The Importance of Municipal Waste Landfill Vegetation for Biological Relevance: A Case Study
Abstract
1. Introduction
2. Materials and Methods
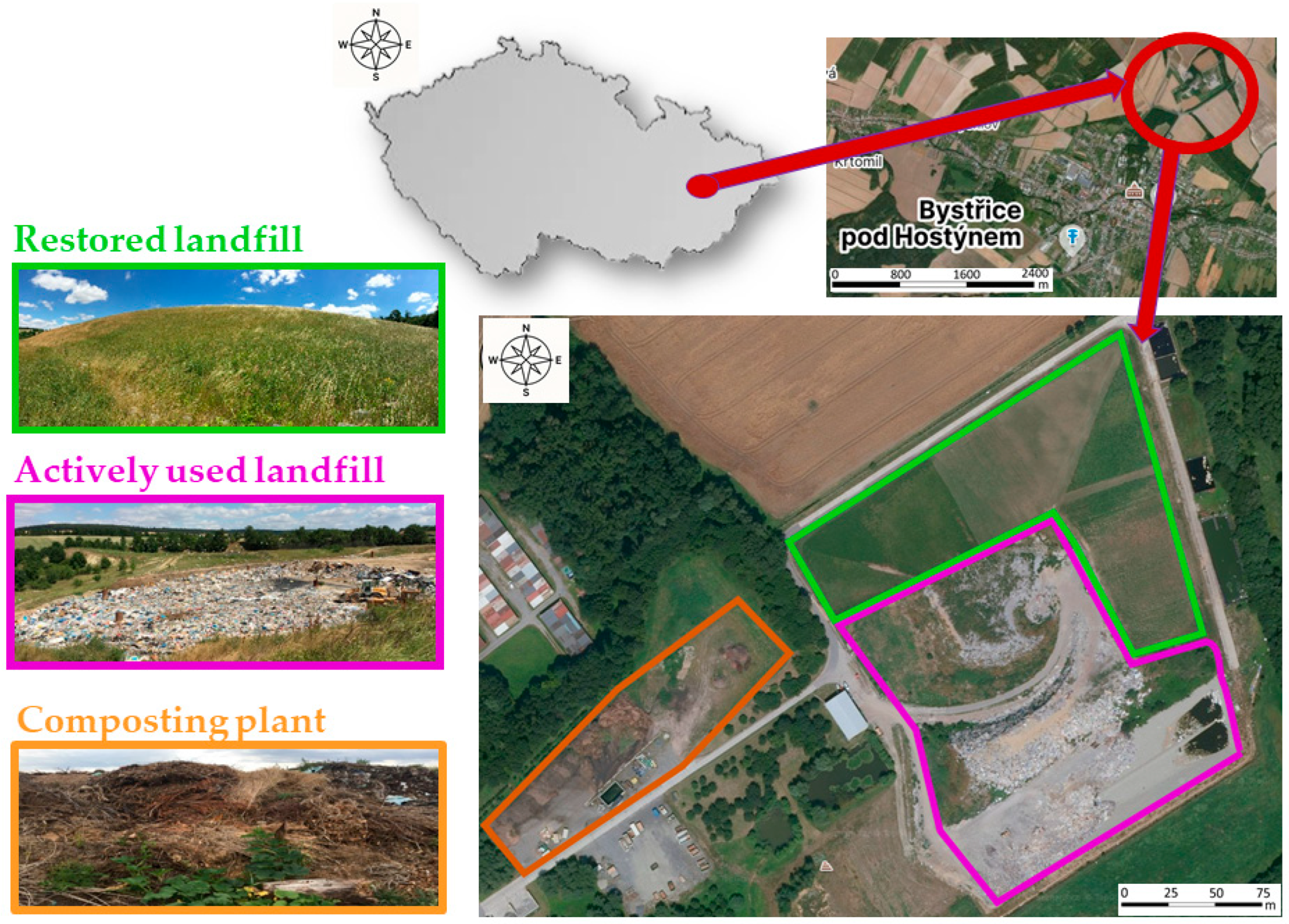
2.1. Study Area
2.2. Description of the Landfill
2.3. Vegetation Assessment Methodology
Characteristics of Selected Sites Within the Landfill
- Restored landfill: It is located on the area of cassette I, which was restored in 2013. After reaching the capacity of this cassette, the area was leveled, covered with geotextiles, a layer of soil was piled up, and the top layer was formed by compost from a local composting plant. Grasses and clovers were planted on the leveled area—specifically Arrhenatherum elatius, Lolium perenne, and Trifolium repens. Regular maintenance takes place during the summer months, including two mowings per year.
- Actively used landfill: These sites, formed by the areas of cassettes II and III, are used for waste disposal. The vegetation of this locality has the least favorable conditions due to intense disturbances, direct contact with landfill waste, and an affected water regime. Waste from surrounding municipalities is continuously deposited at this site. The disposal of waste is conducted in accordance with the prevailing regulations.
- Composting plant: This area includes the composting plant and bio-waste landfill, composting facility, and waste storage. The site shows the highest level of disturbance caused by the regular digging and moving of composted material. In addition, there is an increased nutrient content and sufficient moisture due to irrigation during composting process. The compost produced is primarily utilized for the rehabilitation and restoration of formerly active landfill sites.
