An Expedited Procedure to Highlight Rapid Recharge Processes by Means of Nitrate Pollution Dynamics in the Northern Italy Plain
Abstract
1. Introduction
1.1. Motivation
1.2. Methodological Approach
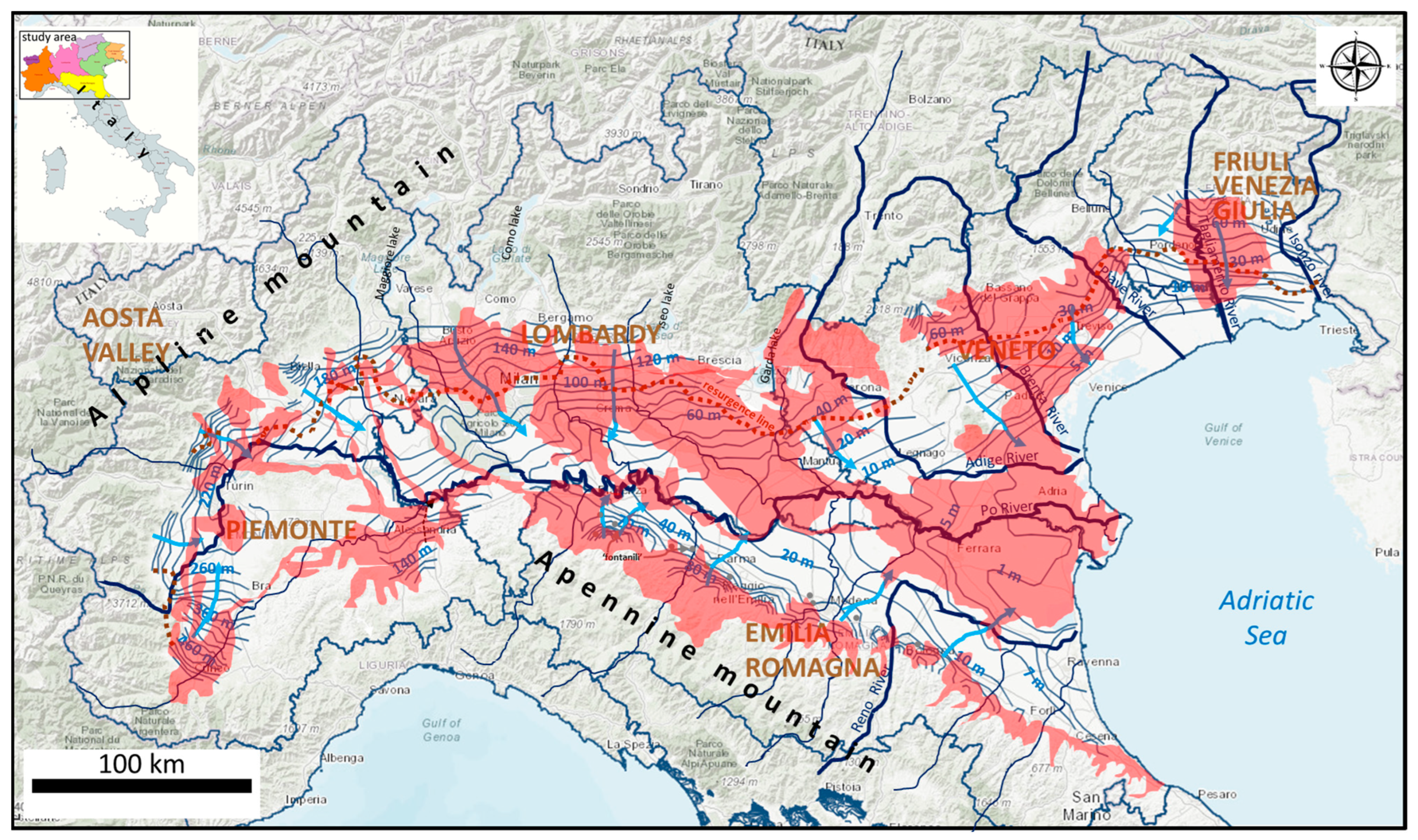
1.3. Study Area
2. Materials and Methods
2.1. Geological Framework
2.2. Hydrogeological Features
- (a)
- In the Friuli region (Figure 2, hydrogeological section A; [31]), north of the resurgence line (High Plain), the conceptual hydrogeological model comprises an unconfined undifferentiated aquifer (A), consisting of approximately 20 to 120 m of coarse-grained gravel and sandy sediments. Due to its high hydraulic conductivity (about 10−3 m/s), this unconfined aquifer is particularly vulnerable to surface pollution sources. Its primary recharge occurs through vertical infiltration of rainfall and lateral infiltration from the Tagliamento River bed (estimated flow of about 1.5 m3/s/km; in [31]). In general, the High Plain, corresponds to the recharge area of aquifers that develop in the Low Plain.
- (b)
- Similarly, in Emilia-Romagna region, as illustrated in hydrogeological section B of Figure 2, there is a transition from the undifferentiated unconfined aquifer system (A), developed in the area of the fluvial fans (High Plain), to the multi-aquifer system of the Lower Plain (aquifers A1 and A2).
- (a)
- the widespread prevalence of intensive agriculture and the extensive use of nitrate fertilizers, improper management of livestock waste, and leakage from urban sewage systems [39]. The areas adjacent to the Alps and the Apennines experience the most severe nitrate pollution, sometimes exceeding 100 mg/L; and
- (b)
- the high intrinsic vulnerability (Figure 3) of the indifferent aquifer, due to its high hydraulic conductivity, and of the unconfined aquifer systems, attributed to the shallow depth to the water table (1–2 m) and the lithological composition of the unsaturated zone (sandy or silty-sandy).
