Limestone Processing Sludge: From Waste to Sustainable Resource
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Moisture Content in the Sludge
2.2.2. Characterization of Starting Dry Sludge Particles and Milled Powders
2.2.3. Mechanosynthesis
3. Results
3.1. Sludge Characterization
3.2. Powder Particles Characterization After Drying
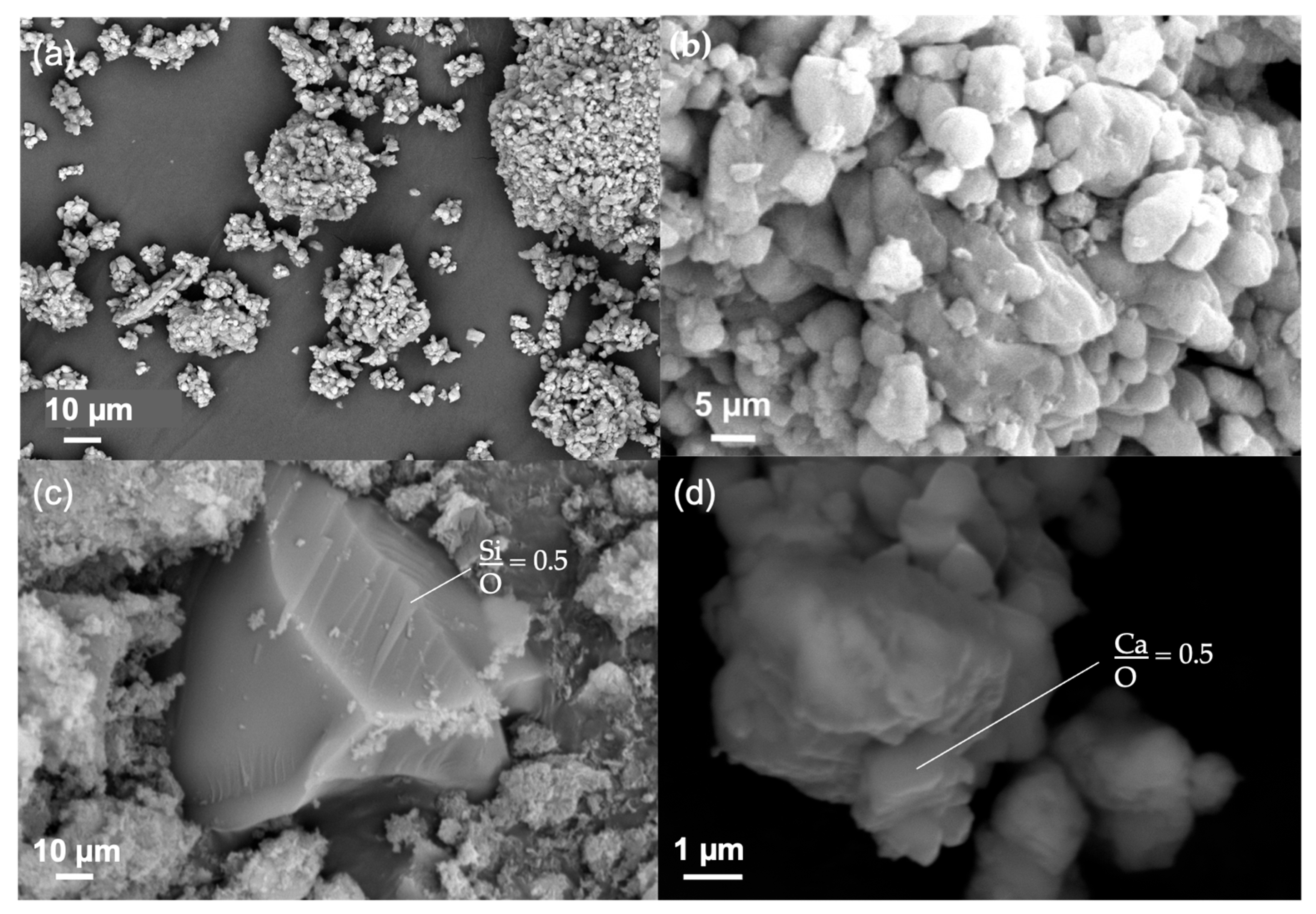
3.2.1. Particle Morphology
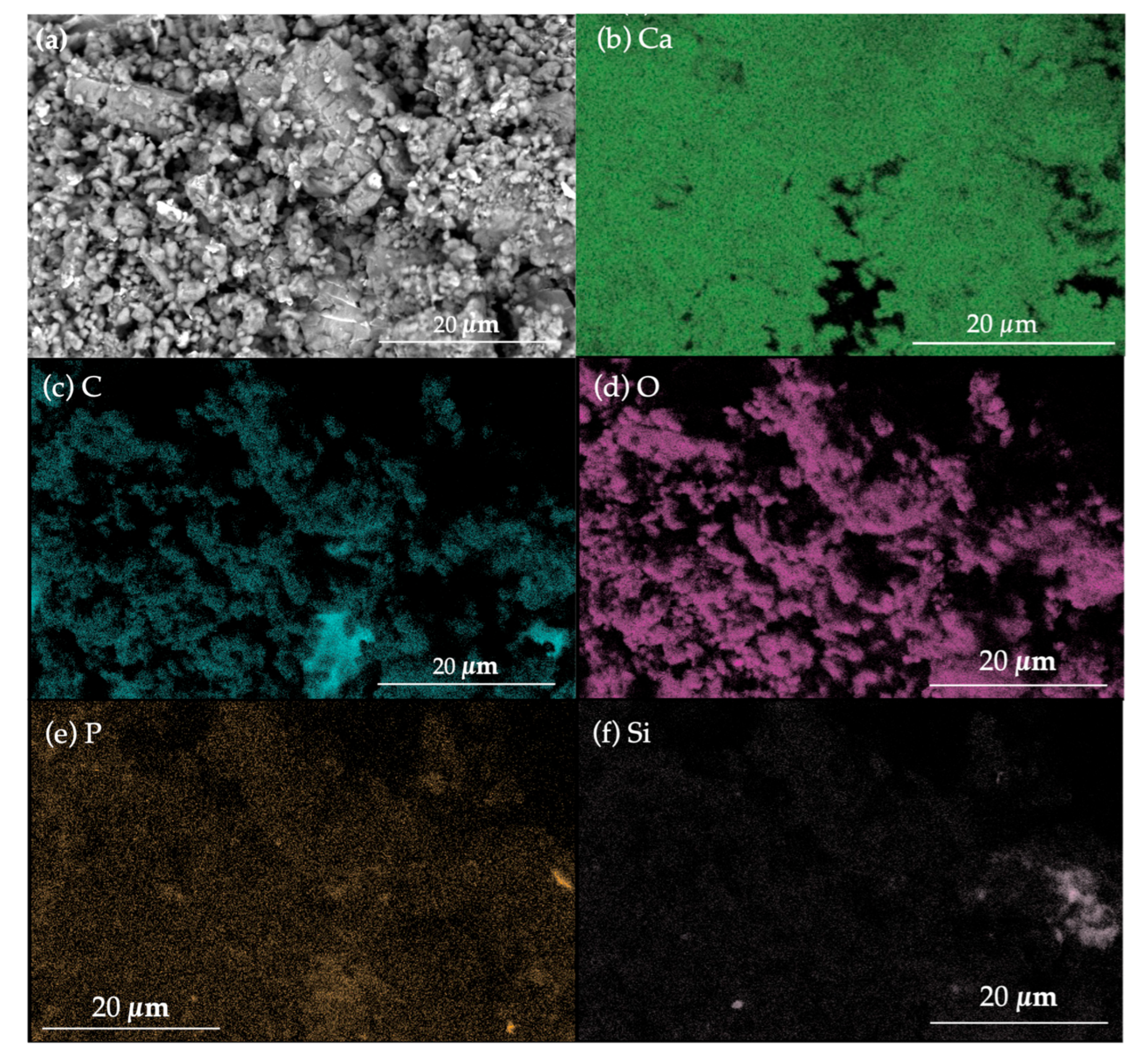
3.2.2. Chemical Composition and Crystalline Phases Present
3.3. Characterization of Milled Powders
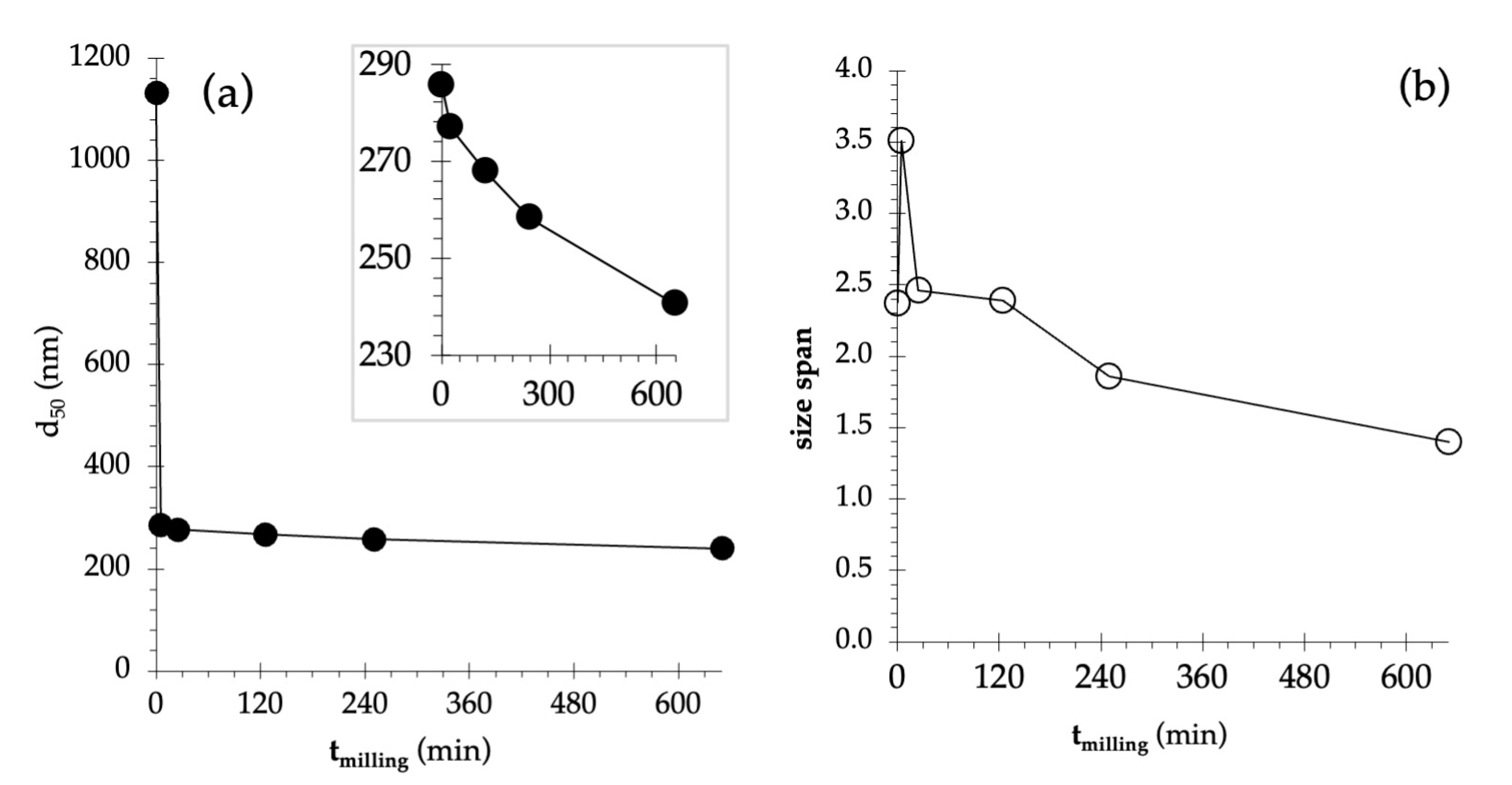
3.3.1. Particles’ Morphology
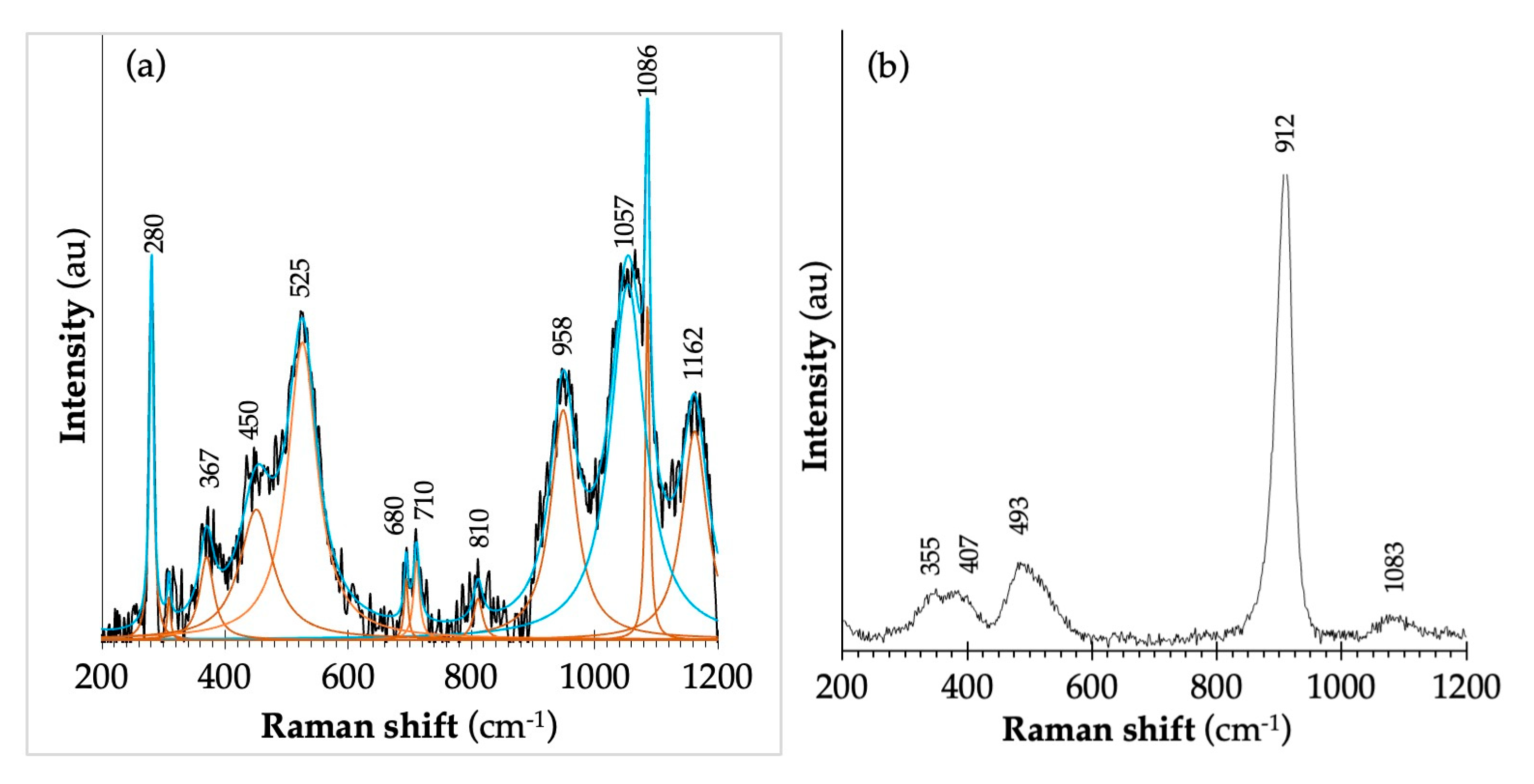
3.3.2. Phase Transformation During Milling
4. Discussion
