Scholar]
- Misenheimer, J.; Nelson, C.; Huertas, E.; Medina-Vera, M.; Prevatte, A.; Bradham, K. Total and Bioaccessible Soil Arsenic and Lead Levels and Plant Uptake in Three Urban Community Gardens in Puerto Rico. Geosciences 2018, 8, 43. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, D.; Proshad, R.; Uwiringiyimana, E.; Wang, Z. Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci. Rep. 2021, 11, 22824. [Google Scholar] [CrossRef]
- Alsafran, M.; Usman, K.; Al Jabri, H.; Rizwan, M. Ecological and Health Risks Assessment of Potentially Toxic Metals and Metalloids Contaminants: A Case Study of Agricultural Soils in Qatar. Toxics 2021, 9, 35. [Google Scholar] [CrossRef]
- Meharg, A.A.; Rahman, M.M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef]
- Demissie, S.; Mekonen, S.; Awoke, T.; Mengistie, B. Assessing Acute and Chronic Risks of Human Exposure to Arsenic: A Cross-Sectional Study in Ethiopia Employing Body Biomarkers. Environ. Health Insights 2024, 18, 11786302241257365. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment (CCME). Scientific Criteria Document for the Development of Soil Quality Guidelines for Nickel: Environmental and Human Health; CCME: Winnipeg, MB, Canada, 2015; Available online: https://ccme.ca/en/chemical/139 (accessed on 23 August 2025).
- Antoniadis, V.; Shaheen, S.M.; Boersch, J.; Frohne, T.; Du Laing, G.; Rinklebe, J. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around mining areas in Egypt. J. Environ. Manag. 2019, 251, 109572. [Google Scholar]
- Charlesworth, S.; Everett, M.; McCarthy, R.; Ordonez, A.; De Miguel, E. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ. Geochem. Health 2011, 33, 103–123. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Arsenic: Fact Sheet; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 23 August 2025).
- World Health Organisation (WHO). Human Health Effects of Benzene, Arsenic, Cadmium, Nickel, Lead and Mercury: Report of an Expert Consultation. 2024. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2023-8983-48755-72523?utm_source=chatgpt.com (accessed on 23 August 2025).
- Tian, Y.; Hou, Q.; Zhang, M.; Gao, E.; Wu, Y. Exposure to arsenic and cognitive impairment in children: A systematic review. PLoS ONE 2025, 20, e0319104. [Google Scholar] [CrossRef]
- Notario-Barandiaran, L.; Compañ-Gabucio, L.M.; Bauer, J.A.; Vioque, J.; Karagas, M.R.; Signes-Pastor, A.J. Arsenic Exposure and Neuropsychological Outcomes in Children: A Scoping Review. Toxics 2025, 13, 542. [Google Scholar] [CrossRef]
- Chmielewski, J.; Wszelaczyńska, E.; Pobereżny, J.; Florek-Łuszczki, M.; Gworek, B. Heavy Metals in Leafy Vegetables and Soft Fruits from Allotment Gardens in the Warsaw Agglomeration: Health Risk Assessment. Sustainability 2024, 17, 6666. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Di Stasio, L.; Gentile, A.; Tangredi, D.N.; Piccolo, P.; Oliva, G.; Vigliotta, G.; Cicatelli, A.; Guarino, F.; Guidi Nissim, W.; Labra, M.; et al. Urban Phytoremediation: A Nature-Based Solution for Environmental Reclamation and Sustainability. Plants 2025, 14, 2057. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).