2.4. Methodology for Assessing Selected Ecosystem Functions
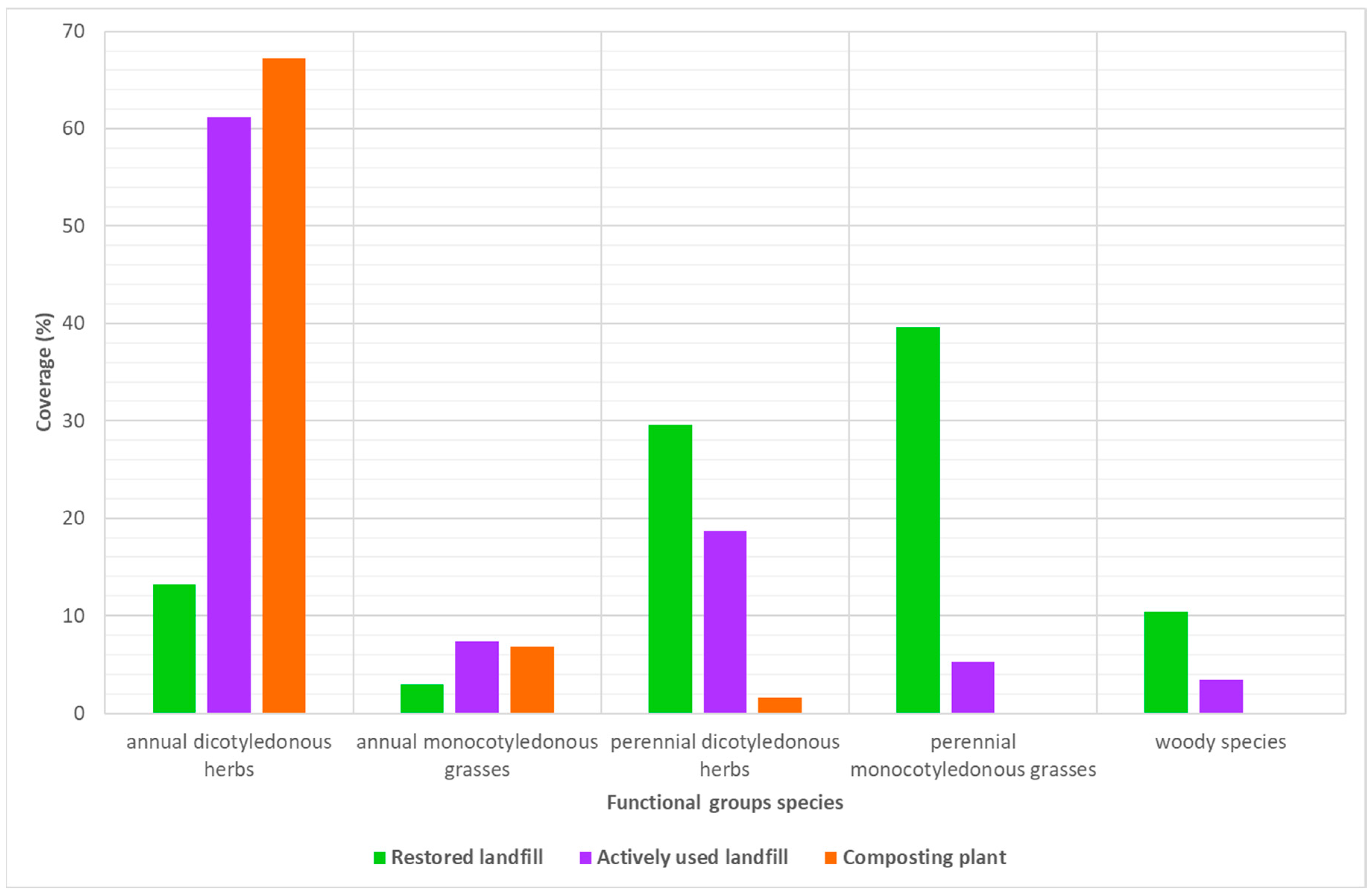
- annual dicotyledonous herbs,
- annual monocotyledonous grasses,
- perennial dicotyledonous herbs,
- perennial monocotyledonous grasses,
- woody species.
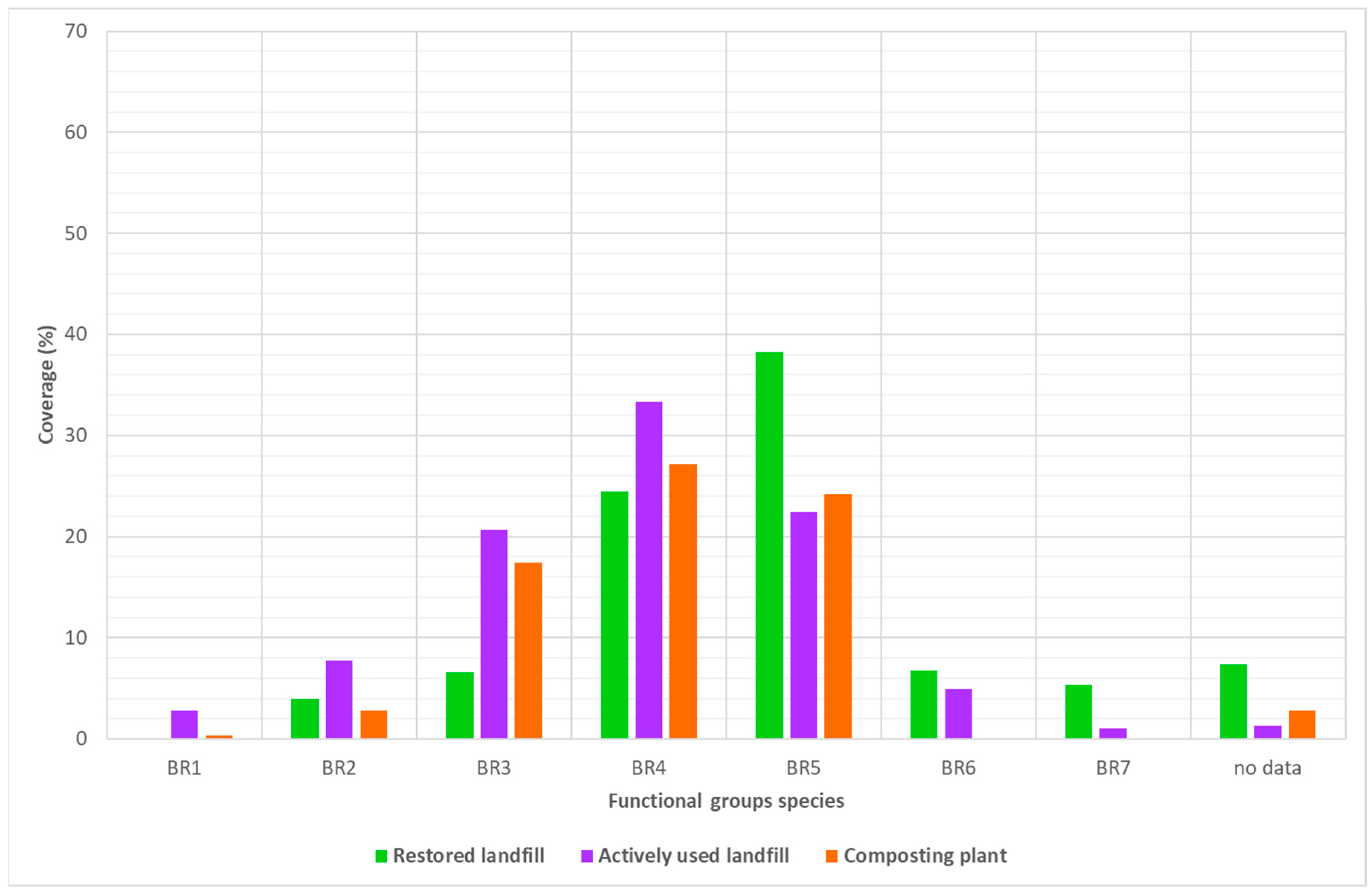
- BR1 = <6 associated species,
- BR2 = 6–12 species,
- BR3 = 13–24 species,
- BR4 = 25–50 species,
- BR5 = 51–100 species,
- BR6 = 101–200 species,
- BR7 = 201–400 species,