4.1. Limestone Sludge as Calcium Source for Bioceramics
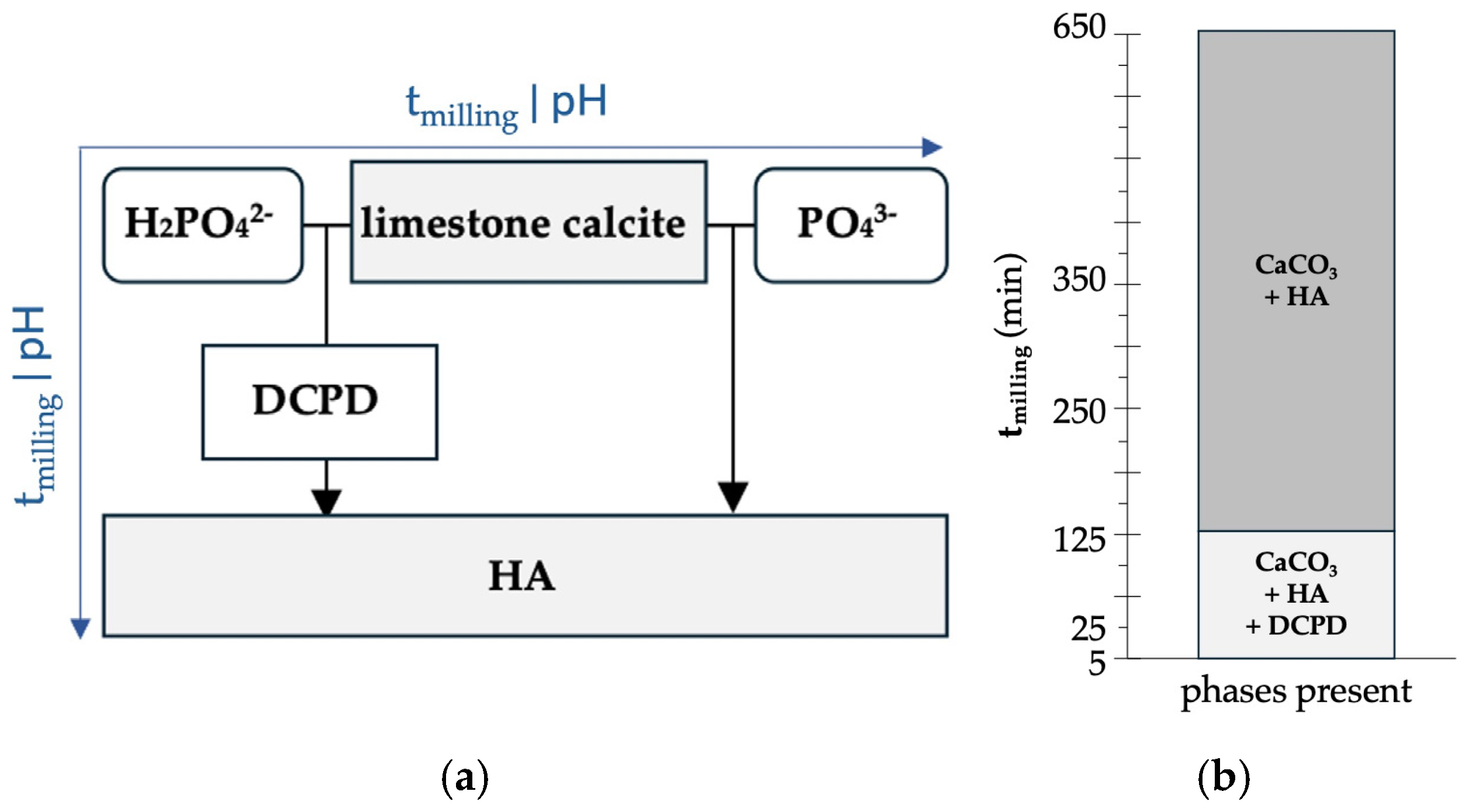
4.2. Mechanochemical Reaction Pathway and Phase Evolution
4.3. Microstructural and Functional Implications
4.4. Economic and Environmental Feasibility
4.5. Broader Context and Future Work
- Quantitative assessment of process scalability and energy demand.