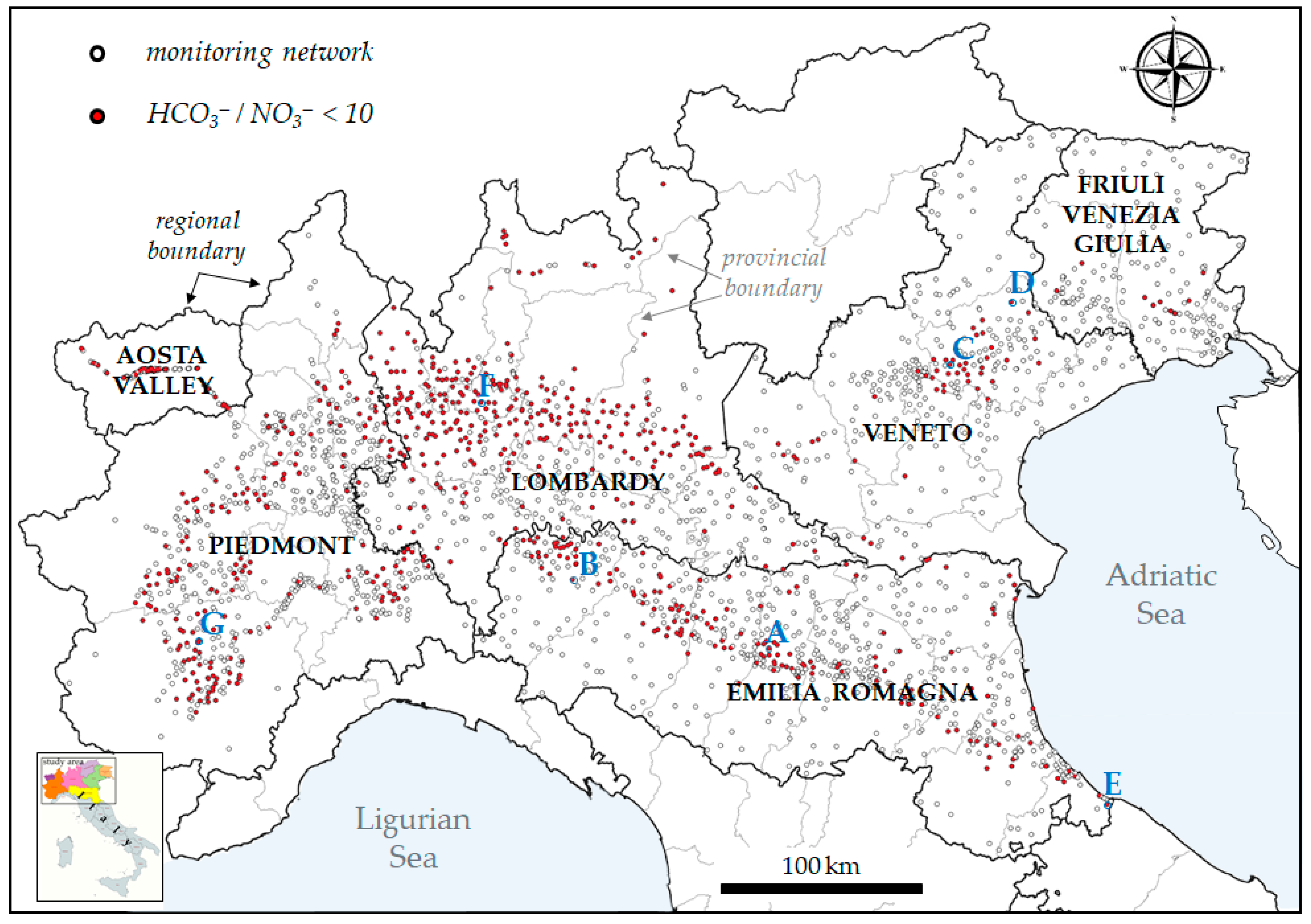
2.3. Sources and Description of Bicarbonate and Nitrate Data
3. Results
3.1. Criteria and Constraints
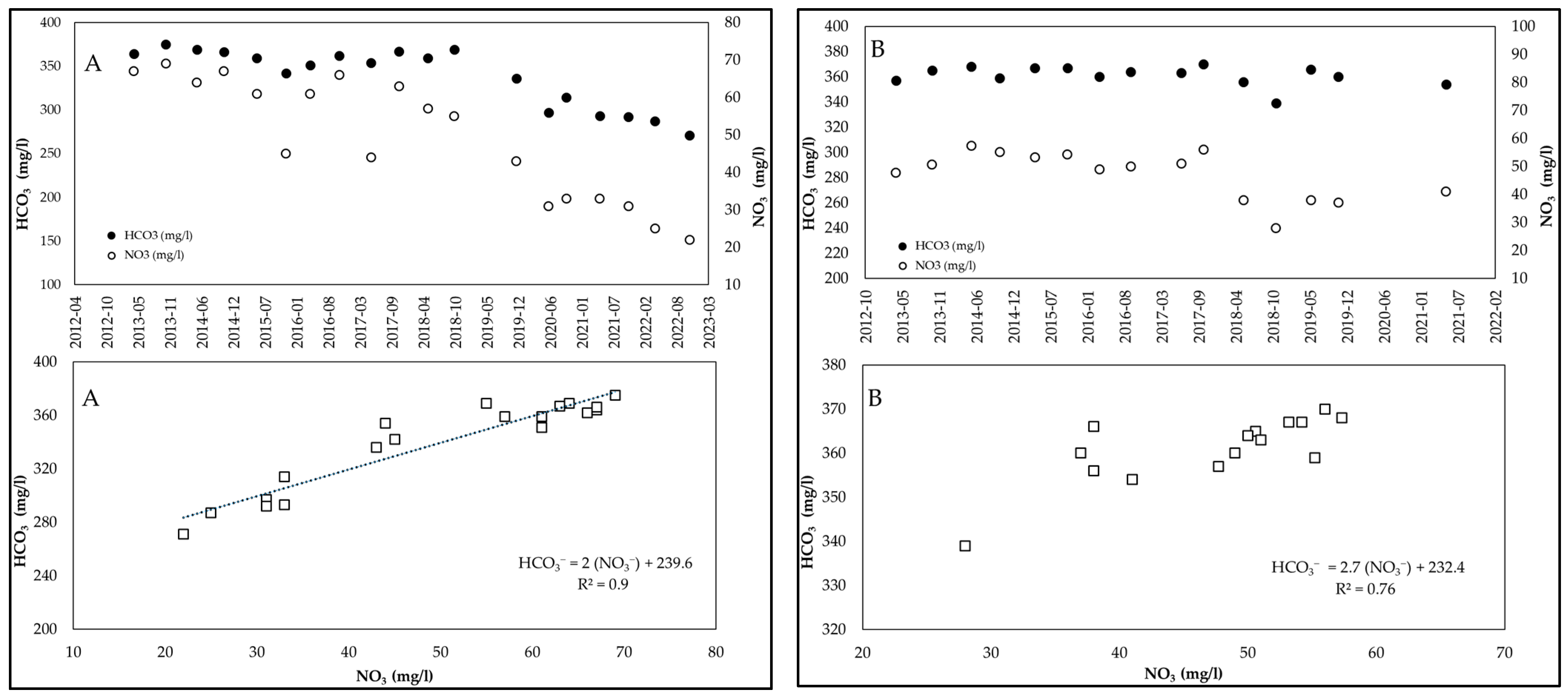
3.2. Examples of HCO3−/NO3− Trends Recorded in Wells of the Po Valley
4. Discussion
4.1. Sensitivity of Bicarbonate and Nitrate to Meteorological Factors: Theoretical Framework
4.2. Nitrate and Tritium in Groundwater Recharge Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. 35p. Available online: https://documents.un.org/doc/undoc/gen/n15/291/89/pdf/n1529189.pdf (accessed on 28 June 2025).
