Abstract
Plant domestication has led to a series of morphological and physiological changes aimed at making species more suitable for human use and consumption. In Vitis vinifera ssp. sativa, these changes include increased sugar content and berry size, modifications in seed morphology, and the transition from dioecy to hermaphroditism. This process, which began approximately 6000–8000 years ago in the Transcaucasian region, unfolded in multiple stages and involved the natural abandonment of wild Vitis populations. While it contributed to the phenotypic diversification of modern grapevine cultivars, it also came at the expense of biodiversity. Selection for yield and quality has resulted in the loss of resilience traits in cultivated grapevines. In this study, 23 Vitis species of American origin were examined, analyzing for each their native range, susceptibility to biotic and abiotic stresses, and their suitability for propagation. The study, characterization, and compilation of these American Vitis species provide a valuable resource for consultation and use in targeted grapevine breeding programs. These efforts aim to recover adaptive traits from wild progenitors, enhance the resilience of cultivated grapevines, and address the challenges posed by modern agriculture and sustainability.
1. Introduction
The Vitaceae family comprises 16 genera and around 950 species, predominantly distributed across tropical regions spanning Africa, Asia, the Pacific Islands, and the Neotropics [1,2], with a limited number of genera extending into temperate zones. Vitaceae is distinguished from other angiosperm families by its leaf-opposed tendrils, which contribute to its success as a climbing liana in diverse tropical and temperate vegetation types [1]. The grape is one of the main horticultural crops worldwide and is taxonomically classified within the genus Vitis [3]. Grape berries are one of the most economically valuable fruit crops. In addition to their use in the production of wine, table grapes, raisins, juices and distillates, in recent years, interest has also extended to the antioxidant properties and health benefits associated with grape products [4]. Their significance is also rooted in their long-standing connection with the evolution of human civilization. Wine, as the principal derivative of grape cultivation, held a revered status in ancient societies, often regarded as a divine beverage and closely linked to mythological deities such as Dionysus and Bacchus [3]. Centers of Vitis spp. diversity are primarily located in the southeastern United States [5,6] and East Asia [7,8]. Eastern Asia—including regions such as China, Japan, and Java—harbors up to 30 native species, while two subspecies, belonging to the widely cultivated Vitis vinifera species, are distributed across Central Asia and Europe [9]. Additionally, up to 28 species are native to a broad area encompassing the eastern and southwestern United States as well as Mexico [9]. Knowledge of these centers of diversity is essential to understand the origins and the domestication process of grapevine.
Plant domestication is an evolutionary process, driven by human intervention, that has led thousands of plant species to diverge morphologically and genetically from their wild ancestors [10,11]. This phenomenon has affected numerous plant species, including the grapevine (V. vinifera), with the aim of selecting characteristics favorable to cultivation and human consumption. In the case of V. vinifera ssp. sativa, domestication has led to substantial modifications compared to the wild form V. vinifera ssp. sylvestris. Key traits selected during grapevine domestication include increased berry and cluster size, elevated sugar content, modifications in seed morphology, and a shift from a dioecious to a hermaphroditic reproductive system [3,12,13]. These changes are typical examples of the so-called ‘domestication syndrome’, a set of common phenotypic and physiological traits acquired by domesticated plant species [14]. Although these traits have favored fruit productivity and quality, they have often been accompanied by genetic bottlenecks, which have reduced the available genetic diversity and compromised resistance to biotic and abiotic stresses [12]. Artificial selection has therefore led to an unintended loss of traits originally present in wild populations. In addition to the domestication syndrome, another critical factor is vegetative propagation, which has been widely used in grapevines since the birth of their cultivation [15]. While this method of reproduction ensures the preservation of desired agronomic traits, it has blocked the adaptive evolution of the species by limiting genetic recombination and preventing the generation of new genotypes [16].
The lack of recombination and the continued preservation of old genotypes have further accentuated the vulnerability of cultivated vines to biotic and abiotic environmental changes and, thus, to the related stresses. Consequently, it is essential to promote genetic improvement strategies that involve the introgression of resistance traits from wild populations [12,17]. Environmental conditions are continuously changing, also as a result of various human activities. Among these, climate change stands out as one of the most significant and widely acknowledged drivers affecting the global distribution and incidence not only abiotic stress but also of plant diseases [18,19,20]. The current global changes increase the abiotic pressures on agriculture and necessitate the development of solutions to maintain crop production [21]. As described by [22], the process of adaptation consists of the formation, over generations, of new allelic combinations that give rise to genotypes that are better suited to the environment. It is well known that grapevine plants are influenced by many environmental stresses. Among these, the most significant are extreme temperatures, excessive or insufficient light exposure, waterlogging, drought, mineral deficiencies in the soil, and elevated soil salinity [23]. In the upcoming years, changes are expected in the distribution range of infectious plant diseases. As these diseases shift geographically, new interactions between abiotic and biotic factors may influence their distribution, leading to unexpected changes in plant disease risk [24,25,26]. Human activities have also historically played a key role in the spread and introduction of various pathogens affecting grapevines, as well as harmful insects, such as the grapevine phylloxera Daktulosphaira vitifoliae. Native to the northeastern United States [27,28], this insect was accidentally introduced into major European wine-growing regions via American grapevines, which had been imported to control grapevine powdery mildew (Erysiphe necator) [29]. Climate change is also impacting grape production in terms of yield, composition, and wine quality. As a result, the geographical landscape of viticulture is shifting due to issues related to drought and other associated abiotic stress factors [30,31,32,33]. Under future climate change scenarios, particularly rising temperatures, a study by [34] projected potential increases in the pressure of the disease Plasmopara viticola in Italy (Europe), associated with a higher incidence of primary infections occurring in May and June. This would require the implementation of additional strategies to control the pathogen, with plant protection efforts needing to focus more heavily on early-season infections. Consequently, the costs associated with managing the disease are also expected to rise in future scenarios [34]. Similar trends have been observed in other wine-growing regions as well [35]. The impacts of infection may vary depending on the region, but in all cases, plants are expected to become more vulnerable due to abiotic stress. Under climate change scenarios, an increase in the frequency of extreme weather events is expected to negatively affect overall plant health [36]. Additionally, environmental changes are expected to exacerbate the epidemiological risk of infections caused by Xylella fastidiosa subsp. fastidiosa, the causal agent of Pierce’s disease [20]. Moreover, the association of certain pests such as the grapevine moth (Lobesia botrana) and rising temperatures may negatively impact crop yields due to greater asynchrony between plant growth stages and the timing of larval emergence [37]. Fortunately, this does not appear to apply to grapevine powdery mildew (E. necator), which, under projected climate change conditions, is expected to show a lower severity of infection in grapevine [37]. These differences in the response of pathogens to environmental changes highlight that the interactions between plants and infectious agents are the result of a long coevolution. The coevolution between plants and pathogens is an important process for maintaining plant species diversity and in the development of plant resistance [38,39,40]. Genetic differences in plant resistance are advantageous because they make it harder for pathogens to adapt and infect new, susceptible plants [40]. Coevolutionary interactions between plants and pathogens show a high level of variability in resistance, both among different populations and within the same population [38,41,42,43,44,45,46,47]. In grapevines, pathogen resistance is inherited across generations, influenced by factors such as geographic origin, species-specific traits, and the nature of host–pathogen interactions [48,49,50].
One of the greatest challenges facing humanity today is how to make agricultural production more sustainable in the face of climate change. The European Union (EU) has implemented various strategies, such as the European Green Deal, which represents a set of policy initiatives with ambitious goals to overcome the challenges of climate change and environmental degradation. One of the most important policy initiatives for European agriculture was the Farm-to-Fork Strategy whose objectives relate to more sustainable food production [51]. The need to develop strategies to reduce pesticide use in viticulture is linked to evidence that although vineyards occupy only about 5% of European agricultural land, they account for almost 70% of all fungicide applications in agriculture [52]. Moreover, due to the high pest sensitivity of the grapevine, 13% in mass of all synthetic pesticides used in Europe are applied in the wine industry [52]. According to EUROSTAT [53], the three largest grape- and fruit-producing countries, Spain, France and Italy, recorded around 50% of the total amount of fungicides in Europe.
The aim of this study is to analyze and characterize resistance traits in wild grapevine species, in order to assess their potential for the introgression of resistance-associated genes through breeding programs, with the ultimate goal of developing cultivars with enhanced resilience to biotic and abiotic stresses.
2. Vitis spp.
We review here the literature concerning 23 species distributed from North to Central America (Figure A1 in Appendix A). Despite their poor grape quality, many of them have interesting adaptive characters, such as the susceptibility to abiotic (Table 1) and biotic (Table 2) stresses and the propagation aptitude, that could be useful in future breeding programs.

Table 1.
Schematic summary of all American Vitis species analyzed in the work in relation to their abiotic tolerances/resistances. The absence of ticks for some of the resistances does not indicate lack of resistance but only that it has not been established in the bibliography.

Table 2.
Schematic summary of all American Vitis species analyzed in the work in relation to their biotic tolerances/resistances. The absence of ticks for some of the resistances does not indicate lack of resistance but only that it has not been established in the bibliography.
2.1. Vitis berlandieri (=Vitis cinerea var. helleri Bailey)
2.1.1. Area of Origin
Named after the French botanist Jean-Louis Berlandier, V. berlandieri is native to the southern regions of the United States (Edwards Plateau area, TX, USA), southern New Mexico and northern Mexico [7,54,144,145,146]. In the wild, this species is predominantly found in clay, dry and calcareous soils, a geographical distribution that has significantly influenced its adaptation [54,62].
2.1.2. Susceptibility to Abiotic Stresses
The species shows a remarkable capacity for thermal adaptation, tolerating both high and cold winter temperatures [79]. The deep root system gives V. berlandieri a relatively high tolerance to drought [146]; however, it is very susceptible to waterlogging [54]. It is one of the species that is best adapted to calcareous soils [79], distinguishing itself from other American species, which show leaf chlorosis symptoms in lime-rich soils. Furthermore, it shows good tolerance to salinity [147].
2.1.3. Susceptibility to Biotic Stresses
The species is known for its resistance to phylloxera (D. vitifoliae) [80,81]. It also has a fair tolerance to powdery mildew (E. necator), downy mildew (P. viticola) and Pierce’s disease (X. fastidiosa) [54,79,82,83]. In particular, the potential of V. berlandieri for the development of rootstocks resistant to X. fastidiosa has been highlighted [84].
2.1.4. Propagation Aptitude
Vitis berlandieri is unsuitable as a rootstock in its pure form due to its poor rhizogenic aptitude, which makes propagation using both cuttings and grafting difficult. This limitation has been overcome through interspecific hybridization with species characterized by a higher rooting and grafting capacity [79,145].
2.2. Vitis riparia
2.2.1. Area of Origin
Also referred to as ‘bank grapes’, V. riparia is a species that mainly grows along watercourses and riverbanks. However, it can grow in a wide range of environments, provided there is sufficient water availability [76]. As a result, the plant does not require a deep root system to survive and prefers deep alluvial soils [54]. Vitis riparia is the most widespread wild grape species in North America, with a range extending from Canada, Manitoba and Quebec to Florida and from Texas to the Colorado Rocky Mountains [54,76,148].
2.2.2. Susceptibility to Abiotic Stresses
Vitis riparia does not tolerate calcareous soils [76] and prefers sandy, moist soils [62]. Its shallow roots make it particularly susceptible to drought, a trait that is also inherited by rootstocks obtained through crossing with other species [54,64]. However, it is highly resistant to the cold, surviving average winter temperatures as low as −40 °C. Several American species are more resistant to cold than V. vinifera, but none of them match the cold tolerance of V. riparia. Furthermore, it can tolerate root flooding for several days [54].
2.2.3. Susceptibility to Biotic Stresses
In its area of origin, V. riparia has co-evolved with the main grapevine pathogens, powdery mildew (E. necator), downy mildew (P. viticola) and phylloxera (D. vitifoliae), thus developing tolerance to these diseases [85,86,121,134,149,150,151,152,153,154]. In particular, V. riparia, which is native to regions where phylloxera is endemic, possesses root systems that are naturally resistant to this pest [64,76,87,88,155]. Vitis riparia is resistant to parasitic nematodes, confirming its suitability as a rootstock in environments prone to such parasites [89]. Despite its resistance to numerous pathogens, V. riparia is susceptible to Pierce’s disease [54].
2.2.4. Propagation Aptitude
Vitis riparia has played a crucial role in the history of rootstocks, directly providing ready-to-use selections for vineyards where they were already adapted, without the need for further crosses or selections between seedlings [76]. Since the 1980s, breeding programs have focused on the development of cultivars, commonly known as ‘northern hybrids’, that are cold- and disease-resistant and suitable for growing in the cold and moist regions of the eastern and central United States [62,156,157,158].
2.3. Vitis rupestris
2.3.1. Area of Origin
By the early 1900s, the population of V. rupestris was distributed throughout much of the central United States [159] and may have been introduced to other geographic areas [160]. Currently, the population of V. rupestris is restricted to specific habitats, with a distribution primarily concentrated in the Ozark Plateau, Missouri and Arkansas, and the Ouachita Mountains, Oklahoma [6,161].
2.3.2. Susceptibility to Abiotic Stresses
Vitis rupestris is a classic chloride excluder capable of maintaining low chloride levels in the aerial organs of the plant when grown in saline soil [147,161,162]. It can grow well in soils with a very high presence of gravel [162] and is highly resistant to salinity [55]. It is considered a species with high chill requirements [163].