- BR8 = >400 species.
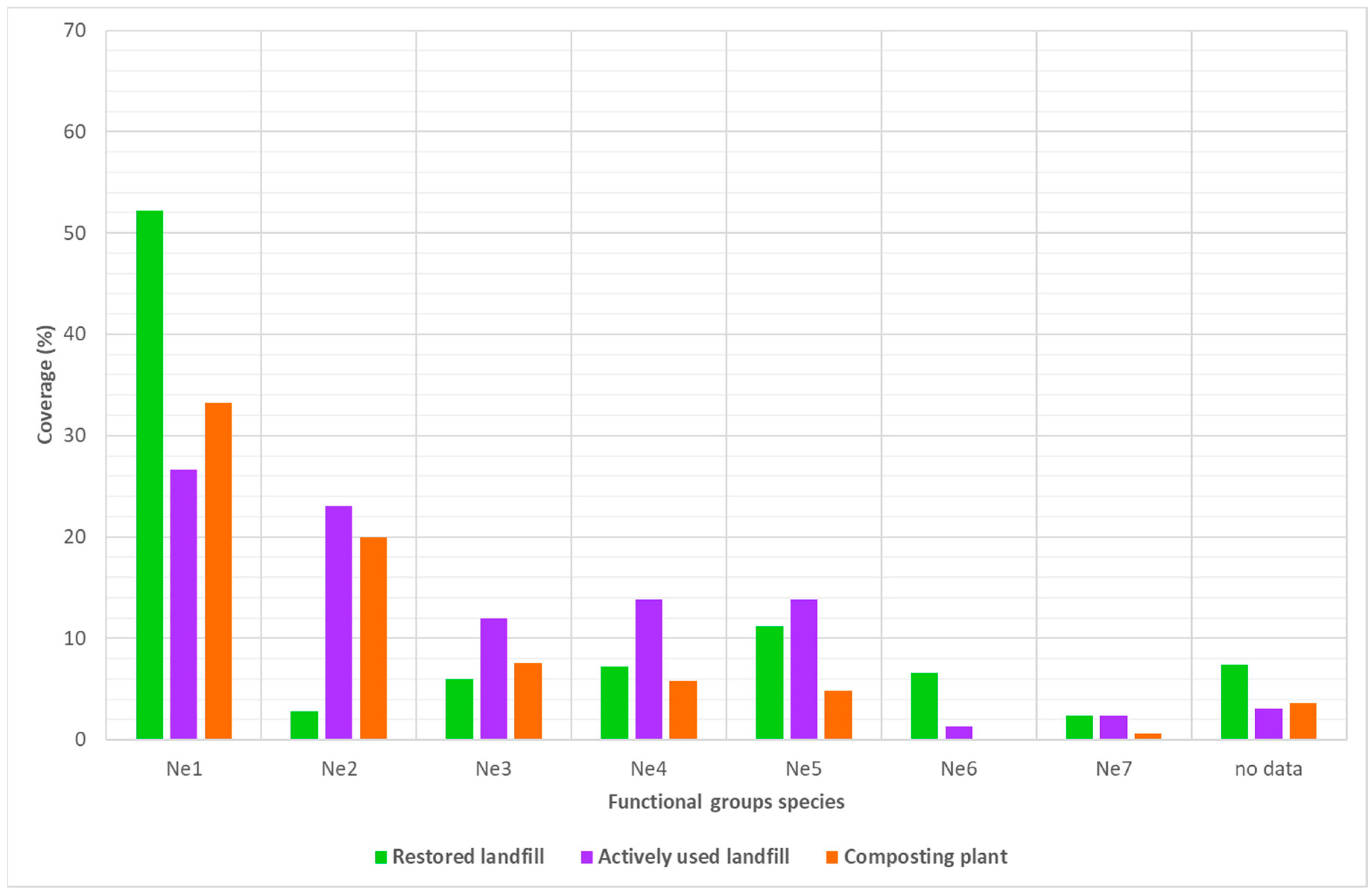
- NE1: no nectar production and no pollen to collect,
- NE2: no or negligible nectar production (<0.2 g) and high pollen production,
- NE3: low nectar production (0.2 to 5 g) and high pollen production,
- NE4: medium nectar production (5 to 20 g),
- NE5: rather high nectar production (20 to 50 g),
- NE6: high nectar production (50 to 200 g),
- NE7: very high nectar production (>200 g).