- Šimůnek, J.; Huang, K.; van Genuchten, M.T. The HYDRUS Code for Simulating the One-Dimensional Movement of Water, Heat, and Multiple Solutes in Variably-Saturated Media; Version 6.0; Department of Environmental Sciences, University of California Riverside: Riverside, CA, USA, 1998. [Google Scholar]
- Niswonger, R.; Prudic, D.; Regan, R. Documentation of the Unsaturated-Zone Flow (UZF1) Package for Modeling Unsaturated Flow Between the Land Surface and the Water Table with MODFLOW-2005; U.S. Geological Techniques and Methods Book 6; U.S. Geological Survey Publication: Reston, VA, USA, 2006; Chapter A19. [Google Scholar]
- Batelaan, O.; De Smedt, F. WetSpass: A Flexible, GIS Based, Distributed Recharge Methodology For Regional Groundwater Modelling; IAHS-AISH Publication: Maastricht, The Netherlands, 2001; Volume 269, pp. 11–18. [Google Scholar]
- Veeranna, J.; Jeet, P. Groundwater Recharges Technology for Water Resource Management: A Case Study. In Groundwater Management and Resources; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Barua, S.; Cartwright, I.; Dresel, P.E.; Daly, E. Using multiple methods to investigate the effects of land-use changes on groundwater recharge in a semi-arid area. Hydrol. Earth Syst. Sci. 2021, 25, 89–104. [Google Scholar] [CrossRef]
- Hartmann, A. The Hydrology of Groundwater Systems-From Recharge to Discharge. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 3, pp. 324–330. ISBN 9780128220412. [Google Scholar] [CrossRef]
- Han, R.; Liu, W.; Zhang, J.; Zhao, T.; Sun, H.; Xu, Z. Hydrogeochemical charac-teristics and recharge sources identification based on isotopic tracing of alpine rivers in the Tibetan Plateau. Environ. Res. 2023, 229, 115981. [Google Scholar] [CrossRef]
- Meadows, C.; Hagedorn, B. Temporal and Spatial Patterns of Groundwater Recharge Across a Small Watershed in the California Sierra Nevada Mountains. Front. Water 2022, 4, 815228. [Google Scholar] [CrossRef]
- Rapti-Caputo, D.; Martinelli, G. The geochemical and isotopic composition of aquifer systems in the deltaic region of the Po River plain (northern Italy). Hydrogeol. J. 2009, 17, 467–480. [Google Scholar] [CrossRef]
- Shi, P.; Huang, Y.; Yang, C.; Li, Z. Quantitative estimation of groundwater recharge in the thick loess deposits using multiple environmental tracers and methods. J. Hydrol. 2021, 603, 126895. [Google Scholar] [CrossRef]
- Yenehun, A.; Nigate, F.; Belay, A.S.; Desta, M.T.; Van Camp, M.; Walraevens, K. Groundwater recharge and water table response to changing conditions for aquifers at different physiography: The case of a semi-humid river catchment, northwestern highlands of Ethiopia. Sci. Total Environ. 2020, 748, 142243. [Google Scholar] [CrossRef]
- Gumuła-Kawęcka, A.; Jaworska-Szulc, B.; Szymkiewicz, A.; Gorczewska-Langner, W.; Pruszkowska-Caceres, M.; Angulo-Jaramillo, R.; Šimůnek, J. Estimation of ground-water recharge in a shallow sandy aquifer using unsaturated zone modeling and water table fluctuation method. J. Hydrol. 2022, 605, 127283. [Google Scholar] [CrossRef]
- Dekongmen, B.W.; Anornu, G.K.; Kabo-Bah, A.T.; Larbi, I.; Sunkari, E.D.; Dile, Y.T.; Agyare, A.; Gyamfi, C. Groundwater recharge estimation and potential recharge mapping in the Afram Plains of Ghana using SWAT and remote sensing techniques. Groundw. Sustain. Dev. 2022, 17, 1000741. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, X.; Wang, C.Y. Hydro-mechanical coupling in the shallow crust—Insight from groundwater level and satellite radar imagery in a mining area. J. Hydrol. 2021, 594, 125649. [Google Scholar] [CrossRef]
- Healy, R.W. Estimating Groundwater Recharge; Cambridge University Press: Cambridge, UK, 2010; 256p, ISBN 978-0521863964. [Google Scholar]
- Scanlon, B.R.; Healy, R.W.; Cook, P.G. Choosing appropriate techniques for quantifying groundwater recharge. Hydrogeol. J. 2002, 10, 18–39. [Google Scholar] [CrossRef]
- Robins, N.S. Recharge: The key to groundwater pollution and aquifer vulnerability. In Groundwater Pollution, Aquifer Recharge and Vulnerability; Robins, N.S., Ed.; Special Publications; Geological Society: London, UK, 1998; Volume 130, 234p, ISBN 978-1897799987. [Google Scholar]
- Simmers, I.; Hendrickx, J.M.H.; Kruseman, G.P.; Rushton, K.R. Recharge of Phreatic Aquifers in (Semi-) Arid Areas. In IAH International Contributions to Hydrogeology 19, 1st ed.; Simmers, I., Ed.; A.A.Balkema: Rotterdam, The Netherlands, 1997; ISBN 90-5410-694-8. [Google Scholar] [CrossRef]
- Smerdon, B.D. A synopsis of climate change effects on groundwater recharge. J. Hydrol. 2017, 555, 125–128. [Google Scholar] [CrossRef]
- Giuliano, G.; Mari, G.; Cavallin, A.; De Amicis, M. Ricerca sulla vulnerabilità naturale e sul rischio di inquinamento delle acque sotterranee nella pianura padana e veneto friulana: Carta della infiltrabilità regionale, carta idrogeologica regionale, Carta della vulnerabilità regionale (scala 1:500,000). Mem. Descr. Della Carta Geol. D’italia 1998, 56, 1–102. [Google Scholar]