2.3.3. Susceptibility to Biotic Stresses
Vitis rupestris exhibits notable tolerance to biotic stresses and has become a valuable genetic resource for breeding vine varieties resistant to diseases and pests, thereby contributing significantly to modern viticulture [156,161]. Vitis rupestris, used both directly as a rootstock and as a parent in the development of new rootstock hybrids, has conferred durable phylloxera (D. vitifoliae) tolerance to grapevines for over 150 years [90]. It is also an important source of resistance to pathogens such as black rot (G. bidwellii) [91], powdery mildew [92,93] and downy mildew (P. viticola). Alleles of the resistance locus Rpv3 (a major determinant of downy mildew resistance) were indeed associated with V. rupestris in more than 50% of the 233 hybrid grape cultivars examined [94].
2.3.4. Propagation Aptitude
The rooting capacity of V. rupestris is notably high, and it also demonstrates a strong grafting compatibility with V. vinifera cultivars [164]. Additionally, studies have shown that V. rupestris exhibits a high level of in vitro regeneration, with somatic embryos and seedlings readily produced from anther tissues [55,165].
2.4. Vitis labrusca
2.4.1. Area of Origin
Vitis labrusca, also known as the “northern fox grape”, is a vigorous climbing plant native to the eastern United States. Its distribution extends from Georgia to southeastern Canada, with Indiana marking the western boundary [54]. As a North American native species, it exhibits excellent adaptability to its natural environment [60]. This vine thrives in climates characterized by mild to high temperatures in spring, hot summers, and abundant rainfall throughout the year, averaging around 1700 mm annually [95].
2.4.2. Susceptibility to Abiotic Stresses
Vitis labrusca demonstrates remarkable cold resistance, as shown in several studies [54,56,57,58,59,60], and moderate drought tolerance [61]. The species is also adapted to acidic soils, which are preferred for cultivation [54,166,167], and, thus, it shows poor tolerance to calcareous soils. In terms of iron uptake efficiency, V. labrusca is similar to V. riparia and V. rotundifolia, being inefficient. This is due to its limited ability to release protons (H+) into the soil to enhance iron solubility. However, under iron deficiency, the plant can produce organic acids to alleviate this stress [168].
2.4.3. Susceptibility to Biotic Stresses
Vitis labrusca is distinguished by its high resistance to various pathogens and pests. It is highly resistant to the fungus B. cinerea [96], downy mildew (P. viticola) [95,97,98,99,169,170,171], and powdery mildew (E. necator) [54,97,98,99,100,172,173]. Additionally, it shows significant resistance to the dagger nematode (Xiphinema index) [101]. However, it is susceptible to Pierce’s disease [54]. A distinctive feature of V. labrusca is the presence of leaf trichomes and the constitutive and inducible expression of genes involved in the biosynthesis of terpenes, flavonoids, and phenylpropanoids. This makes it less palatable to Popillia japonica (Japanese beetle) compared to V. vinifera [60]. Furthermore, the genetic composition of V. labrusca provides an additional advantage in resisting herbivorous insects, demonstrating a better adaptation compared to other grapevine species [54].
2.4.4. Propagation Aptitude
Vitis labrusca has hermaphroditic flowers [167] and is known for the high rooting capacity of its cuttings, making it an ideal species for rapid and efficient propagation [174]. Native to North America, it has been utilized in breeding programs since the 1980s to create hybrids with enhanced resistance to abiotic and biotic stresses [60]. These so-called “northern hybrids” result from crosses between V. vinifera and wild species such as V. labrusca, which has contributed to improved resistance to both abiotic and biotic stresses [62,157].
2.5. Vitis cinerea (=Vitis simpsonii Munson)
2.5.1. Area of Origin
Vitis cinerea, commonly known as “possum grape,” “sweet grape,” or “pigeon grape,” is a native species primarily distributed in the southeastern United States, extending as far west as Texas. Its geographical range is concentrated along the banks of the Mississippi and Missouri rivers, where it thrives in humid environments, particularly woodlands located near watercourses [54].
2.5.2. Susceptibility to Abiotic Stresses
Vitis cinerea prefers relatively acidic soils [54], is drought-tolerant [62,63] and is particularly well adapted to warm-humid climatic conditions [175].
2.5.3. Susceptibility to Biotic Stresses
Vitis cinerea is resistant to powdery mildew (E. necator) [54,102] and, due to its co-evolution with the pathogen, shows complete resistance to downy mildew (P. viticola), like many other North American Vitis species, including V. rotundifolia and V. riparia [54,103]. It also possesses a high level of resistance to the nematode Meloidogyne javanica [104] and total resistance to phylloxera (D. vitifoliae) [105,106].
2.5.4. Propagation Aptitude
Vitis cinerea represents a genetic resource of considerable interest for rootstock selection, not only for its ability to confer complete resistance to phylloxera but also for its potential to improve scion performance. This positive effect is especially evident in shallow soils, soils with high gravel content and dry conditions [176,177]. The adaptation to warm-humid climatic conditions of this species seems to be passed on to its progeny in crosses [175].
2.6. Vitis X champinii
2.6.1. Area of Origin
Vitis champinii is considered a natural hybrid between V. mustangensis and V. rupestris. It is native to some arid and low-rainfall regions of west-central Texas and northern Mexico, where the ranges of these two species overlap [64,65].
2.6.2. Susceptibility to Abiotic Stresses
Vitis champinii is known for its high drought tolerance [65]. Under moderate water deficit conditions, this species maintains higher stomatal conductance and photosynthesis rates than other Vitis species [178,179]. In addition, one study showed that V. champinii develops a larger canopy and a more extensive root system than other species in the genus [180]. It is also particularly tolerant to calcareous and saline soils [64].
2.6.3. Susceptibility to Biotic Stresses
Vitis champinii is resistant to phylloxera and has strong resistance to nematodes [54,64]. In particular, it is resistant to the nematode X. index [101] and contains a xylem sap phytochemical that hinders the development of mature biofilms by X. fastidiosa, significantly inhibiting colony growth compared to susceptible cultivars [181]. Although V. champinii does not possess the PdR1 locus associated with resistance to X. fastidiosa, it has defense mechanisms that affect both the vector insect and the bacterium, including the presence of leaf trichomes [182].
2.6.4. Propagation Aptitude
Vitis champinii is known for its low rooting and grafting ability, often proving difficult to propagate [64,183]. However, the late selection of cuttings has been identified as a promising practice to increase propagation success [184]. Despite these limitations, V. champinii is widely used as a rootstock in vineyards and selection programs, especially in areas with high nematode concentrations or saline soils, such as the Central Valley of California and some regions of Australia [64].
2.7. Vitis aestivalis (=Vitis rufotomentosa Small)
2.7.1. Area of Origin
Vitis aestivalis is native to eastern North America and grows in dry upland areas and ravines. Its distribution extends from southern Ontario to Florida, east to Maine, and west to Oklahoma and Texas [185]. This species evolved by adapting to the sandy habitats of the Appalachian Mountains, a naturally inhospitable environment for the development of the phylloxera root form [54,79].
2.7.2. Susceptibility to Abiotic Stresses
Vitis aestivalis demonstrates remarkable adaptability to temperature extremes, showing less susceptibility to the adverse effects of high root temperatures compared to European Vitis species [186]. Additionally, it exhibits exceptional cold tolerance, withstanding temperatures as low as −30 °C. This cold resistance is notable, even though no specific genes directly associated with this trait have been identified [54,66]. Furthermore, it shows high drought resistance and adapts well to wet and rainy summers, hence the name ‘summer grape’ [54].
2.7.3. Susceptibility to Biotic Stresses
Vitis aestivalis is highly resistant to economically important diseases such as black rot, and downy mildew [107,108,109] and to powdery mildew, but this resistance can be overcome by virulent isolates of the pathogen, suggesting a specific interaction between races of the fungus and resistance genes [110]. A V. aestivalis hybrid called ‘Norton’ also shows tolerance to Pierce’s disease [111,112]. It is resistant to phylloxera [111,113,114] and to B. cinerea bunch rot [109,111]. In addition, accessions of V. aestivalis. resistant to root-knot nematodes have been identified [115].
2.7.4. Propagation Aptitude
It is a species that has difficulty propagating through dormant cuttings, with rooting rates ranging between 9.4% and 22.0% [187,188]. The propagation of V. aestivalis achieved a 40.9% success rate under background heat and rooting hormone treatments, with cuttings treated with indole-3-butyric acid (IBA) showing up to four times greater rooting success than untreated ones [189,190].
2.8. Vitis arizonica
2.8.1. Area of Origin
Vitis arizonica, also known as “Canyon grape,” is a species native to the extreme west of Texas and endemic to the American Southwest [191,192]. This region is characterized by an arid climate and a considerable variety of ecological conditions, to which the species has successfully adapted [72].
2.8.2. Susceptibility to Abiotic Stresses
Vitis arizonica exhibits high cold resistance [67]. Furthermore, this species is tolerant to lime-induced chlorosis, a condition that occurs when it is grown in calcareous soils. This tolerance, shared with other species such as V. californica, V. longii, and V. monticola, suggests the presence of a common protective mechanism that allows these species to mitigate stress from high soil alkalinity [68]. Vitis arizonica shows moderate vulnerability to xylem embolism caused by drought, but is able to repair it rapidly after irrigation, restoring water transport. This adaptation, supported by increased root pressure under water stress, allows the plant to quickly recover transpiration rates and effectively cope with drought conditions [65]. The ability of V. arizonica to tolerate lime-induced chlorosis and recover from drought-induced embolism suggests that it possesses adaptive traits that could be valuable for grapevine rootstock breeding programs aimed at improving tolerance to stresses in calcareous soils and drought-prone environments [65,68].
2.8.3. Susceptibility to Biotic Stresses
Vitis arizonica exhibits high resistance to infection by X. fastidiosa [116]. This resistance is attributed to a single dominant locus, named PdR1, which has been genetically and physically mapped [193,194,195,196]. Native grape phylloxera strains that form leaf galls on V. arizonica in Arizona and New Mexico have not been found on the roots, suggesting a possible intrinsic resistance of V. arizonica to root infestations, as well as the inability of the parasite to induce root galls [82,117]. Additionally, V. arizonica has been identified as resistant to X. index [118], powdery mildew (E. necator) [119,120] and highly resistant to anthracnose [197].
2.8.4. Propagation Aptitude
Vitis arizonica demonstrates excellent rooting ability when propagated from cuttings. In vitro studies have shown a success rate greater than 73% for V. arizonica cuttings, indicating effective propagation through this method [198]. Furthermore, stem cuttings, particularly those from semi-hardwood, exhibited the highest rooting success compared to softwood or hardwood cuttings [199].
2.9. Vitis californica
2.9.1. Area of Origin
Vitis californica, commonly known as the California wild grape, is a species endemic to the northern Central Valley, in California [200]. It is found from the Tehachapi Mountains in the south to southern Oregon and is common in the Central Valley, extending up to about 1000 m in the Coastal Range, Sierra Nevada, Cascade, and Klamath Mountains [200].
2.9.2. Susceptibility to Abiotic Stresses
Vitis californica is categorized as a species tolerant to lime-induced chlorosis [69] when grown in calcareous soil [68]. The leaf movement of V. californica allows the plant to balance light absorption with the risk of thermal stress, helping to protect its photosynthetic components. This adaptive strategy prevents excessive temperatures that could reduce photosynthetic capacity and ensures optimal performance even under high-radiation and -temperature conditions [201].
2.9.3. Susceptibility to Biotic Stresses
Vitis californica did not show sufficient resistance to phylloxera, so it is not suitable for use in rootstocks [114,122,123]. Vitis californica does not exhibit significant resistance to powdery mildew [121]. Some V. californica plants were found to be highly susceptible to nematode attack (M. incognita) and had no relevant natural defenses [202].
2.9.4. Propagation Aptitude
Some studies state that it is possible to easily propagate V. californica [203].
2.10. Vitis rotundifolia (=Muscadinia rotundifolia)
2.10.1. Area of Origin
Vitis rotundifolia, commonly known as ‘Muscadine’, is a species of Vitis primarily associated with the southeastern United States [204]. Its natural distribution range extends from Delaware to central Florida and along the Gulf of Mexico to the eastern part of Texas [205,206,207,208]. The species also extends north along the Mississippi River to Missouri and reaches areas near the Appalachian Mountains from both the east and west [208].
2.10.2. Susceptibility to Abiotic Stresses
Vitis rotundifolia is particularly sensitive to winter freeze and lime-induced chlorosis [54]. This species prefers deep, well-drained soils and thrives in humid, shaded environments, conditions that support optimal vegetative growth [70].
2.10.3. Susceptibility to Biotic Stresses
The species V. rotundifolia has coevolved with the native grapevine diseases and pests of North America, developing resistance or tolerance, although variable, to numerous pathogens and pests. It is particularly resistant to downy mildew (P. viticola) [124,125], powdery mildew (E. necator) [125,126,127], and phylloxera (D. vitifoliae) [125,176]. Additionally, V. rotundifolia exhibits resistance to black rot, the bacterium causing Pierce’s disease, and the dagger nematode X. index, which transmits the grapevine fanleaf virus [54].