2.5. Statistical Data Processing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Ceschin, S.; Kumbaric, A.; Caneva, G.; Zuccarello, V. Testing flora as bioindicator of buried structures in the archaeological area of Maxentius’s Villa (Rome, Italy). J. Archaeol. Sci. 2012, 39, 1288–1295. [Google Scholar] [CrossRef]
- Campos, J.A.; Peco, J.D.; García-Noguero, E. Antigerminative comparison between naturally occurring naphthoquinones and commercial pesticides: Soil dehydrogenase activity used as bioindicator to test soil toxicity. Sci. Total Environ. 2019, 694, 133672. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Sarkar, A.; Basu, A.G.; Roy, S. Effect of plastic pollution on freshwater flora: A meta-analysis approach to elucidate the factors influencing plant growth and biochemical markers. Water Res. 2022, 225, 119114. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef]
- Maire, E.; Villéger, S.; Graham, N.A.J.; Hoey, A.S.; Cinner, J.; Ferse, S.C.A.; Aliaume, C.; Booth, D.J.; Feary, D.A.; Kulbicki, M.; et al. Community-wide scan identifies fish species associated with coral reef services across the Indo-Pacific. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181167. [Google Scholar] [CrossRef] [PubMed]
- Mahaut, L.; Fort, F.; Violle, C.; Freschet, G.T. Multiple facets of diversity effects on plant productivity: Species richness, functional diversity, species identity and intraspecific competition. Funct. Ecol. 2020, 34, 287–298. [Google Scholar] [CrossRef]
- Ellis, E.C. Anthropogenic transformation of the terrestrial biosphere. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 1010–1035. [Google Scholar] [CrossRef]
- Ellis, E.C. Ecology in an anthropogenic biosphere. Ecol. Monogr. 2015, 85, 287–331. [Google Scholar] [CrossRef]
- Waters, C.N.; Zalasiewicz, J.; Summerhayes, C.; Barnosky, A.D.; Poirier, C.; Gałuszka, A.; Cearreta, A.; Edgeworth, M.; Ellis, E.C.; Ellis, M.; et al. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 2016, 351, aad2622. [Google Scholar] [CrossRef]
- Aguilar, R.G.; Owens, R.; Giardino, J.R. The expanding role of anthropogeomorphology in critical zone studies in the Anthropocene. Geomorphology 2020, 366, 107165. [Google Scholar] [CrossRef]
- Benítez-López, A.; Santini, L.; Schipper, A.M.; Busana, M.; Huijbregts, M.A.J. Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS Biol. 2019, 17, e3000247. [Google Scholar] [CrossRef]
- Hu, X.; Næss, J.S.; Iordan, C.M.; Huang, B.; Zhao, W.; Cherubini, F. Recent global land cover dynamics and implications for soil erosion and carbon losses from deforestation. Anthropocene 2021, 34, 100291. [Google Scholar] [CrossRef]
- Dubois, N.; Saulnier-Talbot, É.; Mills, K.; Gell, P.; Battarbee, R.; Bennion, H.; Chawchai, S.; Dong, X.; Francus, P.; Flower, R.; et al. First human impacts and responses of aquatic systems: A review of palaeolimnological records from around the world. Anthr. Rev. 2018, 5, 28–68. [Google Scholar] [CrossRef]
- Reeves, J.M.; Gell, P.A.; Reichman, S.M.; Trewarn, S.M.; Zawadzki, A. Industrial past, urban future: Using palaeo-studies to determine the industrial legacy of the Barwon Estuary, Victoria, Australia. Mar. Freshw. Res. 2016, 67, 837–849. [Google Scholar] [CrossRef]
- Tolotti, M.; Boscaini, A.; Salmaso, N. Comparative analysis of phytoplankton patterns in two modified lakes with contrasting hydrological features. Aquat. Sci. 2010, 72, 213–226. [Google Scholar] [CrossRef]
- Kattel, G.; Gell, P.; Zawadzki, A.; Barry, L. Palaeoecological evidence for sustained change in a shallow Murray River (Australia) floodplain lake: Regime shift or press response? Hydrobiologia 2017, 787, 269–290. [Google Scholar] [CrossRef]
- Dick, J.; Haynes, D.; Tibby, J.; Garcia, A.; Gell, P. A history of aquatic plants in the Ramsar-listed Coorong wetland, South Australia. J. Paleolimnol. 2012, 46, 623–635. [Google Scholar] [CrossRef]
- Davidson, T.A.; Reid, M.A.; Sayer, C.D.; Chilcott, S. Palaeolimnological records of shallow-lake biodiversity change: Exploring the merits of single versus multi-proxy approaches. J. Paleolimnol. 2013, 49, 431–446. [Google Scholar] [CrossRef]
- Kattel, G.; Gell, P.; Perga, M.E.; Jeppesen, E.; Grundell, R.; Weller, S.; Zawadzki, A.; Barry, L. Tracking a century of change in trophic structure and dynamics in a floodplain wetland: Integrating palaeo-ecological and palaeo-isotopic evidence. Freshw. Biol. 2015, 60, 711–723. [Google Scholar] [CrossRef]
- Hossain, M.S.; Santhanam, A.; Norulaini, N.A.N.; Omar, A.K.M. Clinical solid waste management practices and its impact on human health and environment—A review. Waste Manag. 2011, 31, 754–766. [Google Scholar] [CrossRef]