- Martinelli, G.; Chahoud, A.; Dadomo, A.; Fava, A. Isotopic Features of Emilia-Romagna Region (North Italy) Groundwaters: Environmental and Climatological Implications. J. Hydrol. 2014, 519, 1928–1938. [Google Scholar] [CrossRef]
- Castiglioni, G.B.; Biancotti, A.; Bondesan, M.; Cortemiglia, G.C.; Elmi, C.; Favero, V.; Gasperi, G.; Marchetti, G.; Orombelli, G.; Pellegrini, G.B.; et al. Geomorphological map of the Po plain, Italy, at a scale of 1: 250 000. Earth Surf. Proc. Land. 1999, 24, 1115–1120. [Google Scholar] [CrossRef]
- Pavan, V.; Antolini, G.; Barbiero, R.; Berni, N.; Brunier, F.; Cacciamani, C.; Cagnati, A.; Cazzuli, O.; Cicogna, A.; De Luigi, C.; et al. High resolution climate precipitation analysis for north-central Italy, 1961–2015. Clim. Dyn. 2019, 52, 3435–3453. [Google Scholar] [CrossRef]
- AGIP Mineraria. Campi gassiferi Padani. [Gas fields in the Po Plain]. In I Giacimenti Gassiferi Dell’Europa Occidentale [The Gas Fields of Western Europe]; Accademia Nazionale dei Lincei ed ENI, Vol. 2, Atti del Convegno di Milano, 30 September, 5 October 1957; AGIP: Rome, Italy, 1959; pp. 45–497. [Google Scholar]
- Pieri, M.; Groppi, G. Subsurface Geological Structure of the Po Plain, Italy; Progetto finalizzato Geodinamica—Sottoprogetto 5—Modello Strutturale, C.N.R., Pubbl. 414; Verlag Nicht Ermittelbar: Roma, Italy, 1981. [Google Scholar]
- Livani, M.; Petracchini, L.; Benetatos, C.; Marzano, F.; Billi, A.; Carminati, E.; Doglioni, C.; Petricca, P.; Maffucci, R.; Codegone, G.; et al. Subsurface geological and geophysical data from the Po Plain and the northern Adriatic Sea (north Italy). Earth Syst. Sci. Data 2023, 15, 4261–4293. [Google Scholar] [CrossRef]
- Orombelli, G.; Ravazzi, C. The Late Glacial and Early Holocene: Chronology and paleoclimate. Alp. Mediterr. Quat. 1996, 9, 439–444. [Google Scholar]
- Florineth, D.; Schlüchter, C. Alpine evidence for atmospheric circulation patterns in Europe during the last glacial maximum. Quat. Res. 2000, 54, 295–308. [Google Scholar] [CrossRef]
- Ori, G.G. Continental Depositional Systems of the Quaternary of the Po Plain (Northern Italy). Sediment. Geol. 1993, 83, 1–14. [Google Scholar] [CrossRef]
- Rapti-Caputo, D.; Bratus, A.; Santarato, G. Strategic groundwater resources in the Tagliamento River basin (Northern Italy): Hydrogeological investigation integrated with geophysical exploration. Hydrogeol. J. 2009, 17, 1393–1409. [Google Scholar] [CrossRef]
- Rapti Caputo, D. Risorse Idriche Sotterranee a est di Ferrara: Indagini sul Comportamento Idrogeologico e Idrochimico. Proposte per una Gestione Ottimale. Doctoral Thesis, Università degli Studi di Ferrara, Ferrara, Italy, 2000; p. 215. [Google Scholar]
- Amorosi, A.; Farina, M.; Severi, P.; Preti, D.; Caporale, L.; Di Dio, G. Genetically related alluvial deposits across active fault zones: An example of alluvial fan-terrace correlation from the upper Quaternary of the southern Po Basin, Italy. Sediment. Geol. 1996, 102, 275–295. [Google Scholar] [CrossRef]
- Fontana, A.; Mozzi, P.; Marchetti, M. Alluvial Fans and Megafans along the Southern Side of the Alps. Sediment. Geol. 2014, 301, 150–171. [Google Scholar] [CrossRef]
- Marrocchino, E.; Rapti-Caputo, D.; Vaccaro, C. Chemical-mineralogical characterization as useful tool in the assessment of the decay of the Mesola Castle (Ferrara, Italy). Constr. Build. Mater. 2010, 24, 2672–2683. [Google Scholar] [CrossRef]
- Belli, M.; Centioli, D.; de Zorzi, P.; Sansone, U.; Capri, S.; Pagnotta, R.; Pettine, M. Metodi Analitici per le Acque; APAT-IRSA-CNR, Manuali e Linee Guida; I.G.E.R.: Roma, Italy, 2004; Volume 29, 110p, ISBN 88-448-0083-7. [Google Scholar]
- Orecchia, C.; Giambastiani, B.M.S.; Greggio, N.; Campo, B.; Dinelli, E. Geochemical Characterization of Groundwater in the Confined and Unconfined Aquifers of the Northern Italy. Appl. Sci. 2002, 12, 7944. [Google Scholar] [CrossRef]
- Hem, J. Study and Interpretation of the Chemical Characteristics of Natural Water; Geological Survey Water-Supply Paper 1473; United States Government Printing Office: Washington, DC, USA, 1979; 363p. [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po River, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Italian National Institute for Environmental Protection Research—I.S.P.R.A. Progress Status of Action Programs for the Protection and Remediation of Water from Pollution Caused by Nitrates from Agricultural Sources. Mignuoli, M.C., Peleggi, M., Salvati, S., Galanti, V., Martinelli, A., Eds.; 2023. Available online: https://indicatoriambientali.isprambiente.it/it/acque-interne/stato-di-avanzamento-dei-programmi-dazione-la-tutela-e-il-risanamento-delle-acque-dallinquinamento (accessed on 1 October 2025). (In Italian).