2.10.4. Propagation Aptitude
Vitis rotundifolia exhibits three distinct floral types: perfect hermaphrodite, staminate (male), and imperfect hermaphrodite (female) [125]. It has a chromosome set of 2 n = 40, unlike V. vinifera where 2 n = 38 [209,210]. For this reason, it is generally incompatible with Vitis species in both flowering and grafting, but it can produce fertile hybrids with V. rupestris, a characteristic that enables its use in modern rootstock breeding programs [54]. This ability to generate fertile hybrids allows for the incorporation of desirable traits, such as disease resistance, into rootstock varieties, making V. rotundifolia an important resource for improving the resilience and productivity of grapevine cultivation.
2.11. Vitis vulpina (=Vitis cordifolia Michaux)
2.11.1. Area of Origin
Vitis vulpina is also called the ‘winter grape’. Its habitat extends from Pennsylvania to Florida [211]. This species name has unfortunately been misinterpreted by many taxonomists and viticulturists over the years, but some investigations indicate that V. vulpina is a synonym of V. cordifolia [128,212], according to the International Standards of Botanical Nomenclature. Furthermore, Vitis illex is another minor variant of V. vulpina and is therefore treated as a synonym [128,159].
2.11.2. Susceptibility to Abiotic Stresses
Vitis vulpina has shown a lower response to sprouting than other species and is considered a high-chilling species, requiring more than 1000 chilling hours [163,213].
2.11.3. Susceptibility to Biotic stresses
Resistance to root-knot nematode (Meloidogyne spp.) has been reported in grape rootstocks with V. vulpina in their parentage [129]. It has been used in the past in France for breeding phylloxera-resistant rootstocks [128]. Furthermore, it may have some resistance to Pierce’s disease [128].
2.11.4. Propagation Aptitude
Vitis vulpina stands out for its high competitive ability, adapting well to gaps in shrubland contexts, where it maximizes light availability and exhibits a higher propagation density compared to other Vitis species in undisturbed areas [214].
2.12. Vitis mustangensis (=Vitis candicans Engelmann)
2.12.1. Area of Origin
Vitis mustangensis (syn. V. candicans), commonly known as the ‘Mustang grape,’ is a species native to the southern United States, particularly Texas, and northern Mexico [54,70,73,215,216]. It predominantly thrives in floodplains, on the slopes and summits of calcareous and chalk hills in southwestern Texas, as well as in the wooded ravines of the Texas black prairies. It prefers limestone-rich soils, although it grows vigorously in almost any soil type [70].
2.12.2. Susceptibility to Abiotic Stresses
Vitis mustangensis exhibits significant tolerance to drought and extreme heat but shows limited or no resistance to low temperatures [54,70,71]. However, it is vulnerable to chlorosis, a condition that frequently occurs in calcareous soils [73].
2.12.3. Susceptibility to Biotic Stresses
Vitis mustangensis shows significant resistance to numerous pathogens. It is particularly resistant to phylloxera, powdery mildew, and downy mildew. Furthermore, it appears to be capable of transferring resistance to Pierce’s disease to varieties grafted onto it [54,73,217]. It is also resistant to the fungal disease caused by Phymatotrichopsis omnivora, the causal agent of the common root rot found in Texas, Mexico, and the southwestern United States [217]. Additionally, it has been identified as a source of resistance against the nematode M. incognita [130].
2.12.4. Propagation Aptitude
Rootstocks containing V. mustangensis may encounter some rooting difficulties depending on the associated parent [73]. Its root penetration ability is deeper than that of any other species [70]. It is very difficult to propagate by cutting and although interspecific hybridization in Vitis can sometimes be limited by cross-incompatibility this species shows high pollen fertility and good compatibility with other species, allowing the effective transmission of its characteristics through hybridization [70]. Other southern species, such as V. champinii and V. longii, are probably natural hybrids of V. mustangensis, V. rupestris, and other native species [90], which are known to be highly resistant to nematodes [54].
2.13. Vitis girdiana
2.13.1. Area of Origin
Vitis girdiana is a species endemic to Southern California [70,200]. It is found in or near springs and creeks from Baja California to the Tehachapi Mountains and from coastal areas to the desert regions of California and southern Nevada [200].
2.13.2. Susceptibility to Abiotic Stresses
Vitis girdiana has good tolerance to salt and drought [69]. This species has a highly developed palisade layer in its leaves, which are also thicker compared to other wild species of the genus Vitis. This makes it particularly suitable for xeric habitats [72]. Reflective leaf pubescence, as observed in the characteristically gray leaves of V. girdiana, could be adaptive under xeric conditions and could work in reducing leaf temperature and water loss [72].
2.13.3. Susceptibility to Biotic Stresses
Vitis girdiana did not show sufficient resistance to phylloxera [70,114].
2.14. Vitis acerifolia (=Vitis longii Prince)
2.14.1. Area of Origin
Vitis acerifolia has several synonyms including V. longii and V. solonis. It is a vigorous, small-seeded vine native to the United States on the banks of the Red River, north of Denison, Texas [70,133]. It was validly described under the name of V. longii [218,219].
2.14.2. Susceptibility to Abiotic Stresses
It is categorized as a tolerant species to lime-induced chlorosis [69] when grown in calcareous soil [68]. This species shows strong adaptation to calcareous soils, where it tolerates drought exceptionally well. In contrast, its performance declines markedly on deep sandy upland soils during prolonged droughts, as observed in parts of Texas [70,133].
2.14.3. Susceptibility to Biotic Stresses
Vitis acerifolia exhibits high resistance to phylloxera (D. vitifoliae) [70,133]. During wet seasons, young leaves of the species from the Texas Panhandle region are vulnerable to attacks by gray mold (B. cinerea) and anthracnose (Elsinoë ampelina), although the ‘Microsperma’ variety is not affected. In contrast, the species is minimally impacted by black rot (G. bidwellii), with only slight damage observed [70].
2.14.4. Propagation Aptitude
Its roots are thin, highly branched, very fibrous and deeply penetrating [70]. Its flowers show high fruit set, with abundant and viable pollen. Germination and inflorescence are among the earliest, while defoliation is late [70]. It grows easily from cuttings. The pollen of staminate plants is highly prolific and exhibits high fertility, exerting a significant influence on trait expression in interspecific crosses. Due to its great vigor, its resistance and the ease of growth from cuttings, this species offers great incentives for hybridization [70].
2.15. Vitis palmata (=Vitis rubra Michaux)
2.15.1. Area of Origin
Also known as the ‘Cat Bird Grape’, V. palmata (syn. Vitis rubra) discovered by Michaux, it is native to the Illinois region near Chicago and near the Illinois River, a tributary of the Mississippi [70,220].
2.15.2. Susceptibility to Abiotic Stresses
Vitis palmata has a very compact leaf mesophyll to optimize gas exchange and water management [72]. Moderately branched and climbing, it can grow in shallow soils [70], and it is resistant to cold and drought when it is well-rooted. It prefers argillaceous, alluvial, moist bottomlands [70].
2.15.3. Susceptibility to Biotic Stresses
It is resistant to phylloxera and is free from all major diseases [70] and it is considered resistant to downy mildew [134].
2.15.4. Propagation Aptitude
Its roots are rather thick, abundant, hard and penetrating. Germination and inflorescence are very late, and in the first years of growth, it is structurally [54].
2.16. Vitis shuttleworthii (=Vitis coriacea Planchon)
2.16.1. Area of Origin
Vitis shuttleworthii is a species native to the southeast United States [135,221].
2.16.2. Susceptibility to Abiotic Stresses
It is well suited for cultivation in tropical and subtropical regions thanks to its adaptation to environmental stresses such as soil salinity [73,74].
2.16.3. Susceptibility to Biotic Stresses
This species has been useful in developing rootstock cultivars with resistance to Pierce’s disease and nematodes [128]. In particular, it is considered one of the most resistant to Pierce’s disease and has long been used to develop grape varieties resistant to severely infected areas [135,136,137]. This species also shows resistance to downy mildew [134,138], black rot [135] and anthracnose [138]. Most accessions of V. shuttleworthii can be characterized as moderately resistant to powdery mildew [139]. The grapes of this vine exhibit resistance to Colletotrichum acutatum [222].
2.17. Vitis X doaniana
2.17.1. Area of Origin
Vitis doaniana (Doan’s Grape) is a hybrid grape resulting from the natural hybridization of V. mustangensis with V. acerifolia. Its native range includes Oklahoma, Colorado, Texas and New Mexico [70,216].
2.17.2. Susceptibility to Abiotic Stresses
Vitis doaniana exhibits good tolerance to salt and drought, with excellent chloride exclusion, and demonstrates great hardiness in enduring cold and drought, making it one of the best species for dry climates [69,70].
2.17.3. Susceptibility to Biotic Stresses
Some accessions of V. doaniana are considered moderately resistant to powdery mildew [139]. In some experiments on grapevine resistance to nematodes it was found that V. doaniana may have intrinsic resistance to damage caused by nematodes, making it one of the most promising varieties [140].
2.18. Vitis monticola
2.18.1. Area of Origin
Vitis monticola is a vine species endemic to the Edwards Plateau of central Texas [70,221].
2.18.2. Susceptibility to Abiotic Stresses
It is classified as a tolerant species to lime-induced chlorosis when grown in calcareous soils [68]. It can grow in shallow soil [70], and it is considered a xeric species due to its sclerophytic leaves [223], which have smaller but more numerous vascular bundles than those of mesophytic species. In addition, reflective leaf pubescence aids heat dissipation and reduces water loss [72].
2.18.3. Susceptibility to Biotic Stresses
This vine is highly disease-resistant and therefore always appears healthy. Its foliage resists gray mold (B. cinerea) well and the fruit is free from black rot [70]
2.18.4. Propagation Aptitude
The cuttings root with some difficulty, but a little more easily than those of V. berlandieri. Hybrids containing this Vitis species are excellent for grafting because of their vigor and strength [70].
2.19. Vitis popenoei (Muscadinia subgenus)
2.19.1. Area of Origin
Vitis popenoei is a relatively unknown species. It is native to Central America, with its distribution range extending from eastern Maryland to Texas and southward to the Gulf Coast [54]. It is adapted to specific climatic conditions, with a distribution range that extends several hundred kilometers further south than that of the other two known species of the subgenus Muscadinia, V. rotundifolia and V. munsoniana [224].
2.19.2. Susceptibility to Abiotic Stresses
Vitis popenoei grows in environments characterized by humid climate [225]. However, the primary limitation to its geographic distribution is its sensitivity to cold. Regions where minimum winter temperatures consistently drop to −18 °C are considered unsuitable for its cultivation [75].
2.19.3. Susceptibility to Biotic Stresses
Vitis popenoei shows natural resistance to Pierce’s disease (X. fastidiosa) [141]. Like other species belonging to the Muscadinia subgenus, such as V. rotundifolia and V. munsoniana, it is also resistant to many other pathogens, including grapevine downy mildew (P. viticola) and powdery mildew (E. necator) [75].
2.20. Vitis tiliifolia
2.20.1. Area of Origin
The wild grapevine V. tiliifolia is widely distributed in the southern regions of Mexico and the Antilles, with its range extending to Colombia [226,227].
2.20.2. Susceptibility to Biotic Stresses
Vitis tiliifolia exhibits moderate resistance to P. viticola, the causal agent of downy mildew [134].
2.21. Vitis blancoi
2.21.1. Area of Origin
Vitis blancoi grows along waterways in the Sierra Madre mountains in the state of Jalisco, near the city of Guadalajara, and also near Montemorelos, Nuevo Leon state, in Mexico [70].
2.21.2. Susceptibility to Abiotic Stresses
It appears to be very susceptible to spring frosts because just 8–10 degrees of frost are enough to destroy the vitality of the vine above the ground [70].
2.21.3. Propagation Aptitude
This vine is easily propagated and grows by cuttings [70].
2.22. Vitis baileyana
2.22.1. Area of Origin
This species, also known as the ‘Wild ’Grape Possum,’ has been observed along the Kanawha River near Kanawha Falls, as well as in the North Carolina area [70].
2.22.2. Susceptibility to Abiotic Stresses
Vitis baileyana grows well along mountain streams at altitudes up to 900 m (about 3000 feet). It grows well on calcareous clay soils [70].
2.22.3. Susceptibility to Biotic Stresses
Some specimens of V. baileyana were observed with the presence of phylloxera galls on the leaves, but in general, they were in good health [70].
2.23. Vitis nesbittiana
2.23.1. Area of Origin
Vitis nesbittiana is a species native to central Mexico, discovered in 1987 by Comeaux, and found in tropical rainforests near Jalapa, Veracruz, at elevations between 1700 and 2120 m [228].
2.23.2. Susceptibility to Abiotic Stresses
Vitis nesbittiana being native to mountainous areas, it can be used as a rootstock for its ability to develop in tropical climates [76,77,78].
2.23.3. Susceptibility to Biotic Stresses
In some experiments, some resistance to Pierce’s disease has been observed [142]. In addition, some resistance to root-knot nematodes (Meloidogyne spp.) was also observed in seedlings hybridized with V. nesbittiana in other experiments [143].