- Lestari, P.; Trihadiningrum, Y. The impact of improper solid waste management to plastic pollution in Indonesian coast and marine environment. Mar. Pollut. Bull. 2019, 149, 110505. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.-G.; Pandey, A. Assessing the impact of industrial waste on environment and mitigation strategies: A comprehensive review. J. Hazard. Mater. 2020, 398, 123019. [Google Scholar] [CrossRef]
- Li, W.; Achal, V. Environmental and health impacts due to e-waste disposal in China—A review. Sci. Total Environ. 2020, 737, 139745. [Google Scholar] [CrossRef]
- Wong, J.T.-F.; Chen, X.-W.; Mo, W.-Y.; Man, Y.-B.; Ng, C.W.-W.; Wong, M.-H. Restoration of plant and animal communities in a sanitary landfill: A 10-year case study in Hong Kong. Land Degrad. Dev. 2016, 27, 490–499. [Google Scholar] [CrossRef]
- Hamid, H.; Li, L.Y.; Grace, J.R. Review of the fate and transformation of per- and polyfluoroalkyl substances (PFASs) in landfills. Environ. Pollut. 2018, 235, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Rasapoor, M.; Young, B.; Brar, R.; Baroutian, S. Improving biogas generation from aged landfill waste using moisture adjustment and neutral red additive—Case study: Hampton Downs’s landfill site. Energy Convers. Manag. 2020, 216, 112947. [Google Scholar] [CrossRef]
- Sauvé, G.; Van Acker, K. The environmental impacts of municipal solid waste landfills in Europe: A life cycle assessment of proper reference cases to support decision making. J. Environ. Manag. 2020, 261, 110216. [Google Scholar] [CrossRef] [PubMed]
- Paleologos, E.K.; Bunge, R.; Weibel, G.; Vitone, C.; Ploetze, M.; Petti, R.; Koda, E.; O’Kelly, B.C.; Podlasek, A.; Vaverková, M.D.; et al. Paradigm shifts in incinerator ash, sediment material recovery and landfill monitoring. Environ. Geotech. 2023, 10, 501–514. [Google Scholar] [CrossRef]
- Koda, E.; Winkler, J.; Wowkonowicz, P.; Černý, M.; Kiersnowska, A.; Pasternak, G.; Vaverková, M.D. Vegetation changes as indicators of landfill leachate seepage locations: Case study. Ecol. Eng. 2022, 174, 106448. [Google Scholar] [CrossRef]
- Salgueiro, P.A.; Prach, K.; Branquinho, C.; Mira, A. Enhancing biodiversity and ecosystem services in quarry restoration—Challenges, strategies, and practice. Restor. Ecol. 2020, 28, 655–660. [Google Scholar] [CrossRef]
- Winkler, J.; Koda, E.; Skutnik, Z.; Černý, M.; Adamcová, D.; Podlasek, A.; Vaverková, M.D. Trends in the succession of synanthropic vegetation on a reclaimed landfill in Poland. Anthropocene 2021, 35, 100299. [Google Scholar] [CrossRef]
- Robinson, S.L.; Lundholm, J.T. Ecosystem services provided by urban spontaneous vegetation. Urban Ecosyst. 2012, 15, 545–557. [Google Scholar] [CrossRef]
- Guo, P.; Yu, F.; Ren, Y.; Liu, D.; Li, J.; Ouyang, Z.; Wang, X. Response of ruderal species diversity to an urban environment: Implications for conservation and management. Int. J. Environ. Res. Public Health 2018, 15, 2832. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C.B.; Simpson, M.; Casanova, M. Future wet grasslands: Ecological implications of climate change. Ecosyst. Health Sustain. 2016, 2, e01240. [Google Scholar] [CrossRef]
- Ranđelović, D.; Jovanović, S. Understanding the role of ruderal plant species in restoration of degraded lands. In Bio-Inspired Land Remediation; Pandey, V.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 17–35. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Paleologos, E.K.; Adamcová, D.; Podlasek, A.; Pasternak, G.; Červenková, J.; Skutnik, Z.; Koda, E.; Winkler, J. Municipal solid waste landfill: Evidence of the effect of applied landfill management on vegetation composition. Waste Manag. Res. 2022, 40, 1402–1411. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Tree species selection and reaction to mine soil reconstructed at reforested post-mine sites: Central and Eastern European experiences. Ecol. Eng. 2019, 142, 100012. [Google Scholar] [CrossRef]
- Poniatowski, D.; Stuhldreher, G.; Helbing, F.; Hamer, U.; Fartmann, T. Restoration of calcareous grasslands: The early successional stage promotes biodiversity. Ecol. Eng. 2020, 151, 105858. [Google Scholar] [CrossRef]
- Ranđelović, D.; Jakovljević, K.; Šinžar-Sekulić, J.; Kuzmič, F.; Šilc, U. Recognising the role of ruderal species in restoration of degraded lands. Sci. Total Environ. 2024, 938, 173104. [Google Scholar] [CrossRef]
- Czech Geological Society. Geological Map of the Czech Republic, 1: 50 000. C.G.S: Prague, Czech Republic, 2018. Available online: https://mapy.geology.cz/geocr50 (accessed on 20 August 2025).
- Czech Geological Society. Map of Soil Types of the Czech Republic, 1: 50 000. C.G.S: Prague, Czech Republic, 2017. Available online: https://mapy.geology.cz/pudy (accessed on 20 August 2025).