- Cheng, Z.; Su, C.; Zheng, Z.; Chen, Z.; Wei, W. Characterize groundwater vulnerability to intensive groundwater exploitation using tritium time-series and hydrochemical data in Shijiazhuang, North China Plain. J. Hydrol. 2021, 603, 126953. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Amsterdam, The Netherlands, 2009; 649p, ISBN 9780429152320. [Google Scholar] [CrossRef]
- Kessler, T.J.; Harvey, C.F. The global flux of carbon dioxide into groundwater. Geophys. Res. Lett. 2001, 28, 279–282. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1979; 604p, ISBN 978-0133653120. [Google Scholar]
- Rupert, M.G. Decadal-scale changes of nitrate in ground water of the United States, 1988–2004. J. Environ. Qual. 2008, 37, S240–S248. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, Y.; Cao, X.; Xiang, M.; Huang, Y.; Li, H. A Critical Review of Groundwater Table Fluctuation: Formation, Effects on Multifields, and Contaminant Behaviors in a Soil and Aquifer System. Environ. Sci. Technol. 2024, 58, 2185–2203. [Google Scholar] [CrossRef]
- Vicari, L.; Zavatti, A. Idrochimica. In Studi Sulla Vulnerabilità Degli Acquiferi, L’alta e Media Pianura Modenese; Paltrinieri, N., Pellegrini, M., Zavatti, A., Eds.; Pitagora Editrice: Bologna, Italy, 1990; pp. 27–72. ISBN 978-8837107109. [Google Scholar]
- Di Lorenzo, T.; Brilli, M.; Del Tosto, D.; Galassi, D.M.P.; Petitta, M. Nitrate source and fate at the catchment scale of the Vibrata River and aquifer (central Italy): An analysis by integrating component approaches and nitrogen isotopes. Environ. Earth Sci. 2012, 67, 2383–2398. [Google Scholar] [CrossRef]
- Severini, E.; Bartoli, M.; Pinardi, M.; Celico, F. Reactive silica traces manure spreading in alluvial aquifers affected by nitrate contamination: A case study in a high plain of northern Italy. Water 2020, 12, 2511. [Google Scholar] [CrossRef]
- Cocca, D.; Lasagna, M.; De Luca, D.A. Groundwater Chemical Trends Analyses in the Piedmont Po Plain (NW Italy): Comparison with Groundwater Level Variations (2000–2020). Water 2024, 16, 1240. [Google Scholar] [CrossRef]
- Baraza, T.; Cassidy, K.J.; Hasenmueller, E.A. Road salt applications mobilize trace elements from roadside soil to shallow groundwater. Sci. Total Environ. 2024, 942, 173435. [Google Scholar] [CrossRef]
- Broers, H.P. The spatial distribution of groundwater age for different geohydrological situations in the Netherlands: Implications for groundwater quality monitoring at the regional scale. J. Hydrol. 2004, 299, 84–106. [Google Scholar] [CrossRef]
- Pisinaras, V.; Polychronis, C.; Gemitzi, A. Intrinsic groundwater vulnerability determination at the aquifer scale: A methodology coupling travel time estimation and rating methods. Environ. Earth Sci. 2016, 75, 85. [Google Scholar] [CrossRef]
- Santoni, S.; Huneau, F.; Garel, E.; Vergnaud-Ayraud, V.; Labasque, T.; Aquilina, L.; Jaunat, J.; Celle-Jeanton, H. Residence time, mineralization processes and groundwater origin within a carbonate coastal aquifer with a thick unsaturated zone. J. Hydrol. 2016, 540, 50–63. [Google Scholar] [CrossRef]
- Gurdak, J.J.; Qi, S.L. Vulnerability of recently recharged groundwater in principal [corrected] aquifers of the United States to nitrate contamination. Environ. Sci. Technol. 2012, 46, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- Rapti, D.; Martinelli, G.; Zheng, G.; Vincenzi, C. Bottled Mineral Waters as Unconventional Sampling in Hydro-Geological Research. Water 2023, 15, 3466. [Google Scholar] [CrossRef]
- Gerhart, J.M. Ground-Water Recharge and Its Effects on Nitrate Concentration Beneath a Manured Field Site in Pennsylvania. Groundwater 1986, 24, 483–489. [Google Scholar] [CrossRef]
- Hess, L.J.T.; Hinckley, E.-L.; Robertson, G.P.; Matson, P.A. Rainfall intensification increases nitrate leaching from tilled but not no-till cropping systems in the U.S. Midwest. Agric. Ecosyst. Environ. 2020, 290, 106747. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S. Extreme precipitation accelerates the contribution of nitrate sources from anthropogenetic activities to groundwater in a typical headwater area of the North China Plain. J. Hydrol. 2021, 603, 127110. [Google Scholar] [CrossRef]
- Brkić, Z.; Larva, O.; Kuhta, M. Groundwater age as an indicator of nitrate concentration evolution in aquifers affected by agricultural activities. J. Hydrol. 2021, 602, 126799. [Google Scholar] [CrossRef]
- Ravikumar, P.; Somashekar, R.K. Environmental Tritium (3H) and hydrochemical investigations to evaluate groundwater in Varahi and Markandeya River basins, Karnataka, India. J. Environ. Radioact. 2011, 102, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, J.; Deinhart, A.L.; Visser, A.; Bibby, R.K.; Purtschert, R.; Moran, J.E.; Massoudieh, A.; Esser, B.K. Nitrate vulnerability projections from Bayesian inference of multiple groundwater age tracers. J. Hydrol. 2016, 543, 167–181. [Google Scholar] [CrossRef]
- Martinez-Salvador, C.; Moreno-Gomez, M.; Liedl, R. Estimating pollutant residence time and NO3 concentrations in the Yucatan karst aquifer; considerations for an integrated karst aquifer vulnerability methodology. Water 2019, 11, 1431. [Google Scholar] [CrossRef]
- McNeill, G.W.; Anderson, J.; Elliot, T. Nitrate levels and the age of groundwater from the upper Devonian sandstone aquifer in Fife, Scotland. Environ. Geochem. Health 2003, 25, 105–113. [Google Scholar] [CrossRef]
- Castaldo, G.; Visser, A.; Fogg, G.F.; Harter, T. Effect of Groundwater Age and Recharge Source on Nitrate Concentrations in Domestic Wells in the San Joaquin Valley. Environ. Sci. Technol. 2021, 55, 2265–2275. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; ZCai, Z.; Freney, J.R.; Martinelli, L.; Seitzinger, S.P.; Sutton, M. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Zuppi, G.M.; Sacchi, E. Hydrogeology as a climate recorder: Sahara-Sahel (North Africa) and the Po Plain (Northern Italy). Glob. Planet. Change 2004, 40, 79–91. [Google Scholar] [CrossRef]
- Pilla, G.; Sacchi, E.; Zuppi, G.; Braga, G.; Ciancetti, G. Hydrochemistry and Isotope Geochemistry as Tools for Groundwater Hydrodynamic Investigation in Multilayer Aquifers: A Case Study from Lomellina, Po Plain, South-Western Lombardy, Italy. Hydrogeol. J. 2006, 14, 795–808. [Google Scholar] [CrossRef]
- Martinelli, G.; Dadomo, A.; De Luca, D.A.; Mazzola, M.; Lasagna, M.; Pennisi, M.; Pilla, G.; Sacchi, E.; Saccon, P. Nitrate Sources, Accumulation and Reduction in Groundwater from Northern Italy: Insights Provided by a Nitrate and Boron Isotopic Database. Appl. Geochem. 2018, 91, 23–35. [Google Scholar] [CrossRef]
- Martinelli, G.; Cervi, F.; Dadomo, A.; Medioli, G. A preliminary assessment of young water fractions in groundwater from alluvial aquifers facing the northern Italian Apennines. Water 2022, 14, 659. [Google Scholar] [CrossRef]
- ARPAe Database. 2014. Available online: https://dati.arpae.it/dataset/rete-regionale-per-la-qualita-ambientale-acque-sotterranee-dati-2014 (accessed on 28 October 2024).