3. Conclusions
The development of hybrid varieties, thanks to their tolerance to diseases and insects, could be the most promising tool for low-input, low-cost and time-saving viticulture [132,229], especially in the context of global climate change, which has significant implications for food security and agricultural sustainability, affecting crop growth and overall productivity [230,231,232,233]. All around the world, Vitis germplasm repositories represent a valuable resource for discovering and introducing new sources of resistance [169]. Compared to domesticated cultivars, wild crop relatives exhibit greater resilience to the impacts of global climate change and possess a stronger capacity to adapt to challenging environmental conditions [234]. Wild species within the Vitis genus represent valuable genetic resources for breeding programs, offering traits such as resistance to both biotic and abiotic stresses [235,236,237,238]. It is well known how interspecific gene flow has contributed to the origin of crop plants, to the restoration of crop diversity after domestication or genetic erosion and to the adaptation to challenging environments [239]. Plant protection treatments can be reduced by up to 75% without compromising grape health [240]. In recent years, due to the increasingly evident effects of global climate change, the importance of developing new grapevine genotypes capable of effectively adapting to abiotic stresses has grown. Many of the current programs aim to ensure sustainable viticulture through genetic improvement and the introduction of resistance-related genes present in wild grapevine species, obtained through interspecific crosses [241]. The responses of vines to extreme temperatures, drought, heavy metals, and other environmental stresses are the focus of numerous scientific studies, with a clear goal: to create new hybrids that are more resilient and better suited to the challenges of climate change [22]. Our analysis of the scientific literature highlights that within the genetic pool of American Vitis species, there are highly valuable traits of resistance to both biotic and abiotic stresses, which could play a pivotal role in modern grapevine breeding programs. A central step in these programs has always been the selection of suitable parents for resistance transfer. For this reason, considering the resistance or susceptibility traits of each American wild species is of fundamental importance.
Among the species considered, V. aestivalis emerges as one of the most promising for resistance to multiple biotic stresses, including P. viticola, E. necator, B. cinerea, G. bidwellii, D. vitifoliae, X. fastidiosa, and nematodes. Similarly, V. rotundifolia shows a broad spectrum of resistance to most of these pathogens, with the exception of B. cinerea. Other species such as V. berlandieri, V. riparia, V. rupestris, V. labrusca, V. cinerea, and V. shuttleworthii also exhibit interesting resistance patterns, particularly against downy and powdery mildew, which remain major diseases in viticulture. In addition, specific resistance genes such as PdR1 in V. arizonica highlight the importance of focusing on single loci, even when the overall resistance of the species is not extended to other factors.
With regard to abiotic stresses, the potential of wild species is strongly linked to their geographical origin and environmental adaptation. Vitis berlandieri is particularly valuable for its tolerance to drought and low temperatures, while V. aestivalis, V. mustangensis, V. girdiana, and V. shuttleworthii show high levels of adaptation to heat and water scarcity, making them especially relevant for breeding programs in warm and arid viticultural regions.
Overall, the integration of these wild species into modern breeding strategies will enable the development of new grapevine cultivars capable of withstanding both biotic and abiotic pressures, ensuring sustainable viticulture in the face of global climate change. However, a recurrent limitation concerns the accessibility of genetic material for breeding. Although ex situ germplasm collections represent an important reservoir of diversity, their establishment and maintenance are costly and often limited in scope. Even more critical are the barriers related to the exchange of plant material (cuttings, seeds, pollen), which are increasingly subject to phytosanitary regulations and quarantine requirements. While such measures are justified to prevent the spread of pests and diseases, they significantly slow down the circulation of genetic resources and consequently delay breeding progress.
In addition, further challenges arise in the process of transferring resistance. On the one hand, the traceability of resistance genes is often problematic since the loci responsible are not always known or may fail to be effectively transmitted to progenies. On the other hand, many resistance traits do not depend on a single gene but result from complex interactions among multiple genes and physiological mechanisms. As a consequence, it is possible that a plant carrying a known resistance gene does not actually express effective resistance under natural conditions. For this reason, it is essential to complement genetic approaches with direct inoculation tests with pathogens and physiological evaluations on candidate plants, in order to validate resistance traits before their integration into breeding programs.
Author Contributions
Conceptualization, G.R. and L.R.; methodology, L.R.; investigation, M.D., C.R. and G.R.; data curation, M.D. and C.R.; writing—original draft preparation, M.D., C.R. and G.R.; writing—review and editing, M.D., C.R., R.A.A., G.-P.D.S., A.A., G.R. and L.R.; visualization, M.D., C.R., R.A.A., G.-P.D.S., A.A., G.R. and L.R.; supervision, G.R. and L.R.; project administration, L.R.; funding acquisition, L.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare financial support was received for bibliographic research and paper writing of this article. The overall work fulfils some goals of the Project CLEARGENES “ CLimatE chAnge Resilience GENES in Italian fruits and vegetables”—Bandi a Cascata—Programma AGRITECH CN00000022—CUP UNIPD C93C2200279000 -PNRR—M4C2—Inv. 1.4, finanziato dall’Unione europea—NextGenerationEU Codice progetto SP4_WP4.1.1_CLEARGENES and received funding from the PhD fellowship “Cultivar selection for Xylella resistance in viticulture”, PNRR—DM 630/2024, co-funded by Vivai Cooperativi Rauscedo S.C.A.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank Sana Rehman for reviewing the English in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
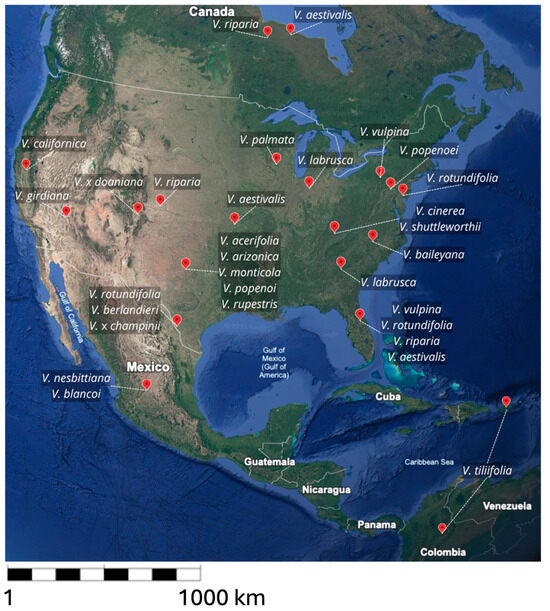
Figure A1.
Geographic distribution of American Vitis species. The map shows the main regions of origin of wild grapevine (Google Earth, 2025).
References
- Wen, J. Vitaceae. Flowering Plants · Eudicots. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 9, pp. 467–479. [Google Scholar]
- Wen, J.; Lu, L.; Nie, Z.; Liu, X.; Zhang, N.; Ickert-Bond, S.; Gerrath, J.; Manchester, S.R.; Boggan, J.; Chen, Z. A New Phylogenetic Tribal Classification of the Grape Family (Vitaceae). J. Syst. Evol. 2018, 56, 262–272. [Google Scholar] [CrossRef]
- This, P.; Lacombe, T.; Thomas, M. Historical Origins and Genetic Diversity of Wine Grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S.; Sharma, D. A Breif Overview on Vitis vinifera. Sch. Acad. J. Pharm. 2021, 10, 231–239. [Google Scholar] [CrossRef]
- Comeaux, B.L.; Nesbitt, W.B.; Fantz, P.R. Taxonomy of the Native Grapes of North Carolina. Castanea 1987, 52, 197–215. [Google Scholar]
- Moore, M.O. Classification and Systematics of Eastern North American Vitis L. (Vitaceae) North of Mexico. SIDA Contrib. Bot. 1991, 14, 339–367. [Google Scholar]
- Galet, P. Cépages et Vignobles de France: Les Vignes Américaines. In Tome 1, Les Vignes Américanes; Dehan, C., Ed.; Imprimerie: Montpellier, France, 1988. [Google Scholar]
- Chen, Z.; Hui, R.; Wen, J. Vitaceae. In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 12. [Google Scholar]
- Reisch, B.I.; Pratt, C. Grapes. In Fruit Breeding, 2nd ed.; Janick, J., Moore, J.N., Eds.; Wiley: New York, NY, USA, 1996; pp. 297–369. [Google Scholar]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and Processes in Crop Domestication: An Historical Review and Quantitative Analysis of 203 Global Food Crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Miller, A.J.; Gross, B.L. From Forest to Field: Perennial Fruit Crop Domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef]
- Zhou, Y.; Muyle, A.; Gaut, B.S. Evolutionary Genomics and the Domestication of Grapes. In The Grape Genome; Springer International Publishing: Cham, Switzerland, 2019; pp. 39–55. [Google Scholar]
- Maghradze, D.; Kikilashvili, S.; Gotsiridze, O.; Maghradze, T.; Fracassetti, D.; Failla, O.; Rustioni, L. Comparison between the Grape Technological Characteristics of Vitis vinifera subsp. sylvestris and subsp. sativa. Agronomy 2021, 11, 472. [Google Scholar] [CrossRef]
- Gepts, P. Crop Domestication as a Long-Term Selection Experiment. Plant Breed. Rev. 2003, 24, 1–44. [Google Scholar] [CrossRef]
- Maghradze, D.; Rehman, S.; Chutlashvili, A.; Kikilashvili, S.; Kikvadze, M.; Shamugia, A.; Charkviani, S.; McGovern, P.; Failla, O.; Gotsiridze, O.; et al. Differences in rooting ability between wild and cultivated Vitis vinifera. OENO One 2025, 59, 9371. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic Structure and Domestication History of the Grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef]
- Grassi, F.; Arroyo-Garcia, R. Origins and Domestication of the Grape. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The Proportion of Soil-Borne Pathogens Increases with Warming at the Global Scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Romero, À.; Iturbide, M.; Moralejo, E.; Gutiérrez, J.M.; Matías, M.A. Global Warming Significantly Increases the Risk of Pierce’s Disease Epidemics in European Vineyards. Sci. Rep. 2024, 14, 9648. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Tucker, E.J.; Tester, M. Genetic Analysis of Abiotic Stress Tolerance in Crops. Curr. Opin. Plant Biol. 2011, 14, 232–239. [Google Scholar] [CrossRef]
- Ollat, N.; Cookson, S.J.; Destrac-Irvine, A.; Lauvergeat, V.; Ouaked-Lecourieux, F.; Marguerit, E.; Barrieu, F.; Dai, Z.; Duchêne, E.; Gambetta, G.A.; et al. Grapevine Adaptation to Abiotic Stress: An Overview. Acta Hortic. 2019, 1248, 497–512. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic Stress Responses in Plants: An Overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Dudney, J.; Willing, C.E.; Das, A.J.; Latimer, A.M.; Nesmith, J.C.B.; Battles, J.J. Nonlinear Shifts in Infectious Rust Disease Due to Climate Change. Nat. Commun. 2021, 12, 5102. [Google Scholar] [CrossRef]
- Chaloner, T.M.; Gurr, S.J.; Bebber, D.P. Plant Pathogen Infection Risk Tracks Global Crop Yields under Climate Change. Nat. Clim. Chang. 2021, 11, 710–715. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate Change Impacts on Plant Pathogens, Food Security and Paths Forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Wapshere, A.J.; Helm, K.F. Phylloxera and Vitis: An Experimentally Testable Coevolutionary Hypothesis. Am. J. Enol. Vitic. 1987, 38, 216–222. [Google Scholar] [CrossRef]
- Benheim, D.; Rochfort, S.; Robertson, E.; Potter, I.D.; Powell, K.S. Grape Phylloxera (Daktulosphaira vitifoliae)—A Review of Potential Detection and Alternative Management Options. Ann. Appl. Biol. 2012, 161, 91–115. [Google Scholar] [CrossRef]
- Gale, G. Saving the Vine from Phylloxera. In Wine; CRC Press: Boca Raton, FL, USA, 2002; pp. 70–91. [Google Scholar]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Sgubin, G.; Bois, B.; Ollat, N.; Swingedouw, D.; Zito, S.; Gambetta, G.A. Climate Change Impacts and Adaptations of Wine Production. Nat. Rev. Earth Environ. 2024, 5, 258–275. [Google Scholar] [CrossRef]
- Zaldea, G.; Nechita, A.; Damian, D.; Ghiur, A.D.; Cotea, V.V. Climate Changes in Recent Decades, the Evolution of the Drought Phenomenon and Their Influence on Vineyards in North-Eastern Romania. Not. Bot. Horti Agrobot. Cluj. Napoca 2021, 49, 12448. [Google Scholar] [CrossRef]
- Meladze, M.; Mamasakhlisashvili, L.; Ujmajuridze, L.; Migliaro, D.; Domanda, C.; Rustioni, L. Neglected Cultivars for the Mtskheta-Mtianeti Region (East Georgia): Ampelography, Phenology, and Agro-Climatology. Vitis 2023, 62, 75–84. [Google Scholar] [CrossRef]
- Salinar, F.; Giosuè, S.; Tubiello, F.N.; Rettori, A.; Rossi, V.; Spanna, F.; Rosenzweig, C.; Gullino, M.L. Downy Mildew (Plasmopara viticola) Epidemics on Grapevine under Climate Change. Glob. Chang. Biol. 2006, 12, 1299–1307. [Google Scholar] [CrossRef]
- Angelotti, F.; Hamada, E.; Magalhães, E.E.; Ghini, R.; Garrido, L.d.R.; Pedro Júnior, M.J. Climate Change and the Occurrence of Downy Mildew in Brazilian Grapevines. Pesqui. Agropecu. Bras. 2017, 52, 426–434. [Google Scholar] [CrossRef]
- Lalic, B.; Jankovic, D.; Ninkov, M. Assessment of Climate Change Impact on Downy Mildew Appearance in Serbia Using ECHAM5 Climate Model Outputs. In Proceedings of the Environmental Changes and Adaptation Strategies, Skalice, Slovakia, 9–11 September 2013; pp. 9–11. [Google Scholar]
- Caffarra, A.; Rinaldi, M.; Eccel, E.; Rossi, V.; Pertot, I. Modelling the Impact of Climate Change on the Interaction between Grapevine and Its Pests and Pathogens: European Grapevine Moth and Powdery Mildew. Agric. Ecosyst. Environ. 2012, 148, 89–101. [Google Scholar] [CrossRef]
- Dinoor, A. Sources of Oat Crown Rust Resistance in Hexaploid and Tetraploid Wild Oats in Israel. Can. J. Bot. 1970, 48, 153–161. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thompson, J.N. Changed Patterns of Resistance in a Population of Linum marginale Attacked by the Rust Pathogen Melampsora lini. J. Ecol. 1995, 83, 199. [Google Scholar] [CrossRef]
- Brown, J.K.M. Little Else But Parasites. Science 2003, 299, 1680–1681. [Google Scholar] [CrossRef]
- De Nooij, M.P.; Van Damme, J.M.M. Variation in Host Susceptibility among and within Populations of Plantago lanceolata L. Infected by the Fungus Phomopsis subordinaria (Desm.) Trav. Oecologia 1988, 75, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A. Polymorphism for Disease Resistance in the Annual Legume Amphicarpaea bracteata. Heredity 1988, 60, 27–31. [Google Scholar] [CrossRef]
- Burdon, J.J.; Jarosz, A.M. Host-pathogen Interactions in Natural Populations of Linum marginale and Melampsora lini: I. Patterns of Resistance and Racial Variation in a Large Host Population. Evolution 1991, 45, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Bevan, J.R.; Clarke, D.D.; Crute, I.R. Resistance to Erysiphe fischeri in Two Populations of Senecio vulgaris. Plant Pathol. 1993, 42, 636–646. [Google Scholar] [CrossRef]
- Antonovics, J.; Thrall, P.H.; Jarosz, A.M.; Stratton, D. Ecological Genetics of Metapopulations: The Silene-Ustilago Plant-Pathogen System. In Ecological genetics; Real, L., Ed.; Princeton University Press: Princeton, NJ, USA, 1994; pp. 146–170. [Google Scholar]
- Thrall, P.H.; Burdon, J.J.; Young, A. Variation in Resistance and Virulence among Demes of a Plant Host-Pathogen Metapopulation. J. Ecol. 2001, 89, 736–748. [Google Scholar] [CrossRef]
- Laine, A.L. Resistance Variation within and among Host Populations in a Plant–Pathogen Metapopulation: Implications for Regional Pathogen Dynamics. J. Ecol. 2004, 92, 990–1000. [Google Scholar] [CrossRef]
- Volynkin, V.A.; Levchenko, S.V. Genetic Regularities Governing the Expression and the Inheritance of Resistance to Pathogens in Grapevine from a Standpoint of Co-Evolution of Biological Objects. Acta Hortic. 2018, 1205, 603–608. [Google Scholar] [CrossRef]
- Schröder, S. Plant Immunity as a Result of Co-Evolution: Using the Pair Grapevine, Downy Mildew as a Model. Doctoral Dissertation, Karlsruher Insitute of Technology (KIT), Karlsruhe, Germany, 2010. [Google Scholar]
- Dick, M.W. Towards and Understanding of the Evolution of the Downy Mildews. In Advances in Downy Mildew Research; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 1–57. [Google Scholar]
- European Commission Farm to Fork Strategy. 2020. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 4 June 2025).