- Culek, M. (Ed.) Biogeographical Division of the Czech Republic (Biogeografické Členění České Republiky), 1st ed.; Enigma: Prague, Czech Republic, 1996; p. 347. (In Czech) [Google Scholar]
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Wild, J.; Holubová, D.; Novotný, P.; Řezníčková, M.; Rohn, M.; Dřevojan, P.; Grulich, V.; et al. Pladias Database of the Czech Flora and Vegetation. Preslia 2021, 93, 1–87. [Google Scholar] [CrossRef]
- Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Tintner, J.; Klug, B. Can vegetation indicate landfill cover features? Flora—Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 559–566. [Google Scholar] [CrossRef]
- Körner, K.; Jeltsch, F. Detecting general plant functional type responses in fragmented landscapes using spatially-explicit simulations. Ecol. Model. 2008, 210, 287–300. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Adler, P.B.; Smull, D.; Beard, K.H.; Choi, R.T.; Furniss, T.; Kulmatiski, A.; Meiners, J.M.; Tredennick, A.T.; Veblen, K.E. Competition and coexistence in plant communities: Intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018, 21, 1319–1329. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Levine, J.M.; Loreau, M.; Hector, A. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecol. Lett. 2013, 16 (Suppl. 1), 116–127. [Google Scholar] [CrossRef]
- Baert, J.M.; Jaspers, S.; Janssen, C.R.; De Laender, F.; Aerts, M. Nonlinear partitioning of biodiversity effects on ecosystem functioning. Methods Ecol. Evol. 2017, 8, 1233–1240. [Google Scholar] [CrossRef]
- Testi, A.; Bisceglie, S.; Guidotti, S.; Fanelli, G. Detecting river environmental quality through plant and macroinvertebrate bioindicators in the Aniene River (Central Italy). Aquat. Ecol. 2009, 43, 477–486. [Google Scholar] [CrossRef]
- Gillet, S.; Ponge, J.F. Humus forms and metal pollution in soil. Eur. J. Soil Sci. 2002, 53, 529–540. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, F.R.; Hamzah, H.A. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Process. 2018, 7, 28. [Google Scholar] [CrossRef]
- Azzazy, M.F. Plant bioindicators of pollution in Sadat City, Western Nile Delta, Egypt. PLoS ONE 2020, 15, e0226315. [Google Scholar] [CrossRef] [PubMed]
- Ingelmo, F.; Canet, R.; Ibañez, M.A.; Pomares, F.; García, J. Use of MSW compost, dried sewage sludge and other wastes as partial substitutes for peat and soil. Bioresour. Technol. 1998, 63, 123–129. [Google Scholar] [CrossRef]
- Uzun, I. Use of spent mushroom compost in sustainable fruit production. J. Fruit Ornam. Plant Res. 2004, 12, 157–165. [Google Scholar]
- Pérez-Gimeno, A.; Navarro-Pedreño, J.; Almendro-Candel, M.B.; Gómez, I.; Zorpas, A.A. The use of wastes (organic and inorganic) in land restoration in relation to their characteristics and cost. Waste Manag. Res. 2019, 37, 502–507. [Google Scholar] [CrossRef]
- Bauerek, A.; Diatta, J.; Pierzchała, Ł.; Więckol-Ryk, A.; Krzemień, A. Development of soil substitutes for the sustainable land reclamation of coal mine-affected areas. Sustainability 2022, 14, 4604. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Ateenyi Basamba, T. How safe is chicken litter for land application as an organic fertilizer? A review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef]
- Winkler, J.; Rypová, I.; Dvořák, J. Influence of manure on weed infestation of sugar beet. Listy Cukrov. A Řepařské 2017, 133, 130–136. Available online: http://www.cukr-listy.cz/on_line/2017/PDF/130-136.pdf (accessed on 20 August 2025).
- Vaverková, M.D.; Adamcová, D.; Winkler, J.; Koda, E.; Petrželová, L.; Maxianová, A. Alternative method of composting on a reclaimed municipal waste landfill in accordance with the circular economy: Benefits and risks. Sci. Total Environ. 2020, 723, 137971. [Google Scholar] [CrossRef] [PubMed]
- Uldrijan, D.; Černý, M.; Winkler, J. Solar park—Opportunity or threat for vegetation and ecosystem. J. Ecol. Eng. 2022, 23, 1–10. [Google Scholar] [CrossRef]
- Winkler, J.; Dvořák, J.; Hosa, J.; Martínez Barroso, P.; Vaverková, M.D. Impact of conservation tillage technologies on the biological relevance of weeds. Land 2023, 12, 121. [Google Scholar] [CrossRef]
- Winkler, J.; Martinová, L.; Kotlánová, B.; Děkanovský, I.; Vaverková, M.D. Biological relevance and ecosystem functions of sugar beet weeds. Listy Cukrov. A Řepařské 2025, 141, 98–103. Available online: http://www.cukr-listy.cz/on_line/2025/PDF/98-103.pdf (accessed on 20 August 2025).