- World Commission on Environment and Development. Our Common Future; Oxford University Press: Oxford, MI, USA, 1987; 247p. [Google Scholar]


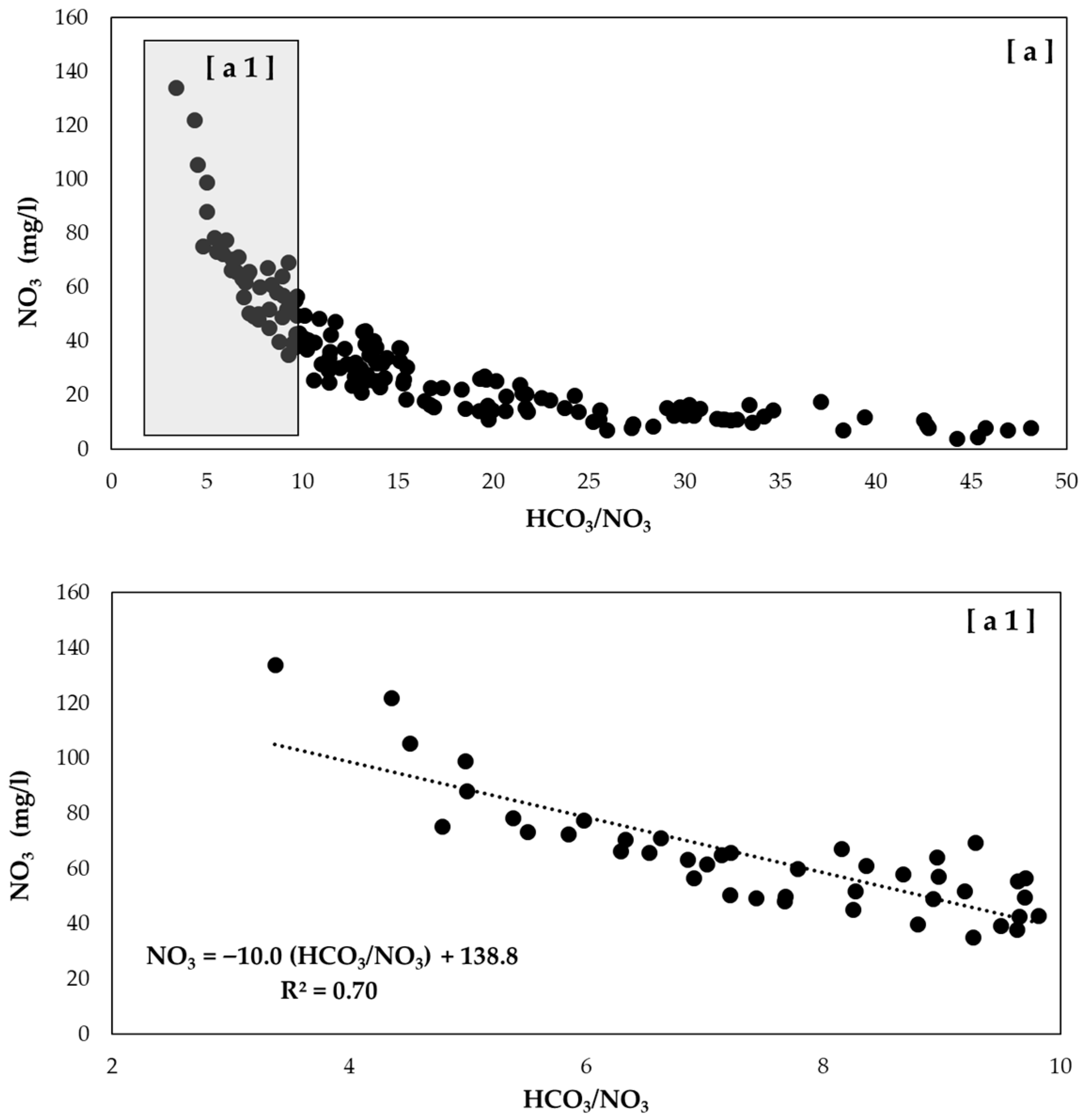

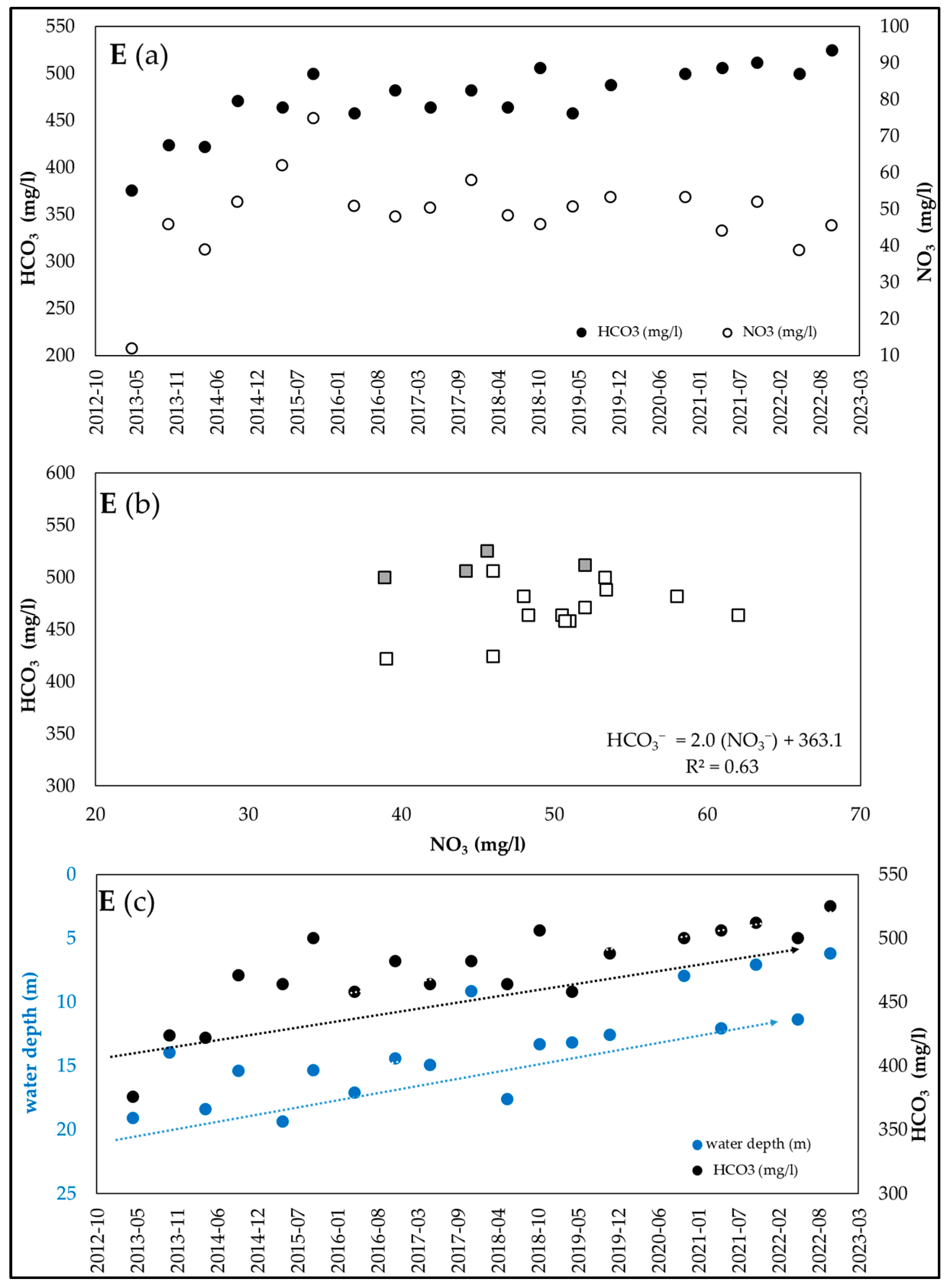
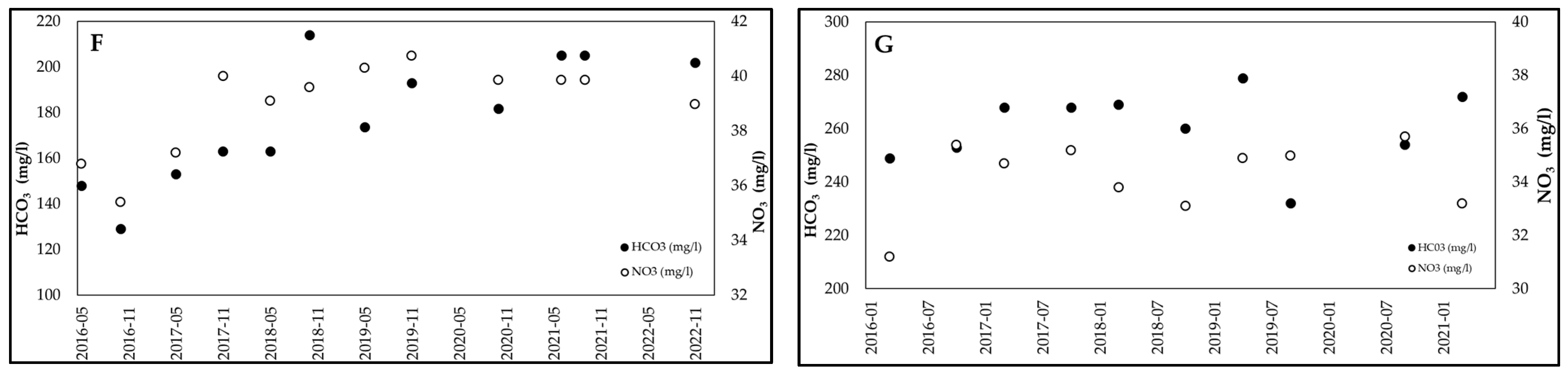
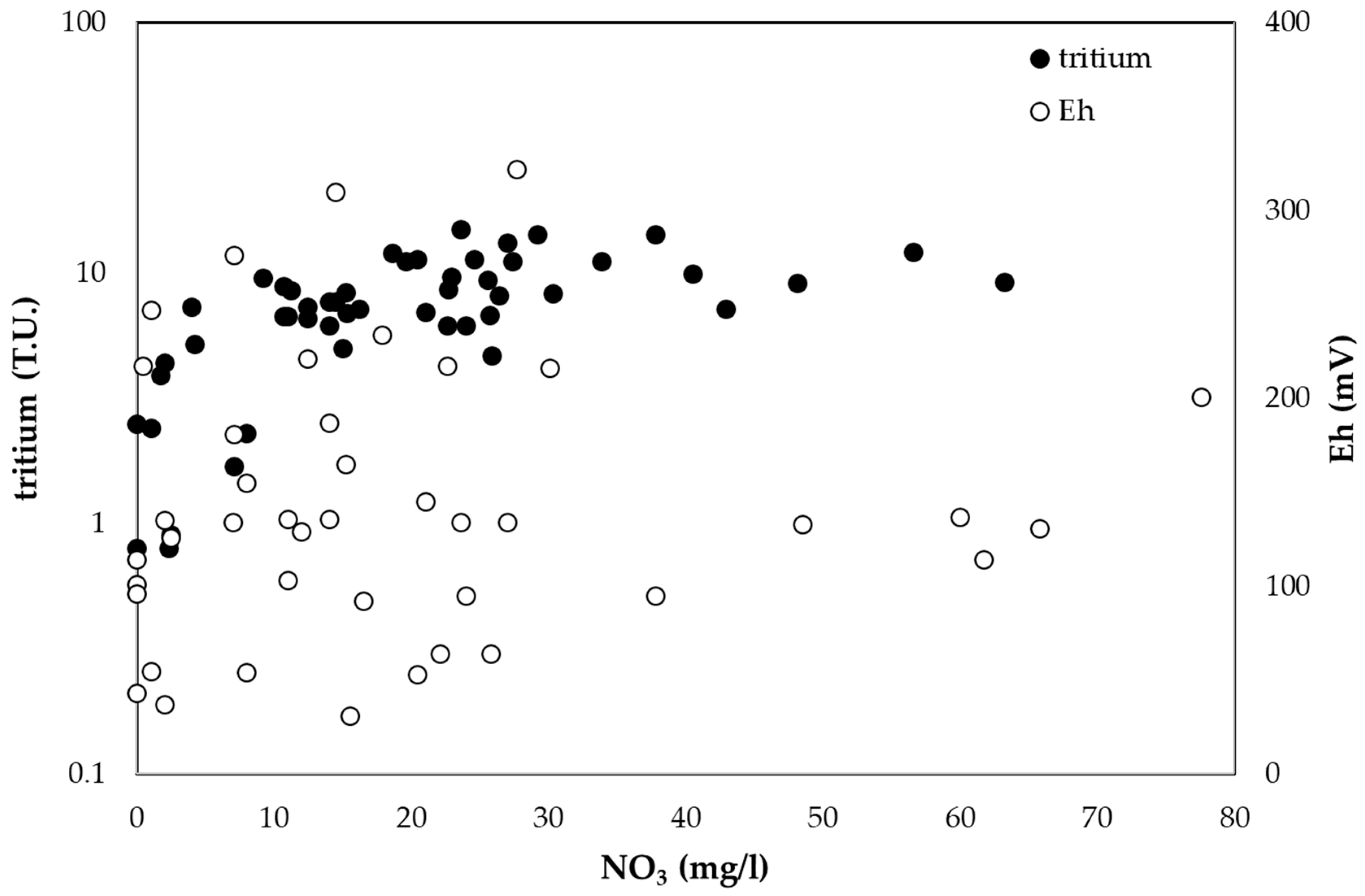
| Equation | R2 (n = 43) | HCO3−/NO3− | NO3− (mg/L) |
|---|---|---|---|
| NO3− = −10.0 (HCO3−/NO3−) + 138.8 | 0.70 | <10 | >30 |
| Well | Parameter | Minimum | Maximum | Mean | CV (%) |
|---|---|---|---|---|---|
| A (n = 19) | HCO3− (mg/L) | 271 | 375 | 345 | 10 |
| NO3− (mg/L) | 31 | 69 | 52 | 27 | |
| HCO3/NO3 | 5.4 | 9.6 | 7.0 | 23 | |
| B (n = 15) | HCO3− (mg/L) | 339 | 370 | 361 | 12 |
| NO3− (mg/L) | 30 | 57.3 | 47.1 | 18 | |
| HCO3/NO3 | 6.4 | 10 | 7.9 | 20 | |
| C (n = 16) | HCO3− (mg/L) | 302 | 371 | 337.9 | 6 |