- Sintering behavior and mechanical performance of HA ceramics derived from sludge.
- In vitro biocompatibility tests to confirm biomedical suitability.
- Detailed techno-economic and lifecycle analyses to validate the industrial potential of the proposed process.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oates, J.A.H. Lime and Limestone: Chemistry and Technology, Production and Uses, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tazzini, A.; Gambino, F.; Casale, M.; Dino, G.A. Managing Marble Quarry Waste: Opportunities and Challenges for Circular Economy Implementation. Sustainability 2024, 16, 3056. [Google Scholar] [CrossRef]
- Murali, G.; Wong, L.S.; Ramkumar, V.R.; Abid, S.R.; Karthik, S. From waste to resource recycled lime sludge: Sustainable low clinker cementitious binder, a comprehensive study on hydration, strength of concrete. J. Build. Eng. 2024, 86, 108935. [Google Scholar] [CrossRef]
- Tennis, P.D.; Thomas, M.D.A.; Weiss, W.J.; Farny, J.A.; Giannini, E.R. State-of-the-Art Report on Use of Limestone in Cements at Levels of Up to 15%; PCA R&D Report SN3148.03; America’s Cement Manufactures: Washington, DC, USA, 2024. [Google Scholar]
- Scioti, A.; Bernardo, G.; Mecca, I.; Fatiguso, F. Characterization of Stone Waste Sludge and Preliminary Investigation on Green Materials Based on Traditional Lime Putty for Sustainable Construction. Sustainability 2024, 16, 9173. [Google Scholar] [CrossRef]
- Mehta, D.; Palwal, D.; Sankhla, V.S. Marble Waste Management for Protection of Ecology and Environment. Int. J. Innov. Sci. Eng. Technol. 2020, 7, 2348–7968. [Google Scholar]
- Baker, R.J.; Leeuwen, J.; White, D.J. Applications for Reuse of Lime Sludge from Water Softening; Final Report for TR-535; Iowa State University: Ames, IA, USA, 2005. [Google Scholar]
- Liu, D.; Wang, Q.; Hu, A.; Wang, Z.; Zhang, Q.; Wang, L. Optimized model of sludge drying characteristics based on an experimental study of thickness, temperature, and humidity. J. Clean. Prod. 2023, 429, 139540. [Google Scholar] [CrossRef]
- Dubey, R.K.; Bhatta, B.D. Chemical and Instrumental Analysis of Limestone and Red Clay from Makwanpur District for Cement Production. J. Nepal Chem. Soc. 2023, 43, 46–52. [Google Scholar] [CrossRef]
- Onodagu, P.D.; Obi, K.O.; Aginam, C.H.; Okoye, M.U.; Chidolue, C.U. Understanding the Influence of Different Limestone Sources on Portland Limestone Cement Characteristics and Advancements in Green Cement. Int. J. Recent Res. Civ. Mech. Eng. 2024, 11, 39–47. [Google Scholar] [CrossRef]
- Kakali, G.; Tsivilis, S.; Kolovos, K. Factors Affecting the Reactivity of the CaO-SiO2-Al2O3-Fe2O3 Mixture. Key Eng. Mater. 2001, 206–213, 1899–1902. [Google Scholar] [CrossRef]
- Chang, Z.; Long, G.; Xie, Y.; Zhou, J.L. Recycling sewage sludge ash and limestone for sustainable cementitious material production. J. Build. Eng. 2022, 49, 104035. [Google Scholar] [CrossRef]
- Ganapathi, H.; Phukan, M. Environmental Hazards of Limestone Mining and Adaptive Practices for Environment Management Plan. In Environmental Processes and Management; Singh, R.M., Shukla, P., Singh, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 121–134. [Google Scholar] [CrossRef]
- Klemm, A.; Wiggins, D. Sustainability of natural stone as a construction material. In Sustainability of Construction Materials; Khatib, J.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 283–308. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; Brant, D.L.; Skousen, J.G. ACID MINE DRAINAGE TREATMENT WITH OPEN LIMESTONE CHANNELS. J. Am. Soc. Min. Reclam. 1996, 1996, 367–374. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; Skousen, J.G.; Simmons, J. Long-term performance of passive acid mine drainage treatment systems. Mine Water Environ. 2003, 22, 118–129. [Google Scholar] [CrossRef]
- Al-Hamaiedeh, H.D. Reuse of Marble Sludge Slime in Ceramic Industry. Jordan J. Civ. Eng. 2010, 4, 264–274. [Google Scholar]
- Amin, S.K.; Abdel Hamid, E.M.; El-Sherbiny, S.A.; Sibak, H.A.; Abadir, M.F. The use of sewage sludge in the production of ceramic floor tiles. HBRC J. 2018, 14, 309–315. [Google Scholar] [CrossRef]
- Careddu, N.; Siotto, G.; Siotto, R.; Tilocca, C. From landfill to water, land and life: The creation of the Centre for stone materials aimed at secondary processing. Resour. Policy 2013, 38, 258–265. [Google Scholar] [CrossRef]
- Khan, Z.; Gul, A.; Shah, S.A.A.; Qazi, S.; Wahab, N.; Badshah, E.; Naqash, T.; Shahzada, K. Utilization of Marble Wastes in Clay Bricks: A Step towards Lightweight Energy Efficient Construction Materials. Civ. Eng. J. 2021, 7, 1488–1500. [Google Scholar] [CrossRef]
- Walker, I.; Bell, R.; Rippy, K. Mineralization of alkaline waste for CCUS. npj Mater. Sustain. 2024, 2, 28. [Google Scholar] [CrossRef]
- Chai, S.Y.W.; How, B.S.; Chin, M.Y.; Ngu, L.H. Utilization of accelerated weathering of limestone captured carbon dioxide (CO2) with cement kiln dust to produce building material. J. Clean. Prod. 2024, 468, 143047. [Google Scholar] [CrossRef]