- Schindler, B.Y.; Blaustein, L.; Lotan, R.; Shalom, H.; Kadas, G.J.; Seifan, M. Green roof and photovoltaic panel integration: Effects on plant and arthropod diversity and electricity production. J. Environ. Manag. 2018, 225, 288–299. [Google Scholar] [CrossRef]
- Blaydes, H.; Potts, S.G.; Whyatt, J.D.; Armstrong, A. Opportunities to enhance pollinator biodiversity in solar parks. Renew. Sustain. Energy Rev. 2021, 145, 111065. [Google Scholar] [CrossRef]
- Tang, A.M.; Hughes, P.N.; Dijkstra, T.A.; Askarinejad, A.; Brenčič, M.; Cui, Y.J.; Diez, J.J.; Firgi, T.; Gajewska, B.; Gentile, F.; et al. Atmosphere–vegetation–soil interactions in a climate change context: Impact of changing conditions impacting on engineered transport infrastructure slopes in Europe. Q. J. Eng. Geol. Hydrogeol. 2018, 1, 156–168. [Google Scholar] [CrossRef]
- Rusch, A.; Binet, D.; Delbac, L.; Thiéry, D. Local and landscape effects of agricultural intensification on Carabid community structure and weed seed predation in a perennial cropping system. Landsc. Ecol. 2016, 31, 2163–2174. [Google Scholar] [CrossRef]
- De Heij, S.E.; Willenborg, C.J. Connected Carabids: Network interactions and their impact on biocontrol by Carabid beetles. Bioscience 2020, 70, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Foffová, H.; Ćavar Zeljković, S.; Honěk, A.; Martinková, Z.; Tarkowski, P.; Saska, P. Which seed properties determine the preferences of Carabid beetle seed predators? Insects 2020, 11, 757. [Google Scholar] [CrossRef]
- Hurajová, E.; Martínez Barroso, P.; Havel, L.; Děkanovský, I.; Winkler, J. Relationship between vegetation succession and earthworm population in vineyards. J. Ecol. Eng. 2024, 25, 134–144. [Google Scholar] [CrossRef]
- Batten, K.M.; Scow, K.M.; Davies, K.F.; Harrison, S.P. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biol. Invasions 2006, 8, 217–230. [Google Scholar] [CrossRef]
- Lutgen, E.R.; Rillig, M.C. Influence of spotted knapweed (Centaurea maculosa) management treatments on arbuscular mycorrhizae and soil aggregation. Weed Sci. 2004, 52, 172–177. [Google Scholar] [CrossRef]
- Marler, M.J.; Zabinski, C.A.; Callaway, R.M. Mycorrhizae indirectly enhance competitive effects of an invasive forb on a native bunchgrass. Ecology 1999, 80, 1180–1186. [Google Scholar] [CrossRef]
- Walston, L.J.; Li, Y.; Hartmann, H.M.; Macknick, J.; Hanson, A.; Nootenboom, C.; Lonsdorf, E.; Hellmann, J. Modeling the ecosystem services of native vegetation management practices at solar energy facilities in the Midwestern United States. Ecosyst. Serv. 2021, 47, 101227. [Google Scholar] [CrossRef]
- Sajwan, K.S.; Twardowska, I.; Punshon, T.; Alva, A.K. Coal Combustion Byproducts and Environmental Issues; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Braga, L.; Furia, E.; Buldrini, F.; Mercuri, A.M. Pollen and flora as bioindicators in assessing the status of polluted sites: The case study of the Mantua Lakes (SIN “Laghi di Mantova e Polo Chimico”; N Italy). Sustainability 2023, 15, 9414. [Google Scholar] [CrossRef]
- Więckol-Ryk, A.; Pierzchała, Ł.; Bauerek, A.; Krzemień, A. Minimising coal mining’s impact on biodiversity: Artificial soils for post-mining land reclamation. Sustainability 2023, 15, 9707. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Peña, V.; Ricker, M.; Campos, A.; Howe, H.F. Restoring tropical biodiversity: Leaf traits predict growth and survival of late-successional trees in early-successional environments. For. Ecol. Manag. 2005, 217, 365–379. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Winkler, J.; Vaverková, M.D.; Havel, L. Anthropogenic life strategy of plants. Anthr. Rev. 2023, 10, 455–462. [Google Scholar] [CrossRef]