| NO3− (mg/L) | 31.4 | 70.3 | 48.9 | 24 | |
| HCO3/NO3 | 5.2 | 9.8 | 7.2 | 20 | |
| D (n = 14) | HCO3− (mg/L) | 112 | 439 | 276.6 | 36 |
| NO3− (mg/L) | 14.3 | 62.6 | 34.5 | 48 | |
| HCO3/NO3 | 5.8 | 10.0 | 8.5 | 23 | |
| E (n = 19) | HCO3− (mg/L) | 376 | 525 | 473.8 | 8 |
| NO3− (mg/L) | 38.9 | 75 | 50.7 | 16 | |
| HCO3/NO3 | 6.6 | 12.8 | 9.5 | 15 | |
| F (n = 12) | HCO3− (mg/L) | 129 | 214 | 177.5 | 15 |
| NO3− (mg/L) | 35.4 | 40.7 | 39.0 | 4 | |
| HCO3/NO3 | 3.6 | 5.4 | 4.55 | 12 | |
| G (n = 10) | HCO3− (mg/L) | 232 | 279 | 260.4 | 5 |
| NO3− (mg/L) | 31.2 | 35.7 | 34.2 | 4 | |
| HCO3/NO3 | 6.6 | 8.2 | 7.6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapti, D.; Martinelli, G. An Expedited Procedure to Highlight Rapid Recharge Processes by Means of Nitrate Pollution Dynamics in the Northern Italy Plain. Environments 2025, 12, 404. https://doi.org/10.3390/environments12110404
Rapti D, Martinelli G. An Expedited Procedure to Highlight Rapid Recharge Processes by Means of Nitrate Pollution Dynamics in the Northern Italy Plain. Environments. 2025; 12(11):404. https://doi.org/10.3390/environments12110404
Chicago/Turabian StyleRapti, Dimitra, and Giovanni Martinelli. 2025. "An Expedited Procedure to Highlight Rapid Recharge Processes by Means of Nitrate Pollution Dynamics in the Northern Italy Plain" Environments 12, no. 11: 404. https://doi.org/10.3390/environments12110404
APA StyleRapti, D., & Martinelli, G. (2025). An Expedited Procedure to Highlight Rapid Recharge Processes by Means of Nitrate Pollution Dynamics in the Northern Italy Plain. Environments, 12(11), 404. https://doi.org/10.3390/environments12110404