- Yurdakul, M. Natural stone waste generation from the perspective of natural stone processing plants: An industrial-scale case study in the province of Bilecik, Turkey. J. Clean. Prod. 2020, 276, 123339. [Google Scholar] [CrossRef]
- Dorozhkin, S. Medical Application of Calcium Orthophosphate Bioceramics. BIO 2011, 1, 1–51. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4) Scaffolds for Bone Tissue Engineering Applications. J. Biotechnol. Biomed. Sci. 2018, 1, 25–93. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Diez-Escudero, A.; Espanol, M.; Ginebra, M.P. High-aspect-ratio nanostructured hydroxyapatite: Towards new functionalities for a classical material. Chem. Sci. 2024, 15, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B. Hydroxyapatite as an advanced adsorbent for removal of heavy metal ions from water: Focus on its applications and limitations. Mater. Today Proc. 2021, 46, 11029–11034. [Google Scholar] [CrossRef]
- Jemli, Y.E.L.; Abdelouahdi, K.; Minh, D.P.; Barakat, A.; Solhy, A. Synthesis and Characterization of Hydroxyapatite and Hydroxyapatite-Based Catalysts. In Design and Applications of Hydroxyapatite-Based Catalysts; Wiley: Hoboken, NJ, USA, 2022; pp. 19–72. [Google Scholar] [CrossRef]
- Munirathinam, R.; Minh, D.P.; Nzihou, A. Calcium phosphates as a novel support material for catalysis in Fischer-Tropsh synthesis. J. Fundam. Renew. Energy Appl. 2017, 7, 51. [Google Scholar] [CrossRef]
- Jaffar, F.H.; Othman, M.H.D.; Ismail, N.J.; Puteh, M.H.; Kurniawan, T.A.; Abu Bakar, S.; Abdullah, H. Hydroxyapatite-based materials for adsorption, and adsorptive membrane process for heavy metal removal from wastewater: Recent progress, bottleneck and opportunities. J. Taiwan Inst. Chem. Eng. 2024, 164, 105668. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of 2’-fucosyllactose/difucosyllactose mixture as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05717. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Mechanochemical synthesis of hydroxyapatite using cuttlefish bone and chicken eggshell as calcium precursors. Mater. Sci. Eng. C 2019, 97, 124–140. [Google Scholar] [CrossRef]
- Ferro, A.C.; Seixas, T.; Guedes, M. Reaction path in the mechanosynthesis of calcium phosphates using a biogenic calcium source. Ceram. Int. 2024, 50, 282–292. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571. [Google Scholar] [CrossRef]
- Pham Minh, D.; Lyczko, N.; Sebei, H.; Nzihou, A.; Sharrock, P. Synthesis of calcium hydroxyapatite from calcium carbonate and different orthophosphate sources: A comparative study. Mater. Sci. Eng. B 2012, 177, 1080–1089. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Hsu, S.K.; Chang, Y.C.; Ho, W.F. Effects of heat treatment on the synthesis of hydroxyapatite from eggshell powders. Ceram. Int. 2015, 41, 10718–10724. [Google Scholar] [CrossRef]
- Brown, P.W.; Hocker, N.; Hoyle, S. Variations in Solution Chemistry During the Low-Temperature Formation of Hydroxyapatite. J. Am. Ceram. Soc. 1991, 74, 1848–1854. [Google Scholar] [CrossRef]
- Peng, S.Y.; Lin, Y.W.; Lin, Y.Y.; Lin, K.L. Hydrothermal synthesis of hydroxyapatite nanocrystals from calcium-rich limestone sludge waste: Preparation, characterization, and application for Pb2+ adsorption in aqueous solution. Inorg. Chem. Commun. 2024, 160, 111943. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- European Commission. LIFE Project. (2011–2014). Sludges from Agglomerated Stones Industry for Environmental Sustainability (LIFE10 ENV/IT/000346). 2025. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE10-ENV-IT-000346 (accessed on 28 September 2025).
- Arif, S. Synthesis of Biomaterial Hydroxyapatite from Limestone by Using Two-Step Conversion. Int. J. Sci. Eng. Inf. Technol. 2020, 5, 236–238. [Google Scholar] [CrossRef]
- El-Mehalawy, N.; Sayed, M.; Abou El-Anwar, E.A.; Soliman, A.A.F.; Naga, S.M. Preparation and characterization of bioceramic composites based on anorthite and β-TCP from dolomitic phosphate and kaolin rocks. Mater. Chem. Phys. 2024, 312, 128625. [Google Scholar] [CrossRef]
- Vu, H.H.T.; Khan, M.D.; Chilakala, R.; Lai, T.Q.; Thenepalli, T.; Ahn, J.W.; Park, D.U.; Kim, J. Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal. Sustainability 2019, 11, 1524. [Google Scholar] [CrossRef]
- Yatongchai, C.; Thavornyutikarn, B. Conversion of lime mud waste to hydroxyapatite biomaterials. Mater. Chem. Phys. 2021, 266, 124544. [Google Scholar] [CrossRef]
- ISO 638:2008; Paper, Board and Pulps—Determination of Dry Matter Content—Oven Drying Method. ISO: Geneva, Switzerland, 2008.
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bruker. X-ray Powder Diffraction in Pharma—Amorphous Content Determination and Degree of Crystallinity. Appl. Rep. XRD 2017, 36, 1–2. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffractom. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Yılmaz, Ö.; Yontem, E.; Ozdogru, Z. D1.3 Inventory of Raw Materials, Waste, and Energy Flows in Industrial Sectors—Summary Report. FISSAC Project, EKODENGE. 2018. Available online: https://fissacproject.eu/wp-content/uploads/2018/06/FISSAC-D1.3-Inventory-of-raw-materials-waste-and-energy-flows-in-industrial-sectors-Summary.pdf (accessed on 28 September 2025).