- Eurostat The Use of Plant Protection Products in the European Union. Data 1992–2003; Euopean Union: Luxenbourg, 2007. [Google Scholar]
- Eurostat Agri-Environmental Indicator—Consumption of Pesticides; Eurostat: Luxembourg, 2023.
- Mullins, M.G. Biology of the Grapevine; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Blois, L.; de Miguel, M.; Bert, P.; Girollet, N.; Ollat, N.; Rubio, B.; Segura, V.; Voss-Fels, K.P.; Schmid, J.; Marguerit, E. Genetic Structure and First Genome-wide Insights into the Adaptation of a Wild Relative of Grapevine, Vitis berlandieri. Evol. Appl. 2023, 16, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. (Ed.) The Science of Grapevines; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Antcliff, A.J.; Newman, H.P.; Barrett, H.C. Variation in Chloride Accumulation in Some American Species of Grapevine. Vitis 1983, 22, 357–362. [Google Scholar]
- Lebrun, L.; Rajasekaran, K.; Mullins, M.G. Selection in Vitro for NaCI-Tolerance in Vitis rupestris Scheele. Ann. Bot. 1985, 56, 733–740. [Google Scholar] [CrossRef]
- Striegler, R.K.; Allen, A.; Bergmeier, E.; Caple, H. Understanding and Preventing Freeze Damage in Vineyards: Workshop Proceedings; University of Missouri Extension: Colombia, MO, USA, 2007. [Google Scholar]
- Fennell, A. Freezing Tolerance and Injury in Grapevines. J. Crop Improv. 2004, 10, 201–235. [Google Scholar] [CrossRef]
- Köse, B.; Uray, Y.; Bayram, K.; Türk, F. Cold Hardiness Degrees of Some Vitis vinifera L. and Vitis labrusca L. Cultivars Grown in Temperate Climate Condition. Rend. Lincei Sci. Fis. Nat. 2024, 35, 253–262. [Google Scholar] [CrossRef]
- Schmid, J.; Manty, F.; Cousins, P. Collecting Vitis berlandieri from Native Habitat Sites. Acta Hortic. 2009, 827, 151–154. [Google Scholar] [CrossRef]
- Pavlousek, P. Evaluation of Drought Tolerance of New Grapevine Rootstock Hybrids. J. Environ. Biol. 2011, 32, 543–549. [Google Scholar] [PubMed]
- Lowe, K.M.; Walker, M.A. Genetic Linkage Map of the Interspecific Grape Rootstock Cross Ramsey (Vitis champinii) × Riparia Gloire (Vitis riparia). Theor. Appl. Genet. 2006, 112, 1582–1592. [Google Scholar] [CrossRef]
- Knipfer, T.; Eustis, A.; Brodersen, C.; Walker, A.M.; Mclerone, A.J. Grapevine Species from Varied Native Habitats Exhibit Differences in Embolism Formation/Repair Associated with Leaf Gas Exchange and Root Pressure. Plant Cell Environ. 2015, 38, 1503–1513. [Google Scholar] [CrossRef]
- Adams, D.B. Genetic Analysis of Cold Hardiness in a Population of Norton (Vitis aestivalis) and Cabernet Sauvignon (Vitis vinifera) Hybrids. Master’s Thesis, The Graduate College of Missouri State University, Springfield, MO, USA, 2017. [Google Scholar]
- Zhang, J.; Wu, X.; Niu, R.; Liu, Y.; Liu, N.; Xu, W.; Wang, Y. Cold-Resistance Evaluation in 25 Wild Grape Species. Vitis 2012, 51, 153–160. [Google Scholar]
- Bavaresco, L.; Fregoni, M.; Perino, A. Physiological Aspects of Lime-Induced Chlorosis in Some Vitis Species. I. Pot Trial on Calcareous Soil. Vitis 1994, 33, 123–126. [Google Scholar]
- Heinitz, C.C.; Fort, K.; Walker, M.A. Developing Drought and Salt Resistant Grape Rootstocks. Acta Hortic. 2015, 1082, 305–312. [Google Scholar] [CrossRef]
- Munson, T.V. Foundations of American Grape Culture; Orange Judd Company: New York, NY, USA, 1909. [Google Scholar]
- Greene, S.L.; Williams, K.A.; Khoury, C.K.; Kantar, M.B.; Marek, L.F. North American Crop Wild Relatives; Greene, S.L., Williams, K.A., Khoury, C.K., Kantar, M.B., Marek, L.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, ISBN 978-3-319-97120-9. [Google Scholar]
- Ickert-Bond, S.M.; Harris, A.; Lutz, S.; Wen, J. A Detailed Study of Leaf Micromorphology and Anatomy of New World Vitis L. Subgenus Vitis within a Phylogenetic and Ecological Framework Reveals Evolutionary Convergence. J. Syst. Evol. 2018, 56, 309–330. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications; Academic press: Oxford, UK, 2020. [Google Scholar]
- Han, Y.; Li, X. Current Progress in Research Focused on Salt Tolerance in Vitis vinifera L. Front Plant Sci. 2024, 15, 1353436. [Google Scholar] [CrossRef]
- Buck, K.; Worthington, M. Genetic Diversity of Wild and Cultivated Muscadine Grapes (Vitis rotundifolia Michx.). Front. Plant Sci. 2022, 13, 852130. [Google Scholar] [CrossRef] [PubMed]
- Cousins, P. Evolution, Genetics, and Breeding: Viticultural Applications of the Origins of Our Rootstocks, in Grapevine Rootstocks: Current Use, Research and Application. In Proceedings of the 2005 Rootstock Symposium, Osage Beach, MO, USA, 5 February 2005; Cousins, P., Striegler, R.K., Eds.; Mid-America Viticulture and Enology Center, Southwest Missouri State University, Mountain Grove Campus: Springfield, MO, USA, 2005; pp. 1–7. [Google Scholar]
- Macías-Gallardo, F.; Castro-Palafox, J.; Ozuna, C. Mexican Wines: Impact of Geography, Climate, and Soil on the Quality of the Grape and Wine─A Review. ACS Food Sci. Technol. 2024, 4, 1598–1609. [Google Scholar] [CrossRef]
- Cruz-Castillo, J.G.; Franco-Mora, O.; Famiani, F. Presence and Uses of Wild Grapevine (Vitis spp.) in the Central Region of Veracruz in Mexico. OENO One 2009, 43, 77. [Google Scholar] [CrossRef]
- Grundler, S.; Schmid, J.; Meßner, J.; Rühl, E.H. Variability in Vitis berlandieri. Acta Hortic. 2015, 1082, 123–129. [Google Scholar] [CrossRef]
- Gu, S. Effect of Rootstocks on Grapevines—Rootstock Review. 2003. Available online: https://www.semanticscholar.org/paper/Effect-of-Rootstocks-on-Grapevines-Gu/89f6307fb00baf15529b7e28e711560264c109e8 (accessed on 7 July 2025).