- Mahaut, L.; Cheptou, P.O.; Fried, G.; Munoz, F.; Storkey, J.; Vasseur, F.; Violle, C.; Bretagnolle, F. Weeds: Against the rules? Trends Plant Sci. 2020, 25, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
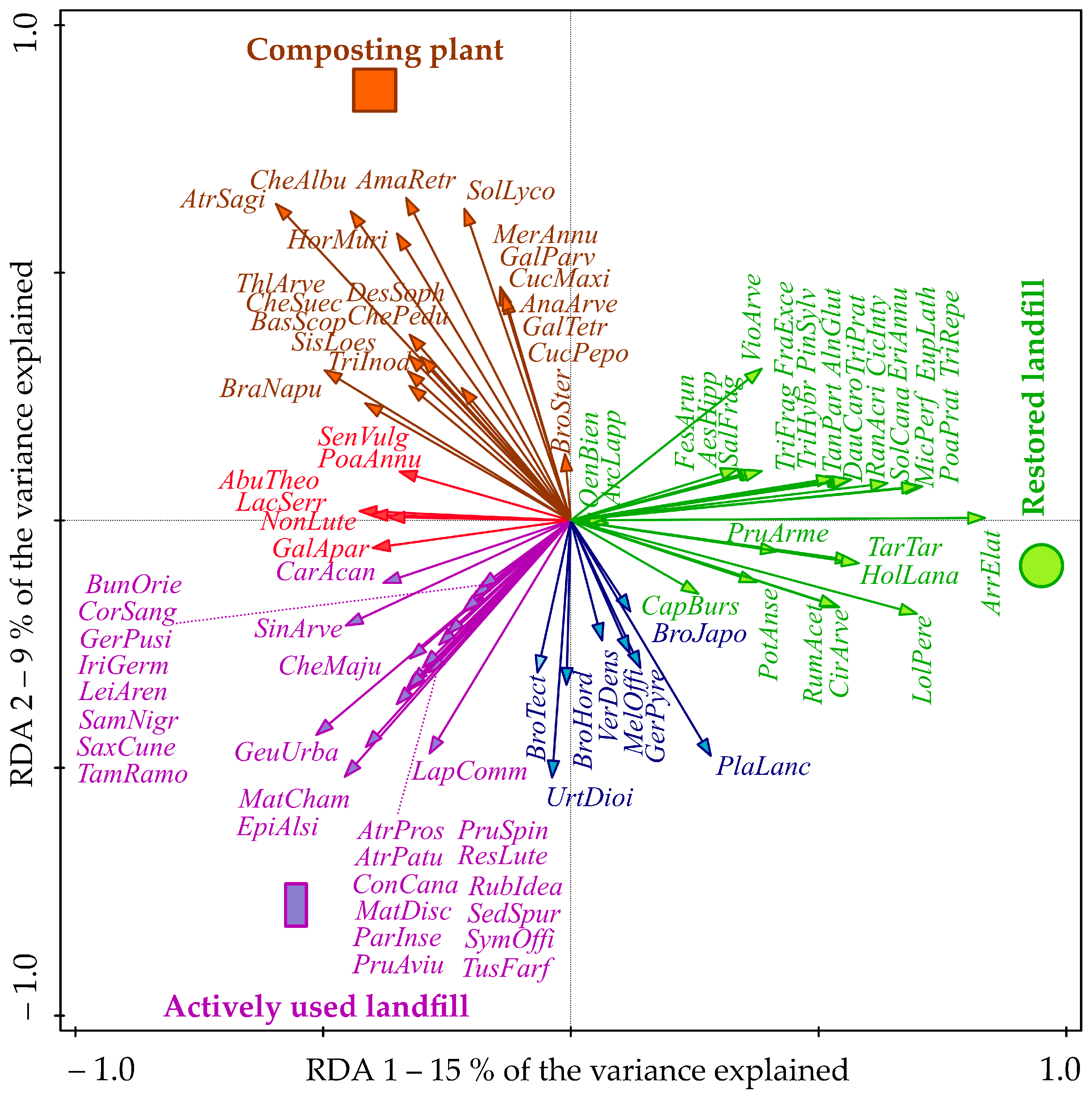
| Preferred Habitat (Color in Figure 5) | Functional Group | Plant Species |
|---|---|---|
| Restored site (green) | Annual dicotyledonous herbs | Capsella bursa-pastoris, Erigeron annuus, Microthlaspi perfoliatum, Viola arvensis |
| Perennial dicotyledonous herbs | Arctium lappa, Cichorium intybus, Cirsium arvense, Daucus carota, Euphorbia lathyris, Oenothera biennis, Potentilla anserina, Ranunculus acris, Rumex acetosa, Solidago canadensis, Tanacetum parthenium, Taraxacum sect. Taraxacum, Trifolium fragiferum, Trifolium pratense, Trifolium hybridum, Trifolium repens | |
| Perennial monocotyledonous grasses | Arrhenatherum elatius, Festuca arundinacea, Holcus lanatus, Lolium perenne, Poa pratensis, | |
| Woody species | Aesculus hippocastanum, Alnus glutinosa, Fraxinus excelsior, Pinus sylvestris, Prunus armeniaca, Salix fragilis | |
| Restored and actively used site (blue) | Annual monocotyledonous herbs | Bromus hordeaceus, Bromus japonicus, Bromus tectorum |
| Perennial dicotyledonous herbs | Geranium pyrenaicum, Melilotus officinalis, Plantago lanceolata, Urtica dioica, Verbascum densiflorum | |
| Actively used site (purple) | Annual dicotyledonous herbs | Atriplex patula, Atriplex prostrata, Conyza canadensis, Geranium pusilum, Lapsana communis, Matricaria chamomilla, Matricaria discoidea, Sinapis arvensis |
| Perennial dicotyledonous herbs | Bunias orientalis, Carduus acanthoides, Chelidonium majus, Geum urbanum, Epilobium alsinifoliu, Parthenocissus inserta, Reseda luteola, Saxifraga cuneifolia, Sedum spurium, Symphytum officinale, Tussilago farfara | |
| Perennial monocotyledonous grasses | Iris germanica, Leimus arenarius | |
| Woody species | Cornus sanguinea, Prunus avium, Prunus spinosa, Rubus ideaus, Sambucus nigra, Tamarix ramosissima | |
| Actively used site and composting plant (red) | Annual dicotyledonous herbs | Abutilon theophrasti, Galium aparine, Lactuca serriola, Nonea lutea, Senecio vulgaris |
| Annual monocotyledonous herbs | Poa annua | |
| Composting plant (brown) | Annual dicotyledonous herbs | Amaranthus retroflexus, Anagallis arvensis, Atriplex sagittata, Bassia scoparia, Brassica napus, Chenopodium album, Chenopodium album subsp. pedunculare, Chenopodium suecicum, Cucurbita maxima, Cucurbita pepo, Descurainia sophia, Galeopsis tetrahit, Galinsoga parviflora, Mercurialis annua, Sisymbrium loeselii, Solanum lycopersicum, Thlaspi arvense, Tripleurospermum inodorum |
| Annual monocotyledonous herbs | Bromus sterilis, Hordeum murinum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, J.; Tomaník, M.; Martínez Barroso, P.; Děkanovský, I.; Sitek, W.; Vaverková, M.D. The Importance of Municipal Waste Landfill Vegetation for Biological Relevance: A Case Study. Environments 2025, 12, 401. https://doi.org/10.3390/environments12110401
Winkler J, Tomaník M, Martínez Barroso P, Děkanovský I, Sitek W, Vaverková MD. The Importance of Municipal Waste Landfill Vegetation for Biological Relevance: A Case Study. Environments. 2025; 12(11):401. https://doi.org/10.3390/environments12110401
Chicago/Turabian StyleWinkler, Jan, Marek Tomaník, Petra Martínez Barroso, Igor Děkanovský, Wiktor Sitek, and Magdalena Daria Vaverková. 2025. "The Importance of Municipal Waste Landfill Vegetation for Biological Relevance: A Case Study" Environments 12, no. 11: 401. https://doi.org/10.3390/environments12110401
APA StyleWinkler, J., Tomaník, M., Martínez Barroso, P., Děkanovský, I., Sitek, W., & Vaverková, M. D. (2025). The Importance of Municipal Waste Landfill Vegetation for Biological Relevance: A Case Study. Environments, 12(11), 401. https://doi.org/10.3390/environments12110401