- Lewis, J.A. Colloidal Processing of Ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Kitanaka, R.; Tsuboi, M.; Ozaki, Y. Biogenic apatite in carbonate concretions with and without fossils investigated in situ by micro-Raman spectroscopy. Sci. Rep. 2023, 13, 9714. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive Ceramics: Physical Chemistry. In Comprehensive Biomaterials; Ducheyne, P., Healy, H.E., Hutmacher, D.W., Grainger, D., kirkpatrcik, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 187–221. [Google Scholar] [CrossRef]
- Rudolph, W.W. Raman- and infrared-spectroscopic investigations of dilute aqueous phosphoric acid solutions. Dalton Trans. 2010, 39, 9642. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X.; King, P.L. Accommodation of the carbonate ion in apatite: An FTIR and X-ray structure study of crystals synthesized at 2–4 GPa. Am. Mineral. 2004, 89, 1422–1432. [Google Scholar] [CrossRef]
- Paquette, J.; Reeder, R.J. Single-crystal X-ray structure refinements of two biogenic magnesian calcite crystals. Am. Mineral. 1990, 75, 1151–1158. [Google Scholar]
- Macphee, D.E.; Lachowski, E.E. Cement Components and Their Phase Relations. In Lea’s Chemistry of Cement and Concrete; Hewlett, P.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 95–129. [Google Scholar] [CrossRef]
- Gabbott, P. Fast Scanning DSC. In Principles and Applications of Thermal Analysis; Wiley: Hoboken, NJ, USA, 2008; pp. 51–86. [Google Scholar] [CrossRef]
- Allen, T. Particle Size Measurement, 2nd ed.; Chapman and Hall: London, UK, 1975. [Google Scholar] [CrossRef]
- Toshima, T.; Hamai, R.; Tafu, M.; Takemura, Y.; Fujita, S.; Chohji, T.; Tanda, S.; Li, S.; Qin, G.W. Morphology control of brushite prepared by aqueous solution synthesis. J. Asian Ceram. Soc. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Lago-Vila, M.; Rodríguez-Seijo, A.; Arenas-Lago, D.; Andrade, L.; Vega, M.F.A. Heavy metal content and toxicity of mine and quarry soils. J. Soils Sediments 2017, 17, 1331–1348. [Google Scholar] [CrossRef]
- ISO 13175-3:2012(E); Implants for Surgery—Calcium Phosphates—Part 3: Hydroxyapatite and Beta-Tricalcium Phosphate Bone Substitutes (13175-3). ISO: Geneva, Switzerland, 2012.
- Thomas, D.B.; McGoverin, C.M.; Fordyce, R.E.; Frew, R.D.; Gordon, K.C. Raman spectroscopy of fossil bioapatite—A proxy for diagenetic alteration of the oxygen isotope composition. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 310, 62–70. [Google Scholar] [CrossRef]
- Burany, S. Scanning Electron Microscopy and X-Ray Microanalysis. J. Goldstein, D. Newbury, D. Joy, C, Lyman, P. Echlin, E. Lifshin, L. Sawyer, and J. Michael. Kluwer Academic, Plenum Publishers, New York. Microsc. Microanal. 2003, 9, 484. [Google Scholar] [CrossRef]
- Ho, W.F.; Hsu, H.C.; Hsu, S.K.; Hung, C.W.; Wu, S.C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Andersen, C.B. Understanding Carbonate Equilibria by Measuring Alkalinity in Experimental and Natural Systems. J. Geosci. Educ. 2002, 50, 389–403. [Google Scholar] [CrossRef]
- Francis, M.D.; Webb, N.C. Hydroxyapatite formation from a hydrated calcium monohydrogen phosphate precursor. Calcif. Tissue Res. 1970, 6, 335–342. [Google Scholar] [CrossRef]
- de Groot, K. Effect of Porosity and Physicochemical Properties on the Stability, Resorption, and Strength of Calcium Phosphate Ceramics. Ann. N. Y. Acad. Sci. 1988, 523, 227–233. [Google Scholar] [CrossRef] [PubMed]
 HA (cod file 9003548). (b) Simultaneous DSC/TG curves of powder heated up to 1000 °C at 10 °C/min, including a magnification of the 280–480 °C temperature range.
HA (cod file 9003548). (b) Simultaneous DSC/TG curves of powder heated up to 1000 °C at 10 °C/min, including a magnification of the 280–480 °C temperature range.
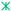 HA (cod file 9003548). (b) Simultaneous DSC/TG curves of powder heated up to 1000 °C at 10 °C/min, including a magnification of the 280–480 °C temperature range.
HA (cod file 9003548). (b) Simultaneous DSC/TG curves of powder heated up to 1000 °C at 10 °C/min, including a magnification of the 280–480 °C temperature range.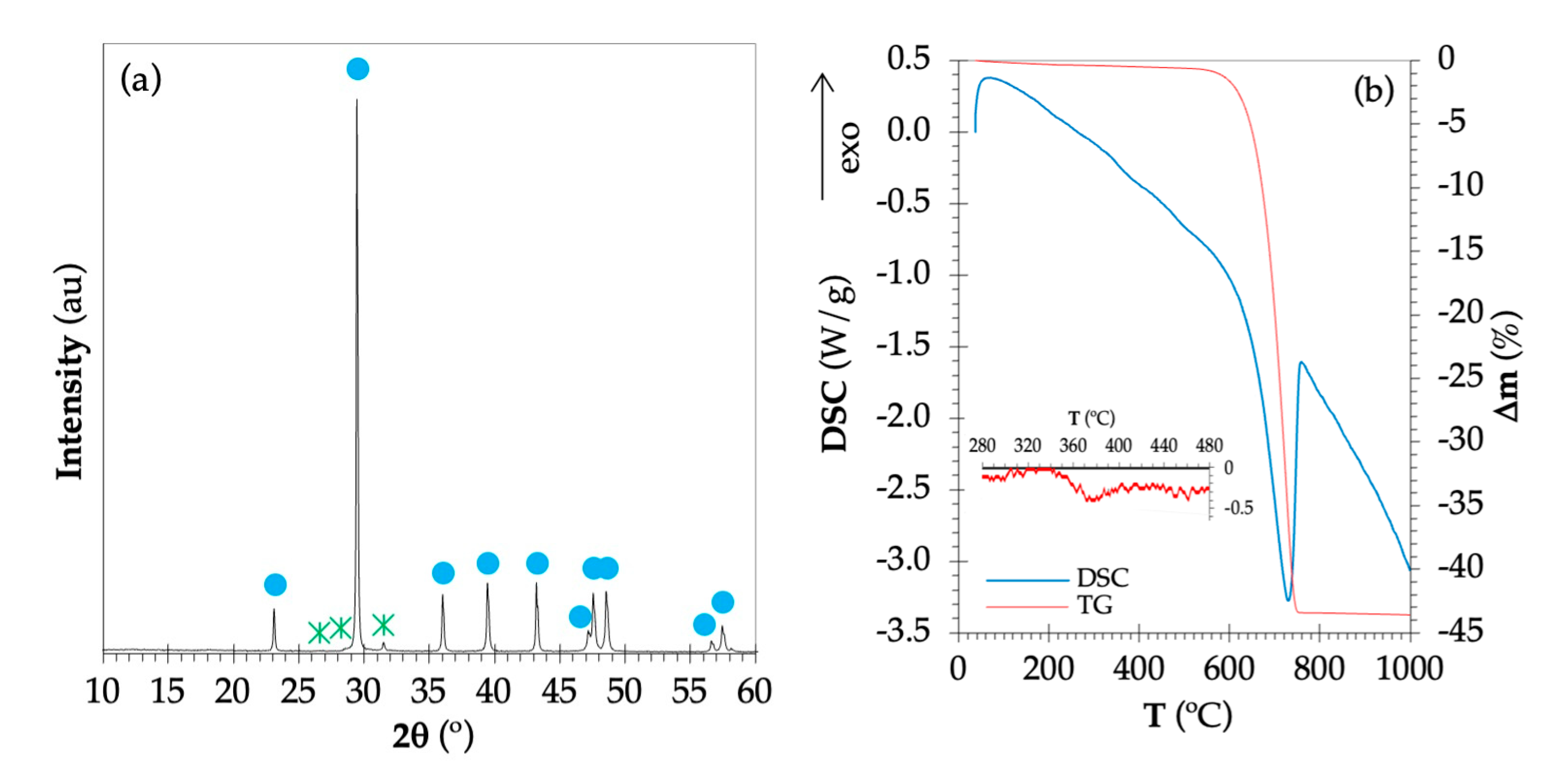
 HA (cod 9003548), and ■ DCPD (cod 1533075).