- Khan, M.M.; Akram, M.T.; Qadri, R.W.K.; Al-Yahyai, R. Role of Grapevine Rootstocks in Mitigating Environmental Stresses: A Review. J. Agric. Mar. Sci. 2020, 25, 1. [Google Scholar] [CrossRef]
- Granett, J.; Walker, M.A.; Kocsis, L.; Omer, A.D. Biology and Management of Grape Phylloxera. Annu. Rev. Entomol. 2001, 46, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Correll, R.L. Rootstock Effects on Salt Tolerance of Irrigated Field-Grown Grapevines (Vitis vinifera L. Cv. Sultana). Ion Concentrations in Leaves and Juice. Aust. J. Grape Wine Res. 2004, 10, 90–99. [Google Scholar] [CrossRef]
- Aguirre-Liguori, J.A.; Morales-Cruz, A.; Gaut, B.S. Evaluating the Persistence and Utility of Five Wild Vitis Species in the Context of Climate Change. Mol. Ecol. 2022, 31, 6457–6472. [Google Scholar] [CrossRef]
- Goheen, A.C.; Pearson, R.C. Compendium of Grape Diseases; APS Press: Saint Paul, MN, USA, 1988. [Google Scholar]
- English-Loeb, G.; Norton, A. Lack of Trade-off between Direct and Indirect Defence against Grape Powdery Mildew in Riverbank Grape. Ecol. Entomol. 2006, 31, 415–422. [Google Scholar] [CrossRef]
- Millardet, A. Histoire Des Principales Variétés et Espèces de Vigne d’Origine Américaine Qui Résistent Au Phylloxéra; Masson, G., Ed.; Univercity of Bordeaux: Paris, France, 1885. [Google Scholar]
- Rahemi, A.; Dale, A.; Fisher, H.; Taghavi, T.; Bonnycastle, A.; Kelly, J. A Report on Vitis riparia in Ontario, Canada. Acta Hortic. 2016, 1136, 33–38. [Google Scholar] [CrossRef]
- Ferris, H.; Zheng, L.; Walker, M.A. Resistance of Grape Rootstocks to Plant-Parasitic Nematodes. J. Nematol. 2012, 44, 377–386. [Google Scholar] [PubMed]
- Pongrácz, D.P. Rootstocks for Grapevines; David Philip Publisher: Cape Town, South Africa, 1983. [Google Scholar]
- Takacs, E.M.; Isby, A.D.; Appleton, P.M.; Reisch, B.I. Delineating the Mechanism and Inheritance of Black Rot Resistance from Vitis rupestris and V. cinerea. In Proceedings of the 11th International Conference on Grapevine Breeding and Genetics, Beijing, China, 28 July–2 August 2014. [Google Scholar]
- Barba, P.; Cadle-Davidson, L.; Harriman, J.; Glaubitz, J.C.; Brooks, S.; Hyma, K.; Reisch, B. Grapevine Powdery Mildew Resistance and Susceptibility Loci Identified on a High-Resolution SNP Map. Theor. Appl. Genet. 2014, 127, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Barba, P.; Cadle-Davidson, L.; Galarneau, E.; Reisch, B. Vitis rupestris B38 Confers Isolate-Specific Quantitative Resistance to Penetration by Erysiphe necator. Phytopathology 2015, 105, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Di Gaspero, G.; Copetti, D.; Coleman, C.; Castellarin, S.D.; Eibach, R.; Kozma, P.; Lacombe, T.; Gambetta, G.; Zvyagin, A.; Cindrić, P.; et al. Selective Sweep at the Rpv3 Locus during Grapevine Breeding for Downy Mildew Resistance. Theor. Appl. Genet. 2012, 124, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Gavioli, M.C.; Rockenbach, M.F.; Welter, L.J.; Guerra, M.P. Histopathological Study of Resistant (Vitis labrusca L.) and Susceptible (Vitis vinifera L.) Cultivars of Grapevine to the Infection by Downy Mildew. J. Hortic. Sci. Biotechnol. 2020, 95, 521–531. [Google Scholar] [CrossRef]
- Gabler, F.M.; Smilanick, J.L.; Mansour, M.; Ramming, D.W.; Mackey, B.E. Correlations of Morphological, Anatomical, and Chemical Features of Grape Berries with Resistance to Botrytis cinerea. Phytopathology 2003, 93, 1263–1273. [Google Scholar] [CrossRef]
- Atak, A.; Akkurt, M.; Polat, Z.; Çelik, H.; Kahraman, K.A.; Akgül, D.S.; Özer, N.; Söylemezoğlu, G.; Şire, G.G.; Eibach, R. Susceptibility to Downy Mildew (Plasmopara viticola) and Powdery Mildew (Erysiphe necator) of Different Vitis Cultivars and Genotypes. Ciência E Técnica Vitivinícola 2017, 32, 23–32. [Google Scholar] [CrossRef]
- Yıldırım, Z.; Atak, A.; Akkurt, M. Determination of Downy and Powdery Mildew Resistance of Some Vitis spp. Ciência E Técnica Vitivinícola 2019, 34, 15–24. [Google Scholar] [CrossRef]
- Wan, Y.; Schwaninger, H.; He, P.; Wang, Y. Comparison of Resistance to Powdery Mildew and Downy Mildew in Chinese Wild Grapes. Vitis 2007, 46, 132. [Google Scholar]
- Barros, L.B.; Biasi, L.A.; Carisse, O.; De Mio, L.L.M. The Influence of Table Grape Rootstock and Cultivar Combinations on Susceptibility to Downy Mildew. Australas. Plant Pathol. 2018, 47, 171–179. [Google Scholar] [CrossRef]
- Schurig, J.; Ipach, U.; Helmstätter, B.; Kling, L.; Hahn, M.; Trapp, O.; Winterhagen, P. Selected Genotypes with the Genetic Background of Vitis aestivalis and Vitis labrusca Are Resistant to Xiphinema index. Plant Dis. 2021, 105, 4132–4137. [Google Scholar] [CrossRef] [PubMed]
- Dalbó, M.A.; Ye, G.N.; Weeden, N.F.; Wilcox, W.F.; Reisch, B.I. Marker-Assisted Selection for Powdery Mildew Resistance in Grapes. J. Am. Soc. Hortic. Sci. 2001, 126, 83–89. [Google Scholar] [CrossRef]
- Ochssner, I.; Hausmann, L.; Töpfer, R. Rpv14, a New Genetic Source for Plasmopara Viticola Resistance Conferred by Vitis Cinerea. VITIS-J. Grapevine Res. 2016, 55, 79–81. [Google Scholar]
- Smith, H.M.; Smith, B.P.; Morales, N.B.; Moskwa, S.; Clingeleffer, P.R.; Thomas, M.R. SNP Markers Tightly Linked to Root Knot Nematode Resistance in Grapevine (Vitis cinerea) Identified by a Genotyping-by-Sequencing Approach Followed by Sequenom MassARRAY Validation. PLoS ONE 2018, 13, e0193121. [Google Scholar] [CrossRef]
- Pavloušek, P. Screening of Rootstock Hybrids with Vitis cinerea Arnold for Phylloxera Resistance. Open Life Sci. 2012, 7, 708–719. [Google Scholar] [CrossRef]
- Schmid, J.; Manty, F.; Rühl, E.H. Utilizing the Complete Phylloxera Resistance of Vitis cinerea Arnold in Rootstock Breeding. Acta Hortic. 2003, 604, 393–400. [Google Scholar] [CrossRef]
- Reisch, B.I.; Goodman, R.N.; Martens, M.H.; Weeden, N.F. The Relationship between Norton and Cynthiana, Red. Wine Cultivars Derived from Vitis aestivalis. Am. J. Enol. Vitic. 1993, 44, 441–444. [Google Scholar] [CrossRef]
- Fung, R.W.M.; Qiu, W.; Su, Y.; Schachtman, D.P.; Huppert, K.; Fekete, C.; Kovács, L.G. Gene Expression Variation in Grapevine Species Vitis vinifera L. and Vitis aestivalis Michx. Genet. Resour. Crop Evol. 2007, 54, 1541–1553. [Google Scholar] [CrossRef]
- Sapkota, S.D.; Chen, L.L.; Yang, S.; Hyma, K.E.; Cadle-Davidson, L.E.; Hwang, C.F. Quantitative Trait Locus Mapping of Downy Mildew and Botrytis Bunch Rot Resistance in a Vitis aestivalis -Derived ‘Norton’-Based Population. Acta Hortic. 2019, 1248, 305–312. [Google Scholar] [CrossRef]
- Ramming, D.W.; Gabler, F.; Smilanick, J.L.; Margosan, D.A.; Cadle-Davidson, M.; Barba, P.; Mahanil, S.; Frenkel, O.; Milgroom, M.G.; Cadle-Davidson, L. Identification of Race-Specific Resistance in North American Vitis Spp. Limiting Erysiphe necator Hyphal Growth. Phytopathology 2012, 102, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Chen, L.-L.; Schreiner, K.; Ge, H.; Hwang, C.-F. A Phenotypic Study of Botrytis Bunch Rot Resistance in Vitis aestivalis-Derived ‘Norton’ Grape. Trop. Plant Pathol. 2015, 40, 279–282. [Google Scholar] [CrossRef]
- Kamas, J.; Appel, D.; Black, M.; Hellman, E.; Lauziere, I.; Mitchell, F.; Morano, L.; Wendel, L. Unraveling Pierce’s Disease in Its Native Environment. Wine Bus. Mon. 2004, 11, 35–38. [Google Scholar]
- Hedrick, U.P. The Grapes of New York. State of New York; Albany, J.R., Ed.; Lyon Co.: Albany, NY, USA, 1908; Volume 3. [Google Scholar]
- Grzegorczyk, W.; Walker, M.A. Evaluating Resistance to Grape Phylloxera in Vitis Species with an in Vitro Dual Culture Assay. Am. J. Enol. Vitic. 1998, 49, 17–22. [Google Scholar] [CrossRef]
- Boyden, L.E.; Cousins, P. Evaluation of Vitis aestivalis and Related Taxa as Sources of Resistance to Root-Knot Nematodes. Acta Hortic. 2003, 623, 283–290. [Google Scholar] [CrossRef]
- Riaz, S.; Tenscher, A.C.; Graziani, R.; Krivanek, A.F.; Ramming, D.W.; Walker, M.A. Using Marker-Assisted Selection to Breed Pierce’s Disease-Resistant Grapes. Am. J. Enol. Vitic. 2009, 60, 199–207. [Google Scholar] [CrossRef]
- Downie, D.; Granett, J. A Life Cycle Variation in Grape Phylloxera, Daktulosphaira vitifoliae (Fitch). Southwest. Entomol. 1998, 23, 11–16. [Google Scholar]
- Van Zyl, S.; Vivier, M.A.; Riaz, S.; Walker, M.A. The Genetic Mapping of Xiphinema index Resistance Derived from Vitis arizonica. Acta Hortic. 2014, 1046, 165–168. [Google Scholar] [CrossRef]
- Boubals, D. Etude des causes de la résistance des Vitacées à l’oïdium de la Vigne et de leur mode de transmission héréditaire. Ann. Amélior. Plantes 1961, 11, 401–500. [Google Scholar]
- Galet, P. Les Maladies et Les Parasites de La Vigne; Impr. du Paysan du Midi: Lattes, France, 1977; Volume 1. [Google Scholar]
- Fayyaz, L.; Tenscher, A.; Viet Nguyen, A.; Qazi, H.; Walker, M.A. Vitis Species from the Southwestern United States Vary in Their Susceptibility to Powdery Mildew. Plant Dis. 2021, 105, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Viala, P.; Ravaz, L. American Vines. In Complete Translation, 2nd ed.; Dubois, R., Twight, E.H., Eds.; Freygang-Leary: San Francisco, CA, USA, 1903. [Google Scholar]
- Granett, J.; De Benedictis, J.; Marston, J. Host Suitability of Vitis Californica Bentham to Grape Phylloxera, Daktulosphaira vitifoliae (Fitch). Am. J. Enol. Vitic. 1992, 43, 249–252. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Wiedeman-Merdinoglu, S.; Coste, P.; Dumas, V.; Haetty, S.; Butterlin, G.; Greif, C. Genetic Analysis of Downy Mildew Resistance Derived from Muscadinia rotundifolia. Acta Hortic. 2003, 603, 451–456. [Google Scholar] [CrossRef]
- Hickey, C.C.; Smith, E.D.; Cao, S.; Conner, P. Muscadine (Vitis rotundifolia Michx., Syn. Muscandinia rotundifolia (Michx.): The Resilient, Native Grape of the Southeastern U.S. Agriculture 2019, 9, 131. [Google Scholar] [CrossRef]
- Barker, C.L.; Donald, T.; Pauquet, J.; Ratnaparkhe, M.B.; Bouquet, A.; Adam-Blondon, A.-F.; Thomas, M.R.; Dry, I. Genetic and Physical Mapping of the Grapevine Powdery Mildew Resistance Gene, Run1, Using a Bacterial Artificial Chromosome Library. Theor. Appl. Genet. 2005, 111, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Blanc, S.; Wiedemann-Merdinoglu, S.; Dumas, V.; Mestre, P.; Merdinoglu, D. A Reference Genetic Map of Muscadinia rotundifolia and Identification of Ren5, a New Major Locus for Resistance to Grapevine Powdery Mildew. Theor. Appl. Genet. 2012, 125, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Mortensen, J.A. The Native Grape Species of Florida. Proc. Fla. State Hortic. Soc. 1979, 92, 286–289. [Google Scholar]
- Cousins, P.; Lauver, M. Segregation of Resistance to Root-Knot Nematodes in a Vitis vulpina Hybrid Population. Acta Hortic. 2003, 623, 313–318. [Google Scholar] [CrossRef]
- Lider, L.A. Inheritance of Resistance to Root-Knot Nematode (Meloidogyne incognita, Kofoid and White) in Vitis spp. Doctoral Dissertation, University of California, Davis, CA, USA, 1952. [Google Scholar]
- Botos, E.P.; Hajdú, E.; Borbás, É. Genetic analysis of root-knot nematode resistance derived from Vitis mustangensis. In Proceedings of the 8th International Conference on Grape Genetics and Breeding, Keeskemét, Hungary, 26–31 August 2020; Proceedings of the No. 603. International Society for Horticultural Science: Toronto, Canada, 2002; pp. 149–155. [Google Scholar]
- Rombough, L. The Grape Grower: A Guide to Organic Viticultur; Chelsea Green Publishing: White River Junction, VT, USA, 2002. [Google Scholar]
- André, M. Vitis acerifolia Rafinesque et Ses Principaux Hybrides Postculturaux En France, Contribution à Leur Caractérisation. Les Nouv. Arch. De La Flore Jurassienne Et Du Nord.-Est De La Fr. 2022, 20, 173–188. [Google Scholar]
- Staudt, G.; Kassemeyer, H.H. Evaluation of Downy Mildew Resistance in Various Accessions of Wild Vitis Species. VITIS-J. Grapevine Res. 2015, 34, 225–228. [Google Scholar]
- Lu, J.; Hunter, W.; Dang, P. Towards Identifying Pierce’s Disease Resistant Genes from a Native American Grape Species (Vitis shuttleworthii)—A Genomic Approach. In Proceedings of the Pierce’s Disease Research Symp, Coronado, CA, USA, 7–10 December 2004; pp. 31–34. [Google Scholar]
- Mortensen, J.A.; Stover, L.H.; Balerdi, C.F. Sources of Resistance to Pierce’s Disease in Vitis. J. Am. Soc. Hortic. Sci. 1977, 102, 695–697. [Google Scholar] [CrossRef]
- Cheng, D.W.; Lin, H.; Andrew Walker, M.; Stenger, D.C.; Civerolo, E.L. Effects of Grape Xylem Sap and Cell Wall Constituents on in Vitro Growth, Biofilm Formation and Cellular Aggregation of Xylella fastidiosa. Eur. J. Plant Pathol. 2009, 125, 213–222. [Google Scholar] [CrossRef]
- Lu, J.; Huang, H.; Ren, Z.; Bradeley, F.; Hunter, W. Towards Identification, Isolation and Characterization of Disease Resistant Genes from the Native North American Grape Species Vitis shuttleworthii. Acta Hortic. 2007, 738, 767–772. [Google Scholar] [CrossRef]
- Staudt, G. Evaluation of Resistance to Grapevine Powdery Mildew (Uncinula necator [Schw.] Burr., Anamorph Oidium tuckeri Berk.) in Accessions of Vitis Species. VITIS-J. Grapevine Res. 2015, 36, 151. [Google Scholar]
- Snyder, E. Susceptibility of Grape Rootstocks to Root Knot Nematode (No. 405); US Department of Agriculture: Washington, DC, USA, 1936.