HA (cod 9003548), and ■ DCPD (cod 1533075).
 HA (cod 9003548), and ■ DCPD (cod 1533075).
HA (cod 9003548), and ■ DCPD (cod 1533075).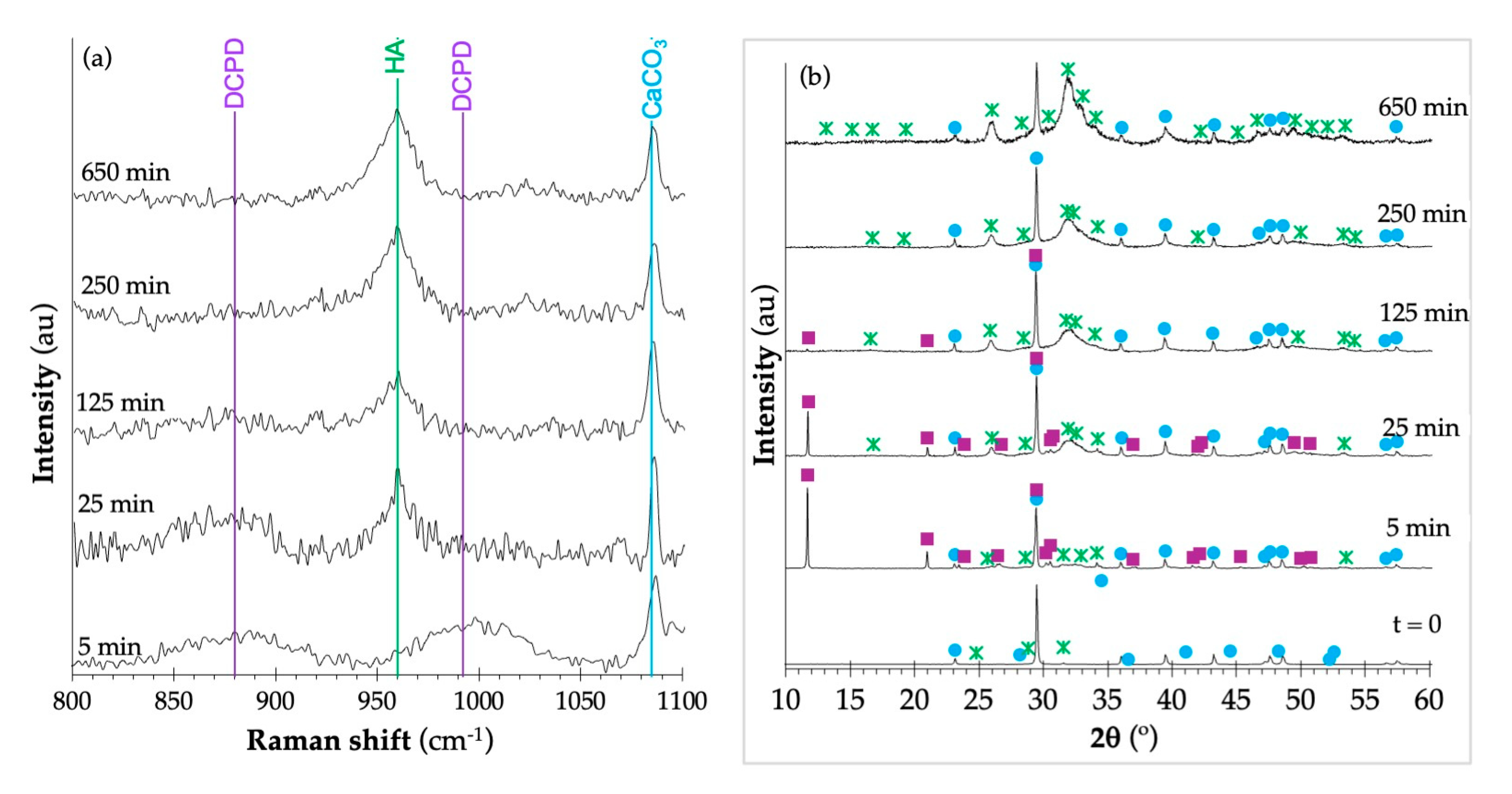
| Concentration | ||
|---|---|---|
| Sample 1 (mg/g) | Sample 2 (mg/g) | |
| Aluminum | 0.0877 ± 0.0027 | 0.0314 ± 0.0003 |
| Sulfur | 1.0928 ± 0.0588 | 3.582 ± 0.0260 |
| Mercury | <0.0014 | <0.00059 |
| Cadmium | <0.0004 | <0.00042 |
| Iron | 0.1865 ± 0.0306 | 0.233 ± 0.0007 |
| tmilling (min) | Phase (wt%) | Reliability Factors | |||||
|---|---|---|---|---|---|---|---|
| Calcite | HA | DCPD | Rwp | Rexp | χ2 | GoF | |
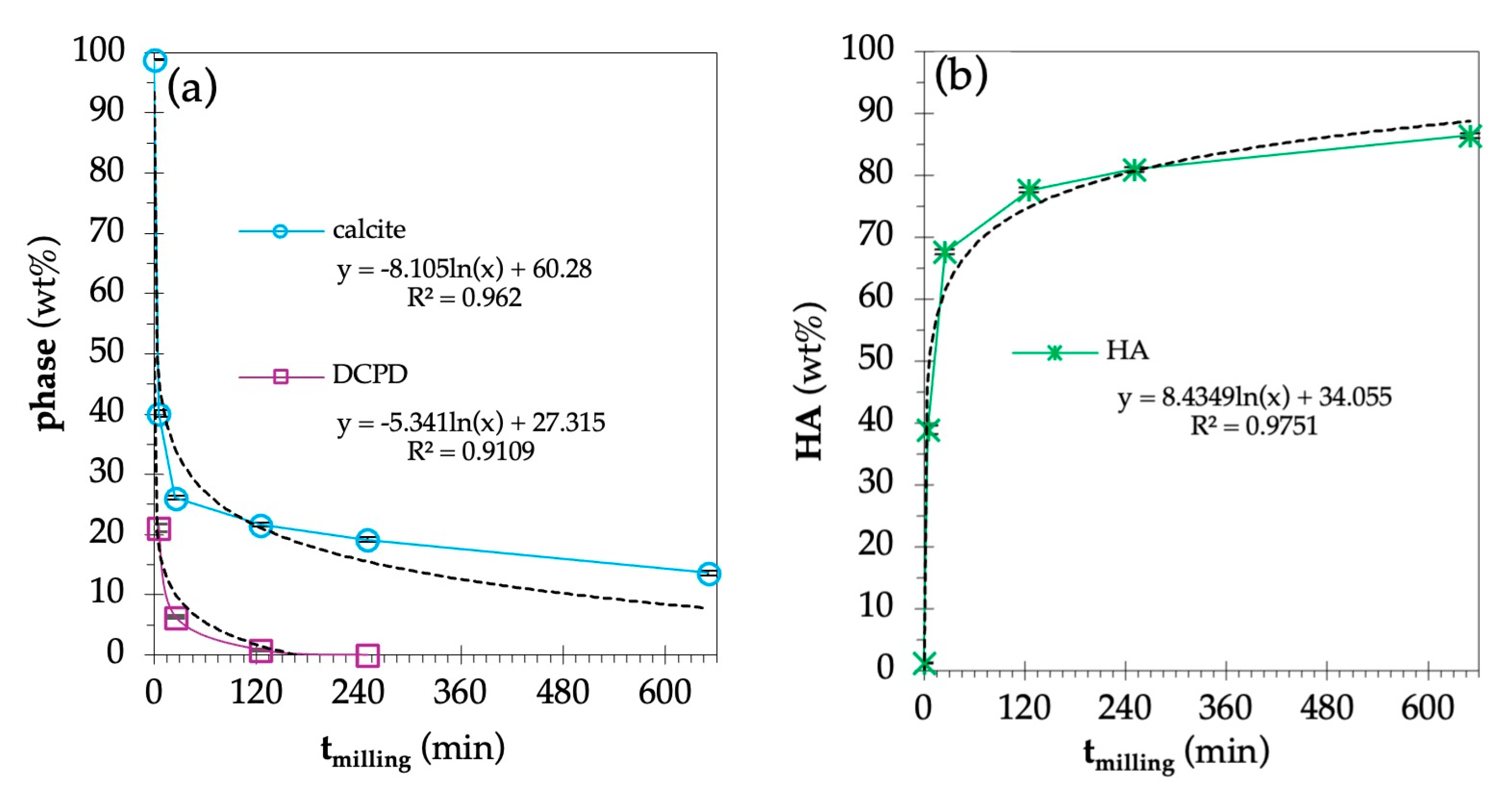
| 0 | 98.8 ± 0.1 | 1.2 ± 0.1 | - | 8.06 | 5.74 | 1.9717 | 1.4012 |
| 5 | 40.1 ± 0.6 | 38.8 ± 0.7 | 21.1 ± 0.7 | 12.1 | 6.35 | 4.0063 | 2.0016 |
| 25 | 26.1 ± 0.4 | 67.6 ± 0.4 | 6.3 ± 0.2 | 9.08 | 6.61 | 1.8870 | 1.3717 |
| 125 | 21.6 ± 0.3 | 77.6 ± 0.4 | 0.8 ± 0.1 | 7.75 | 6.58 | 1.3872 | 1.1778 |
| 250 | 19.1 ± 0.4 | 80.9 ± 0.4 | - | 7.60 | 6.40 | 1.4102 | 1.1875 |