- Andersen, P.C.; Sarkhosh, A.; Huff, D.; Breman, J. The Muscadine Grape (Vitis rotundifolia Michx). EDIS 2020, 2020, HS100–HS763. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Lin, H.; Walker, M.A. Xylella fastidiosa Population Dynamics in Grapevine Genotypes Differing in Susceptibility to Pierce’s Disease. Am. J. Enol. Vitic. 2007, 58, 326–332. [Google Scholar] [CrossRef]
- Boyden, L.E.; Cousins, P. Segregation of Resistance to Root-Knot Nematodes in a Vitis nesbittiana Hybrid Population. HortScience 2004, 39, 804. [Google Scholar] [CrossRef]
- Dami, I.E.; Ennahli, S.; Zhang, Y. Assessment of Winter Injury in Grape Cultivars and Pruning Strategies Following a Freezing Stress Event. Am. J. Enol. Vitic. 2012, 63, 106–111. [Google Scholar] [CrossRef]
- Dixon, C.W.; Gschwend, A.R. Trichomes and Unique Gene Expression Confer Insect Herbivory Resistance in Vitis labrusca Grapevines. BMC Plant Biol. 2024, 24, 609. [Google Scholar] [CrossRef]
- Bilir Ekbic, H.; Gecene, İ.; Ekbic, E. Determination of the Tolerance of Fox Grapes (Vitis labrusca L.) to Drought Stress by PEG Application in Vitro. Erwerbs-Obstbau 2022, 64, 87–94. [Google Scholar] [CrossRef]
- Atak, A. Vitis Species for Stress Tolerance/Resistance. Genet. Resour. Crop Evol. 2025, 72, 2425–2444. [Google Scholar] [CrossRef]
- Remaily, G.W. Diversity of North American Species of Vitis. IBPGR Plant Genet. Resour. Newsl. 1987, 71, 25–30. [Google Scholar]
- Alleweldt, G.; Possingham, J.V. Progress in Grapevine Breeding. Theoretical and Applied Genetics. Theor. Appl. Genet. 1988, 75, 669–673. [Google Scholar] [CrossRef]
- Dry, I.B.; Feechan, A.; Anderson, C.; Jermakow, A.M.; Bouquet, A.; Adam-Blondon, A.-F.; Thomas, M.R. Molecular Strategies to Enhance the Genetic Resistance of Grapevines to Powdery Mildew. Aust. J. Grape Wine Res. 2010, 16, 94–105. [Google Scholar] [CrossRef]
- van Heerden, C.J.; Burger, P.; Vermeulen, A.; Prins, R. Detection of Downy and Powdery Mildew Resistance QTL in a ‘Regent’ × ‘RedGlobe’ Population. Euphytica 2014, 200, 281–295. [Google Scholar] [CrossRef]
- Boso, S.; Alonso-Villaverde, V.; Gago, P.; Santiago, J.L.; Martínez, M.C. Susceptibility to Downy Mildew (Plasmopara viticola) of Different Vitis Varieties. Crop Prot. 2014, 63, 26–35. [Google Scholar] [CrossRef]
- Polesani, M.; Bortesi, L.; Ferrarini, A.; Zamboni, A.; Fasoli, M.; Zadra, C.; Lovato, A.; Pezzotti, M.; Delledonne, M.; Polverari, A. General and Species-Specific Transcriptional Responses to Downy Mildew Infection in a Susceptible (Vitis vinifera) and a Resistant (V. riparia) Grapevine Species. BMC Genom. 2010, 11, 117. [Google Scholar] [CrossRef]
- Alleweldt, G.; Spiegel-Roy, P.; Reisch, B. Genetic Resources of Temperate Fruit and Nut Crops. Int. Soc. Hortic. Sci. 1991, 290, 291–330. [Google Scholar]
- Cui, X.; Xue, J.; Zhang, B.; Chen, C.; Tang, Y.; Zhang, P.; Zhang, J. Physiological Change and Screening of Differentially Expressed Genes of Wild Chinese Vitis Yeshanensis and American Vitis riparia in Response to Drought Stress. Sci. Hortic. 2020, 266, 109140. [Google Scholar] [CrossRef]
- Reisch, B.I.; Owens, L.C.; Cousins, S.P. Grape. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer Science: Philadelphia, PA, USA, 2012. [Google Scholar]
- Smiley, L.; Cochran, D.; Domoto, P.; Nonnecke, G.; Miller, W.W. A Review of Cold Climate Grape Cultivars; Iowa State University Extension Publisher: Ames, IA, USA, 2016. [Google Scholar]
- Svyantek, A.; Stenger, J.; Auwarter, C.; Shikanai, A.; Köse, B.; Wang, Z.; Kadium, V.R.; Brooke, M.; Delavar, H.; Pilli, R.; et al. This Is How We Chill from 23 Til: Breeding Cold Hardy Grapevines for Unprecedented and Unpredictable Climate Challenges. Acta Hortic. 2024, 1385, 127–138. [Google Scholar] [CrossRef]
- Bailey, L.H. Vites Peculiares Ad Americum Borealem. Gentes Herbarum. Gentes Herbarum 1934, 3, 149–244. [Google Scholar]
- Steyermark, J.A. Flora of Missouri; The Iowa State University Press: Ames, IA, USA, 1963. [Google Scholar]
- Pap, D.; Miller, A.J.; Londo, J.P.; Kovács, L.G. Population Structure of Vitis rupestris, an Important Resource for Viticulture. Am. J. Enol. Vitic. 2015, 66, 403–410. [Google Scholar] [CrossRef]
- Morano, L.; Walker, M.A. Soils and Plant Communities Associated with Three Vitis Species. Am. Midl. Nat. 1995, 134, 254. [Google Scholar] [CrossRef]
- Londo, J.P.; Johnson, L.M. Variation in the Chilling Requirement and Budburst Rate of Wild Vitis Species. Environ. Exp. Bot. 2014, 106, 138–147. [Google Scholar] [CrossRef]
- Riaz, S.; Pap, D.; Uretsky, J.; Laucou, V.; Boursiquot, J.-M.; Kocsis, L.; Andrew Walker, M. Genetic Diversity and Parentage Analysis of Grape Rootstocks. Theor. Appl. Genet. 2019, 132, 1847–1860. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Mullins, M.G. Influence of Genotype and Sex-Expression on Formation of Plantlets by Cultured Anthers of Grapevines. Agronomie 1983, 3, 233–238. [Google Scholar] [CrossRef]
- Hardie, W.J.; Cirami, R.M. Grapevine Rootstock. In Viticulture; Coombe, B.G., Dry, P.R., Eds.; Winetitles, Adelaide Press: Adelaide, Australia, 1997; Volume 1, pp. 154–176. [Google Scholar]
- Celik, H.; Kose, B.; Cangi, R. Determination of Fox Grape Genotypes (Vitis labrusca L.) Grown in Northeastern Anatolia. Hortic. Sci. 2008, 35, 162–170. [Google Scholar] [CrossRef]
- Ollat, N.; Laborde, B.; Neveux, M.; Diakou-Verdin, P.; Renaud, C.; Moing, A. Organic Acid Metabolism in Roots of Various Grapevine (Vitis) Rootstocks Submitted to Iron Deficiency and Bicarbonate Nutrition. J. Plant Nutr. 2003, 26, 2165–2176. [Google Scholar] [CrossRef]
- Cadle-Davidson, L. Variation Within and Between Vitis Spp. for Foliar Resistance to the Downy Mildew Pathogen Plasmopara viticola. Plant Dis. 2008, 92, 1577–1584. [Google Scholar] [CrossRef]
- Brown, M.V.; Moore, J.N.; McNew, R.W.; Fenn, P. Inheritance of Downy Mildew Resistance in Table Grapes. J. Am. Soc. Hortic. Sci. 1999, 124, 262–267. [Google Scholar] [CrossRef]
- Kortekamp, A.; Zyprian, E. Leaf Hairs as a Basic Protective Barrier against Downy Mildew of Grape. J. Phytopathol. 1999, 147, 453–459. [Google Scholar] [CrossRef]
- Gee, C.T.; Gadoury, D.M.; Cadle-Davidson, L. Ontogenic Resistance to Uncinula Necator Varies by Genotype and Tissue Type in a Diverse Collection of Vitis Spp. Plant Dis. 2008, 92, 1067–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cadle-Davidson, L.; Chicoine, D.R.; Consolie, N.H. Variation Within and Among Vitis Spp. for Foliar Resistance to the Powdery Mildew Pathogen Erysiphe necator. Plant Dis. 2011, 95, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Köse, B.; Uray, Y.; Karabulut, B.; Türk, F.; Bayram, K.; Çelik, H. Determination of Rooting and Vine Sapling Rates of Single-Bud Cuttings Prepared from Vitis labrusca L. Grape Cultivars. Erwerbs-Obstbau 2023, 65, 2005–2016. [Google Scholar] [CrossRef]
- Barrett, H.C. Vitis cinerea as a Source of Desirable Characters in Grape Breeding. Proc. Amer. Soc. Hort. Sci. 1957, 70, 165–168. [Google Scholar]
- Schmid, J.; Rühl, H. Performance of Vitis cinerea Hybrids in Motherblock and Nursery-Preliminary Results. Acta Hortic. 2003, 117, 141–145. [Google Scholar] [CrossRef]
- Börner, C. Die Ersten Reblausimmunen Rebenkreuzungen. Angew. Bot. 1943, 25, 126–143. [Google Scholar]
- Cochetel, N.; Ghan, R.; Toups, H.S.; Degu, A.; Tillett, R.L.; Schlauch, K.A.; Cramer, G.R. Drought Tolerance of the Grapevine, Vitis champinii Cv. Ramsey, Is Associated with Higher Photosynthesis and Greater Transcriptomic Responsiveness of Abscisic Acid Biosynthesis and Signaling. BMC Plant Biol. 2020, 20, 55. [Google Scholar] [CrossRef]
- Patil, S.G.; Karkamkar, S.P.; Deshmukh, M.R. Evaluation of Grape Varieties for Their Drought Tolerance. J. Maharashtra Agric. Univ. 2003, 28, 250–251. [Google Scholar]
- Perry, R.L.; Lyda, S.D.; Bowen, H.H. Root Distribution of Four Vitis Cultivars. Plant Soil. 1983, 71, 63–74. [Google Scholar] [CrossRef]
- Hao, L.; Zaini, P.A.; Hoch, H.C.; Burr, T.J.; Mowery, P. Grape Cultivar and Sap Culture Conditions Affect the Development of Xylella fastidiosa Phenotypes Associated with Pierce’s Disease. PLoS ONE 2016, 11, e0160978. [Google Scholar] [CrossRef] [PubMed]
- Backus, E.A.; Shugart, H.J.; Gutierrez, J.; Ebert, T.A.; Walker, M.A. Field-Collected Glassy-Winged Sharpshooters (Hemiptera: Cicadellidae) Perform More Xylella fastidiosa Inoculating Behaviors on Susceptible Vitis vinifera Cv. ‘Chardonnay’ Than on Resistant Vitis champinii Grapevines. J. Econ. Entomol. 2021, 114, 1991–2008. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.P.; Hussey, E.E. The Value of Plant Growth Regulators in the Propagation of Vitis champini Rootstocks. Am. J. Enol. Vitic. 1980, 31, 250–253. [Google Scholar] [CrossRef]
- Blennerhassett, R.M.; Considine, J.A. Propagation of Vitis champini Planchon Cv Ramsey, Storage and Field Practices. Am. J. Enol. Vitic. 1979, 30, 79–80. [Google Scholar] [CrossRef]
- Jolley, N. Investigation into Timing of Cuttings for Maximum Propagation Efficiency of Vitis aestivalis ‘Norton/Cynthiana’Grapevines. Doctoral Dissertation, University Honors College, Middle Tennessee State University, Murfreesboro, TN, USA, 2017. [Google Scholar]
- Kadir, S.A. Growth of Vitis vinifera L. and Vitis aestivalis Michx. as Affected by Temperature. Int. J. Fruit. Sci. 2005, 5, 69–82. [Google Scholar] [CrossRef]
- Uhls, A.L.; Jolley, N.; Johnston, T.V.; DuBois, J.D. The Effect of Sample Date and Timing of Cuttings for Maximum Propagation Efficiency of the Grape, Vitis aestivalis “Norton/Cynthiana. ” Food Nutr. Sci. 2018, 09, 268–276. [Google Scholar] [CrossRef][Green Version]
- Portz, D.; Domoto, P.; Nonnecke, G. Propagation Treatment Effects on Rooting of “Cynthiana” Grape Hardwood Cuttings; Iowa State University Department of Horticulture: Ames, IA, USA, 2004. [Google Scholar][Green Version]
- Enderton, D.; Dilley, C.; Domoto, P.; Nonnecke, G. Cynthiana Grape Cultivar Propagation Study; Iowa State University Extension: Ames, IA, USA, 2002. [Google Scholar][Green Version]
- Keeley, K.; Preece, J.E.; Taylor, B.H. Increased Rooting of “Norton” Grape Cuttings Using Auxins and Gibberellin Biosynthesis Inhibitors. HortScience 2003, 38, 281–283. [Google Scholar] [CrossRef]
- Johnson, P.J.; Hilsenbeck, R.A. An Investigation of Vitis arizonica (the Canyon Grape) as a Potential Rootstock in West Texas. Tex. J. Agric. Nat. Resour. 1989, 3, 34–36. [Google Scholar]
- Fiscus, C.J.; Aguirre-Liguori, J.A.; Gaut, G.R.J.; Gaut, B.S. Climate, Population Size, and Dispersal Influences Mutational Load across the Landscape in Vitis Arizonica. BioRxiv 2024. [Google Scholar] [CrossRef]
- Krivanek, A.F.; Famula, T.R.; Tenscher, A.; Walker, M.A. Inheritance of Resistance to Xylella fastidiosa within a Vitis rupestris × Vitis arizonica Hybrid Population. Theor. Appl. Genet. 2005, 111, 110–119. [Google Scholar] [CrossRef]
- Krivanek, A.F.; Riaz, S.; Walker, M.A. Identification and Molecular Mapping of PdR1, a Primary Resistance Gene to Pierce’s Disease in Vitis. Theor. Appl. Genet. 2006, 112, 1125–1131. [Google Scholar] [CrossRef]
- Riaz, S.; Krivanek, A.F.; Xu, K.; Walker, M.A. Refined Mapping of the Pierce’s Disease Resistance Locus, PdR1, and Sex on an Extended Genetic Map of Vitis rupestris × V. arizonica. Theor. Appl. Genet. 2006, 113, 1317–1329. [Google Scholar] [CrossRef]
- Agüero, C.B.; Riaz, S.; Tenscher, A.C.; Bistué, C.; Walker, M.A. Molecular and Functional Characterization of Two RGA Type Genes in the PdR1b Locus for Pierce’s Disease Resistance in Vitis arizonica/candicans. Plant Cell Tissue Organ. Cult. 2022, 151, 497–510. [Google Scholar] [CrossRef]
- Patil, S.G.; Honrao, B.K.; Rao, V.G.; Patil, V.P. Field Evaluation of Grape Germplasm for Resistance against Anthracnose. Biovigyanam 1990, 16, 69–72. [Google Scholar]
- Basinger, A.; Durham, R. In Vitro Rooting of Vitis Species Native to Texas and New Mexico. Small Fruits Rev. 2001, 1, 29–34. [Google Scholar] [CrossRef]
- Everett, R.L.; Meeuwig, R.O.; Robertson, J.H. Propagation of Nevada Shrubs by Stem Cuttings. J. Range Manag. 1978, 31, 426. [Google Scholar] [CrossRef]
- Dangl, G.S.; Mendum, M.L.; Yang, J.; Walker, M.A.; Preece, J.E. Hybridization of Cultivated Vitis vinifera with Wild V. californica and V. girdiana in California. Ecol. Evol. 2015, 5, 5671–5684. [Google Scholar] [CrossRef]
- Gamon, J.A.; Pearcy, R.W. Leaf Movement, Stress Avoidance and Photosynthesis in Vitis californica. Oecologia 1989, 79, 475–481. [Google Scholar] [CrossRef]
- Walker, M.A. Resistance in Vitis and Muscadinia Species to Meloidogyne incognita. Plant Dis. 1994, 78, 1055. [Google Scholar] [CrossRef]
- Robbins, J.; Burger, D. Propagating California Wild Grapes. Calif. Agric. 1986, 40, 9–10. [Google Scholar]
- Bouquet, A. La Muscadine (Vitis rotundifolia) et Sa Culture Aux Etats-Unis. OENO One 1978, 12, 1. [Google Scholar] [CrossRef]
- Bailey, L.H. The Standard Encyclopedia of Horticulture; Macmillan: New York, NY, USA, 1937. [Google Scholar]
- Dearing, C. New Muscadine Grapes; U.S. Department of Agriculture: Washington, DC, USA, 1948; p. 769.