| 650 | 13.6 ± 0.4 | 86.4 ± 0.4 | - | 7.89 | 6.05 | 1.7008 | 1.3041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, M.; Carrasqueira, J.; Seixas, T.; Afonso, C.; Gil, M.M.; Bernardino, R.; Gamboa, R.; Bernardino, S. Limestone Processing Sludge: From Waste to Sustainable Resource. Environments 2025, 12, 405. https://doi.org/10.3390/environments12110405
Guedes M, Carrasqueira J, Seixas T, Afonso C, Gil MM, Bernardino R, Gamboa R, Bernardino S. Limestone Processing Sludge: From Waste to Sustainable Resource. Environments. 2025; 12(11):405. https://doi.org/10.3390/environments12110405
Chicago/Turabian StyleGuedes, Mafalda, Joana Carrasqueira, Tomás Seixas, Clélia Afonso, Maria Manuel Gil, Raul Bernardino, Roberto Gamboa, and Susana Bernardino. 2025. "Limestone Processing Sludge: From Waste to Sustainable Resource" Environments 12, no. 11: 405. https://doi.org/10.3390/environments12110405
APA StyleGuedes, M., Carrasqueira, J., Seixas, T., Afonso, C., Gil, M. M., Bernardino, R., Gamboa, R., & Bernardino, S. (2025). Limestone Processing Sludge: From Waste to Sustainable Resource. Environments, 12(11), 405. https://doi.org/10.3390/environments12110405