- Weaver, R.J. Grape Growing. In Wiley-Interscience; Wiley-Interscience: New York, NY, USA, 1976. [Google Scholar]
- Olien, W.C. The Muscadine Grape: Botany, Viticulture, History, and Current Industry. HortScience 1990, 25, 732–739. [Google Scholar] [CrossRef]
- Ivasișin, D. The Karyotypes of Interspecific Grapevine Hybrids (Vitis vinifera L. × Vitis rotundifolia Michx.) BC4. J. Nativ. Alien. Plant Stud. 2024, 20, 51–61. [Google Scholar] [CrossRef]
- Patel, G.I.; Olmo, H.P. Cytogenetics of Vitis: I. The Hybrid V. vinifera × V. rotundifolia. Am. J. Bot. 1955, 42, 141–159. [Google Scholar] [CrossRef]
- Hithcock, A.S. The Distribution of the Genus Vitis in Kansas. Trans. Kans. Acad. Sci. 1893, 13, 79–80. [Google Scholar]
- Moore, M.O.C. A Systematic Study of Eastern North American Vitis (Vitaceae) North of Mexico; University of Georgia: Athens, GA, USA, 1990. [Google Scholar]
- Wylie, R.B. The Role of the Epidermis in Foliar Organization and Its Relations to the Minor Venation. Am. J. Bot. 1943, 30, 273–280. [Google Scholar] [CrossRef]
- Luken, J.O.; Kuddes, L.M.; Tholemeier, T.C. Response of Understory Species to Gap Formation and Soil Disturbance m Lonicera Maackii Thickets. Restor. Ecol. 1997, 5, 229–235. [Google Scholar] [CrossRef]
- Engelmann, G.; Gray, A.; Blankinship, J.W. Plantae Lindheimerianae: An Enumeration of F. Lindheimer’s Collection of Texan Plants, with Remarks and Descriptions of New Species, Etc; Printed by Freeman and Bolles: Boston, MA, USA, 1845. [Google Scholar]
- La Cruz, A.A.D.; Hilbert, G.; Mengin, V.; Rivière, C.; Ollat, N.; Vitrac, C.; Bordenave, L.; Decroocq, S.; Delaunay, J.; Mérillon, J.; et al. Anthocyanin Phytochemical Profiles and Anti-oxidant Activities of Vitis candicans and Vitis doaniana. Phytochem. Anal. 2013, 24, 446–452. [Google Scholar] [CrossRef]
- Rombough, L. The Grape Grower: A Guide to Organic Viticulture; Chelsea Green Publishing: Hartford, VT, USA, 2002. [Google Scholar]
- Prince, W.R.; Prince, W. A Treatise on the Vine: Embracing Its History from the Earliest Ages to the Present Day, with Descriptions of Above Two Hundred Foreign and Eighty American Varieties; Together with a Complete Dissertation on the Establishment, Culture, and Management of Vineyards; Swords, T.J., Ed.; William Prince: New York, NY, USA, 1830. [Google Scholar]
- Rafinesque, C.S. American Manual of the Grape Vines and the Art of Making Wine: Including an Account of 62 Species of Vines, with Nearly 300 Varieties. An Account of the Principal Wines, American and Foreign. Properties and Uses of Wines and Grapes. Cultivation of Vines in America; and the Art to Make Good Wines; Printed for the Author: Philadelphia, PA, USA, 1830; 84p. [Google Scholar]
- Engelmann, G. Vitis palmata, Vahl. Bot. Gaz. 1883, 8, 254–255. [Google Scholar] [CrossRef]
- Pavek, D.S.; Lamboy, W.F.; Garvey, E.J. Selecting in Situ Conservation Sites for Grape Genetic Resources in the USA. Genet. Resour. Crop Evol. 2003, 50, 165–173. [Google Scholar] [CrossRef]
- Hilha, A.; Andrade, C.E.L.; Burin, M.R.; Medeiros, R.F.; Almeida, M.P.; Orlandi, F.B.; Freitas, F.R.; Dalbó, M.A.; Souza, A.L.K.; May-de-Mio, L.L.; et al. Vitis Species and Varietal Resistance of Mature Berries against Inoculation of an Isolate of the Colletotrichum acutatum Complex That Causes Grape Ripe Rot. Acta Hortic. 2024, 1385, 95–102. [Google Scholar] [CrossRef]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of Leaf Anatomy in Determining Mesophyll Diffusion Conductance to CO2 across Species: Quantitative Limitations and Scaling up by Models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Fennell, J.L. Two New North American Species of Vitis. Two New North American Species of Vitis. J. Wash. Acad. Sci. 1940, 30, 15–19. [Google Scholar]
- Graham, C.D.K.; Forrestel, E.J.; Schilmiller, A.L.; Zemenick, A.T.; Weber, M.G. Evolutionary Signatures of a Trade-off in Direct and Indirect Defenses across the Wild Grape Genus, Vitis. Evol. 2023, 77, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Juárez, N.; Iménez-Fernández, V.M.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A. Phenolic Compounds and Antioxidant Activity of Wild Grape (Vitis tiliifolia). Phenolic Compounds and Antioxidant Activity of Wild Grape (Vitis tiliifolia). Ital. J. Food Sci. 2018, 30, 128–144. [Google Scholar]
- Mata-Alejandro, H.; Galindo-Tovar, M.E.; Famiani, F.; Leyva-Ovalle, O.R.; Cruz-Castillo, J.G. Environmental Conditions, and Phenolic Compounds Potential in the Leaves of Vitis tiliifolia Humb.; Bonpl. Ex Schult. Genet. Resour. Crop Evol. 2021, 68, 3435–3444. [Google Scholar] [CrossRef]
- Comeaux, B.L. A New Vitis (Vitaceae) from Veracruz, Mexico. SIDA Contrib. Bot. 1987, 12, 273–277. [Google Scholar]
- Lisek, J. Yielding and Healthiness of Selected Grape Cultivars for Processing in Central Poland. J. Fruit. Ornam. Plant Res. 2010, 37, 265–272. [Google Scholar]
- Yang, Y.; Tilman, D.; Jin, Z.; Smith, P.; Barrett, C.B.; Zhu, Y.-G.; Burney, J.; D’Odorico, P.; Fantke, P.; Fargione, J.; et al. Climate Change Exacerbates the Environmental Impacts of Agriculture. Science 2024, 385, eadn3747. [Google Scholar] [CrossRef]
- You, Y.; Yu, J.; Nie, Z.; Peng, D.; Barrett, R.L.; Rabarijaona, R.N.; Lai, Y.; Zhao, Y.; Dang, V.-C.; Chen, Y.; et al. Transition of Survival Strategies under Global Climate Shifts in the Grape Family. Nat. Plants 2024, 10, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.S.; Redden, R.; Maxted, N.; Dulloo, M.E.; Guarino, L.; Smith, P. Crop Wild Relatives and Climate Change; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pironon, S.; Etherington, T.R.; Borrell, J.S.; Kühn, N.; Macias-Fauria, M.; Ondo, I.; Tovar, C.; Wilkin, P.; Willis, K.J. Potential Adaptive Strategies for 29 Sub-Saharan Crops under Future Climate Change. Nat. Clim. Chang. 2019, 9, 758–763. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B. Back into the Wild—Apply Untapped Genetic Diversity of Wild Relatives for Crop Improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cruz, A.; Aguirre-Liguori, J.A.; Zhou, Y.; Minio, A.; Riaz, S.; Walker, A.M.; Cantu, D.; Gaut, B.S. Introgression among North American Wild Grapes (Vitis) Fuels Biotic and Abiotic Adaptation. Genome Biol. 2021, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cao, S.; Liu, Z.; Xiao, H.; Hu, J.; Xu, X.; Chen, P.; Ma, Z.; Ye, J.; Chai, L.; et al. Genomic Conservation of Crop Wild Relatives: A Case Study of Citrus. PLoS Genet. 2023, 19, e1010811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xin, H.; Fan, P.; Zhang, J.; Liu, Y.; Dong, Y.; Wang, Z.; Yang, Y.; Zhang, Q.; Ming, R.; et al. The Genome of Shanputao (Vitis amurensis) Provides a New Insight into Cold Tolerance of Grapevine. The Plant Journal 2021, 105, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Long, R.; Ma, Z.; Xiao, H.; Xu, X.; Liu, Z.; Wei, C.; Wang, Y.; Peng, Y.; Yang, X.; et al. Evolutionary Genomics of Climatic Adaptation and Resilience to Climate Change in Alfalfa. Mol. Plant 2024, 17, 867–883. [Google Scholar] [CrossRef]
- Foria, S.; Magris, G.; Jurman, I.; Schwope, R.; De Candido, M.; De Luca, E.; Ivanišević, D.; Morgante, M.; Di Gaspero, G. Extent of Wild–to–Crop Interspecific Introgression in Grapevine (Vitis Vinifera) as a Consequence of Resistance Breeding and Implications for the Crop Species Definition. Hortic. Res. 2022, 9, uhab010. [Google Scholar] [CrossRef]
- Wingerter, C.; Eisenmann, B.; Weber, P.; Dry, I.; Bogs, J. Grapevine Rpv3-, Rpv10- and Rpv12-Mediated Defense Responses against Plasmopara viticola and the Impact of Their Deployment on Fungicide Use in Viticulture. BMC Plant Biol. 2021, 21, 470. [Google Scholar] [CrossRef]
- Atak, A. New Perspectives in Grapevine (Vitis Spp.) Breeding. In Case Studies of Breeding Strategies in Major Plant Species; IntechOpen: London, UK, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).